Background: Electroconvulsive therapy (ECT) is the gold-standard treatment for refractory depression. Borderline personality disorder (BPD) is generally considered a poor predictor of treatment response. We sought to assess symptom-severity outcomes among depressed patients with (BPD+) and without (BPD-) comorbid BPD undergoing acute phase ECT.
Methods: The study sample consisted of at least moderately depressed patients who received an acute course of ECT from January 2011 to December 2016 at an academic, freestanding psychiatric hospital. Participants completed a DSM-IV-validated BPD screening instrument at baseline. Measures of DSM-IV depressive symptom severity from the Quick Inventory of Depressive Symptomatology-Self Report (QIDS-SR) were taken serially on 4 occasions. Outcomes of interest comprised total QIDS-SR score trajectory, QIDS-SR suicidality subscore, and symptom cluster subscores posited to differentiate response among antidepressant treatments.
Results: Of the 693 individuals who met study inclusion criteria, 145 (20.9%) screened positive for BPD. Overall, ECT was associated with significant improvement of depressive symptoms (χ21 = 504.8, P < .0001). Despite differing from BPD- individuals on key baseline features, BPD+ individuals responded to ECT with similar improvement in overall depression severity (χ21 = 0.22, P = .64), suicidality (χ21 = 1.63, P = .20), and core emotional (χ21 = 0.63, P = .43), sleep (χ21 = 0.20, P = .65), and atypical (χ21 = 1.30, P = .25) symptoms after 15 treatments. Post hoc analysis indicated a slightly less robust overall response among the BPD+ group by the 15th treatment.
Conclusions: Acute course ECT benefits depressed patients with or without comorbid BPD, although patients with BPD may exhibit less pronounced improvement over time.
See commentary by Zimmerman.
ABSTRACT
Background: Electroconvulsive therapy (ECT) is the gold-standard treatment for refractory depression. Borderline personality disorder (BPD) is generally considered a poor predictor of treatment response. We sought to assess symptom-severity outcomes among depressed patients with (BPD+) and without (BPD-) comorbid BPD undergoing acute phase ECT.
Methods: The study sample consisted of at least moderately depressed patients who received an acute course of ECT from January 2011 to December 2016 at an academic, freestanding psychiatric hospital. Participants completed a DSM-IV-validated BPD screening instrument at baseline. Measures of DSM-IV depressive symptom severity from the Quick Inventory of Depressive Symptomatology-Self Report (QIDS-SR) were taken serially on 4 occasions. Outcomes of interest comprised total QIDS-SR score trajectory, QIDS-SR suicidality subscore, and symptom cluster subscores posited to differentiate response among antidepressant treatments.
Results: Of the 693 individuals who met study inclusion criteria, 145 (20.9%) screened positive for BPD. Overall, ECT was associated with significant improvement of depressive symptoms (χ21 = 504.8, P < .0001). Despite differing from BPD- individuals on key baseline features, BPD+ individuals responded to ECT with similar improvement in overall depression severity (χ21 = 0.22, P = .64), suicidality (χ21 = 1.63, P = .20), and core emotional (χ21 = 0.63, P = .43), sleep (χ21 = 0.20, P = .65), and atypical (χ21 = 1.30, P = .25) symptoms after 15 treatments. Post hoc analysis indicated a slightly less robust overall response among the BPD+ group by the 15th treatment.
Conclusions: Acute course ECT benefits depressed patients with or without comorbid BPD, although patients with BPD may exhibit less pronounced improvement over time.
J Clin Psychiatry 2021;82(2):19m13202
To cite: Yip AG, Ressler KJ, Rodriguez-Villa F, et al. Treatment outcomes of electroconvulsive therapy for depressed patients with and without borderline personality disorder: a retrospective cohort study. J Clin Psychiatry. 2021;82(2):19m13202.
To share: https://doi.org/10.4088/JCP.19m13202
© Copyright 2021 Physicians Postgraduate Press, Inc.
aMcLean Hospital/Harvard Medical School, Belmont, Massachusetts
bBeth Israel Deaconess Medical Center, Boston, Massachusetts
*Corresponding author: Agustin G. Yip, MD, PhD; McLean Hospital, 115 Mill St, Belmont MA 02478 ([email protected]).
Electroconvulsive therapy (ECT) is a safe and highly effective treatment for major depressive disorder (MDD), with and without psychotic features, and for other indications such as mania and catatonia.1 It is not without potentially serious side effects, however, including confusion; memory loss, which may be persistent; physical side effects; and medical complications.2
Clinical features associated with better treatment response include depression with psychotic features,3 shorter duration of and medication failure in the current episode,4 older age,5 and catatonia.6 Individuals with borderline personality disorder (BPD), many of whom present with refractory depressive symptoms, are generally considered suboptimal responders to ECT.7-10 This prevailing collective understanding (eg, 2010 APA Treatment Guidelines for BPD: “‘ ¦patients with major depression and a personality disorder have a less favorable outcome with ECT than depressed patients without a personality disorder”11) is based on a limited number of uncontrolled studies.
Notably, most of the clinical and empirical studies that describe experience with ECT in patients with comorbid personality disorders do not report results specifically for BPD,11 are small case series,12-14 or, with few exceptions,8,10 use non-standardized outcome measures.8,11,15,16 A systematic review7 presented conflicting results concerning the treatment-modifying effect of BPD on ECT for depression but concluded that depressed patients with BPD can be effectively treated with ECT.
There is clearly a need to expand the limited evidence base to help clinicians and patients weigh the harms and benefits of ECT; on the one hand, patients should not needlessly be exposed to its risks without the prospect of benefit, and on the other, a potentially life-saving treatment should not be withheld from them. Our primary aim for this study was to assess the trajectory of depressive symptom reduction among a large, well-characterized cohort of depressed patients with (BPD+) and without (BPD-) BPD undergoing acute-phase ECT in an academic, freestanding psychiatric hospital.
