Abstract
Objective: To conduct a targeted literature review to examine the impact of cognitive impairment and negative symptoms among patients with schizophrenia treated in the United States across a range of outcomes pertinent to the US health care system decision-makers, such as payers and policy-makers.
Data Sources: The authors searched EMBASE and PubMed from January 2012 to January 2024. Search terms included schizophrenia, cognitive impairment and negative symptoms, and direct medical and nonmedical, indirect, and societal outcomes.
Study Selection: Considered for inclusion were US-based studies reporting on the relationship between cognitive impairment or negative symptoms and direct medical and nonmedical, indirect, and societal outcomes in patients with schizophrenia. A total of 4,212 articles were initially identified for screening.
Data Extraction: One reviewer extracted data and another reviewer ensured studies met Population, Intervention, Comparison, Outcomes, Study Design-Time Period (PICOS-T) criteria for inclusion and exclusion.
Results: Eight studies (n = 262,683) were included that reported specifically on associations between cognitive impairment or negative symptoms and targeted outcomes. Patients with schizophrenia and moderate/severe cognitive impairment had a 100% increase in relapse-related hospitalizations (0.6 vs 0.3, adjusted incidence rate ratio = 1.85, P < .05) and ER visits (0.4 vs 0.2, adjusted odds ratio = 1.77, P < .05) vs patients with no/mild cognitive impairment. Additionally, there was an almost 50% increase in outpatient visits (8.4 vs 5.5, P < .001) and inpatient admissions (6.8 vs 4.5, P < .001) over the study period (2014 Q1–2017 Q4) for patients with negative symptoms vs without negative symptoms. Direct nonmedical, indirect, and societal outcomes are described.
Conclusions: This review highlights the economic burden of cognitive impairment and negative symptoms by focusing on outcomes relevant to health care decision-makers in the United States.
J Clin Psychiatry 2024;85(3):24r15316
Author affiliations are listed at the end of this article.
Schizophrenia is a chronic mental health condition that can occur at any age, with the average age of diagnosis for men in the late teens to early 20s and for women between their late 20s to early 30s.1 The annual prevalence of schizophrenia among US adults is around 1%,2 which equates to around 3 million adults in the US aged 18 or older.3 Approximately 100,000 Americans are newly diagnosed with schizophrenia each year.4
Schizophrenia is characterized by positive, cognitive, and negative symptoms,1 and these symptoms contribute to suboptimal functional outcomes.5–7 Positive symptoms, which constitute hallucinations, delusions, disorganized thinking, and behavior,8 are treated with antipsychotic medications to ensure patients achieve clinical stability and avoid relapses.9 Cognitive symptoms affect essentially all domains of cognition, with most salient impairments in processing speed, attention, working, and episodic memory as well as executive functioning and social cognition.10 Negative symptoms include loss of motivation, interest, and enjoyment in daily activities as well as social withdrawal and difficulties in displaying emotions.11
The prevalence of cognitive impairment and negative symptoms in the schizophrenia population is quite high with more than 80% of patients experiencing significant cognitive impairment12 and up to 60% of patients showing clinically relevant negative symptoms.11 Despite substantial advancement in the scientific understanding of the illness and in the treatment of positive symptoms, cognitive and negative symptoms still have no approved treatments.12,13 Cognitive impairment is a significant factor in functional disability.12 Functional disability is mediated by factors such as functional capacity, defined as the ability to perform critical everyday living skills.14–19 Additionally, a lower treatment response and poor functional outcomes are predicted by a significant negative symptom burden.11
The chronic, long-term nature of schizophrenia is relevant to patients, caregivers and family members, society, medical providers, policy-makers, and payers. Although achieving clinical stability is currently the primary goal due to the nature of available treatments, direct medical outcomes (inpatient, outpatient, and emergency room [ER] costs, and utilization), direct nonmedical outcomes (costs and utilization associated with the criminal justice system), and indirect outcomes (lost productivity) are of additional importance to stakeholders.20 There are additional, harder-to-quantify societal outcomes that are crucial in assessing functional disability related to schizophrenia.12 Putting these pieces together, we developed a conceptual framework (Figure 1) to demonstrate the potential association between cognitive and negative symptoms and outcomes of interest in a variety of functional domains.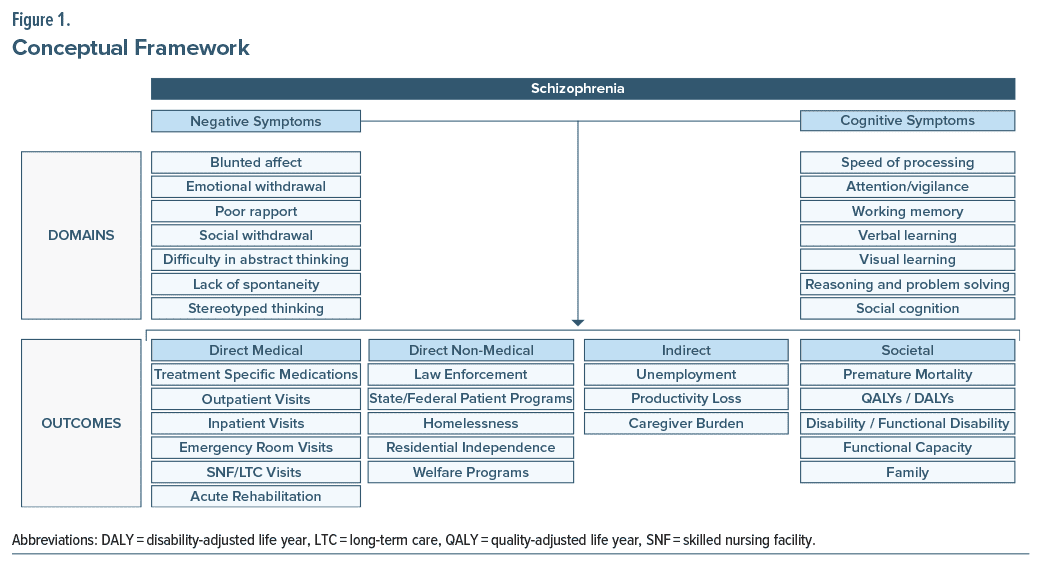
The association between negative symptoms and health care costs and resource utilization (HCRU) has been conducted in an earlier systematic literature review by Weber et al21 Our review adds additional outcomes of interest as well as focuses on both cognitive impairment and negative symptoms from a US perspective. The primary objective of this literature review was to examine the effect of cognitive impairment and negative symptoms associated with schizophrenia on a wide range of outcomes beyond endpoints used in evaluating efficacy in clinical trials.
METHODOLOGY
The study followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement guidelines for conducting systematic reviews and meta-analyses.22 The search was conducted in EMBASE and PubMed. When this review began, a 10-year time horizon (January 2013–December 2022) was chosen to ensure the outcomes of interest such as health care resource use and costs were still applicable to the schizophrenia population today. However, during the finalization of our review, new evidence was identified in 2023. To ensure our review reflected the most recent data, we chose to update the review to cover evidence published between January 2012 and January 2024. Additional desktop searches were conducted in Google and Google Scholar for any grey literature including conference proceedings or abstracts. The review ensured selected studies matched the Population, Intervention, Comparison, Outcomes, Study Design-Time Period (PICOS-T) criteria23 for inclusion and exclusion.
Eligibility Criteria
Inclusion of eligible studies followed PICOS-T criteria:
- Population: Patients diagnosed with schizophrenia and cognitive impairment or negative symptoms
- Intervention: Any
- Comparison: Any
- Outcomes:
- Outcomes focusing on direct medical, direct nonmedical, indirect, and societal impact, specifically any mention of their rates, HCRU, and costs.
- Costs include direct costs of treatment and other expenses directly induced by the illness, including the legal system, indigent assistance including public assistance payments (supplementary security income or food stamps), homelessness or residential support costs, vocational assistance services, as well as direct (Veterans Affairs or social security) payments for disability compensation.
- Study Design: US-specific prospective and retrospective cohort and cross-sectional studies were included. Randomized controlled trials, case reports, and case series were excluded.
