Objective: Although most patients with schizophrenia are not aggressive, individuals with the disorder have increased risk of hostile behavior. Cariprazine, a dopamine D3 and D2 receptor partial agonist antipsychotic with preferential binding to D3 receptors, was evaluated for antihostility effects in patients with schizophrenia.
Method: Post hoc analyses were conducted using pooled data from 3 positive randomized, placebo-controlled, phase 2/3 studies in inpatients (18-60 years) with acute exacerbation of schizophrenia according to DSM-IV-TR criteria; data were collected between 2008 and 2011. The principal post hoc outcome was mean change from baseline to week 6 on the Positive and Negative Syndrome Scale (PANSS) hostility item (P7); separate analyses adjusted for certain PANSS positive symptoms and sedation covariates. Analyses were based on the pooled intent-to-treat population (N = 1,466) using a mixed-effects model for repeated measures approach; separate analyses were conducted in subgroups categorized by baseline hostility item scores (P7: ≥ 2, ≥ 3, ≥ 4).
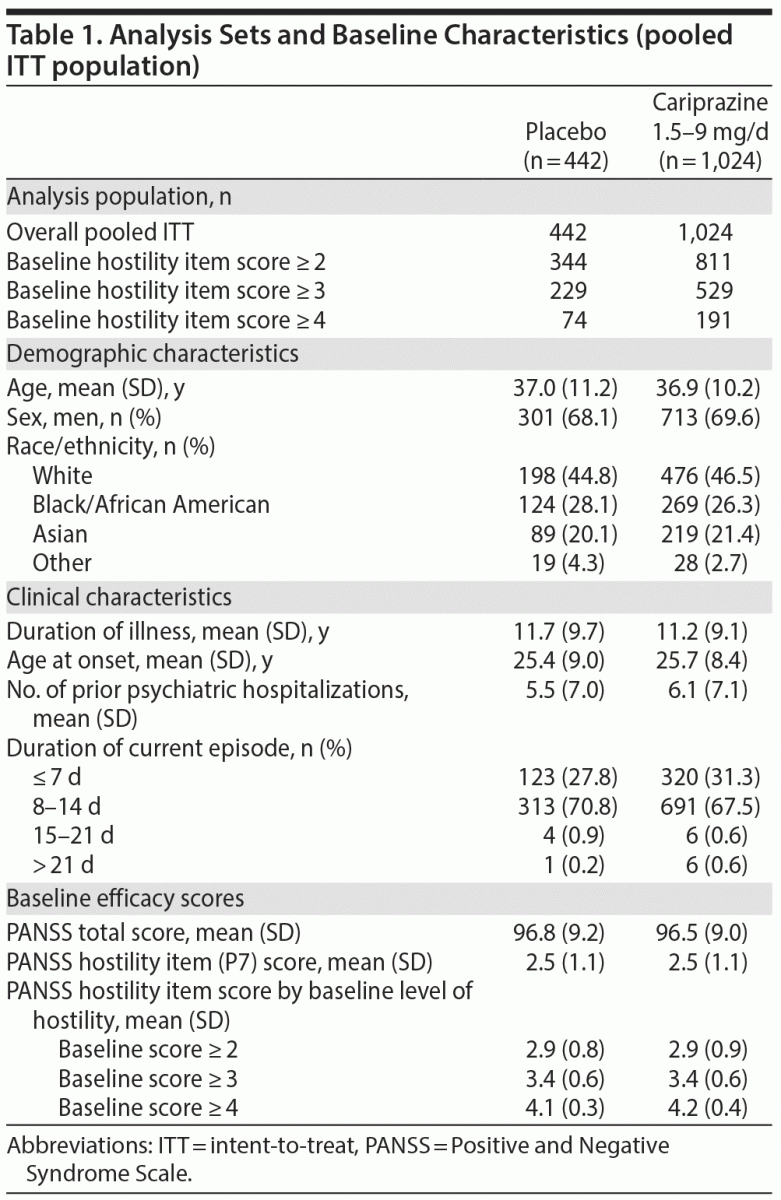
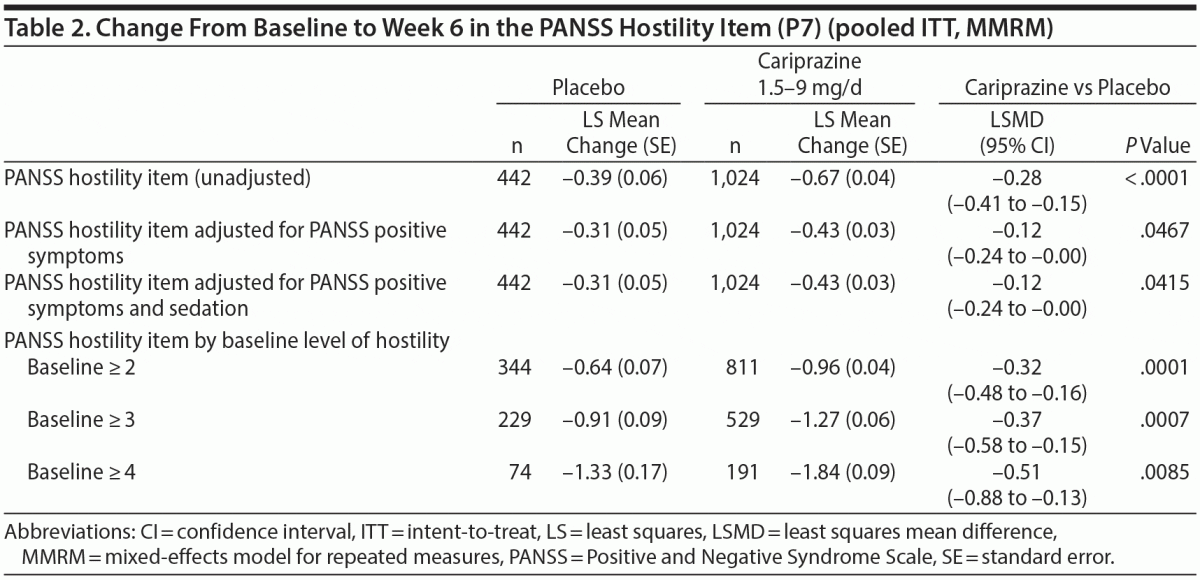
Results: The least squares mean difference (LSMD) in change from baseline to week 6 was statistically significant on all PANSS hostility item analyses in favor of cariprazine versus placebo: unadjusted (-0.28; P < .0001), adjusted for PANSS positive symptoms (-0.12; P < .05), adjusted for positive symptoms plus sedation (-0.12; P < .05). The magnitude of change for cariprazine increased with greater baseline hostility (LSMD vs placebo for ≥ 2, ≥ 3, ≥ 4 subgroups: -0.32, -0.37, -0.51, respectively; P < .01 all).
Conclusions: Significant improvement on the hostility item was seen in cariprazine- versus placebo-treated patients with schizophrenia; the effect of cariprazine increased with greater levels of baseline hostility.