METHODS
Setting
This study was a retrospective cohort study of psychiatric patients (aged 16-88 years) who received an index (ie, first) course of ECT during the study period, January 2011-December 2016, at McLean Hospital. This study was approved by the hospital organization’s Institutional Review Board. Patients who received ECT completed the hospital’s Clinical Measurement Initiative (CMI)17 self-reports, which include the McLean Screening Instrument for Borderline Personality Disorder (MSI-BPD),18 the key predictor variable of interest, collected at baseline, and the 16-item Quick Inventory of Depressive Symptomatology-Self Report (QIDS-SR),19 the key outcome depression measure of interest (globally and separated into symptom clusters), collected at baseline (before the first ECT treatment) and at 5-treatment intervals thereafter. We excluded participants for whom we had incomplete baseline CMI assessments. We also excluded patients whose baseline disease severity was not in at least the moderate range according to QIDS-SR criteria (score ≥ 11).19
Participants
Study participants comprised patients referred to the Neurotherapeutics Program at McLean Hospital primarily but not exclusively from the hospital’s inpatient services. All patients referred for ECT received a consultation from one of the ECT providers to determine appropriateness of this treatment. Patients underwent ECT after completing a thorough informed consent process and were not treated against their will without a court order or guardianship with ECT treatment plan in place. Prior to treatment, all patients received medical clearance from the hospital’s Internal Medicine service with further workup pursued as warranted.
Treatments
All patients received ECT using a spECTrum 5000Q device (MECTA Corporation). Seizure threshold was determined during the first treatment by dose titration. Most patients were started with ultrabrief pulse (0.3 ms) right unilateral electrode placement at 6 times the seizure threshold and continued with this modality through at least the first 6 treatments. Dosage could be increased if seizures were felt to be inadequate in morphology or if the patient was not progressing clinically. If no improvement was seen, patients generally received higher-power ultrabrief pulse unilateral treatment or were switched to brief pulse or bilateral setting (using the half-age method) depending on illness severity and patient preference after discussion of risks and benefits. Occasionally, patients were started on bilateral (at twice the seizure threshold) or brief pulse unilateral settings if there were an acute need for a faster response (eg, severe suicidality). Generally, methohexital was used as the anesthetic agent but may have been replaced by etomidate or ketamine if seizures were inadequate. Succinylcholine was used as the muscle relaxant, and a small dose of propofol was typically used to prevent posttreatment agitation.
Acute phase of ECT treatment usually consisted of 6 to 12 treatments, typically occurring within a 3- to 4-week period (ie, 3 treatments/wk). We examined treatment outcomes serially at 5-treatment intervals from baseline through the third QIDS-SR assessment (after treatment 15, approximately), including all individuals who would have completed what is generally considered a complete course of acute phase treatment.
BPD Defined
The MSI-BPD18 is a 10-item screening instrument based on Diagnostic and Statistical Manual for Mental Disorders, Fourth Edition (DSM-IV)20 criteria for BPD. It consists of binary questions about the respondent’s feelings of dissociation, problems with impulsivity, chronic feelings of emptiness, unstable and intense interpersonal relationships, identity disturbance, deliberate self-harm, affective instability, frantic efforts to avoid real or imagined abandonment, recurrent suicidal behavior, and paranoid ideation. A cutoff MSI-BPD score of ≥ 7 was used to define BPD+ as this score optimized sensitivity (0.81) and specificity (0.85) against the Diagnostic Interview for DSM-IV Personality Disorders DIPD-IV21 BPD module.18
Outcome Variables
The QIDS-SR19,22 consists of 16 items that correspond to DSM-IV major depressive episode (MDE) symptom criterion domains including sleep disturbance, sad/low mood, decrease/increase in appetite or weight, concentration, self-criticism, suicidal ideation, interest, energy/fatigue, and psychomotor agitation or retardation. The total score ranges between 0 and 27; we used a cutoff score of ≥ 11 to define an MDE of at least moderate severity (in accordance with QIDS-SR criteria19) for which ECT would be considered. We also examined the patients’ QIDS-SR suicidality item score trajectory specifically as chronic suicidality is one of the cardinal features of BPD. Additionally, we examined the trajectories of 3 key empirically derived symptom clusters shown to differ in their responsiveness to antidepressant medications23: sleep disturbance, core emotional (energy/fatigability, concentration, loss of interest, mood, feelings of worthlessness), and atypical (psychomotor slowing/agitation, suicidal ideation, hypersomnia) symptom clusters.
Statistical Analysis
We calculated univariate summary statistics of patient demographic and clinical characteristics at baseline for BPD+ and BPD- groups.
For the main outcome measure, we used a generalized linear mixed effects model.24,25 As fixed effects, we entered BPD screening status, treatment interval, age, sex, psychiatric hospitalization within the previous 6 months, self-rated physical health, substance misuse, and baseline QIDS-SR score. As random effects, we used intercepts for participants and by-participant random slopes for the effect of BPD. These models do not discard incomplete observations, and they address missing data by assuming that missing values are missing at random (MAR).26 All the models described were fitted using the lme4 package27 in R version 3.5.2 (R Foundation, 2018).28 This likelihood-based estimation incorporates all observed information on the repeated measures of the outcome to produce estimates that are valid under a MAR assumption; no explicit imputation is involved.29 We did not replace missing response values with simple-imputed values (eg, last observation carried forward [LOCF]).30,31 We nonetheless conducted sensitivity analyses using complete-case-only and LOCF data.

- Prevailing beliefs about depressed patients with borderline personality disorder (BPD) being suboptimal candidates for electroconvulsive therapy (ECT) are based on limited data.
- Results from this large cohort indicate that ECT alleviates depressive symptoms, regardless of BPD comorbidity. However, nondepressive BPD core features may lead to a less durable response to treatment.
We used analogous linear mixed models to regress the secondary outcomes against the same main predictor variable (BPD) and covariates.