- Time Horizon: January 2012–January 2024
Search Terms/Strategy
The following search items were used, which were all combined using the logic operator AND:
- Terms that identify study population: Schizophrenia AND cognitive impairment OR negative symptoms.
- Terms that identify outcomes: Cost OR economic OR resource OR law enforcement OR incarceration OR violence OR crime OR judicial OR legal OR police protection OR state program OR federal program OR patient support OR job program OR homelessness OR homeless status OR residential independence OR independence in residence OR independent living OR assisted living OR housing OR food assistance OR food stamp program OR food stamp OR welfare OR tax credit OR unemployment OR productivity OR suicide OR caregiver OR societal OR intangible OR quality-adjusted life year OR disability-adjusted life year OR disability OR disability compensation OR functional disability OR functional capacity OR social stigma OR relationship.
- Term that identifies the country: United States.
- Filters applied: Classical Article, Clinical Study, Comparative Study, Multicenter Study, Observational Study, Humans, English.
Study Selection and Data Extraction
The review began with screening of titles and abstracts. Screening criteria included patient population (patients with schizophrenia and cognitive impairment or negative symptoms), outcomes of interest (any publication evaluating key outcomes of interest in the schizophrenia population was included), and study design (prospective and retrospective cohort studies, cross-sectional studies were included; randomized controlled trials, case reports, and case series were excluded). Another reviewer independently assessed the studies that were selected after initial screening of titles and abstracts to ensure they matched the PICOS-T criteria for inclusion and exclusion.
Data on the variables listed below were extracted by a single reviewer and checked by a second independent reviewer for accuracy and quality:
- Study characteristics: study design, data source, study period, population of interest, inclusion/exclusion criteria, and number of patients.
- Definitions of outcomes of interest: we included a total of 18 outcomes of interest and the definitions are described below:
- Direct Medical Outcomes: any rates, utilization, and costs associated with the following 6 outcomes:
- Medications (for illness-specific medications, we focused on the number of prescriptions per year and associated costs to treat schizophrenia).
- Outpatient
- Inpatient
- Emergency room or urgent care
- Skilled nursing facility or long-term care
- Acute rehabilitation
- Direct Nonmedical Outcomes: Any rates, utilization, and costs associated with the following 5 outcomes:
- Law enforcement or incarceration
- State or federal programs (focused on the costs of incorporating these programs)
- Homelessness
- Residential independence
- Welfare programs (focused on the costs of incorporating these programs)
- Indirect Outcomes: Any rates and costs associated with the following 3 outcomes:
- Unemployment or reduced wages
- Productivity loss
- Caregiving or unpaid labor for caregiver
- Societal Outcomes: Any rates and costs associated with the following 4 outcomes:
- Premature mortality or suicide (suicide and suicide attempt were included but not suicidal behavior or suicidal ideation).
- Quality-adjusted life years (QALYs) or disability-adjusted life years (DALYs).
- A person’s health status is evaluated using a QALY, in which the benefits of a longer life are modified to consider the quality of that life. The calculation of DALYs is based on the total number of years lost to premature death and years lost to disability.24 For QALYs and DALYs, the review focused on total life years.
- Disability or functional disability or functional capacity and its predictors.
- Family-related issues such as divorce and custody of children.
- Direct Medical Outcomes: any rates, utilization, and costs associated with the following 6 outcomes:
Quality Assessment
Given the assumption that a limited number of studies would be identified, we included all studies regardless of quality. However, when interpreting the data and results, the strengths and weaknesses of the included studies were taken into consideration.
RESULTS
Study Inclusion
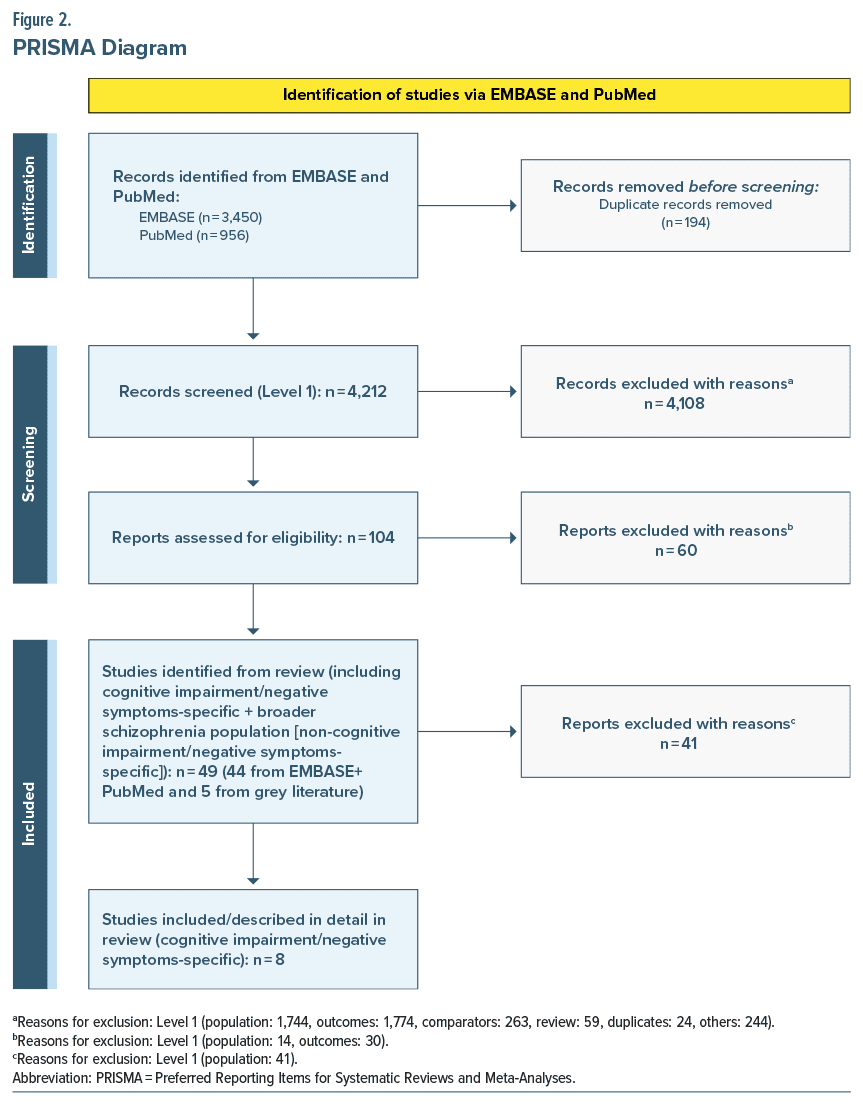
Using predetermined search criteria, we identified 49 studies reporting on the outcomes of interest in the schizophrenia population. After excluding 41 studies that reported on association between the outcomes of interest and schizophrenia in general or symptoms other than cognitive impairment or negative symptoms, 8 studies (n = 262,683, mean age = 46 years, and male = 66%) were included that specifically reported on associations between outcomes of interest and cognitive impairment or negative symptoms (Figure 2).
