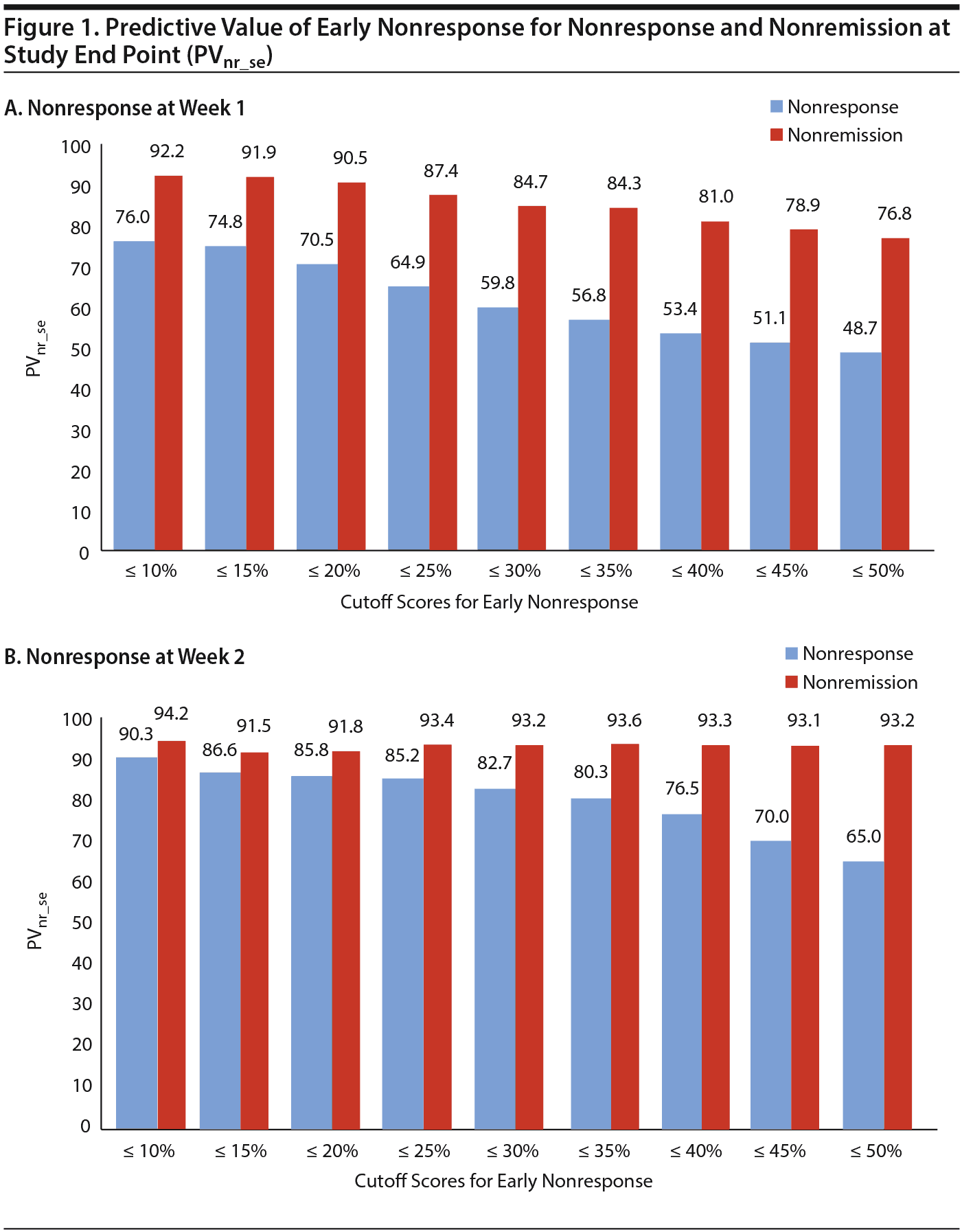Objective: To investigate whether early nonresponse to antipsychotic treatment of acute mania predicts treatment failure and, if so, to establish the best definition or criterion of an early nonresponse.
Data Sources: Short-term efficacy studies assessing antipsychotics that were submitted to the Dutch Medicines Evaluation Board during an 11-year period as part of the marketing authorization application for the indication of acute manic episode of bipolar disorder. Pharmaceutical companies provided their raw patient data, which enabled us to perform an individual patient data meta-analysis.
Study Selection: All double-blind, randomized, placebo-controlled trials assessing the efficacy of antipsychotics for acute manic episode of bipolar disorder were included (10 trials).
Data Extraction: All patients with data available for completer analysis (N = 1,243), symptom severity scores on the Young Mania Rating Scale (YMRS) at weeks 0, 1, and 2 and at study end point (week 3 or 4).