RESULTS
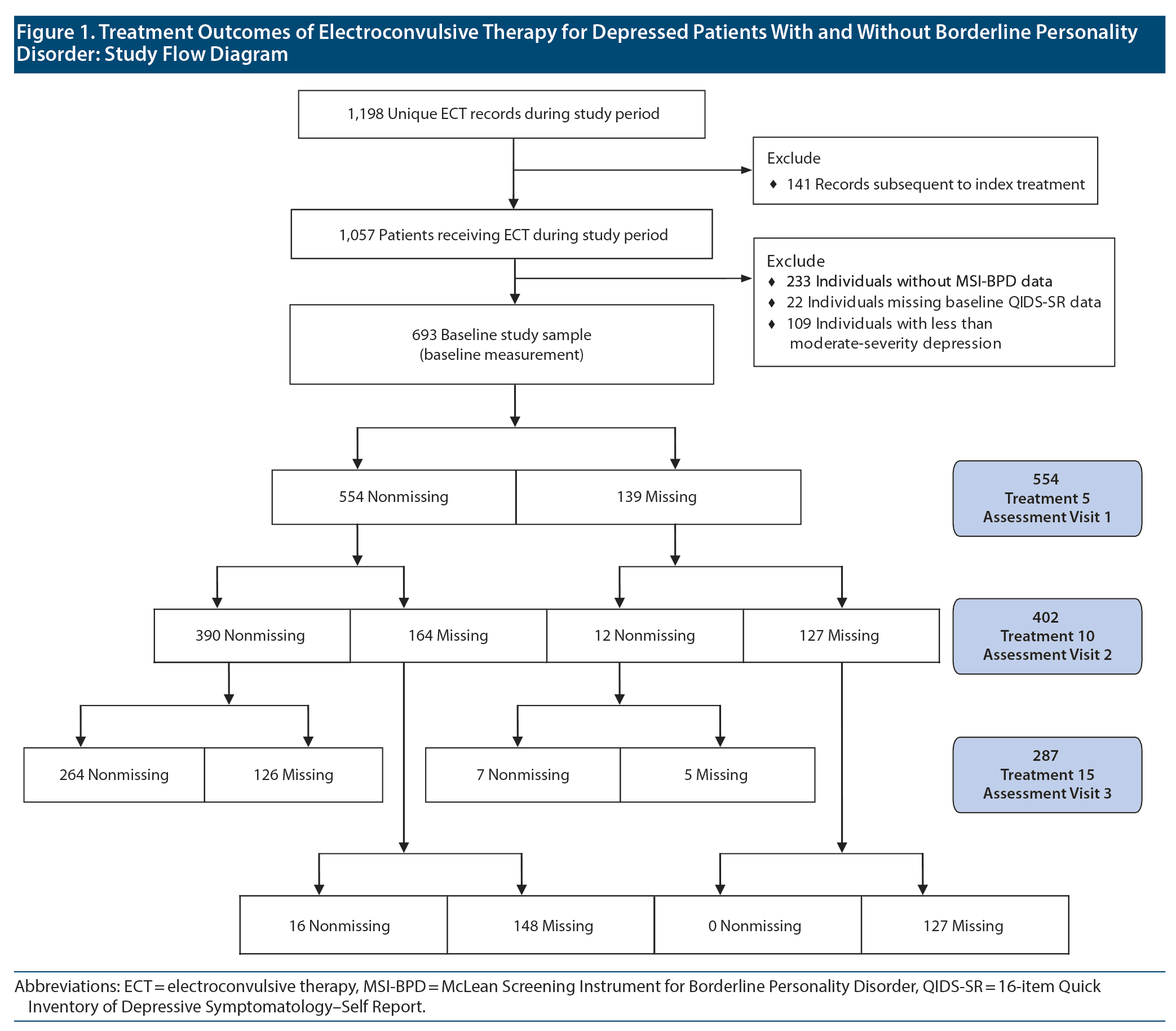
One thousand fifty-seven individuals received ECT during the study period (141 of whom received > 1 course of treatment, in which case only their first, or “index,” course was included). Among these, 824 also had MSI-BPD data. Twenty-two were excluded from the analysis because of missing baseline QIDS-SR data, and 109 were excluded because they did not report at least moderate-severity QIDS-SR depressive symptoms at baseline. Thus, our study sample consisted of the remaining 693 subjects.
Of the study sample, 127 were lost to follow-up after the baseline measurement, 148 did not return after the first follow-up assessment, and 126 did not return after the second assessment visit. Two hundred sixty-four individuals completed all 3 follow-up assessments. Only 28 exhibited nonmonotonic missingness patterns. In summary, 693, 554, 402, and 287 subjects contributed data to baseline and first, second, and third follow-up assessments, respectively (Figure 1). Missing data patterns did not differ between BPD groups (Fisher exact P = .60). Neither was missingness associated with baseline or preceding QIDS-SR score: At visit 1, the nonmissing group had a baseline score of 18.1 vs 17.5 among the missing group (P = .14); at visit 2, nonmissing observations had a mean percentage QIDS-SR score reduction from baseline to visit 1 of 34.4% vs 37.1% among the missing (P = .31); and at visit 3 neither percent change between baseline and visit 2 (40.2% vs 42.9% reduction, P = .55) nor percent change between visit 1 and visit 2 (1.1% increase vs 4.0% reduction, P = .41) were different between the nonmissing and missing groups.