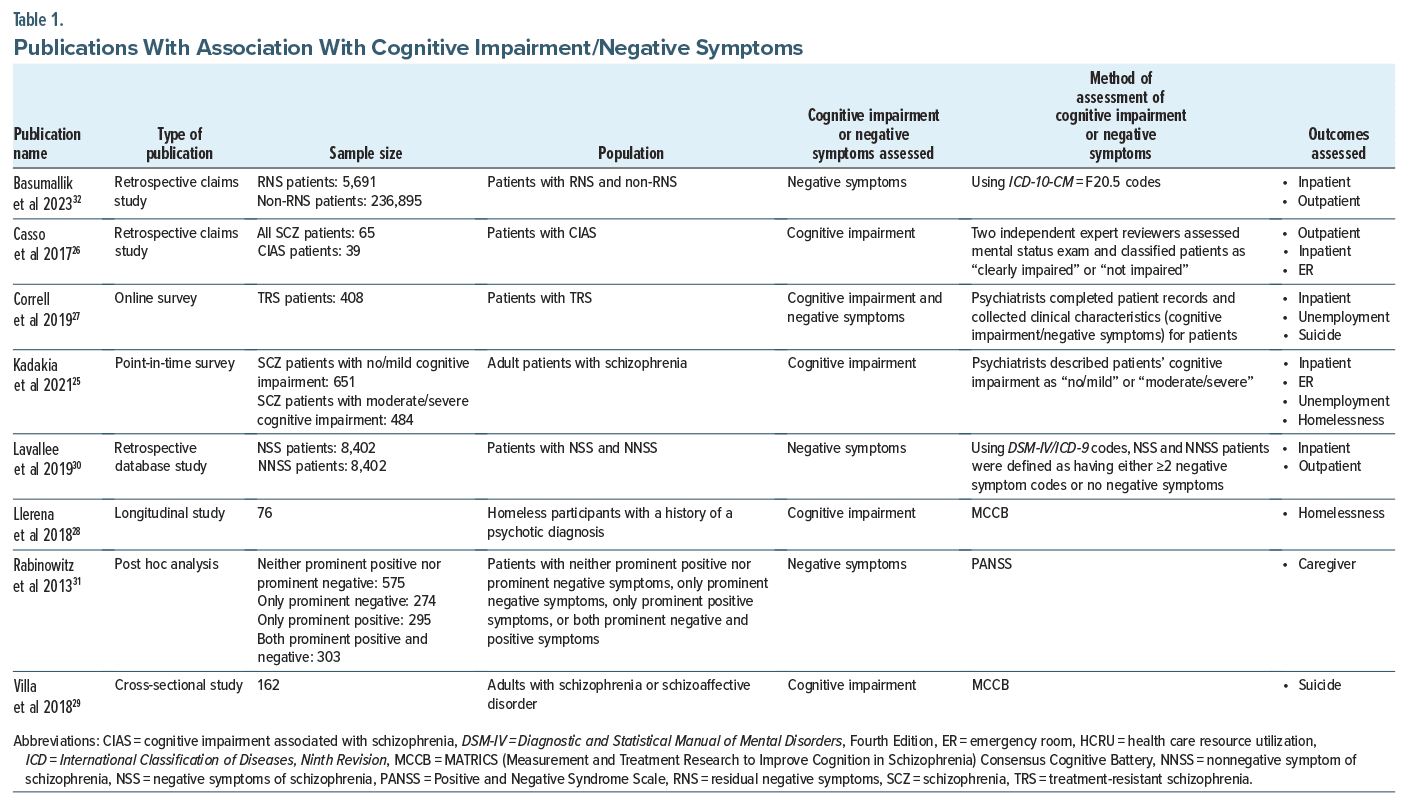
Table 1 summarizes the 8 publications25–32 that reported on associations between outcomes of interest and cognitive impairment or negative symptoms. Among these, 5 studies25–29 reported on associations between outcomes of interest (outpatient visits, inpatient admissions, ER visits, unemployment, homelessness, and suicide) and cognitive impairment. Four studies27,30–32 reported on associations between outcomes of interest (outpatient visits, inpatient admissions, unemployment, caregiver burden, and suicide) and negative symptoms. The study by Correll et al27 was the only study that reported on associations between outcomes of interest in patients with cognitive impairment and negative symptoms and schizophrenia. An assessment of these studies was conducted to determine the distribution and availability of evidence across the outcomes of interest. Based on the 18 outcomes of interest described above, 6 outcomes of interest were available for patients with cognitive impairment and 5 outcomes of interest were available for patients with negative symptoms. The following section provides a detailed summary on the associations of outcomes of interest in patients with cognitive impairment and negative symptoms, respectively.
Studies With Associations With Cognitive Impairment
Five studies (n = 1,846, mean age = 44 years, and male = 67%) reported on the association between some of the relevant outcomes of interest and cognitive impairment in patients with schizophrenia.25–29
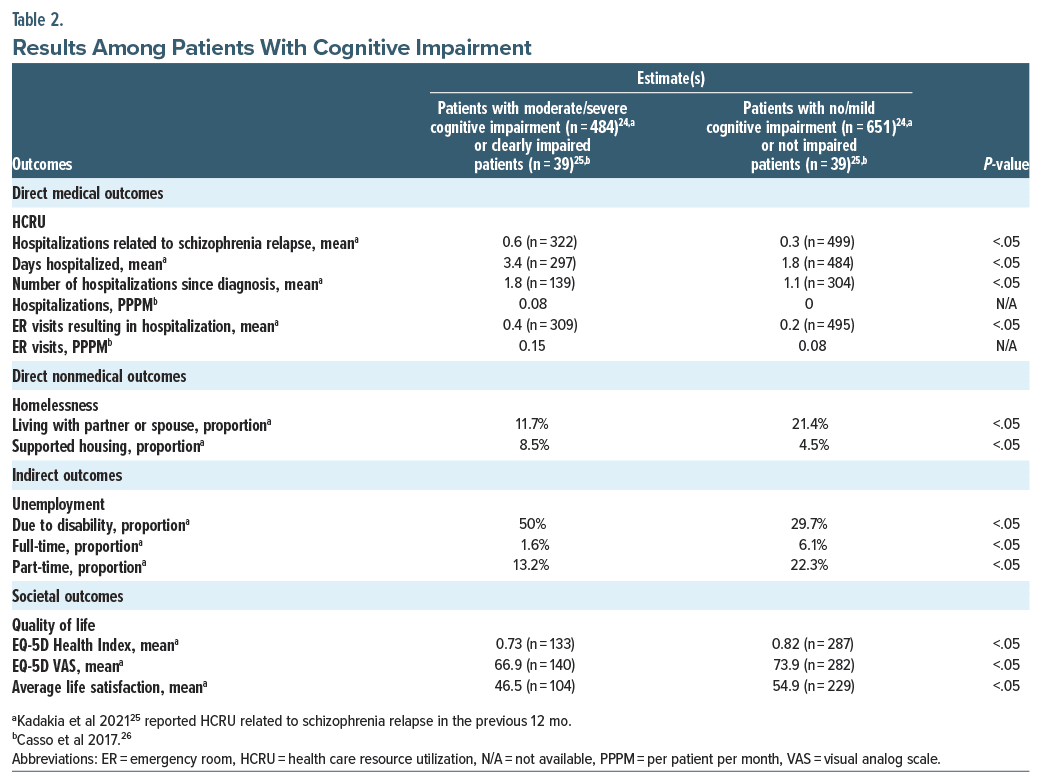
Direct medical outcomes. Health care resource utilization. Kadakia et al25 assessed the relationship between cognitive impairment (not at all cognitively impaired, borderline, or mild cognitive impairment were classified as no/mild; moderate, marked, severe, or among the most extreme were classified as moderate/severe) and HCRU among patients with schizophrenia (n=1,135, mean age=40.0 years, and male=58.6%) and found statistically significant differences in patients whose cognitive impairment was moderate/severe vs those who had no/ mild cognitive impairment. Patients with schizophrenia and moderate/severe cognitive impairment showed a 100% increase in resource utilization for relapse-related hospitalizations (0.6 vs 0.3, adjusted incidence rate ratio=1.85, P<.05) and ER visits leading to hospitalization (0.4 vs 0.2, adjusted odds ratio (aOR)=1.77, P <.05) (Table 2).25 Casso et al’s26 study in 2017 analyzed the relationship between cognitive impairment associated with schizophrenia and HCRU (n=65, mean age=43 years, and male=59%). The findings revealed a trend where patients with cognitive impairment had twice as many ER visits compared to nonimpaired patients, although the results were not statistically significant (0.15 vs 0.08 per patient per month).26 In their 2019 study,27 Correll et al investigated the HCRU in treatment-resistant schizophrenia (TRS) patients, categorized by their hospitalization history: those with ≥2 hospitalizations (n=322, mean age=39.7 years, and male=73.9%) and ≤1 hospitalization (n=79, mean age=37.7 years, and male=69.6%). Cognitive impairment severity was assessed based on a scale ranging from 1 (minimal) to 6 (extreme) in patients with cognitive impairment. The study found that patients with schizophrenia who experienced any cognitive impairment had more ≥2 hospitalizations than ≤1 hospitalization (41.3% vs 22.8%, P <.01). Additionally, patients who experienced cognitive impairment daily had more ≥2 hospitalizations than ≤1 hospitalization (65.4% vs 27.8%), although the results were not statistically significant.27
Direct nonmedical outcomes. Homelessness. Kadakia et al25 also assessed the relationship between cognitive impairment and homelessness among patients with schizophrenia and found statistically significant differences in patients with moderate/severe cognitive impairment vs those with no/mild cognitive impairment. The proportion of patients with moderate/severe cognitive impairment who lived in supported housing was approximately 90% greater than patients with no/mild cognitive impairment (8.5% vs 4.5%, aOR =1.99, and P <.05) (Table 2). In another study by Llerena et al,28 the authors found that almost half of patients with schizophrenia (n=30, mean age=49.33 years, and male=100%) were susceptible to unsheltered homelessness with days unsheltered being significantly correlated with overall cognition such that poorer cognition was associated with more days unsheltered.