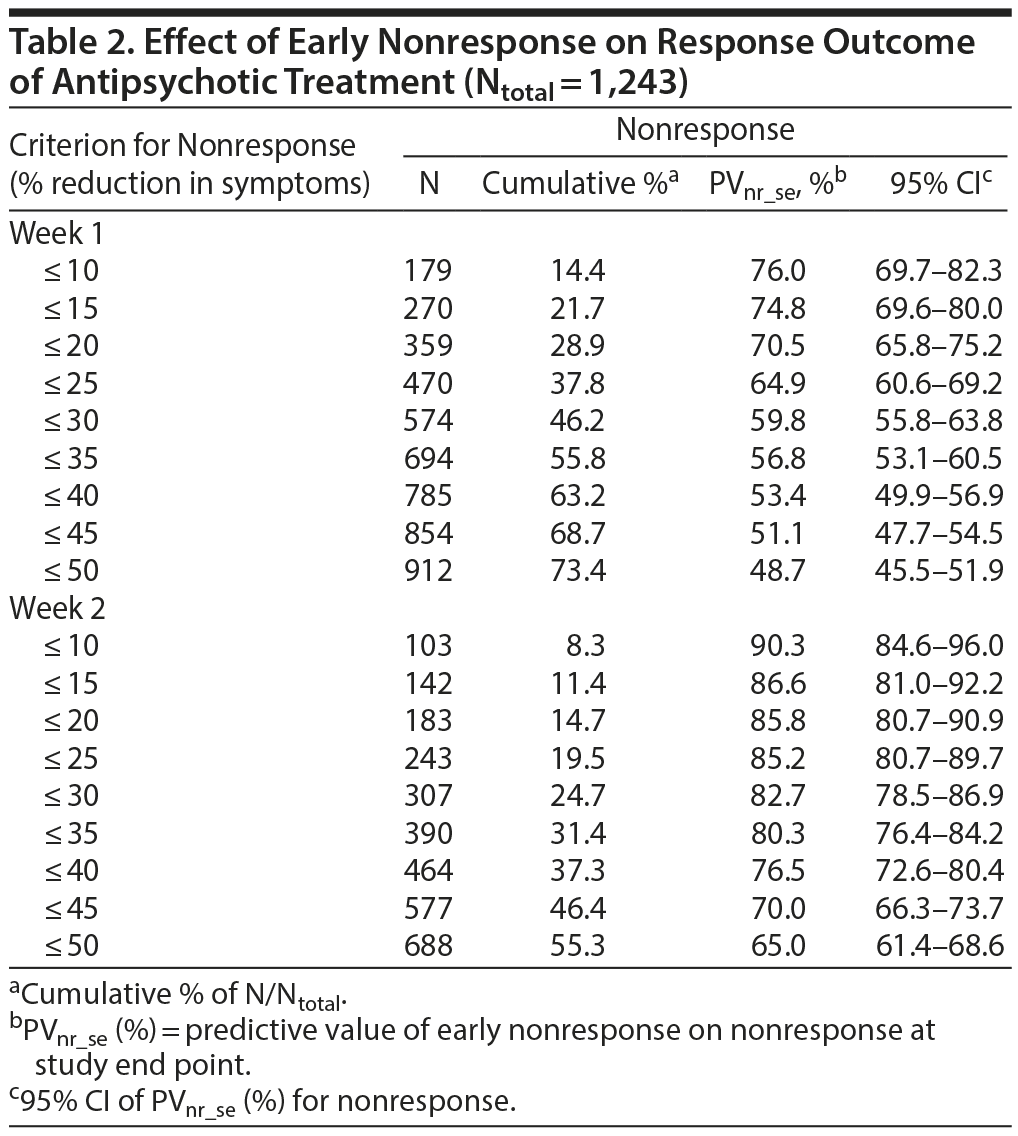
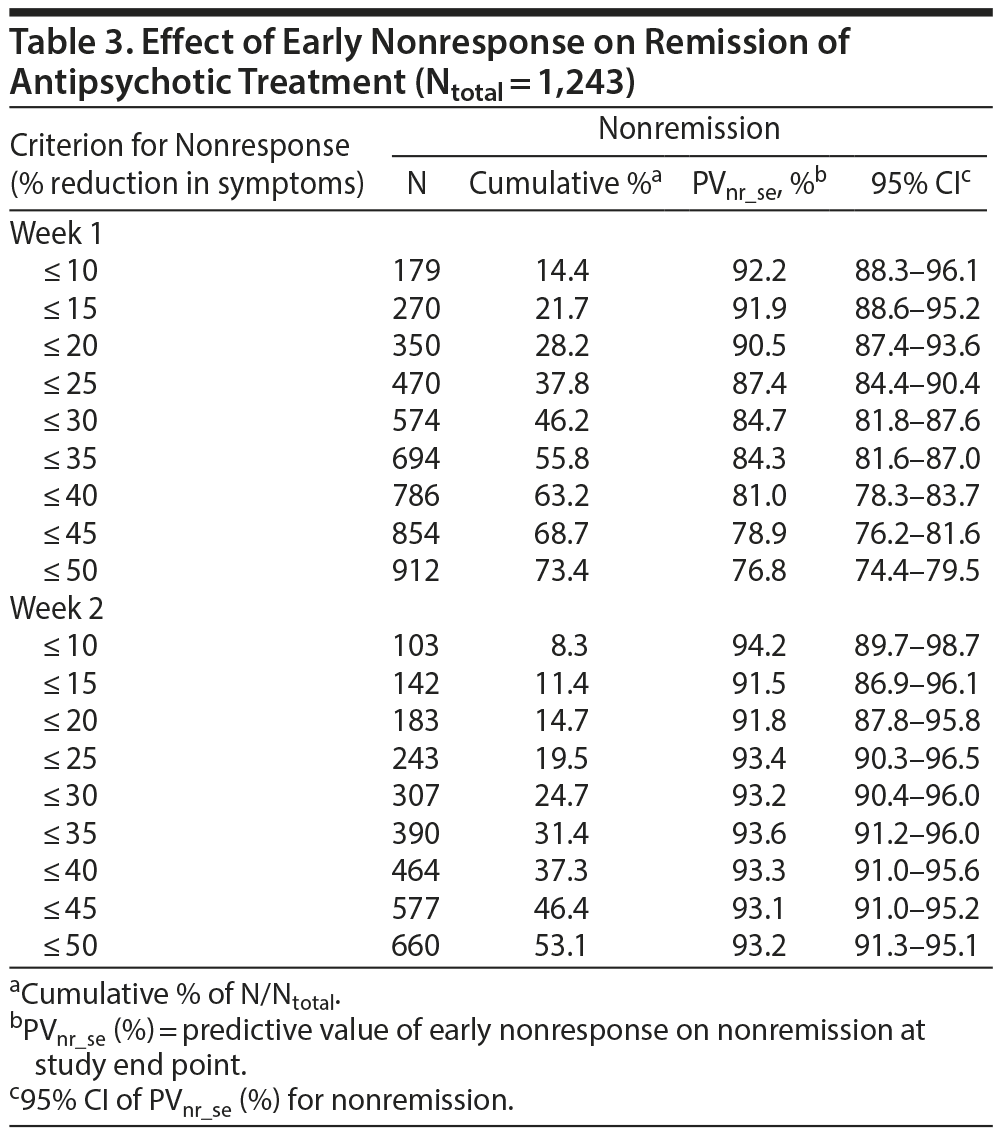
Results: The a priori chances of nonresponse and nonremission at study end point were 40.9% (95% CI, 38.2%-43.6%) and 65.3% (95% CI, 62.0%-68.6%), respectively. Early nonresponse in weeks 1 and 2, defined by cutoff scores ranging from a ≤ 10% to a ≤ 50% reduction in symptoms compared to baseline on the YMRS, significantly predicted nonresponse (≤ 0% symptom reduction) and nonremission (YMRS score higher than 8) in week 3. The predictive value of early nonresponse (PVnr_se) at week 1 for both nonresponse and nonremission at study end point declined linearly with increasing cutoff scores of early nonresponse; nonresponse: 76.0% (95% CI, 69.7%-82.3%) for a ≤ 10% response to 48.7% (95% CI, 45.5%-51.9%) for a ≤ 50% response; nonremission: 92.2% (95% CI, 88.3%-96.1%) for a ≤ 10% response to 76.8% (95% CI, 74.4%-79.5%) for a ≤ 50% response. A similar linear decline was observed for increasing cutoff scores of early nonresponse at week 2 for nonresponse, but not for nonremission at end point: nonresponse 90.3% (95% CI, 84.6%-96.0%) for a ≤ 10% response to 65.0% (95% CI, 61.4%-68.6%) for a ≤ 50% response; nonremission: 94.2% (95% CI, 89.7%-98.7%) for a ≤ 10% response and 93.2% (95% CI, 93.1%-95.1%) for a ≤ 50% response. Specific antipsychotic characteristics did not modify these findings at either time point (week 1: P = .127; week 2: P = .213).