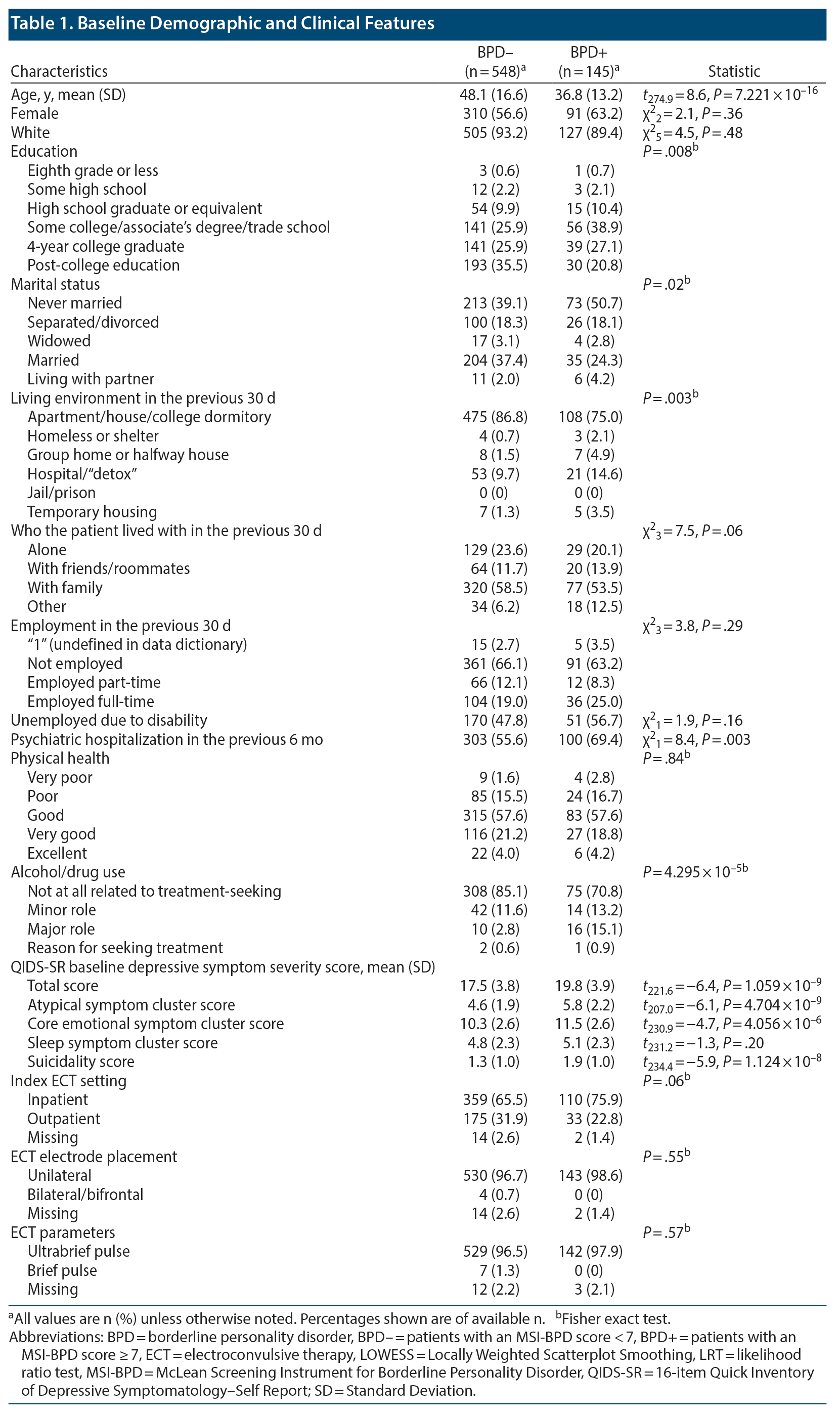
In the study sample, the BPD+ group (n = 145) differed from BPD- group (n = 548) on a number of key demographic and clinical features, namely age (the BPD+ group was younger by an average of 11 years), education (a lower proportion of patients in the BPD+ group completed college or higher level), marital status (patients in the BPD+ group were less likely to be married), living situation within 30 days prior to baseline measurement (individuals in the BPD+ group were less likely to be in permanent accommodation), psychiatric hospitalization within 6 months prior to baseline measurement (patients in the BPD+ group were more likely to have been), and substance misuse (16% of the BPD+ group vs 3.4% of the BPD- group). In particular, the BPD+ group had higher total QIDS-SR scores on admission (mean [SD] = 19.8 [3.9] vs 17.5 [3.8]; P < .0001), driven by significantly higher atypical, core emotional, and suicidality symptom (but not sleep) cluster scores. Sex, race/ethnicity, current living situation, current employment status, disability being reason for unemployment, and physical health status were not substantially different between groups (Table 1).
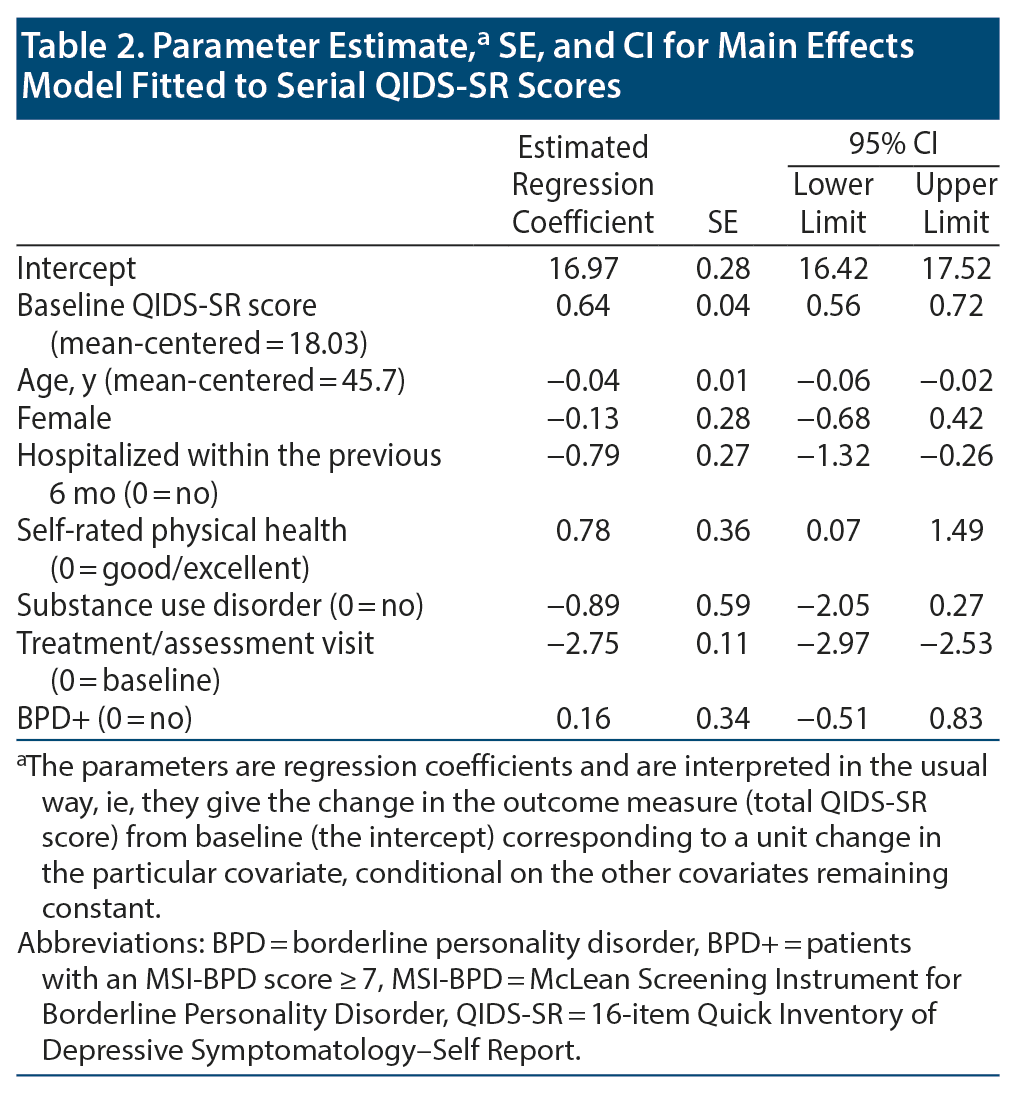
Age, recent psychiatric hospitalization, physical health, and baseline symptom severity were associated with ECT treatment response adjusting for all other covariates, but not sex, physical health, and substance misuse (see Table 2).