Trials Registration: ClinicalTrials.gov identifiers: NCT00694707, NCT01104766, and NCT01104779
The Effect of Cariprazine on Hostility
Associated With Schizophrenia:
Post Hoc Analyses From 3 Randomized Controlled Trials
ABSTRACT
Objective: Although most patients with schizophrenia are not aggressive, individuals with the disorder have increased risk of hostile behavior. Cariprazine, a dopamine D3 and D2 receptor partial agonist antipsychotic with preferential binding to D3 receptors, was evaluated for antihostility effects in patients with schizophrenia.
Method: Post hoc analyses were conducted using pooled data from 3 positive randomized, placebo-controlled, phase 2/3 studies in inpatients (18–60 years) with acute exacerbation of schizophrenia according to DSM-IV-TR criteria; data were collected between 2008 and 2011. The principal post hoc outcome was mean change from baseline to week 6 on the Positive and Negative Syndrome Scale (PANSS) hostility item (P7); separate analyses adjusted for certain PANSS positive symptoms and sedation covariates. Analyses were based on the pooled intent-to-treat population (N = 1,466) using a mixed-effects model for repeated measures approach; separate analyses were conducted in subgroups categorized by baseline hostility item scores
(P7: ≥ 2, ≥ 3, ≥ 4).
Results: The least squares mean difference (LSMD) in change from baseline to week 6 was statistically significant on all PANSS hostility item analyses in favor of cariprazine versus placebo: unadjusted (–0.28; P < .0001), adjusted for PANSS positive symptoms (–0.12; P < .05), adjusted for positive symptoms plus sedation (–0.12; P < .05). The magnitude of change for cariprazine increased with greater baseline hostility (LSMD vs placebo for ≥ 2, ≥ 3, ≥ 4 subgroups: –0.32, –0.37, –0.51, respectively; P < .01 all).
