Because this piece does not have an abstract, we have provided for your benefit the first 3 sentences of the full text.
To the Editor: We thank Dr Lozano and colleagues for their comments on our study “Recombinant Human Erythropoietin to Target Cognitive Dysfunction in Bipolar Disorder: A Double-Blind, Randomized, Placebo-Controlled Phase 2 Trial,” which give us the opportunity to address some conceptually important points regarding the effects of erythropoietin (EPO) on cognition.
Lozano et al note that it is a potential confound that patients remained on their mood-stabilizing treatment including lithium for the duration of the study for 2 reasons: first, lithium has dose-dependent procognitive effects in patients with amnestic mild cognitive impairment in doses from 150 mg to 600 mg but harmful effects on cognition at doses ≥ 1 g; second, lithium and EPO may activate similar signaling pathways and thus exert synergistic actions on neuroplasticity. Lozano et al point out that the EPO-associated improvement of cognition could have therefore been influenced by patients’ lithium treatment during or prior to the study and request (1) explicit data regarding lithium exposure for each study group and (2) analysis of whether EPO and lithium have independent cognitive effects, or whether the beneficial effects attributed to EPO could be partially due to lithium or an interaction between EPO and lithium.
See letter by Lozano et al and article by Miskowiak et al
This work may not be copied, distributed, displayed, published, reproduced, transmitted, modified, posted, sold, licensed, or used for commercial purposes. By downloading this file, you are agreeing to the publisher’s Terms & Conditions.
To the Editor: We thank Dr Lozano and colleagues for their comments on our study “Recombinant Human Erythropoietin to Target Cognitive Dysfunction in Bipolar Disorder: A Double-Blind, Randomized, Placebo-Controlled Phase 2 Trial,”1 which give us the opportunity to address some conceptually important points regarding the effects of erythropoietin (EPO) on cognition.
Lozano et al note that it is a potential confound that patients remained on their mood-stabilizing treatment including lithium for the duration of the study for 2 reasons: first, lithium has dose-dependent procognitive effects in patients with amnestic mild cognitive impairment in doses from 150 mg to 600 mg2 but harmful effects on cognition at doses ≥ 1 g; second, lithium and EPO may activate similar signaling pathways3,4 and thus exert synergistic actions on neuroplasticity. Lozano et al point out that the EPO-associated improvement of cognition could have therefore been influenced by patients’ lithium treatment during or prior to the study and request (1) explicit data regarding lithium exposure for each study group and (2) analysis of whether EPO and lithium have independent cognitive effects, or whether the beneficial effects attributed to EPO could be partially due to lithium or an interaction between EPO and lithium.
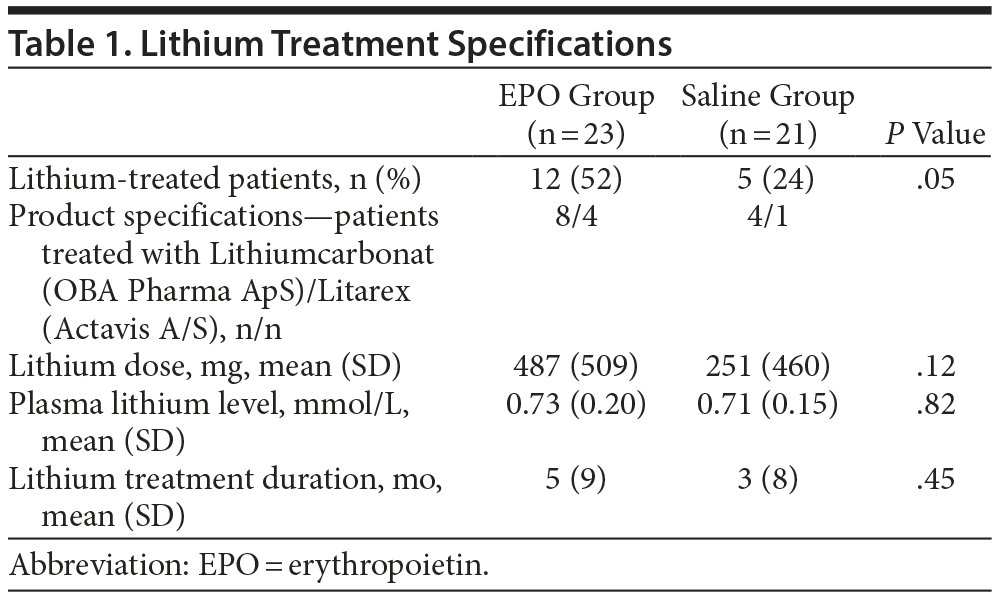
In response to point 1, we have included information about lithium treatment doses (which remained unchanged throughout the trial), duration of lithium treatment, and baseline plasma lithium levels for each study group in Table 1. As mentioned in the original article,1 there was a higher number of lithium-treated patients in the EPO vs saline groups (n = 12 vs n = 5; P = .05). However, within the lithium-treated patients (n = 17), there was no difference in the lithium dose, treatment duration, or plasma lithium level between the EPO and saline groups (P values ≥ 0.12).