Conclusions: When patients fail to respond early (1-2 weeks) after the initiation of antipsychotic treatment for acute mania, clinicians should reconsider their treatment choice using a 2-stage strategy.

Early Nonresponse in the Antipsychotic Treatment of Acute Mania: A Criterion for Reconsidering Treatment?
Results From an Individual Patient Data Meta-Analysis

ABSTRACT
Objective: To investigate whether early nonresponse to antipsychotic treatment of acute mania predicts treatment failure and, if so, to establish the best definition or criterion of an early nonresponse.
Data Sources: Short-term efficacy studies assessing antipsychotics that were submitted to the Dutch Medicines Evaluation Board during an 11-year period as part of the marketing authorization application for the indication of acute manic episode of bipolar disorder. Pharmaceutical companies provided their raw patient data, which enabled us to perform an individual patient data meta-analysis.
Study Selection: All double-blind, randomized, placebo-controlled trials assessing the efficacy of antipsychotics for acute manic episode of bipolar disorder were included (10 trials).
Data Extraction: All patients with data available for completer analysis (N = 1,243), symptom severity scores on the Young Mania Rating Scale (YMRS) at weeks 0, 1, and 2 and at study end point (week 3 or 4).
Results: The a priori chances of nonresponse and nonremission at study end point were 40.9% (95% CI, 38.2%-43.6%) and 65.3% (95% CI, 62.0%-68.6%), respectively. Early nonresponse in weeks 1 and 2, defined by cutoff scores ranging from a ≤ 10% to a ≤ 50% reduction in symptoms compared to baseline on the YMRS, significantly predicted nonresponse (≤ 0% symptom reduction) and nonremission (YMRS score higher than 8) in week 3. The predictive value of early nonresponse (PVnr_se) at week 1 for both nonresponse and nonremission at study end point declined linearly with increasing cutoff scores of early nonresponse; nonresponse: 76.0% (95% CI, 69.7%-82.3%) for a ≤ 10% response to 48.7% (95% CI, 45.5%-51.9%) for a ≤ 50% response; nonremission: 92.2% (95% CI, 88.3%-96.1%) for a ≤ 10% response to 76.8% (95% CI, 74.4%-79.5%) for a ≤ 50% response. A similar linear decline was observed for increasing cutoff scores of early nonresponse at week 2 for nonresponse, but not for nonremission at end point: nonresponse 90.3% (95% CI, 84.6%-96.0%) for a ≤ 10% response to 65.0% (95% CI, 61.4%-68.6%) for a ≤ 50% response; nonremission: 94.2% (95% CI, 89.7%-98.7%) for a ≤ 10% response and 93.2% (95% CI, 93.1%-95.1%) for a ≤ 50% response. Specific antipsychotic characteristics did not modify these findings at either time point (week 1: P = .127; week 2: P = .213).
Conclusions: When patients fail to respond early (1-2 weeks) after the initiation of antipsychotic treatment for acute mania, clinicians should reconsider their treatment choice using a 2-stage strategy.
J Clin Psychiatry 2016;77(9):e1117-e1123
dx.doi.org/10.4088/JCP.15r10051
© Copyright 2016 Physicians Postgraduate Press, Inc.
aDepartment of Psychiatry, Academic Medical Centre, University of Amsterdam, Amsterdam, the Netherlands
bMedicines Evaluation Board, Utrecht, the Netherlands
cNetherlands Institute for Neuroscience, Royal Netherlands Academy of Arts and Sciences, Amsterdam
*Corresponding author: Carlijn C. M. Welten, MD, Department of Psychiatry, Academic Medical Centre, Room PA 2-179, Meibergdreef 9, 1105AZ, Amsterdam, the Netherlands ([email protected]).
Acute manic episodes of bipolar disorder cause patients severe emotional turmoil. These episodes may result in excess morbidity and even mortality.1-4 The aim of acute treatment is to rapidly reduce symptom severity in an attempt to counteract these risks. Contrary to what was earlier believed, first-5-7 and second-generation8-10 antipsychotics have an antimanic effect soon after treatment initiation.11 This response profile makes it important to establish whether patients who do not have an early response ultimately respond to treatment. This issue has been investigated in small studies of 112 or 2 antipsychotic agents.13,14 In a pooled post hoc analysis, Szegedi et al14 state that lack of improvement in the first week of treatment was significantly associated with a lack of improvement at study end point. Kemp et al12 also found that manic patients treated with an antipsychotic without sufficient improvement (< 25% symptom reduction) at week 1 were less likely to reach response or remission by week 3. Similar findings have been reported in patients treated with an antidepressant for bipolar depression or major depressive disorder (MDD).15-18 With regard to bipolar depression, Kemp et al15 found that the absence of early improvement was a highly reliable predictor of eventual nonresponse. For MDD, Nierenberg and colleagues18 found in 1995 that absence of early improvement after 3 weeks of antidepressant treatment reliably predicted nonresponse and nonremission at study end point. This result was recently confirmed by Kudlow et al,19 who found a high negative predictive value of nonresponse to antidepressants at weeks 2-4 for treatment success at end point.