Conclusions: Significant improvement on the hostility item was seen in cariprazine- versus placebo-treated patients with schizophrenia; the effect of cariprazine increased with greater levels of baseline hostility.
Trials Registration: ClinicalTrials.gov identifiers: NCT00694707, NCT01104766, and NCT01104779
J Clin Psychiatry 2016;77(1):109–115
dx.doi.org/10.4088/JCP.15m10192
© Copyright 2016 Physicians Postgraduate Press, Inc.
aNew York Medical College, Valhalla, New York
bForest Research Institute, an Allergan affiliate, Jersey City, New Jersey
cPrescott Medical Communications Group, Chicago, Illinois
dGedeon Richter Plc, Budapest, Hungary
*Corresponding author: Leslie Citrome, MD, MPH, 11 Medical Park Dr, Ste 106, Pomona, NY 10970 ([email protected]).
Although most patients with schizophrenia are not aggressive, individuals with this disorder have an increased risk of hostile and aggressive behavior.1,2 In their most severe form, these behaviors are a medical emergency that require intervention to ensure the safety of the agitated individual, caretakers, medical personnel, and others.3 Hostile and aggressive behavior associated with mental illness is heterogeneous in origin and presentation. The etiology of aggression in schizophrenia is diverse, with causes as wide ranging as substance abuse, medication noncompliance, mistreatment in childhood, conduct disorder, and economic disadvantage.4 Managing the spectrum of aggressive behaviors in patients with schizophrenia is a significant treatment challenge.
Hostility is characterized by irritability, anger, resentment, or aggression.5 Although not the same thing as violent behavior itself, hostility has the potential to escalate into violence and is strongly associated with increased risk of violence in psychotic patients.6 For clinical research purposes, hostility is operationally defined by scores on rating scales. As such, the Positive and Negative Syndrome Scale (PANSS)7 hostility item (P7) is frequently used as a proxy to assess the antihostility effects of antipsychotics in schizophrenia studies.8–10 Aggression, whether impulsive or premeditated, is an overt action that is intended to cause harm.5 Agitated, hostile, and aggressive behaviors in patients with schizophrenia greatly contribute to the burden of disease through increased frequency and duration of hospitalizations, involvement with the criminal justice system, physical victimization as a result of retaliation, and social stigma.11
Atypical antipsychotics are an important treatment option for patients with schizophrenia who exhibit hostile or aggressive behavior.12 Cariprazine, a dopamine D3 and D2 receptor partial agonist antipsychotic with preferential binding to D3 receptors, is approved by the US Food and Drug Administration for the treatment of schizophrenia and mixed or manic episodes of bipolar I disorder. The efficacy and safety of cariprazine in the treatment of patients with schizophrenia have been evaluated in 3 positive randomized, placebo-controlled, phase 2/3 clinical studies.13–15 Post hoc analyses of pooled data from these studies were conducted to investigate the effect of cariprazine on hostility in patients with acute exacerbation of schizophrenia.
METHOD
Study Design
Data were extracted and pooled from 3 positive, 6-week, double-blind, randomized, controlled studies of cariprazine that were conducted in patients with schizophrenia between 2008 and 2011. Two of the studies were fixed-dose and included active and placebo controls. In RGH-MD-16 (ClinicalTrials.gov identifier: NCT00694707),13 patients were randomized to cariprazine 1.5 mg/d, 3 mg/d, or 4.5 mg/d; risperidone 4 mg/d; or placebo; in RGH-MD-04 (NCT01104766),15 patients were randomized to cariprazine 3 mg/d or 6 mg/d, aripiprazole 10 mg/d, or placebo. Risperidone and aripiprazole were included as active controls to ensure assay sensitivity. The third study, RGH-MD-05 (NCT01104779),14 had a fixed/flexible-dose design, and patients were randomized to cariprazine 3–6 or 6–9 mg/d or placebo.
Methods of the constituent studies have been detailed previously.13–15 Briefly, the studies consisted of a washout period of up to 1 week, 6 weeks of double-blind treatment, and a 2-week safety follow-up. The primary efficacy measure in each study was the PANSS; assessments were conducted at screening, baseline, and at the end of each double-blind treatment week (weeks 1–6). Each protocol was approved by an institutional review board (US sites) or ethics committee (non-US sites), and ICH-E6 Good Clinical Practice guidelines were followed; written informed consent was obtained from all participants.