In response to point 2, we have conducted the following analyses:
- We reanalyzed the effects of EPO on speed of complex cognitive processing across attention, memory, and executive function (cognitive composite score) and on sustained attention (Rapid Visual Processing [RVP] speed for correct responses and accuracy) with repeated-measures analysis of covariance (ANCOVA) including the following covariates: lithium dose (mg) and lithium treatment duration (months) in addition to mania and depression symptoms at week 9, measured with the Hamilton Depression Rating Scale 17-items (HDRS-17) and the Young Mania Rating Scale (YMRS) (for original analyses, see Miskowiak et al1). This analysis showed that the beneficial effects of EPO versus saline on cognition remained significant (cognitive composite: F1,37 = 4.96, P = .03; RVP speed: F1,34 = 9.76, P = .004), also at the week 14 follow-up assessment (cognitive composite: F1,37 = 4.59, P = .04; RVP speed: F1,34 = 7.17, P = .01; RVP accuracy: F1,34 = 7.78, P = .009). In additional analyses in which plasma lithium level was entered as a covariate in addition to HDRS-17 and YMRS scores, the effect of EPO on cognition also prevailed at both week 9 (cognitive composite: F1,35 = 4.57, P = .04; RVP speed: F1,33 = 7.52, P = .01) and week 14 (cognitive composite: F1,36 = 3.99, P = .05; RVP speed: F1,33 = 5.39, P = .03; RVP accuracy: F1,33 = 6.33, P = .02).
- We performed an additional exploratory analysis in the subgroup of patients who were not receiving lithium treatment (N = 26; EPO: n = 11, saline: n = 15), of course expecting a loss of statistical power due to the substantial reduction in numbers of patients. This subgroup analysis showed a strong trend toward cognitive improvement in EPO- versus saline-treated patients despite the reduced statistical power (cognitive composite: F1,22 = 3.58, P = .07; RVP speed: F1,20 = 2.98, P = .10).
- Finally, we assessed any potential interaction effects of lithium and EPO on cognition using 2-way ANCOVA with group (EPO/saline) and lithium treatment (yes/no) as fixed factors, cognitive change from baseline to week 9 as the dependent variable, and HDRS-17 and YMRS scores at week 9 as covariates. This analysis revealed no significant 2-way interactions between group and lithium treatment on cognitive change (cognitive composite: F1,37 = 0.24, P = .63; RVP speed: F1,34 = 0.33, P=.57).
In conclusion, the beneficial effects of EPO on cognition in bipolar disorder demonstrated by Miskowiak et al1 were independent of lithium treatment and cannot be attributed to lithium or to an interaction between EPO and lithium.
References
1. Miskowiak KW, Ehrenreich H, Christensen EM, et al. Recombinant human erythropoietin to target cognitive dysfunction in bipolar disorder: a double-blind, randomized, placebo-controlled phase 2 trial. J Clin Psychiatry. 2014;75(12):1347-1355. PubMed doi:10.4088/JCP.13m08839
2. Nunes MA, Viel TA, Buck HS. Microdose lithium treatment stabilized cognitive impairment in patients with Alzheimer’s disease. Curr Alzheimer Res. 2013;10(1):104-107. PubMed
3. Chiu CT, Scheuing L, Liu G, et al. The mood stabilizer lithium potentiates the antidepressant-like effects and ameliorates oxidative stress induced by acute ketamine in a mouse model of stress [published online ahead of print December 28, 2014]. Int J Neuropsychopharmacol. PubMed doi:10.1093/ijnp/pyu102
4. Xiong Y, Mahmood A, Meng Y, et al. Delayed administration of erythropoietin reducing hippocampal cell loss, enhancing angiogenesis and neurogenesis, and improving functional outcome following traumatic brain injury in rats: comparison of treatment with single and triple dose. J Neurosurg. 2010;113(3):598-608. PubMed doi:10.3171/2009.9.JNS09844
Author affiliations: Department of Psychiatry, Copenhagen Psychiatric Centre, Copenhagen University Hospital, Rigshospitalet, Copenhagen, Denmark (Drs Miskowiak, Kessing, and Vinberg); and Department of Clinical Neuroscience, Max Planck Institute of Experimental Medicine, Göttingen, Germany (Dr Ehrenreich).
Potential conflicts of interest: Dr Miskowiak has received consultancy fees from Lundbeck. Dr Ehrenreich has submitted and holds user patents for erythropoietin in stroke, schizophrenia, and multiple sclerosis. Within the last 3 years, Dr Kessing has been a consultant for Lundbeck, AstraZeneca, and Servier. Dr Vinberg has been a consultant to Eli Lilly, Lundbeck, Servier, and AstraZeneca.
Funding/support: The study discussed in this letter [J Clin Psychiatry 2014;75(12):1347-1355] was funded by the Danish Ministry of Science, Innovation, and Higher Education; Novo Nordisk Foundation; Beckett Foundation; and Savvצrksejer Juhl’s Mindefond. The Lundbeck Foundation has provided half of Dr Miskowiak’s postdoctorate salary (2012-2015) for her to do full-time research in this period. The sponsors had no role in the planning or conduct of the study or in the interpretation of the results.
J Clin Psychiatry 2015;76(6):e835-e836 (doi:10.4088/JCP.15lr09809a).
© Copyright 2015 Physicians Postgraduate Press, Inc.
Save
Cite
Advertisement
GAM ID: sidebar-top