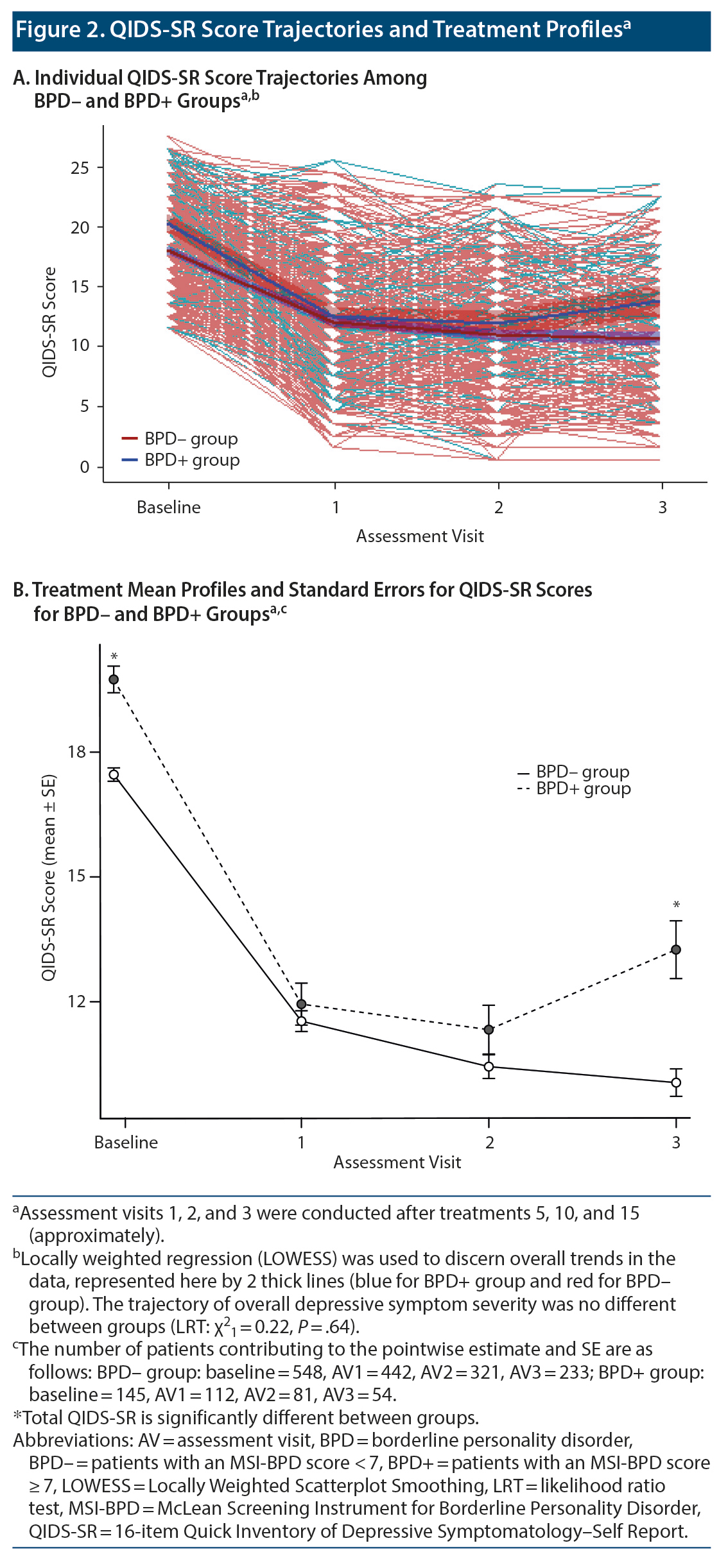
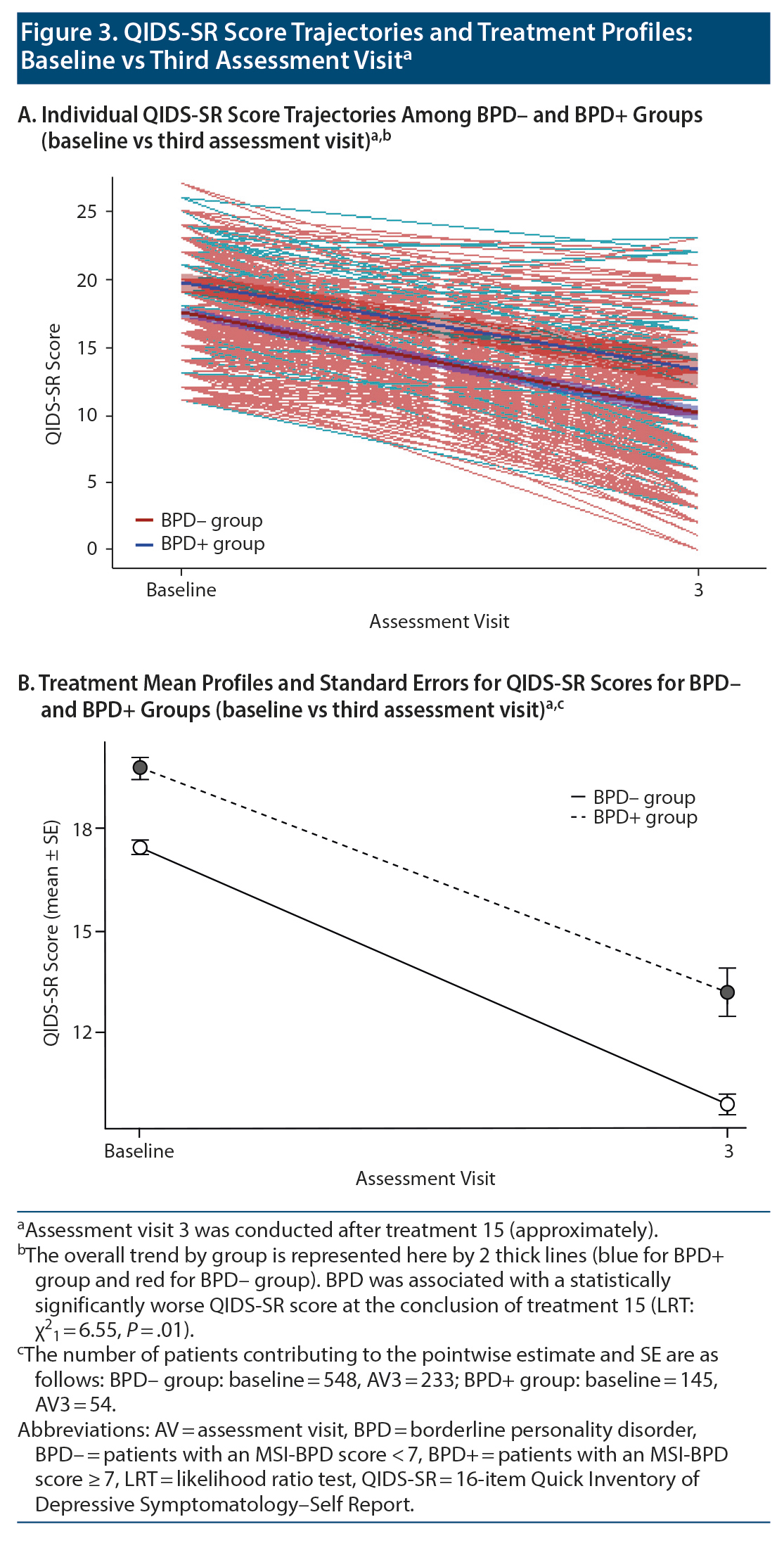
Electroconvulsive therapy was associated with significant clinical improvement in overall QIDS-SR score independent of BPD status (linear mixed model [LMM] regression coefficient ± SE = −2.75 ± 0.11, P < .0001). Mean (SD) total score among individuals in the BPD- group declined from 17.5 (3.8) at baseline to 11.5 (5.3) at the fifth treatment to 10.4 (5.1) at the tenth treatment to 10.0 (5.1) at the 15th treatment. The BPD+ group showed a similar overall pattern that diverged slightly at treatment endpoint: 19.8 (3.9) at baseline vs 11.9 (5.3) at the fifth treatment vs 11.3 (5.3) at the tenth treatment vs 13.2 (5.1) at the 15th treatment (Figure 2). Despite the mean QIDS-SR total score’s being significantly different between groups at treatment endpoint, the trajectory of overall depressive symptom severity was not different (likelihood ratio test [LRT] χ21 = 0.22, P = .64; Figure 2A)—see Table 2. Of note, results from post hoc analysis considering only baseline vs the third assessment visit (ie, 15th treatment) indicate