Patients
Patients were 18–60 years of age, inclusive, and had a diagnosis of schizophrenia based on Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, Text Revision (DSM-IV-TR)16 criteria, with a current psychotic episode < 2 weeks’ duration. The clinical measures required for inclusion were a baseline score ≥ 4 on the Clinical Global Impressions-Severity (CGI-S) Scale (moderately ill or worse),17 PANSS total score ≥ 80 and ≤ 120, and a score ≥ 4 (moderate or higher) on at least 2 of the following PANSS items: delusions, hallucinatory behavior, suspiciousness/persecution, and conceptual disorganization. Patients were hospitalized during screening and for a minimum of 4 weeks during double-blind treatment. Exclusionary criteria were typical of clinical studies in schizophrenia and included various DSM-IV-TR diagnoses (eg, schizoaffective disorder, bipolar disorders, cognitive disorders), treatment-resistant schizophrenia (no response to at least 2 antipsychotic trials of ≥ 6 weeks at a therapeutic dose), substance abuse, and suicidal or homicidal intent (active or past attempt in past 2 years).
Assessment of Hostility
The principal post hoc efficacy assessment of hostility was mean change from baseline to week 6 on the PANSS hostility item, which assesses verbal and nonverbal expressions of anger and resentment, including sarcasm, passive-aggressive behavior, verbal abuse, and assaultiveness.18 Scores range from 1 (no hostility) to 7 (extreme hostility characterized by marked anger that results in extreme uncooperativeness, preventing other interactions, or physical assault). Mean change from baseline on the PANSS hostility item was assessed in the overall patient population and in subgroups of patients categorized by increasing baseline hostility level. Baseline hostility subgroups were defined by cutoff scores and categorized as minimally severe (item score at least 2: questionable pathology; may be at the upper extreme of normal limits), mildly severe (item score at least 3: indirect or restrained communication of anger such as sarcasm, disrespect, hostile expressions, and occasional irritability), or moderately severe (item score at least 4: overtly hostile attitude, showing frequent irritability and direct expression of anger or resentment).
Supportive analyses were conducted using mean change from baseline to week 6 on the PANSS-Excited Component (PANSS-EC), a subscale used to detect differences between drug and placebo when evaluating acute agitation and aggression in psychiatric patients. PANSS-EC items (tension [G4], uncooperativeness [G8], poor impulse control [G14], excitement [P4], and hostility [P7]) are rated from 1 (not present) to 7 (extremely severe).
Statistical Analyses
Post hoc analyses were based on the pooled intent-to-treat (ITT) population, which consisted of all patients who received study medication and had ≥ 1 postbaseline PANSS assessment; additional analyses were conducted in patient subgroups defined by the hostility item score at baseline (item score ≥ 2, ≥3, or ≥ 4). Cariprazine dose groups were combined for post hoc analyses. Change from baseline to week 6 on the hostility item was analyzed using a mixed-effects model for repeated measures (MMRM) approach with study, treatment group, time, and treatment group–by-time interaction as categorical fixed effects; baseline value and baseline-by-time interaction were included as covariates. An unstructured covariance matrix was used to model the covariance of within-patient scores; the Kenward-Roger approximation19 was used to estimate the denominator degrees of freedom on all postbaseline scores using observed cases without imputation of missing values. The least squares mean difference (LSMD) with 95% confidence interval (CI) was reported at each postbaseline time point. All tests were 2-sided at the 5% significance level; P values for post hoc analyses were not adjusted for multiple comparisons.
The hostility item analysis was first conducted without adjustment for covariates. In order to test the specific antihostility effect of cariprazine, separate analyses to correct for possible confounder variables were conducted. The effect of other symptoms of schizophrenia was controlled by adjusting for the change in the sum of certain PANSS positive symptom items (delusions [P1], conceptual disorganization [P2], hallucinatory behavior [P3], grandiosity [P5], suspiciousness/persecution [P6], and unusual thought content [G9]). The effect of sedation was also introduced as a covariate. Sedation was measured by the presence of patient-reported or investigator-observed treatment-emergent adverse events of sedation, somnolence, or hypersomnia. The PANSS-EC was analyzed using an MMRM approach with study, treatment group, time, and treatment group–by-time interaction as categorical fixed effects; baseline value and baseline-by-time interaction were included as covariates.