Due to limited knowledge about the early response (or lack thereof) to antipsychotics, current guidelines do not provide consensus about how and when to determine whether treatment for acute mania is effective.20 Most guidelines propose a period of 2 weeks in which to decide whether to interrupt, switch, or continue current medication,20,21 although a 1-week period has also been found to be long enough to assess whether a patient responds to antipsychotic treatment.12,14 Surprisingly, the National Institute for Health and Care Excellence (NICE) guideline on bipolar disorder does not propose a time period at all.22 To increase our knowledge on how to predict whether patients with acute mania will respond to treatment, our aims were to investigate whether an early nonresponse to antipsychotic treatment is predictive of a later lack of response or remission and, if so, which criterion of an early nonresponse is the best predictor of later treatment failure.
METHODS
Selection of Studies
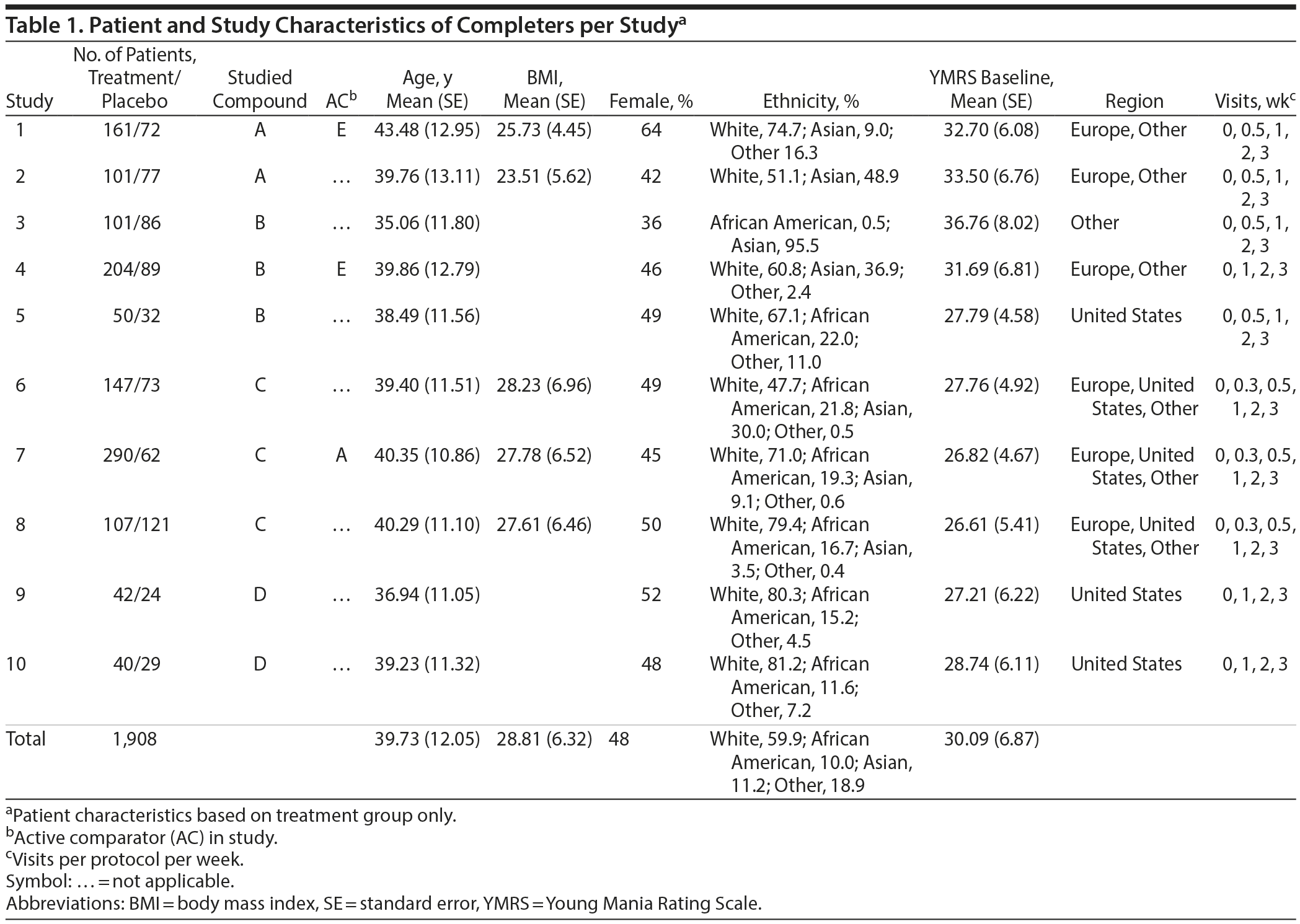
We included all short-term efficacy studies assessing antipsychotics that were submitted to the Dutch Medicines Evaluation Board during an 11-year period as part of the marketing authorization application for the indication of acute manic episode of bipolar disorder. All studies were double-blind, randomized, placebo-controlled trials involving patients with a DSM-IV-diagnosed acute manic episode of bipolar disorder. Pharmaceutical companies provided their raw patient data, which enabled us to perform an individual patient data meta-analysis.
The studies investigated 5 different antipsychotics; active antipsychotic comparators were included and analyzed as treatment. To protect the interests of participating companies, no drug names are mentioned. We restricted the analyses to the data of patients who were prescribed medication in an effective dose according to the Summary of Product Characteristics (SmPC) if the drug had been granted a license for the treatment of an acute manic episode; if the drug had not been granted a license for this indication, expert consensus was established on the effective dose, mainly based on the doses mentioned in the SmPC for related disorders.
Assessments
The Young Mania Rating Scale (YMRS), an interview-based questionnaire, was used to assess the severity of the acute manic episode of bipolar disorder. The YMRS comprises 11 items: 7 items scored on a 0-4 scale and 4 items scored on a 0-8 scale. Total scores range from 0 (no symptoms) to 60 (most severe symptoms).23

- Although antipsychotics are the first-line treatment for an acute manic episode of bipolar disorder, success rates are not very high.