Indirect outcomes. Unemployment. Kadakia et al25 also assessed the relationship between cognitive impairment and unemployment among patients with schizophrenia and found statistically significant differences in patients with moderate/severe cognitive impairment vs those with no/mild cognitive impairment. Patients with no/mild cognitive impairment were almost twice (13.2 vs 22.3%, aOR =0.53, P <.05) and 4 times (1.6 vs 6.1%, aOR =0.25, P <.05) more likely to have part-time and full-time employment compared to patients with moderate/severe cognitive impairment, respectively (Table 2). Correll et al’s27 described unemployment in TRS patients (not employed or not in education [n=304, mean age=40.6 years, and male=74.3%] vs employed or in education [n=96, mean age=35.6 years, and male=68.8%]) who had cognitive impairment. The study found that patients with schizophrenia who experienced cognitive impairment were more likely to be unemployed or not in education than be employed or in education (41.8% vs 25%, P<.01). Additionally, patients who experienced cognitive impairment daily were more likely to be unemployed or not in education than be employed or in education (68.5% vs 20.8%), although the results were not statistically significant.
Societal outcomes. Suicide. Results from Villa et al29 in chronic and treated patients with schizophrenia (n=162, mean age=50.6 years, and male=53%) showed that 41% of the patients attempted suicide (41%). However, the study found no meaningful relationship between cognition and suicide-related history. The study by Correll et al27 described suicide in TRS patients with at least 1 suicide attempt (≥1 suicide attempt [n=153, mean age=38.3 years, and male=72.5%] vs 0 suicide attempt [n=204, mean age=39.8 years, and male=74.5%]) who had cognitive impairment. The study found that patients with schizophrenia who experienced cognitive impairment were more likely to attempt suicide than not attempt one (37.3% vs 36.8%), although the results were not statistically significant. Additionally, patients who experienced cognitive impairment daily were more likely to attempt suicide than not attempt one (59.6% vs 57.3%), although the results were not statistically significant.27
Quality of life. Kadakia et al25 assessed the relationship between cognitive impairment and quality of life (QOL) among patients with schizophrenia and found statistically significant differences in patients with moderate/severe cognitive impairment vs those with no/mild cognitive impairment. Patients with moderate/severe cognitive impairment had significantly lower QOL vs those with no/mild cognitive impairment (EQ-5D Health Index: 0.73 vs 0.82, difference=−0.09, P<.05; EQ-5D visual analogue scale: 66.9 vs 73.9, difference=−7.0, P <.05) (Table 2).
Studies With Associations With Negative Symptoms
Four studies (n = 261,245, mean age = 46 years, and male = 66%) reported on the association between some of the relevant outcomes of interest and negative symptoms in patients with schizophrenia.27,30–32
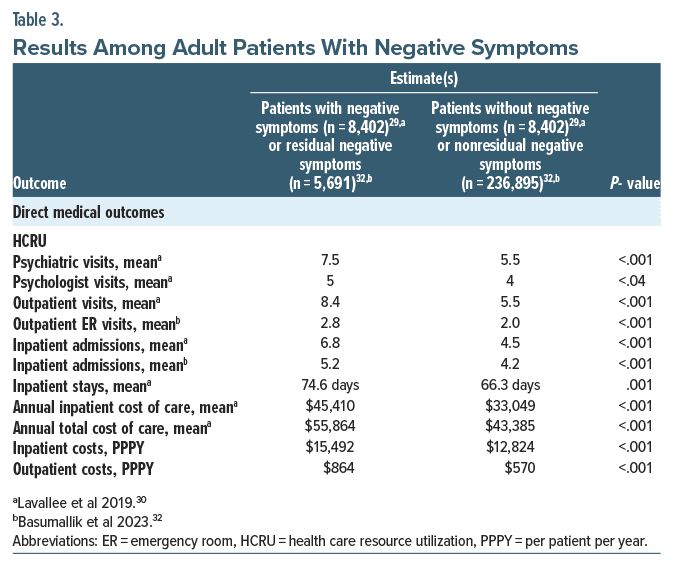
Direct medical outcomes. Health care resource utilization. Lavallee et al30 investigated whether patients with negative symptoms utilized more HCRU (and the associated expenditure) than patients without negative symptoms (n=8,402 for negative symptoms and n=8,402 for those without negative symptoms, mean age=54 years, and male=59%). Using the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV) and International Classification of Diseases, Ninth Revision (ICD-9) code for Schizophrenia Residual Type or with the specifier With Prominent Negative Symptoms, patients with and without negative symptoms were defined as having either ≥2 negative symptoms codes or no negative symptoms, respectively. There was almost a 50% increase in outpatient visits (8.4 vs 5.5, P<.001) and inpatient admissions (6.8 vs 4.5, P <.001) which were statistically significant for patients with negative symptoms compared to patients without negative symptoms (Table 3). Additionally, there was almost 40% and 30% statistically significant increases in annual inpatient ($45,410 vs $33,049, P<.001) and total ($55,864 vs $43,385, P <.001) cost of care in patients with negative symptoms compared to patients without negative symptoms (Table 3).30 Correll et al’s27 study described the utilization of resources in TRS patients (≥2 hospitalizations [n=322, mean age=39.7 years, and male=73.9%] vs ≤1 hospitalization [n=79, mean age=37.7 years, and male=69.6%]) who had negative symptoms which was assessed based on severity levels. The study found that patients with schizophrenia who experienced any negative symptoms had more ≥2 hospitalizations than ≤1 hospitalization (75.8% vs 67.1%), although the results were not statistically significant. Additionally, patients who experienced negative symptoms daily had more ≥2 hospitalizations than ≤1 hospitalization (50.8% vs 20.8%, P≤.001). A more recent 2023 retrospective claims database study by Basumallik et al,32 which estimated the HCRU and health care costs among patients with residual negative symptoms, found that patients with residual negative symptoms (n=5,691, mean age=50 years, and male=60.7%) had significantly greater all-cause inpatient admissions (5.2 vs 4.2, P<.001) and greater outpatient ER visits (2.8 vs 2.0, P<.001) compared to patients with nonresidual negative symptoms (n=236,895, mean age=47.7 years, and male=57.3%). Additionally, the residual negative symptoms cohort had greater average schizophrenia-related inpatient stay costs ($15,492 vs $12,842, P<.001) and greater ER costs ($864 vs $570, P<.001) compared to the nonresidual negative symptoms’ cohort (Table 3).32
Indirect outcomes. Unemployment. Correll et al’s27 study described unemployment in TRS patients (not employed or not in education [n = 304, mean age = 40.6 years, and male = 74.3%] vs employed or in education [n = 96, mean age = 35.6 years, and male = 68.8%]) who had negative symptoms. The study found that patients with schizophrenia who experienced any negative symptoms were more likely to be unemployed or not in education than be employed or in education (75.3% vs 70.8%), although the results were not statistically significant. Additionally, patients who experienced negative symptoms daily were more likely to be unemployed or not in education than be employed or in education (52.0% vs 23.5%, P ≤ .001).