RESULTS
A total of 1,466 patients had baseline and postbaseline PANSS assessments and were included in the overall pooled ITT population; baseline demographics and disease characteristics were similar between the placebo- and cariprazine-treatment groups (Table 1). The majority of patients were white males, the mean age was ~37 years, and most patients had at least a minimal level of hostility at baseline (item score ≥ 2). In the pooled population, the incidence of sedation was 1.8% and 3.7%, somnolence was 1.6% and 2.4%, and hypersomnia was 0 and 0.1% for placebo- and cariprazine-treated patients, respectively.
Efficacy Analyses
In the overall pooled population, statistically significant improvement in mean change from baseline to week 6 was observed for cariprazine- versus placebo-treated patients on each PANSS hostility item analysis: change in PANSS hostility item without accounting for any covariates, change in PANSS hostility item adjusted for PANSS positive symptoms, and change in PANSS hostility item adjusted for PANSS positive symptoms and sedation (Table 2). On the PANSS hostility item analysis without adjustment for covariates, the difference in mean change from baseline was statistically significant in favor of cariprazine versus placebo at every analysis point from week 1 through week 6 (Figure 1). After adjusting for changes in PANSS positive symptoms, improvement in mean change from baseline remained statistically significant for cariprazine versus placebo from week 1 through week 6 (Figure 2). The LSMD (95% CI) values for cariprazine versus placebo were almost identical from week 1 through week 6 when adjustments were made for positive symptom change alone and when adjustments were made for positive symptom change and sedation combined.
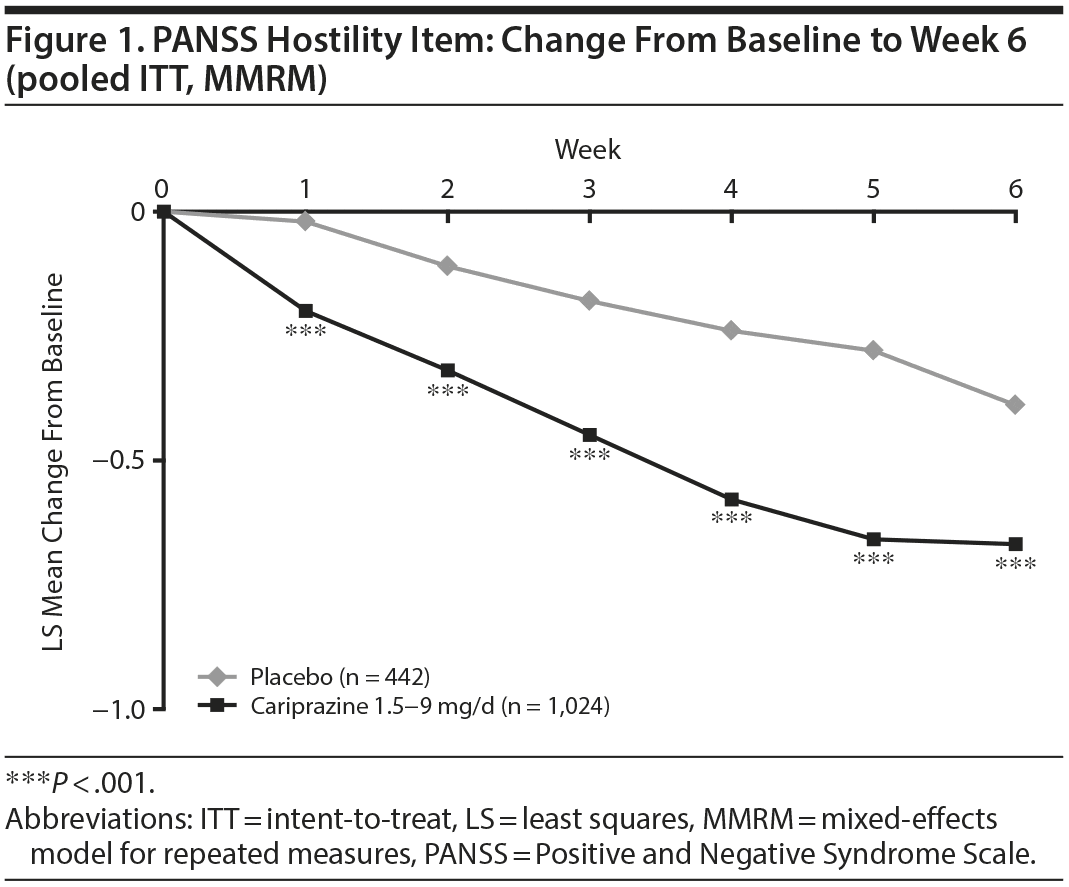
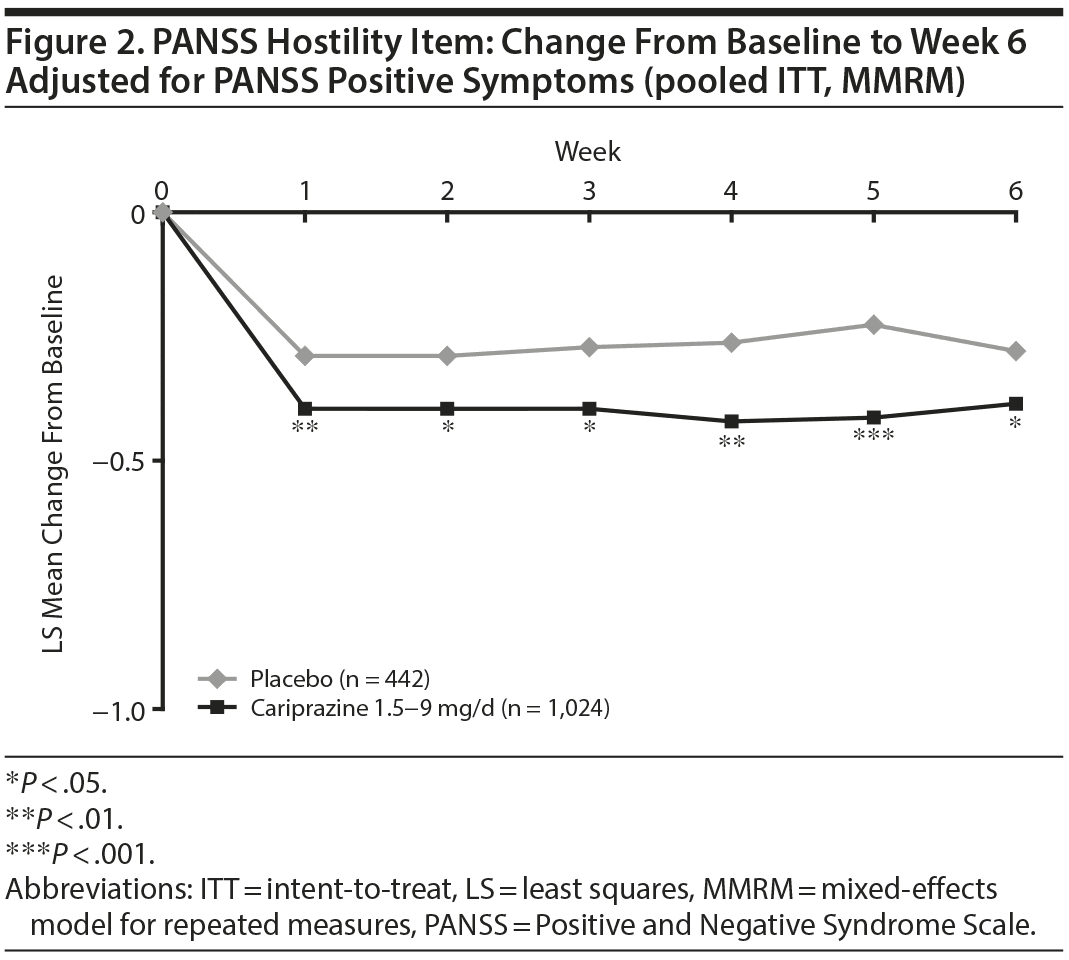
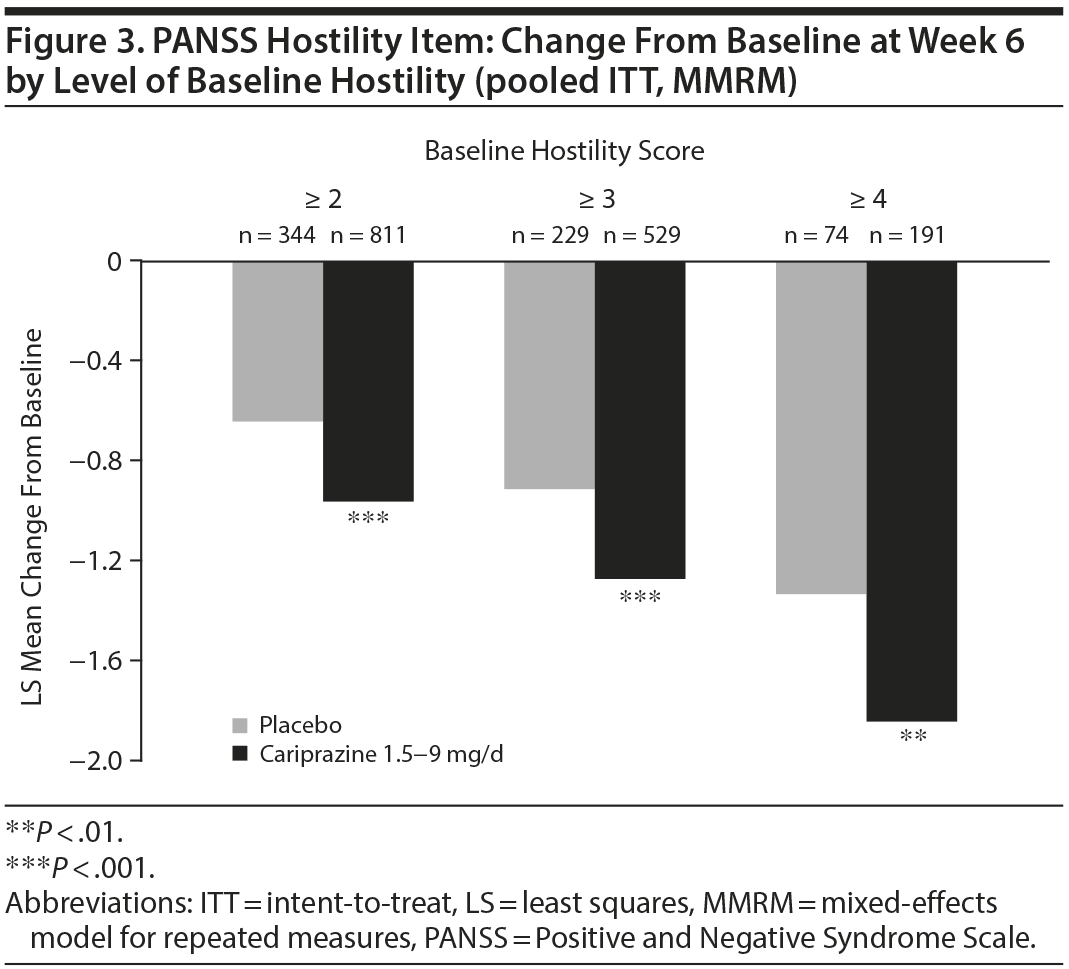
In baseline hostility subgroup analyses, statistically significant improvement in mean change from baseline to week 6 was seen in cariprazine- compared with placebo-treated patients in each of the baseline hostility cohorts analyzed (Table 2). For patients with a PANSS baseline hostility item score ≥ 2 or ≥ 3, the difference in mean change from baseline was statistically significant in favor of cariprazine versus placebo at every time point from week 1 through week 6, while in the group with a baseline hostility item score ≥ 4, statistically significant improvement for cariprazine was seen from week 3 onward. At week 6, higher levels of baseline hostility were associated with greater magnitude of change in the hostility item, with the greatest effect seen in cariprazine patients with baseline hostility ≥ 4 (Figure 3).
A statistically significant difference in change from baseline to week 6 on the PANSS-EC subscale in favor of cariprazine versus placebo (LSMD [95% CI]: –1.5 [–2.0 to –0.9], P < .0001) supported the principal PANSS hostility item analysis.
DISCUSSION
In these post hoc analyses, significantly greater improvement in hostility assessed by mean change in the PANSS hostility item was seen in favor of cariprazine-treated patients compared with placebo-treated patients. The improvement associated with cariprazine appeared to be partially independent of improvement in PANSS positive symptoms, independent of the presence or absence of sedation, and greater in cariprazine patients with higher versus lower levels of hostility at baseline. In a supportive analysis, significant improvement in mean change from baseline to week 6 was also seen for cariprazine versus placebo on the PANSS-EC subscale. These results suggest that cariprazine may be associated with specific antihostility effects that are independent of a general antipsychotic effect; as such, cariprazine may be a useful treatment option for hostility in patients with schizophrenia.