- Nonresponse to antipsychotic treatment after 1 or 2 weeks is a strong predictor of nonresponse and nonremission after 3 to 4 weeks.
- Therefore, existing antipsychotic treatments of acute mania should be seriously reconsidered in the case of nonresponse after 1 or 2 weeks.
To identify the best definition of early nonresponse and when to assess it, we used different cutoff scores for nonresponse (≤ 10%, ≤ 15%, ≤ 20%, ≤ 25%, ≤ 30%, ≤ 35%, ≤ 40%, ≤ 45%, and ≤ 50% reduction in the YMRS score compared to baseline) measured at weeks 1 and 2 after treatment initiation and calculated whether these scores were predictive of subsequent treatment nonresponse (defined as ≤ 50% reduction in YMRS score at study end point compared with baseline) or nonremission (YMRS score > 8 at end point). The definitions of response and remission are based on the Task Force Report of the International Society for Bipolar Disorders (ISBD), because there is no consensus about these terms in the European Medicines Agency (EMA) Committee for Proprietary Medicinal Products (CPMP) guideline on the clinical investigation of medicinal products for the treatment and prevention of bipolar disorder.24,25 The study end point was 3 weeks after the baseline measurement. This is the time point recommended by the CPMP guideline for investigating the short-term efficacy of drugs for use in the acute manic episodes of bipolar disorder.24 If outcome data at week 3 were missing, we used data at week 4, if available.
Statistical Analysis
Analyses were restricted to the treatment groups, since our study objective was to determine the predictive value of early nonresponse to the antipsychotic treatment of acute mania. Results of similar analyses from the placebo groups are presented briefly in Supplementary eTable 1. Two different analyses were considered: (1) an intention-to-treat (ITT) analysis including all patients with data available at least for week 0 and study end point (N = 1,264) and (2) a completer analysis including only patients with data available for week 0, week 1, week 2, and study end point (N = 1,243). Because the difference between these 2 study groups was only 21 patients, we decided to provide data for the completer analysis (N = 1,243).
To answer the first research question “Is an early nonresponse to antipsychotic treatment predictive of a later lack of response or remission?” we used individual patient data to calculate treatment response at 1 and 2 weeks. To identify the most adequate criterion of early nonresponse, we calculated the predictive value for treatment failure (PVnr_se) of each cutoff score for early nonresponse. The PVnr_se was defined as the probability that early nonresponders become nonresponders or nonremitters at study end point, ie, PV (nonresponse or nonremission at end point / early nonresponse).
To assess the potential effect of differences in antipsychotics on response and remission rates, we performed 2 multilevel mixed-effects logistic regression analyses with a random intercept for study and performed a likelihood ratio test to investigate the effect of specific antipsychotics on response and remission.
RESULTS
Study Characteristics
Data were analyzed from 10 studies involving 2,666 patients, of which 1,908 patients met criteria per the completer analyses. Of these patients, 1,243 were treated with antipsychotics. Their mean (SE) age was 39.79 (12.11) years, mean (SE) body mass index (BMI) was 26.81 (6.08), and mean (SE) severity score at baseline was 30.02 (6.62); 47% of patients were women. The included patients were 59.9% white, 10.0% African American, and 11.2% Asian, and 18.9% patients were another ethnic background (Table 1).
Effect of Early Nonresponse Cutoff Scores on Treatment Failure
The a priori chances of nonresponse and nonremission were 40.9% (95% CI, 38.2%-43.6%) and 65.3% (95% CI, 62.0%-68.6%), respectively. Table 2 and Figure 1A and 1B show that the predictive value of early nonresponse at weeks 1 and 2 for treatment nonresponse at end point (PVnr_se) decreased linearly with increasing cutoff score for early nonresponse at week 1 and week 2. Table 3 and Figure 1A and 1B show that a similar linear decreasing pattern was observed for the prediction of nonremission by increasing early nonresponse cutoffs at week 1, whereas early nonresponse cutoffs at week 2 were not related to nonremission at end point due to the overall very high nonremission rates (ceiling effect). More patients at week 1 than at week 2 met the definition of nonresponse/nonremission at end point, regardless of the cutoff score used, whereas the predictive value of nonresponse at week 1 for treatment failure was lower than that at week 2 (Tables 2 and 3).
For example, in week 1, early nonresponse defined as a ≤ 10% reduction in YMRS score resulted in a PVnr_se of 76.0% (95% CI, 69.7%-82.3%; N = 179) for nonresponse, whereas early nonresponse defined as a ≤ 25% reduction in YMRS score resulted in a PVnr_se of 64.9% (95% CI, 60.6%-69.2%; N = 470), and nonresponse defined as a ≤ 50% reduction in YMRS score resulted in a PVnr_se of 48.7% (95% CI, 45.5%-51.9%; N = 912) (Table 2). Similarly, for nonremission at end point, week 1 early nonresponse defined as a ≤ 10% reduction in YMRS score resulted in a PVnr_se of 92.2% (95% CI, 88.3%-96.1%; N = 179), whereas early nonresponse defined as a ≤ 25% reduction in YMRS score resulted in a PVnr_se of 87.4% (95% CI, 84.4%-90.4%; N = 470), and nonresponse defined as a ≤ 50% reduction in YMRS score resulted in a PVnr_se of 76.8% (95% CI, 74.4%-79.5%; N = 912). Contrary, in week 2, the PVnr_se was 94.2% (95% CI, 89.7%-98.7%; N = 103) for early nonresponse defined as a ≤ 10% reduction in YMRS score, 93.4% (95% CI, 90.3%-96.5%; N = 243) for early nonresponse defined as a ≤ 25% reduction in YMRS score, and 93.2% (95% CI, 91.3%-95.1%; N = 660) for early nonresponse defined as a ≤ 50% reduction in YMRS score (Table 3). The positive predictive values for early response at week 1 and week 2 and the positive and negative predictive values for the placebo group are presented in Supplementary eTables 1 and 2, respectively.