Caregiver burden. Using the Family/Caregiver Baseline Interview, Rabinowitz et al31 assessed the number of lost workdays outside or within the household in the past month due to a family member’s illness. They estimated that caregivers taking care of patients with prominent negative symptoms (n = 274) experienced more lost workdays compared to caregivers responsible for patients with neither prominent positive nor prominent negative symptoms (n = 575) (1.81 vs 1.73, overall P-value < .001).
Societal outcomes. Suicide. The 2019 study by Correll et al27 described suicide in TRS patients [≥1 suicide attempt (n=153, mean age=38.3 years, and male=72.5%) vs 0 suicide attempt (n=204, mean age=39.8 years, and male=74.5%)] who had negative symptoms. The study found that patients with schizophrenia who experienced any negative symptoms had similar results with respect to attempting suicide than not attempting one (75.2% vs 75.5%).
QOL. Rabinowitz et al31 analyzed Short Form-12 mental health scores as well as functioning items using Lehman’s and Heinrich’s health-related QOL scales. The study found that caregivers taking care of patients with prominent negative symptoms (n=274) had lower scores on both instruments compared to caregivers responsible for patients with neither prominent positive nor prominent negative symptoms (n=575) (SF-12 Mental Health [40.5 vs 43.3, overall P-value<.001] and Lehman’s and Heinrich’s health related quality of life Scale [−0.75 vs 0.99, overall P-value<.001]).
DISCUSSION
To our knowledge, this is the first literature review that summarizes outcomes that are relevant to the US health care system and assess the impact of cognitive impairment and negative symptoms on these outcomes of interest in patients diagnosed with schizophrenia. A systematic literature review conducted by Weber et al21 identified 6 studies with literature on HCRU and costs associated with negative symptoms of schizophrenia and was not country-specific. Our US-centric review contributes to the literature by expanding on additional outcomes of interest and focusing both on cognitive impairment and negative symptoms. Putting the burden of cognitive impairment and negative symptoms into the context to that of positive symptoms, Correll et al27 observed that a greater proportion of patients with schizophrenia who experienced cognitive impairment and negative symptoms daily had >2 hospitalizations (65.4% and 50.8%, respectively) compared to those patients who experienced positive symptoms daily (32.5%).27 While positive symptoms are generally responsible for hospitalization and are also related to caregiver burden and cost, negative and cognitive symptoms, alone or in combination with positive symptoms, are related to HCRU, caregiver burden, and cost even more than positive symptoms21,31,33,34 for which targeted treatments currently exist.
Compared to positive symptoms, cognitive impairment and negative symptoms generally receive much less attention and are also less responsive to currently available pharmacotherapeutic agents, such as dopamine D2 antagonists and D2 partial agonists,11 including various psychopharmacologic augmentation strategies.35 The few impactful studies that evaluated some of these outcomes found that patients with schizophrenia with more severe cognitive impairment had worse outcomes compared to patients with no/mild cognitive impairment.25 With regard to negative symptoms, there was a significant increase in HCRU and direct costs in patients with schizophrenia who had negative symptoms compared to those who did not.30
There was a lack of research regarding the association between cognitive impairment and negative symptoms on other direct medical (medications and long-term care), direct nonmedical (law enforcement and residential independence), indirect (disability-related unemployment and caregiver burden), and societal outcomes (family issues such as divorce and public stigma). However, a few studies reported on these associations among the general schizophrenia population. Bessonova et al36 evaluated the treatment costs of second-generation oral antipsychotics and found mean annual direct medical costs ranging between $17,115 and $28,101. Pilon et al37 assessed the economic burden in recently relapsed Medicaid patients with schizophrenia and found that recently relapsed patients had $21,862 higher mean total health care costs compared to patients without schizophrenia ($37,424 vs $15,563, P < .001), driven by $8,486 higher mean long-term care costs (P < .001). Lin et al38 assessed incarceration rates among US veterans who had a schizophrenia relapse and found patients in the relapse cohort having greater incarceration (0.6% vs 0.4%) compared to patients in the nonrelapse cohort. In another study, a greater proportion of remitted patients lived in independent housing compared to nonremitted patients (72% vs 65%, P < .05).39 In a survey analysis, relapsed patients with schizophrenia were 38% more likely to experience disability-related unemployment compared to patients in remission.40 In a study that evaluated caregiver burden of patients with TRS (n = 27, mean age = 48 years, and male = 22%), Brain et al41 found caregivers administering direct care for an average of 37 hours per week. Almost 80% reported being on call the whole day. In a study on primary caregivers, Csoboth et al42 estimated the schizophrenia caregiver group had higher direct ($28,314 vs $19,750), indirect ($8,999 vs $8,085), and total costs ($32,905 vs $23,930) compared to the nonschizophrenia caregiver group. Lin et al38 observed divorce rates in US veterans with schizophrenia and a history of relapse, finding similar rates of separation (36% vs 34%) in the relapse (n=16,862, mean age=56.4 years, and male=91.95) vs nonrelapse cohort (n=16,862, mean age=56.6 years, and male=92.1). Pescosolido et al43 evaluated public perceptions of violence and support for coercive treatment over 2 decades and found over 60% of people felt that patients with schizophrenia were dangerous, and 44%–59% were in support of using coercive treatment.
Most of the data from ex-US countries on cognitive impairment and negative symptoms align with what we have found in our review of US-specific data, in that the focus is on the impact of cognitive impairment and negative symptoms in patients with schizophrenia on direct medical outcomes. A study by Millier et al44 which explored HCRU by health states (defining health states by the level of impairment across positive, cognitive, and negative symptoms) found that the highest burden was for the use of day clinics for those with a predominance of positive and cognitive symptoms. In another population-based, retrospective review of medical records in Spain, patients with negative symptoms had significantly higher average/unit health care costs than patients without negative symptoms (€2,170.00 vs €1,765.30, respectively; P < .001).45
Limitations
This review has several limitations. First, despite the employed systematic search strategy, it is possible that not all eligible studies were included owing to some not being captured by the selected inclusion criteria and/or search terms. For example, we may have missed studies (unpublished reports or grey literature) owing to the wide variation in how cognitive impairment is defined. Second, the generalizability of our findings may be limited since our review focused only on US-based studies. Nevertheless, this restriction was the result of an explicit focus on the US health care system, as it also has unique features that make a focused review relevant, such as ethnic and socio-economic characteristics, payer and payer system characteristics, and funding as well as access to care barriers. Third, since the primary focus of our review was to address the economic burden of cognitive impairment and negative symptoms in patients with schizophrenia, we were unable to identify data on missed appointments and frequency of outpatient visits scheduled or attended. These outcomes should be investigated in future studies, as they could be crucial in understanding potential downstream associations between visit frequency, adherence to visits, and the total cost of care for schizophrenia patients. Fourth, the included studies were heterogeneous and often reported only partial data. For example, 5 of the 825–32 studies that we identified and relied heavily on in this review did not use validated instruments to assess either cognitive impairment or negative symptoms. Three studies utilized opinions or judgments from expert reviewers26 or psychiatrists25,27 to assess cognitive impairment and/or negative symptoms. Two studies30,32 evaluated negative symptoms by using DSM-IV/ICD-9/ICD-10 codes, which has reliability and validity issues. Although cognitive impairment is one of the core symptoms of schizophrenia, neither the ICD nor the DSM have included cognitive impairment in their diagnostic criteria for schizophrenia to date. Fifth, although some studies controlled for baseline patient characteristics,25,29,30,32 they did not assess and control for other potentially relevant confounders, such as medication side effects or nonadherence, potential collinearity between cognitive impairment and negative symptoms, illness chronicity, and protracted psychosocial impairment. It is possible that patients with schizophrenia experience cognitive impairment and negative symptoms due to side effects from the antidopaminergic and anticholinergic medications they are on.11,46 Moreover, although less illness insight and knowledge about schizophrenia have been associated with higher medication nonadherence in schizophrenia,47–49 negative and cognitive symptoms have also been identified as factors driving antipsychotic nonadherence,49–53 with cognitive dysfunction even potentially being related to poor illness insight.54 Despite these relationships, none of the identified studies included medication nonadherence in their analyses. Furthermore, there could also be interactions between the various symptom domains of schizophrenia. In this context, 4 studies only assessed the burden caused due to cognitive impairment; 3 studies only evaluated the burden caused due to negative symptoms. Only one study27 covered the burden of all symptom domains. None of the studies controlled for any collinearity that could potentially exist between positive symptoms, cognitive impairment, and negative symptoms. Regarding illness chronicity, only Correll et al27 in their study investigated whether time of onset of TRS had any impact on outcomes and found that patients with late onset TRS (TRS after >5 years of treatment) were associated with poorer outcomes compared to patients with early-onset TRS (TRS within the first 5 years of treatment). The other studies only assessed outcomes in the prevalent population and did not mention or control for chronicity. Therefore, it is difficult to gauge whether outcomes are equally robust in patients with early-episode vs later-episode schizophrenia. Similarly, despite potential interactions between negative and cognitive symptoms and health care cost with psychosocial impairment, none of the 8 reviewed studies looked at confounding effects of protracted psychosocial impairment, such as loss of social supports and occupational disability. Finally, there is currently no clinically useful bedside test for cognitive impairment. While the Montreal Cognitive Assessment and Mini-Mental Status Examination have been tested as quick screening tools for severe cognitive dysfunction in schizophrenia,55,56 these tests by no means assess the breadth and depth of the cognitive dysfunction that is mostly much below the profound level as found in patients with dementia where these tests are generally used.57–59 Similarly, negative symptoms are often not measured or quantified. Therefore, the classification of patients with vs without cognitive impairment, or with prominent/predominant negative symptoms11 in clinical care settings and population, is difficult or impossible. This complexity makes the identification of such subpopulations and understanding their impact on HCRU outcomes a challenging task.