that BPD was associated with a statistically significantly worse QIDS-SR score (LMM regression coefficient ± SE = 0.70 ± 0.28, P = .01; LRT χ21 = 6.55, P = .01; Figure 3).
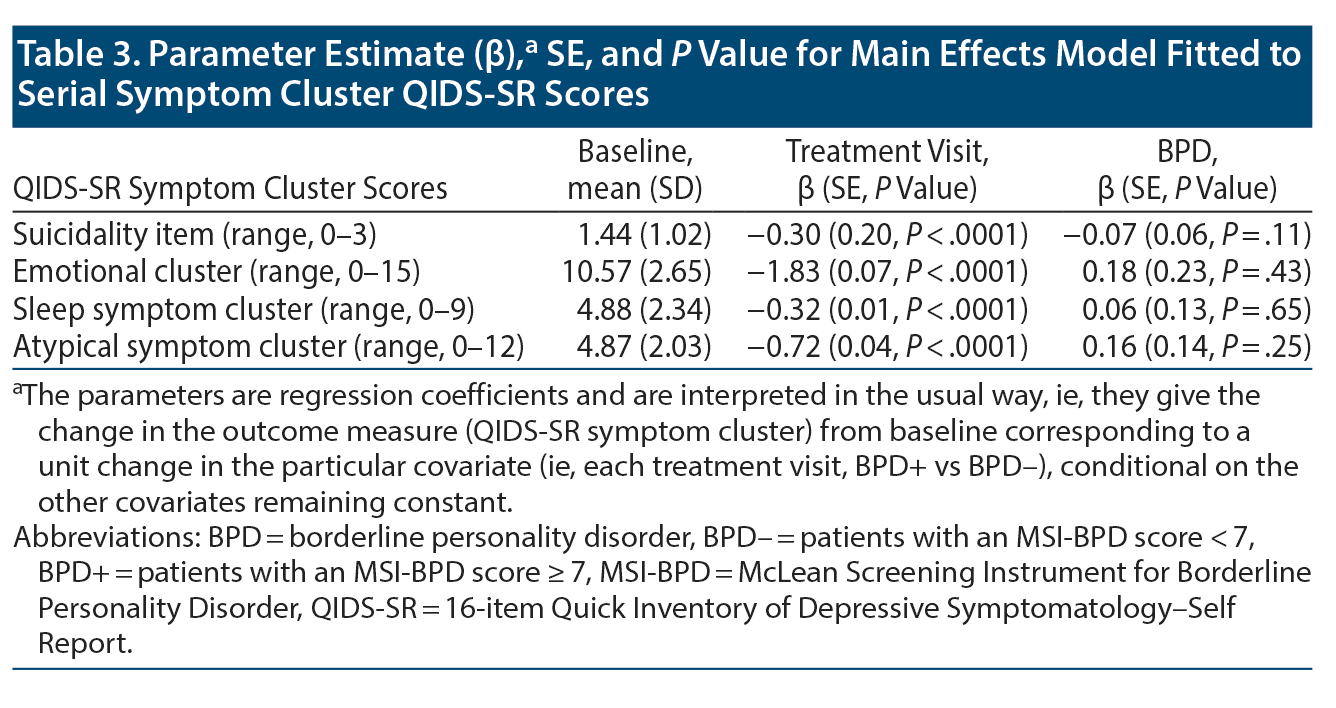
Change in QIDS-SR suicidality score (range, 0-3) was not significantly different between groups (LRT χ21 = 1.63, P = .20). The cohort had significantly reduced scores throughout the ECT treatment course (LMM regression coefficient ± SE = −0.30 ± 0.20, P < .0001; Table 3). Baseline vs 15th treatment mean (SD) scores for BPD- and BPD+ groups were 1.3 (1.0) vs 0.6 (0.8) and 1.9 (1.0) vs 1.0 (0.9), respectively.
There was a significant improvement in the QIDS-SR core emotional symptom cluster severity (score range, 0-15) in the cohort (LMM regression coefficient ± SE = −1.83 ± 0.07, P < .0001). Mean (SD) scores among individuals in the BPD- group declined from 10.3 (2.6) at baseline to 5.3 (3.5) by the 15th treatment and among individuals in the BPD+ group, from 11.5 (2.6) to 7.6 (3.5). Score trajectory was not significantly different between groups (LRTχ21 = 0.63, P = .43). See Table 3 for details.