Optimal Combination of Cutoff Score for Early Nonresponse and Time Point
The most adequate criterion for early nonresponse could not be determined because of the linear relationship between cutoff score and PVnr_se and the linear relationship between the cutoff score and the number of patients who met criteria for nonresponse or nonremission (Figure 1A and 1B). To illustrate the potential effect of a change in treatment strategy based on early nonresponse, we provide a brief example, defining early nonresponse as a ≤ 25% reduction in YMRS score in week 1 and a ≤ 50% reduction in YMRS score in week 2 for nonresponse at study end point. These cutoff scores have been used in previous studies.12-14
The a priori chance of nonresponse at study end point was 40.9%. At week 1, a nonresponder (≤ 25% reduction in YMRS score) had a 64.9% chance of treatment failure, resulting in an increase in the PVnr_se of 24.0%. A clinician can now decide to change the existing treatment (eg, dose elevation, adjuvant medication) or switch to another medication. If the patient is switched to another antipsychotic, he or she again has an a priori chance of treatment failure of 40.9%. Overall, when switched to an alternative antipsychotic treatment, this patient now has “only” a 40.9 ×— 40.9 = 16.7% chance of treatment failure. Patients who do not meet the definition for nonresponse should be reevaluated in week 2. If the criterion for nonresponse (≤ 50%) is met in week 2, there again is a 64.9% chance of nonresponse at study end point.
Effect of Type of Antipsychotic
Potential differences in the type of antipsychotic used did not affect our findings. When nonresponse in week 1 (defined as ≤ 25% reduction in YMRS score) was entered in a multilevel mixed-effects logistic regression analyses with a random intercept for study, the likelihood ratio test (LRχ28) = 12.60, P = .127. For nonresponse in week 2 (≤ 50% reduction in YMRS score), LRχ28 = 10.80, P = .213.
DISCUSSION
The aim of this study was to investigate whether a lack of response to antipsychotic treatment after 1 or 2 weeks predicts future treatment failure and to determine the optimal criterion (cutoff score to define nonresponse, measured at 1 or 2 weeks) for prompting reevaluation of treatment choices. We found early nonresponse at weeks 1 and 2, defined as a ≤ 10% to a ≤ 50% reduction in symptom scores from baseline, respectively, to be a significant and clinically relevant predictor of treatment failure in terms of both nonresponse and nonremission. At both 1 and 2 weeks, the predictive value for treatment failure (PVnr_se) and the number of patients meeting criteria for nonresponse were linearly related to the cutoff score. A higher cutoff score (eg, ≤ 50% vs ≤ 10% reduction in symptom score) resulted in a lower PVnr_se and an increased number of patients meeting criteria for nonresponse (weeks 1 and 2) and nonremission (week 1). Because of this linear relationship, we could not determine the best combination of criteria (cutoff score and evaluation in week 1 or 2) for prompting treatment reconsideration. However, our data do suggest that if patients fail to improve after 1 or 2 weeks of antipsychotic treatment for acute manic episodes, clinicians should reconsider a change in treatment strategy.
Although this may not seem surprising, this finding may have important clinical implications and could be helpful for clinicians when deciding on treatment strategy; waiting for a treatment response might not be the best strategy, especially in outpatients and patients with serious self-defeating behaviors (eg, money spending, promiscuity) or lack of support from their social network. However, the question remains, How long should one wait to change treatment strategy? Two considerations are important: (1) A very poor response in week 1 (eg, ≤ 10% symptom reduction) increases the chance of nonresponse at end point from 41% to 76% and may call for an immediate change in treatment strategy, whereas a weak response in week 1 (≤ 50%) only increases the chance of nonresponse at end point from 41% to 49%, and, thus, an immediate change in treatment strategy is not needed; and (2) potential nonresponders can still be identified in week 2 with very high predictive power. Therefore, a 2-stage follow-up strategy, with monitoring of illness severity in weeks 1 and 2 after treatment initiation is recommended in patients with acute mania. For remission, these data look rather different; a very poor response in week 1 increases the chance of nonremission from 65% to 92%, whereas a weak response increases the chance of nonremission to 77%. One may conclude either that any nonresponse calls for immediate change in treatment strategy or that remission in such a short term is not a realistic treatment goal. We feel that the latter conclusion is clinically more accurate.
Our findings are in line with those of earlier studies on early nonresponse and the prediction of outcome in acute mania. For example, Ketter et al13 found a significant early treatment response with ziprasidone at day 4 after treatment initiation. Subsequently, Kemp et al12 and Szegedi et al14 studied 2 different antipsychotics and found that, at week 1 (≤ 25% symptom reduction), the negative predictive values for nonresponse were 75% in Kemp et al12 and 67% and 80% in Szegedi et al,14 whereas the negative predictive values for nonremission at week 2 were 95% and 85% and 76%, respectively. These findings are very similar to our findings of the negative predictive values for early nonresponse (≤ 25% symptom reduction) for nonresponse (65%) and for nonremission (87%).
One of the strengths of our study is that we conducted an individual patient data meta-analysis of 5 different antipsychotic drugs and were able to include 1,243 patients for complete case analyses. We now know that the type of drug does not modify the effect of early nonresponse on treatment success or failure, and, thus, findings can be generalized to all antipsychotics. Furthermore, contrary to Szegedi and colleagues,14 we were also able to assess early nonresponse rates in week 2 and therefore could assess the most parsimonious time frame (1 or 2 weeks) to reconsider antipsychotic treatment. Considering these strengths, our findings could help to promote agreement in the psychiatric field about the recommendation of the most adequate time frame to reconsider antipsychotic treatment in patients with acute mania.20 Most guidelines (eg, the guideline of the World Federation of Societies of Biological Psychiatry [WFSBP] and the Dutch Guideline for Bipolar Disorder) now recommend a time frame of 2 weeks,21,26 although 1 recent guideline does not make any recommendation at all (NICE guideline).22 In contrast, most of the empirical literature recommend 1 week.12-14 We recommend a 2-stage strategy, taking into account the level of nonresponse in weeks 1 and 2 and the need to achieve a rapid improvement in patients’ symptoms, given the nature of the manic behavior and the presence or absence of a support network.