Implications for Future Research
Positive, cognitive, and negative symptoms are distinct domains of schizophrenia. Despite some correlation, cognitive impairment and negative symptoms often persist even after positive symptoms stabilize during antipsychotic treatment, as negative symptoms and cognitive impairment are less responsive to current treatment options.60 Additionally, treatment with current antipsychotic medications may cause or exacerbate cognitive impairment and secondary negative symptoms.46 Future research focusing on or controlling for these confounding effects as well as focusing on factors, such as the potential associations between various symptom domains, and between various symptom domains and nonadherence and illness chronicity, would be highly valuable. Additionally, the relationship between symptom domains and scheduled, but missed appointments should be investigated. Nonpharmacologic interventions, such as cognitive behavioral therapies, social skills groups, cognitive remediation, and exercise interventions, have been shown to improve cognitive impairment, negative symptoms, and overall functioning to varying degrees, especially when combined with rehabilitation methods.61–66 However, several barriers toward the widespread implementation and consistent use of these nonpharmacologic options remain, including a lack of capacity to support training, inconsistent providers’ abilities within organizations, and time constraints.66–68 Finally, clinicians continue to primarily assess and diagnose schizophrenia without using scales to measure cognitive impairment and negative symptoms. These instruments can be used to measure treatment efficacy and track the severity of cognitive impairment and negative symptoms in the schizophrenia population. In our review, the MATRICS Consensus Cognitive Battery (MCCB), the Beck Cognitive Insight Scale, and the Clinical Assessment Interview for Negative Symptoms have been utilized as assessment tools by Llerena et al28 and Villa et al29 for cognitive impairment and negative symptoms, respectively. Outcomes that capture functioning are also rarely or insufficiently employed in clinical care and are only beginning to gain traction in research on patients with schizophrenia. In general, accurate, valid, and effective assessment methodologies are needed to evaluate cognitive impairment and negative symptoms and to measure the functional and health care utilization outcomes in patients with schizophrenia and of different treatment modalities.10 Research into appropriate nonpharmacologic and novel-mechanism of action pharmacologic treatment options13 that target domains specific to cognitive impairment and negative symptoms should be an area of active investigation.
CONCLUSION
This review highlights the importance of cognitive impairment and negative symptoms in people with schizophrenia, focusing on outcomes that are relevant to health care decision-makers in the United States. While not substantial, some literature exists that points to the increasing burden associated with cognitive impairment and negative symptoms, which is also in line with international publications. Additional studies with more recent and different cohorts are needed to reiterate the clinical and economic burden of cognitive impairment and negative symptoms. There is an ever-growing need for future research that focuses on pharmacologic and nonpharmacologic interventions that can help alleviate the important cognitive impairment and negative symptom domains of schizophrenia.
Article Information
Published Online: August 21, 2024. https://doi.org/10.4088/JCP.24r15316
© 2024 Physicians Postgraduate Press, Inc.
Submitted: February 29, 2024; accepted June 20, 2024.
To Cite: Correll CU, Xiang P, Sarikonda K, et al. The economic impact of cognitive impairment and negative symptoms in schizophrenia: a targeted literature review with a focus on outcomes relevant to health care decision-makers in the United States.
J Clin Psychiatry. 2024;85(3):24r15316.
Author Affiliations: Department of Psychiatry, The Zucker Hillside Hospital, Northwell Health, Glen Oaks, New York; Department of Psychiatry and Molecular Medicine, Donald and Barbara Zucker School of Medicine at Hofstra/Northwell, Hempstead, New York; Department of Child and Adolescent Psychiatry, Charité–Universitätsmedizin Berlin, Berlin, Germany; German Center for Mental Health (DZPG), Partner Site Berlin, Berlin, Germany (Correll); Boehringer Ingelheim, Ridgefield, Connecticut (Xiang); BluePath Solutions, Los Angeles, California (Sarikonda, Bhagvandas, Gitlin).
Corresponding Author: Christoph U. Correll, MD, Psychiatry Research, The Zucker Hillside Hospital, 75-59 263rd St, Glen Oaks, NY 11004 ([email protected]).
Author Contributions: All authors fulfill ICMJE authorship criteria, having each significantly contributed to the design of the study, interpretation of the results, and critical revision of the content.
Relevant Financial Relationships: Dr Correll has been a consultant and/or advisor to or has received honoraria from AbbVie, Acadia, Adcock Ingram, Alkermes, Allergan, Angelini, Aristo, Biogen, Boehringer-Ingelheim, Bristol-Myers Squibb, Cardio Diagnostics, Cerevel, CNX Therapeutics, Compass Pathways, Darnitsa, Delpor, Denovo, Gedeon Richter, Hikma, Holmusk, IntraCellular Therapies, Jamjoom Pharma, Janssen/J&J, Karuna, LB Pharma, Lundbeck, MedInCell, Merck, Mindpax, Mitsubishi Tanabe Pharma, Maplight, Mylan, Neumora Therapeutics, Neurocrine, Neurelis, Newron, Noven, Novo Nordisk, Otsuka, PPD Biotech, Recordati, Relmada, Reviva, Rovi, Sage, Saladax, Seqirus, SK Life Science, Sumitomo Pharma America, Sunovion, Sun Pharma, Supernus, Tabuk, Takeda, Teva, Tolmar, Vertex, Viatris, and Xenon Pharmaceuticals; provided expert testimony for Janssen and Otsuka; served on a Data Safety Monitoring Board for Compass Pathways, Denovo, Lundbeck, Relmada, Reviva, Rovi, Supernus, and Teva; has received grant support from Janssen and Takeda; received royalties from UpToDate; and is a stock option holder of Cardio Diagnostics, Kuleon Biosciences, LB Pharma, Mindpax, and Quantic. Dr Xiang is an employee of Boehringer Ingelheim. Dr Sarikonda, Bhagvandas, and Dr Gitlin are employees of BluePath Solutions who received financial support from Boehringer Ingelheim.
Funding/Support: Boehringer Ingelheim (the sponsor) has provided financial support to BluePath Solutions for conducting the targeted literature review and in the preparation of this manuscript. Dr Correll has received consulting fees from Boehringer Ingelheim.
Role of the Sponsor: Boehringer Ingelheim participated in the design, analysis, and interpretation of the evidence as well as in the review and approval of the manuscript.