Change in score on the QIDS-SR sleep symptom cluster subscale (range, 0-9) was not significantly different between groups (LRT χ21 = 0.20, P = .65). Both groups had significantly reduced insomnia severity following ECT (LMM regression coefficient ± SE = -0.32 ± 0.01, P < .0001): baseline vs 15th treatment mean (SD) scores for BPD- and BPD+ groups were 4.8 (2.4) vs 3.0 (1.9) and 5.1 (2.3) vs 3.3 (2.3), respectively. See Table 3 for details.
There was a significant improvement in the QIDS-SR atypical symptom cluster (score range, 0-12) in the cohort (LMM regression coefficient ± SE = −0.72 ± 0.04, P < .0001). Mean (SD) scores among individuals in the BPD- group declined from 4.6 (1.9) at baseline to 2.8 (2.0) by the 15th treatment and among individuals in the BPD+ group, from 5.8 (2.2) to 3.9 (2.2). Score trajectory was not significantly different between groups (LRT χ21 = 1.30; P = .25); see Table 3. Also see Supplementary Figure 1A-1D for symptom cluster trajectories.
Sensitivity analyses comprising LOCF and complete-case analyses did not lead to different results or conclusions (Supplementary Tables 1A-1C and Supplementary Tables 2A-2C).
In summary, screening positive for BPD was associated with higher baseline levels of global depressive and symptom cluster (except for sleep disturbance) severity, but not with serial change in total QIDS-SR score during acute-phase ECT, nor for suicidality, core emotional, sleep, or atypical symptom clusters. Results from post hoc analysis, however, suggest that BPD+ individuals may do less well compared with BPD- counterparts by the end of treatment.
DISCUSSION
In this retrospective (chart review) cohort study involving standardized baseline and outcome data collection at an academic psychiatric hospital, we followed global and symptom-cluster QIDS-SR score trajectories of moderately to severely depressed patients with and without comorbid BPD undergoing a 15-treatment course of ECT. Our results indicate clinically significant improvement in depression and its key symptom (suicidality, sleep, core emotional, and atypical) clusters, as measured using the QIDS-SR, in patients both with and without BPD who received ECT. No significant differences in treatment response trajectory were noted between the BPD+ and BPD- groups. It is worth noting, however, that the mean QIDS-SR total score was significantly different between groups by the end of treatment, with an estimated post hoc LMM mean score difference of 0.7 between groups.
While there was clear improvement with the BPD+ group in our setting, there was an uptick in QIDS-SR score between the 10th and 15th treatments that was not evident in the comparison group. This increase may be an artifact of the dropout of patients (although neither BPD nor precedent QIDS-SR score was associated with dropout), and one could speculate that it is due to a rebound in affective lability as part of the natural course of BPD or that it might represent a lack of durability in the ECT response among BPD patients. Further study of the post-ECT period and continuation ECT for BPD patients would therefore be of great interest.
To our knowledge, our study is the largest ECT cohort study published to date that specifically examined the potential modification of treatment effect by BPD among depressed patients. Firstly, our use of standardized, validated, and systematically collected baseline, predictor, and outcome measures as part of routine clinical care enabled us to efficiently accrue and analyze data in a reproducible way. In addition, our observation most closely approximates adequate ECT delivery as currently practiced. Lastly, we used statistical techniques that appropriately handled our data’s longitudinal structure and missing observations, as well as reinforced primary findings with sensitivity analyses.
There are a number of limitations to this study worth noting. Inherent in the observational nature of our study design, treatment visit (time) was a proxy for treatment effect of ECT. Data coming from a single site, as they do here, with its particular setting, patient population, and ECT delivery, may limit generalizability of our results. Both predictor and outcome measures rely on self-report, and the MSI-BPD is a screening tool. In common with most longitudinal studies and case series, an extensive amount of data are missing (with significant loss at each subsequent assessment interval), though they are transparently presented as well as dealt with using mixed models and sensitivity analyses. In addition, these results do not address any potential between-group difference in the durability of treatment effect, which is because our data do not capture outcomes after the end of ECT. Perhaps most importantly, the QIDS-SR does not interrogate the core affective, cognitive, behavioral, and interpersonal features of BPD.
Despite the sparse previously published evidence base, it is worth putting our findings in the context of those of 2 methodologically sound studies. Feske et al8 followed 139 patients (n = 20 BPD+ vs n = 42 other personality disorder [PD] vs n = 77 no PD) with unipolar depression scoring at least 20 on the Hamilton Depression Rating Scale treated with ECT and assessed their symptom improvement within 3 days and after 4-8 days after ECT completion. They found lower remission rates among the BPD+ group than the other PD group, which in turn had a worse outcome compared with individuals without comorbid PD (22.2% vs 55.6% vs 71.7%, respectively). More recently, Lee et al10 used a study design closely matching ours (a retrospective chart review from the Mayo Clinic comprising 137 patients with MDD, of whom 29 had screened positive for BPD) and found no difference in Patient Health Questionnaire-9 scores before, immediately after, and a month after ECT treatment.
In conclusion, depression and BPD exhibit phenotypic divergence and yet are highly comorbid, suggesting overlapping underlying liabilities and longitudinal course.32 Our findings add to the evidence base that provides the clinician with a rationale for proceeding with ECT among depressed patients, notwithstanding comorbid BPD. While these data suggest that BPD+ patients show improvement in depression symptoms from ECT, they do not speak to whether there is similar improvement in the BPD core symptoms that led patients and their doctors to turn to ECT in the first place.
Submitted: December 9, 2019; accepted July 22, 2020.
Published online: January 19, 2021.
Potential conflicts of interest: None.
Funding/support: We gratefully acknowledge the funding support of McLean Hospital for the Clinical Measurement Initiative (CMI) program and the Frazier Institute. There was no external funding for this project.
Role of the sponsor: None.
Acknowledgments: The authors thank Alisa B. Busch, MD, MS (McLean Hospital/Harvard Medical School, Belmont, Massachusetts) for insights on CMI data collection and management. Dr Busch has no conflicts of interest to declare.
Supplementary material: See accompanying pages.