This study had some limitations. The first limitation is that our study end point at 3 or 4 weeks could have led to an overestimation of nonresponse and nonremission rates, since some patients may take longer to achieve symptom reduction or remission.27 With a priori chances of nonresponse and nonremission at study end point of 40.9% and 65.3%, respectively, one could argue that treatment for 3 weeks may be too short to assess efficacy and that longer study periods are needed to test the efficacy of treatments for an acute manic episode. However, our end point is in accordance with the EMA CPMP guideline on the clinical investigation of medicinal products for the treatment and prevention of bipolar disorder.24 Nevertheless, we think that future studies are needed to predict nonresponse and nonremission at week 6, week 8, or even later. Second, our study was a post hoc analysis of existing randomized controlled trials, suggesting that early nonresponse can be used to predict nonresponse and nonremission at end point and that early nonresponse could be used for timely switches in treatment strategy. However, future prospective studies using the switching recommendations are needed to test the predictive validity of these recommendations in clinical practice. Third, it has to be emphasized that our findings were based only on efficacy results and did not take into account tolerability, patient compliance, and/or patient preference.28 Unfortunately, we had no data on these variables. However, we do not think that these potential reasons for early nonresponse would change our conclusions with regard to the right time to consider switching to another treatment strategy.
Lastly, if patients do not respond to antipsychotic treatment, clinicians should consider changing the drug used. The next questions are, Which changes are likely to be most effective in these patients? and How should the changes be implemented? There is little evidence with regard to resistance to treatment for episodes of acute mania, and although switching antipsychotic medication in patients with acute mania is common clinical practice, little is known about the best switching strategy (eg, abrupt switch, cross-taper switch, and plateau cross-taper switch).29-31
In conclusion, early nonresponse to antipsychotic treatment is a clinically relevant predictor of treatment outcome and could help clinicians to decide whether their pharmacologic treatment strategy for acute mania should be changed. If a patient fails to improve in the first 2 weeks of treatment, waiting for treatment to become effective may not be the right option. We advise reconsidering treatment options before week 3, using a 2-stage strategy that takes into account the level of nonresponse in weeks 1 and 2 and the need to achieve a rapid improvement in symptoms given the nature of manic behavior and the presence or absence of a support network. However, future prospective studies using these switching recommendations are needed to test the predictive validity of these recommendations in clinical practice.
Submitted: April 15, 2015; accepted September 21, 2015.
Drug names: ziprasidone (Geodon and others).
Potential conflicts of interest: Dr van den Brink has received honoraria from Lundbeck, Merck Serono, Schering-Plough, Reckitt Benckiser, Pfizer, and Eli Lilly; speakers fees from Lundbeck; investigator-initiated industry grants from Alkermes, Neurotech, and Eli Lilly; is a consultant to Lundbeck, Merck Serono, Schering-Plough, and Teva; and has performed paid expert testimony for Schering-Plough. Dr Leufkens has received unrestricted research funding from the Netherlands Organisation for Health Research and Development (ZonMw), the private-public funded Top Institute Pharma (which includes co-funding from universities, government, and industry), Innovative Medicines Initiative, the EU 7th Framework Program (FP7), the Dutch Medicines Evaluation Board, the Dutch National Health Care Institute, and the Dutch Ministry of Health. Dr Denys is a member of the advisory board of Lundbeck and receives occasional consultant fees from Medtronic for educational purposes. Drs Welten, Koeter, Wohlfarth, Storosum, and Gispen-de Wied have nothing to declare.
Funding/support: No funding was received for this study; the pharmaceutical companies of the studies submitted to the Dutch Medicines Evaluation Board (CBG-MEB) provided raw individual patient data.
Previous presentation: The data were presented at the Wetenschapsdag of the MEB, February 6, 2015, Utrecht, the Netherlands.
Acknowledgments: We thank Dr Armand Voorschuur at Nefarma, the association for innovative medicines in the Netherlands, for facilitation of the current collaboration. We also thank the pharmaceutical companies AstraZeneca, Eli Lilly, and Johnson & Johnson for making their raw individual patient data available for secondary analyses.
Supplementary material: See accompanying pages.
REFERENCES
1. Black DW, Winokur G, Hulbert J, et al. Predictors of immediate response in the treatment of mania: the importance of comorbidity. Biol Psychiatry. 1988;24(2):191-198. PubMed doi:10.1016/0006-3223(88)90274-0
2. Andrade C. The risk of harm in mania and the very early time course of improvement: important but neglected variables in treatment research. Bipolar Disord. 2004;6(5):446-447. PubMed doi:10.1111/j.1399-5618.2004.00141.x
3. Clements C, Morriss R, Jones S, et al. Suicide in bipolar disorder in a national English sample, 1996-2009: frequency, trends and characteristics. Psychol Med. 2013;43(12):2593-2602. PubMed doi:10.1017/S0033291713000329
4. Khan A, Faucett J, Morrison S, et al. Comparative mortality risk in adult patients with schizophrenia, depression, bipolar disorder, anxiety disorders, and attention-deficit/hyperactivity disorder participating in psychopharmacology clinical trials. JAMA Psychiatry. 2013;70(10):1091-1099. PubMed doi:10.1001/jamapsychiatry.2013.149
5. Garfinkel PE, Stancer HC, Persad E. A comparison of haloperidol, lithium carbonate and their combination in the treatment of mania. J Affect Disord. 1980;2(4):279-288. PubMed doi:10.1016/0165-0327(80)90029-4
6. Brown D, Silverstone T, Cookson J. Carbamazepine compared to haloperidol in acute mania. Int Clin Psychopharmacol. 1989;4(3):229-238. PubMed doi:10.1097/00004850-198907000-00005
7. Segal J, Berk M, Brook S. Risperidone compared with both lithium and haloperidol in mania: a double-blind randomized controlled trial. Clin Neuropharmacol. 1998;21(3):176-180. PubMed
8. Tohen M, Sanger TM, McElroy SL, et al; Olanzapine HGEH Study Group. Olanzapine versus placebo in the treatment of acute mania. Am J Psychiatry. 1999;156(5):702-709. PubMed
9. Potkin SG, Keck PE Jr, Segal S, et al. Ziprasidone in acute bipolar mania: a 21-day randomized, double-blind, placebo-controlled replication trial. J Clin Psychopharmacol. 2005;25(4):301-310. PubMed doi:10.1097/01.jcp.0000169068.34322.70
10. Keck PE Jr, Versiani M, Potkin S, et al; Ziprasidone in Mania Study Group. Ziprasidone in the treatment of acute bipolar mania: a three-week, placebo-controlled, double-blind, randomized trial. Am J Psychiatry. 2003;160(4):741-748. PubMed doi:10.1176/appi.ajp.160.4.741
11. Tohen M, Jacobs TG, Feldman PD. Onset of action of antipsychotics in the treatment of mania. Bipolar Disord. 2000;2(3 pt 2):261-268. PubMed doi:10.1034/j.1399-5618.2000.20307.x
12. Kemp DE, Johnson E, Wang WV, et al. Clinical utility of early improvement to predict response or remission in acute mania: focus on olanzapine and risperidone. J Clin Psychiatry. 2011;72(9):1236-1241. PubMed doi:10.4088/JCP.09m05874yel
13. Ketter TA, Agid O, Kapur S, et al. Rapid antipsychotic response with ziprasidone predicts subsequent acute manic/mixed episode remission. J Psychiatr Res. 2010;44(1):8-14. PubMed doi:10.1016/j.jpsychires.2009.07.006
14. Szegedi A, Zhao J, McIntyre RS. Early improvement as a predictor of acute treatment outcome in manic or mixed episodes in bipolar-I disorder: a pooled, post hoc analysis from the asenapine development program. J Affect Disord. 2013;150(3):745-752. PubMed doi:10.1016/j.jad.2013.01.024
15. Kemp DE, Ganocy SJ, Brecher M, et al. Clinical value of early partial symptomatic improvement in the prediction of response and remission during short-term treatment trials in 3369 subjects with bipolar I or II depression. J Affect Disord. 2011;130:171-179. doi:10.1016/j.jad.2010.10.026.