Clinical Points
- From a US health care perspective, limited literature of high quality indicates a relevant economic burden of cognitive impairment and negative symptoms in patients with schizophrenia with respect to relevant health care utilization and personal as well as societal cost outcomes.
- For example, presence of moderate/severe cognitive dysfunction almost doubled relapse-related hospitalizations and emergency room visits, while presence of relevant negative symptoms increased inpatient admissions and outpatient visits by about 50%.
- This study reiterates the importance of cognitive impairment and negative symptoms and related outcomes of interest and the need for future research on nonpharmacologic and novel-mechanism pharmacologic interventions that can specifically address these symptom domains of schizophrenia and help alleviate this burden.
References (68)

- Kahn RS, Sommer IE, Murray RM, et al. Schizophrenia. Nat Rev Dis Primers. 2015;1:15067. PubMed CrossRef
- National Alliance on Mental Illness (NAMI). Mental health by the numbers. 2023. Accessed July 23, 2023. https://www.nami.org/mhstats
- United States Census Bureau. Population projections. 2023. Accessed July 31, 2023. https://www.census.gov/programs-surveys/popproj.html.
- MentalHelp.net. Schizophrenia symptoms, patterns, and statistics. 2023. Accessed July 31, 2023. https://www.mentalhelp.net/schizophrenia/statistics/
- Carbon M, Correll CU. Clinical predictors of therapeutic response to antipsychotics in schizophrenia. Dialogues Clin Neurosci. 2014;16(4):505–524. PubMed CrossRef
- Hansen HG, Speyer H, Starzer M, et al. Clinical recovery among individuals with a first-episode schizophrenia an updated systematic review and meta-analysis. Schizophr Bull. 2023;49(2):297–308. PubMed CrossRef
- Catalan A, Richter A, Salazar de Pablo G, et al. Proportion and predictors of remission and recovery in first-episode psychosis: systematic review and meta analysis. Eur Psychiatry. 2021;64(1):e69. PubMed CrossRef
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition, Text Revision (DSM-5-TR). 2022. https://doi.org/10.1176/appi.books.9780890425787
- Ostuzzi G, Bertolini F, Tedeschi F, et al. Oral and long-acting antipsychotics for relapse prevention in schizophrenia-spectrum disorders: a network meta-analysis of 92 randomized trials including 22,645 participants. World Psychiatry. 2022;21(2):295–307. PubMed CrossRef
- Bakkour N, Samp J, Akhras K, et al. Systematic review of appropriate cognitive assessment instruments used in clinical trials of schizophrenia, major depressive disorder and bipolar disorder. Psychiatry Res. 2014;216(3):291–302. PubMed CrossRef
- Correll CU, Schooler NR. Negative symptoms in schizophrenia: a review and clinical guide for recognition, assessment, and treatment. Neuropsychiatr Dis Treat. 2020;16:519–534. PubMed CrossRef
- Harvey PD, Bosia M, Cavallaro R, et al. Cognitive dysfunction in schizophrenia: an expert group paper on the current state of the art. Schizophr Res Cogn. 2022;29:100249. PubMed CrossRef
- Correll CU, Solmi M, Cortese S, et al. The future of psychopharmacology: a critical appraisal of ongoing phase 2/3 trials, and of some current trends aiming to de-risk trial programmes of novel agents. World Psychiatry. 2023;22(1):48–74. PubMed
- Galderisi S, Rossi A, Rocca P, et al. The influence of illness-related variables, personal resources and context-related factors on real-life functioning of people with schizophrenia. World Psychiatry. 2014;13(3):275–287. PubMed CrossRef
- Mucci A, Galderisi S, Gibertoni D, et al. Factors associated with real-life functioning in persons with schizophrenia in a 4-year follow-up study of the Italian network for research on psychoses. JAMA psychiatry. 2021;78(5):550–559. PubMed
- Strassnig MT, Raykov T, O’Gorman C, et al. Determinants of different aspects of everyday outcome in schizophrenia: the roles of negative symptoms, cognition, and functional capacity. Schizophr Res. 2015;165(1):76–82. PubMed CrossRef
- Bowie CR, Depp C, McGrath JA, et al. Prediction of real-world functional disability in chronic mental disorders: a comparison of schizophrenia and bipolar disorder. Am J Psychiatry. 2010;167(9):1116–1124. PubMed CrossRef
- Strassnig M, Cornacchio D, Harvey PD, et al. Health status and mobility limitations are associated with residential and employment status in schizophrenia and bipolar disorder. J Psychiatr Res. 2017;94:180–185. PubMed CrossRef
- Strassnig M, Kotov R, Fochtmann L, et al. Associations of independent living and labor force participation with impairment indicators in schizophrenia and bipolar disorder at 20-year follow-up. Schizophr Res. 2018;197:150–155. PubMed CrossRef
- Desai PR, Lawson KA, Barner JC, et al. Estimating the direct and indirect costs for community-dwelling patients with schizophrenia. J Pharm Health Serv Res. 2013;4(4):187–194.
- Weber S, Scott JG, Chatterton ML. Healthcare costs and resource use associated with negative symptoms of schizophrenia: a systematic literature review. Schizophr Res. 2022;241:251–259. PubMed CrossRef
- Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med. 2009;6(7):e1000100. PubMed CrossRef
- Methley AM, Campbell S, Chew-Graham C, et al. PICO, PICOS and SPIDER: a comparison study of specificity and sensitivity in three search tools for qualitative systematic reviews. BMC Health Serv Res. 2014;14(1):579. PubMed CrossRef
- Davidović M, Zielonke N, Lansdorp-Vogelaar I, et al. Disability-adjusted life years averted versus quality-adjusted life years gained: a model analysis for breast cancer screening. Value Health. 2021;24(3):353–360. PubMed
- Kadakia A, Fan Q, Shepherd J, et al. Healthcare resource utilization and quality of life by cognitive impairment in patients with schizophrenia. Schizophr Res Cogn. 2021;28:100233. PubMed CrossRef
- Casso D, Phillips S, Woodcroft K, et al. SA38. Assessment of cognitive impairment, treatment adherence, and healthcare resource utilization among treated schizophrenia patients. Schizophr Bull. 2017;43(suppl 1):S127.
- Correll CU, Brevig T, Brain C. Exploration of treatment-resistant schizophrenia subtypes based on a survey of 204 US psychiatrists. Neuropsychiatr Dis Treat. 2019;15:3461–3473. PubMed CrossRef
- Llerena K, Gabrielian S, Green MF. Clinical and cognitive correlates of unsheltered status in homeless persons with psychotic disorders. Schizophr Res. 2018;197:421–427. PubMed CrossRef
- Villa J, Choi J, Kangas JL, et al. Associations of suicidality with cognitive ability and cognitive insight in outpatients with schizophrenia. Schizophr Res. 2018;192:340–344. PubMed CrossRef
- Lavallee C, Maughn K, Gyanani A, et al. PMH25 real-world evidence investigation on healthcare utilization and cost among negative symptom patients and NON negative symptom patients with schizophrenia in the US. Value Health. 2019;22(suppl 2):S230.
- Rabinowitz J, Berardo CG, Bugarski-Kirola D, et al. Association of prominent positive and prominent negative symptoms and functional health, well-being, healthcare-related quality of life and family burden: a CATIE analysis. Schizophr Res. 2013;150(2–3):339–342. PubMed CrossRef
- Basumallik S, Semwal M, Afolabi M, et al. Healthcare resource use and cost associated with negative symptoms of schizophrenia. Psych Congress Annual Meeting; 2023.
- Kotzeva A, Mittal D, Desai S, et al. Socioeconomic burden of schizophrenia: a targeted literature review of types of costs and associated drivers across 10 countries. J Med Econ. 2023;26(1):70–83. PubMed
- Cechnicki A, Nowak A, Arciszewska-Leszczuk A, et al. Burden of caregivers of people suffering from schizophrenia versus symptom severity, social functioning and treatment satisfaction of patients treated by a CMHT. Psychiatr Pol. 2023;57(1):19–33. PubMed
- Correll CU, Rubio JM, Inczedy-Farkas G, et al. Efficacy of 42 pharmacologic cotreatment strategies added to antipsychotic monotherapy in schizophrenia: systematic overview and quality appraisal of the meta-analytic evidence. JAMA Psychiatry. 2017;74(7):675–684. PubMed CrossRef
- Bessonova L, Martin A, Doane M, et al. Real-world treatment patterns and costs of oral antipsychotics for treatment of schizophrenia in the United States. Manag Care Spec Pharm. 2019;25(10–a suppl):S1–S100.