REFERENCES
1.Weiner RD, Reti IM. Key updates in the clinical application of electroconvulsive therapy. Int Rev Psychiatry. 2017;29(2):54-62. PubMed CrossRef
2.Andrade C, Arumugham SS, Thirthalli J. Adverse effects of electroconvulsive therapy. Psychiatr Clin North Am. 2016;39(3):513-530. PubMed CrossRef
3.Petrides G, Fink M, Husain MM, et al. ECT remission rates in psychotic versus nonpsychotic depressed patients: a report from CORE. J ECT. 2001;17(4):244-253. PubMed CrossRef
4.Haq AU, Sitzmann AF, Goldman ML, et al. Response of depression to electroconvulsive therapy: a meta-analysis of clinical predictors. J Clin Psychiatry. 2015;76(10):1374-1384. PubMed CrossRef
5.O’ Connor MK, Knapp R, Husain M, et al. The influence of age on the response of major depression to electroconvulsive therapy: a CORE Report. Am J Geriatr Psychiatry. 2001;9(4):382-390. PubMed CrossRef
6.Luchini F, Medda P, Mariani MG, et al. Electroconvulsive therapy in catatonic patients: efficacy and predictors of response. World J Psychiatry. 2015;5(2):182-192. PubMed CrossRef
7.DeBattista C, Mueller K. Is electroconvulsive therapy effective for the depressed patient with comorbid borderline personality disorder? J ECT. 2001;17(2):91-98. PubMed CrossRef
8.Feske U, Mulsant BH, Pilkonis PA, et al. Clinical outcome of ECT in patients with major depression and comorbid borderline personality disorder. Am J Psychiatry. 2004;161(11):2073-2080. PubMed CrossRef
9.Rasmussen KG. Do patients with personality disorders respond differentially to electroconvulsive therapy? a review of the literature and consideration of conceptual issues. J ECT. 2015;31(1):6-12. PubMed CrossRef
10.Lee JH, Kung S, Rasmussen KG, et al. Effectiveness of electroconvulsive therapy in patients with major depressive disorder and comorbid borderline personality disorder. J ECT. 2019;35(1):44-47. PubMed CrossRef
11.American Psychiatric Association Practice Guidelines. Practice guideline for the treatment of patients with borderline personality disorder. Am J Psychiatry. 2001;158(suppl):1-52. PubMed
12.Flint V, Hill-Johnes S. How effective is ECT for those with borderline personality disorder? Nurs N Z. 2008;14(9):12-14. PubMed
13.Kramer BA. Poor response to electroconvulsive therapy in patients with a combined diagnosis of major depression and borderline personality disorder. Lancet. 1982;2(8306):1048. PubMed CrossRef
14.Zimmerman M, Coryell W, Pfohl B, et al. ECT response in depressed patients with and without a DSM-III personality disorder. Am J Psychiatry. 1986;143(8):1030-1032. PubMed CrossRef
15.Black DW, Goldstein RB, Nasrallah A, et al. The prediction of recovery using a multivariate model in 1,471 depressed inpatients. Eur Arch Psychiatry Clin Neurosci. 1991;241(1):41-45. PubMed CrossRef
16.Sareen J, Enns MW, Guertin JE. The impact of clinically diagnosed personality disorders on acute and one-year outcomes of electroconvulsive therapy. J ECT. 2000;16(1):43-51. PubMed CrossRef
17.Busch A, Laband A, Kos A, et al. Developing and implementing an electronic patient-reported outcomes measurement using REDCap in usual care psychiatric settings. iproc. 2016;2(1):e45. CrossRef
18.Zanarini MC, Vujanovic AA, Parachini EA, et al. A screening measure for BPD: the McLean Screening Instrument for Borderline Personality Disorder (MSI-BPD). J Pers Disord. 2003;17(6):568-573. PubMed CrossRef
19 Rush AJ, Trivedi MH, Ibrahim HM, et al. The 16-Item Quick Inventory of Depressive Symptomatology (QIDS), clinician rating (QIDS-C), and self-report (QIDS-SR): a psychometric evaluation in patients with chronic major depression. Biol Psychiatry. 2003;54(5):573-583. PubMed CrossRef
20.American Psychiatric Association. Diagnostic and Statistical Manual for Mental Disorders. Fourth Edition. Washington, DC: American Psychiatric Association; 1994.
21.Zanarini MC, Frankenburg FR, Sickel AE, et al. The Diagnostic Interview for DSM-IV Personality Disorders (DIPD-IV). Belmont, MA: McLean Hospital; 1996.
22.Reilly TJ, MacGillivray SA, Reid IC, et al. Psychometric properties of the 16-item Quick Inventory of Depressive Symptomatology: a systematic review and meta-analysis. J Psychiatr Res. 2015;60:132-140. PubMed CrossRef
23.Chekroud AM, Gueorguieva R, Krumholz HM, et al. Reevaluating the efficacy and predictability of antidepressant treatments: a symptom clustering approach. JAMA Psychiatry. 2017;74(4):370-378. PubMed CrossRef
24.Gelman A, Hill J. Data Analysis Using Regression and Multilevel/Hierarchical Models. Cambridge, NY: Cambridge University Press; 2007.
25.Gueorguieva R. Statistical Methods in Psychiatry and Related Fields: Longitudinal, Clustered, and Other Repeated Measures Data. Boca Raton, FL: CRC Press; 2017.
26.Matthews JN, Altman DG, Campbell MJ, et al. Analysis of serial measurements in medical research. BMJ. 1990;300(6719):230-235. PubMed CrossRef
27.Bates D, Maechler M, Bolker B, et al. Fitting linear mixed-effects models using lme4. J Stat Softw. 2015;67(1):1-48. CrossRef
28.R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing; 2013. https://cran.r-project.org/doc/manuals/r-release/fullrefman.pdf
29.Sullivan TR, White IR, Salter AB, et al. Should multiple imputation be the method of choice for handling missing data in randomized trials? Stat Methods Med Res. 2018;27(9):2610-2626. PubMed CrossRef
30.National Research Council (USA) Panel on Handling Missing Data in Clinical Trials. The Prevention and Treatment of Missing Data in Clinical Trials. Washington, DC: National Academies Press; 2010.
31.Little RJ, D’ Agostino R, Cohen ML, et al. The prevention and treatment of missing data in clinical trials. N Engl J Med. 2012;367(14):1355-1360. PubMed CrossRef
32.Choi-Kain LW, Rodriguez-Villa AM. Borderline personality disorder, atypical depression, and clycothymia: diagnostic distinctions crossing mood and personality disorders borders. In: Choi-Kain LW, Gunderson JG, eds. Borderline Personality and Mood Disorders: Comorbidity and Controversy. New York, NY: Springer; 2015:39-64.
Enjoy free PDF downloads as part of your membership!
Save
Cite
Advertisement
GAM ID: sidebar-top