16. Nierenberg AA, McLean NE, Alpert JE, et al. Early nonresponse to fluoxetine as a predictor of poor 8-week outcome. Am J Psychiatry. 1995;152(10):1500-1503. doi:10.1176/ajp.152.10.1500 PubMed.
17. Kudlow PA, McIntyre RS, Lam RW. Early switching strategies in antidepressant non-responders: current evidence and future research directions. CNS Drugs. 2014;28(7):601-609. doi:10.1007/s40263-014-0171-5 PubMed
18. Nierenberg AA, McLean NE, Alpert JE, et al. Early nonresponse to fluoxetine as a predictor of poor 8-week outcome. Am J Psychiatry. 1995;152(10):1500-1503. PubMed doi:10.1176/ajp.152.10.1500
19. Kudlow PA, McIntyre RS, Lam RW. Early switching strategies in antidepressant non-responders: current evidence and future research directions. CNS Drugs. 2014;28(7):601-609. PubMed doi:10.1007/s40263-014-0171-5
20. Nivoli AM, Murru A, Goikolea JM, et al. New treatment guidelines for acute bipolar mania: a critical review. J Affect Disord. 2012;140(2):125-141. PubMed doi:10.1016/j.jad.2011.10.015
21. Nolen WA, Kupka RW, Schulte PFJ, et al. Richtlijn bipolaire stoornissen – Tweede, herziene versie. Utrecht, the Netherlands: Nederlandse Vereniging voor Psychiatrie; 2008.
22. NICE. Bipolar Disorder: The NICE Guideline on the Assessment and Management of Bipolar Disorder in Adults, Children and Young People in Primary and Secondary Care. NICE clinical guideline 185. London, UK: National Collaborating Centre for Mental Health; 2014.
23. Young RC, Biggs JT, Ziegler VE, et al. A rating scale for mania: reliability, validity and sensitivity. Br J Psychiatry. 1978;133(5):429-435. PubMed doi:10.1192/bjp.133.5.429
24. Committee for Proprietary Medicinal Products (CPMP). Note for guidance on clinical investigation of medicinal products for the treatment and prevention of bipolar disorder. CPMP/EWP/567/98. European Medicines Agency Web site. http://www.ema.europa.eu/docs/en_GB/document_library/
Scientific_guideline/2009/09/WC500003528.pdf. April 26, 2001.
25. Tohen M, Frank E, Bowden CL, et al. The International Society for Bipolar Disorders (ISBD) Task Force report on the nomenclature of course and outcome in bipolar disorders. Bipolar Disord. 2009;11(5):453-473. PubMed doi:10.1111/j.1399-5618.2009.00726.x
26. Grunze H, Vieta E, Goodwin GM, et al. The World Federation of Societies of Biological Psychiatry (WFSBP) guidelines for the biological treatment of bipolar disorders: update 2009 on the treatment of acute mania. World J Biol Psychiatry. 2009;10(2):85-116. PubMed doi:10.1080/15622970902823202
27. Houston JP, Ketter TA, Case M, et al. Early symptom change and prediction of subsequent remission with olanzapine augmentation in divalproex-resistant bipolar mixed episodes. J Psychiatr Res. 2011;45(2):169-173. PubMed doi:10.1016/j.jpsychires.2010.05.016
28. Bernardo M, Vieta E, Saiz Ruiz J, et al; Grupo RECAP. Recommendations for switching antipsychotics: a position statement of the Spanish Society of Psychiatry and the Spanish Society of Biological Psychiatry. Rev Psiquiatr Salud Ment. 2011;4(3):150-168. PubMed doi:10.1016/j.rpsm.2011.07.003
29. Grande I, Bernando M, Bobes J, et al. Antipsychotic switching in bipolar disorder: a systematic review. Int J Neuropsychopharmacol. 2014;17(03):497-507. PubMed doi:10.1017/S1461145713001168
30. Pae CU, Lee KU, Kim JJ, et al. Switching to quetiapine in patients with acute mania who were intolerant to risperidone. Hum Psychopharmacol. 2004;19(1):47-51. PubMed doi:10.1002/hup.549
31. Lee HB, Yoon BH, Kwon YJ, et al. The efficacy and safety of switching to ziprasidone from olanzapine in patients with bipolar I disorder: an 8-week, multicenter, open-label study. Clin Drug Investig. 2013;33(10):743-753. PubMed doi:10.1007/s40261-013-0120-y
Please sign in or purchase this PDF for $40.00.
Save
Cite