- Pilon D, Patel C, Lafeuille MH, et al. Economic burden in medicaid beneficiaries with recently relapsed schizophrenia or with uncontrolled symptoms of schizophrenia not adherent to antipsychotics. J Manag Care Spec Pharm. 2021;27(7):904–914. PubMed CrossRef
- Lin D, Kim H, Wada K, et al. Unemployment, homelessness, and other societal outcomes among US veterans with schizophrenia relapse: a retrospective cohort study. Prim Care Companion CNS Disord. 2022;24(5):21m03173. PubMed CrossRef
- Haynes VS, Zhu B, Stauffer VL, et al. Long-term healthcare costs and functional outcomes associated with lack of remission in schizophrenia: a post-hoc analysis of a prospective observational study. BMC Psychiatry. 2012;12:222. PubMed CrossRef
- Kadakia A, Martins R, Fan A, et al. Assessing the risk of health, social, and fiscal events in schizophrenia according to remission or relapse status using real world data from a SCZ survey in the US. CNS spectrums. 2022;27(2):225–226.
- Brain C, Kymes S, DiBenedetti DB, et al. Experiences, attitudes, and perceptions of caregivers of individuals with treatment-resistant schizophrenia: a qualitative study. BMC Psychiatry. 2018;18(1):253. PubMed CrossRef
- Csoboth C, Witt EA, Villa KF, et al. The humanistic and economic burden of providing care for a patient with schizophrenia. Int J Soc Psychiatry. 2015;61(8):754–761. PubMed CrossRef
- Pescosolido BA, Manago B, Monahan J. Evolving public views on the likelihood of violence from people with mental illness: stigma and its consequences. Health Aff (Millwood). 2019;38(10):1735–1743. PubMed CrossRef
- Millier A, Toumi M, Horváth M, et al. Healthcare resource use in schizophrenia sufferers–findings from the EuroSC cohort. European Neuropsychopharmacol. 2017;27:S908–S909.
- Sicras-Mainar A, Maurino J, Ruiz-Beato E, et al. Impact of negative symptoms on healthcare resource utilization and associated costs in adult outpatients with schizophrenia: a population-based study. BMC psychiatry. 2014;14(1):225. PubMed CrossRef
- Carbon M, Correll CU. Thinking and acting beyond the positive: the role of the cognitive and negative symptoms in schizophrenia. CNS Spectr. 2014;19(suppl 1):38–52. PubMed CrossRef
- Kim J, Ozzoude M, Nakajima S, et al. Insight and medication adherence in schizophrenia: an analysis of the CATIE trial. Neuropharmacology. 2020;168:107634. PubMed CrossRef
- Chan KWS, Hui LMC, Wong HYG, et al. Medication adherence, knowledge about psychosis, and insight among patients with a schizophrenia-spectrum disorder. J Nerv Ment Dis. 2014;202(1):25–29. PubMed CrossRef
- Kane JM, Kishimoto T, Correll CU. Non-adherence to medication in patients with psychotic disorders: epidemiology, contributing factors and management strategies. World Psychiatry. 2013;12(3):216–226. PubMed CrossRef
- Vega D, Acosta FJ, Saavedra P. Testing the hypothesis of subtypes of nonadherence in schizophrenia and schizoaffective disorder: a prospective study. World J Psychiatry. 2020;10(11):260–271. PubMed CrossRef
- Settem VVJ, Karanadi H, Praharaj SK. Cognitive deficits, depressive symptoms, insight, and medication adherence in remitted patients with schizophrenia. Indian J Psychiatry. 2019;61(4):335–341. PubMed CrossRef
- Velligan DI, Sajatovic M, Hatch A, et al. Why do psychiatric patients stop antipsychotic medication? A systematic review of reasons for nonadherence to medication in patients with serious mental illness. Patient Prefer Adherence. 2017;11:449–468. PubMed CrossRef
- El-Missiry A, Elbatrawy A, El Missiry M, et al. Comparing cognitive functions in medication adherent and non-adherent patients with schizophrenia. J Psychiatr Res. 2015;70:106–112. PubMed CrossRef
- Boyer L, Cermolacce M, Dassa D, et al. Neurocognition, insight and medication nonadherence in schizophrenia: a structural equation modeling approach. PLoS One. 2012;7(10):e47655. PubMed CrossRef
- Daderwal MC, Sreeraj VS, Suhas S, et al. Montreal cognitive assessment (MoCA) and digit symbol substitution test (DSST) as a screening tool for evaluation of cognitive deficits in schizophrenia. Psychiatry Res. 2022;316:114731. PubMed CrossRef
- Rosca EC, Cornea A, Simu M. Montreal cognitive assessment for evaluating the cognitive impairment in patients with schizophrenia: a systematic review. Gen Hosp Psychiatry. 2020;65:64–73. PubMed CrossRef
- Nuechterlein KH, Green MF, Kern RS, et al. The MATRICS consensus cognitive battery, part 1: test selection, reliability, and validity. Am J Psychiatry. 2008;165(2):203–213. PubMed CrossRef
- Green MF, Nuechterlein KH, Gold JM, et al. Approaching a consensus cognitive battery for clinical trials in schizophrenia: the NIMH-MATRICS conference to select cognitive domains and test criteria. Biol Psychiatry. 2004;56(5):301–307. PubMed CrossRef
- Millan MJ, Agid Y, Brüne M, et al. Cognitive dysfunction in psychiatric disorders: characteristics, causes and the quest for improved therapy. Nat Rev Drug Discov. 2012;11(2):141–168. PubMed CrossRef
- Kaneko K. Negative symptoms and cognitive impairments in schizophrenia: two key symptoms negatively influencing social functioning. Yonago Acta Med. 2018;61(2):91–102. PubMed CrossRef
- Harvey PD, McGurk SR, Mahncke H, et al. Controversies in computerized cognitive training. Biol Psychiatry Cogn Neurosci Neuroimaging. 2018;3(11):907–915. PubMed CrossRef
- Vita A, Barlati S, Ceraso A, et al. Effectiveness, core elements, and moderators of response of cognitive remediation for schizophrenia: a systematic review and meta-analysis of randomized clinical trials. JAMA Psychiatry. 2021;78(8):848–858. PubMed CrossRef
- Prikken M, Konings MJ, Lei WU, et al. The efficacy of computerized cognitive drill and practice training for patients with a schizophrenia-spectrum disorder: a meta analysis. Schizophr Res. 2019;204:368–374. PubMed CrossRef
- Firth J, Stubbs B, Rosenbaum S, et al. Aerobic exercise improves cognitive functioning in people with schizophrenia: a systematic review and meta-analysis. Schizophr Bull. 2017;43(3):546–556. PubMed CrossRef
- Stubbs B, Vancampfort D, Hallgren M, et al. EPA guidance on physical activity as a treatment for severe mental illness: a meta-review of the evidence and position statement from the European Psychiatric Association (EPA), supported by the International Organization of Physical Therapists in Mental Health (IOPTMH). Eur Psychiatry. 2018;54:124–144. PubMed CrossRef
- Vita A, Gaebel W, Mucci A, et al. European Psychiatric Association guidance on treatment of cognitive impairment in schizophrenia. Eur Psychiatry. 2022;65(1):e57. PubMed CrossRef
- Giordano GM, Caporusso E, Pezzella P, et al. Updated perspectives on the clinical significance of negative symptoms in patients with schizophrenia. Expert Rev Neurother. 2022;22(7):541–555. PubMed CrossRef
- Galderisi S, Kaiser S, Bitter I, et al. EPA guidance on treatment of negative symptoms in schizophrenia. Eur Psychiatry. 2021;64(1):e21. PubMed CrossRef
This PDF is free for all visitors!
Save
Cite