There is a strong evidence base suggesting that the atypical antipsychotic clozapine is the most effective treatment available for the treatment of hostility8 and aggression in schizophrenia.12,20–22 However, small effect sizes for antiaggressive activity,8 modest between-drug differences,12 and serious safety concerns23 with clozapine indicate that there is a need for new treatment options that have efficacy against hostility and better safety profiles. Although several atypical antipsychotics, including risperidone,9 quetiapine,24 olanzapine,8,25 and ziprasidone,26 have shown antihostility effects in patients with schizophrenia, evidence can be difficult to interpret because of differing study methodologies, including some open-label investigations, patient selection, and problems with accurately measuring aggressiveness in a clinical setting. Despite these limitations, our findings of an antihostility effect for cariprazine appear to be consistent with post hoc reports of other atypical antipsychotics compared with placebo in patients with schizophrenia.5
In our analyses, a robust and persistent antihostility effect for cariprazine relative to placebo was detected early in the course of treatment. Statistically significant improvement for cariprazine versus placebo on the PANSS hostility item was seen as early as week 1. Although the treatment effect on hostility was reduced after adjusting for PANSS positive symptoms, differences from placebo remained statistically significant at each week, indicating that improvement associated with cariprazine was partially independent of improvement in positive symptoms. This suggests that cariprazine may have a specific antihostility effect that is independent of the general antipsychotic effect.
Of further interest, when the hostility analysis was adjusted for the presence of sedation in addition to change in the PANSS positive items, the results indicated that the effect of cariprazine on hostility was independent of the presence or absence of sedation. This finding, along with the low incidence of sedation, somnolence, and hypersomnia adverse events for cariprazine-treated patients (< 4% for each) during the double-blind treatment period, suggests that the reduction in hostility for cariprazine-treated patients was not due to nonspecific sedative effects associated with cariprazine. Although the goals of managing acute agitation and hostility include calming the patient to decrease the likelihood of injury to self or others, decreasing the need for seclusion or restraint, and attenuating psychosis, inducing sleep is not a desired outcome because it increases the need for constant patient observation and staff intervention.3 As such, antihostility effects without sedation are an important cariprazine finding.
To examine the relationship between the level of hostility at baseline and the effect of cariprazine, we performed analyses on subgroups in which patients had at least minimal, mild, or moderate hostility at baseline. Although statistically significant improvement in favor of cariprazine versus placebo was observed in each patient subgroup analyzed by the level of baseline hostility, the magnitude of change on the hostility item was greater in cariprazine-treated patients with higher baseline hostility than in patients with lower baseline hostility. Additionally, statistically significant differences in mean change from baseline in favor of cariprazine versus placebo were seen at every time point from week 1 through 6 in patients with a PANSS hostility item score ≥ 2 or ≥ 3 at baseline and from week 3 onward in patients with a hostility item score ≥ 4 at baseline. These findings suggest that although an antihostility effect for cariprazine was apparent sooner in patients with lower levels of baseline hostility, it was persistent in patients regardless of baseline hostility level. Greater efficacy with higher levels of baseline hostility and persistent effect for cariprazine are notable findings in light of results from a meta-analysis that found hostile behavior to be a modifiable factor that was significantly associated with a risk of violence in patients with schizophrenia.6
Although schizophrenia elevates the risk for aggressive behavior, the etiology of violence associated with schizophrenia is heterogeneous, and the causes of aggression are complex.4 Causal pathways leading to aggressive behaviors may be created by a complicated interplay of predisposing biological, environmental, and psychiatric elements in some patients with schizophrenia.4 As shown in the Clinical Antipsychotic Trials of Intervention Effectiveness study, the presence of specific symptoms increases the risk of violence in patients with schizophrenia; hostility was shown to have the most direct relationship to serious violence, while suspiciousness/persecution, hallucinatory behavior, grandiosity, and excitement were also significantly associated with an increased risk.27 Aggression in schizophrenia is additionally influenced by factors as diverse as substance abuse, medication nonadherence, comorbid antisocial personality disorder, and current stress.4 Given this complex set of variables, a clinician’s knowledge of the patient’s history as it pertains to risk factors for aggression may help inform treatment decisions.
Since the nature and pathways leading to aggression and hostility in patients with schizophrenia are so diverse and atypical antipsychotics have different pharmacologic profiles, the potential for variations in response to treatment of hostility may be anticipated. Among available atypical antipsychotics, cariprazine is unique in that it has shown almost 10-fold greater affinity for D3 than D2 receptors in vitro28 and high and balanced in vivo occupancy of both D2 and D3 receptors.29,30 The dopamine D3 receptor is thought to be an important factor in modulating mood and cognition,31–35 and preclinical evidence suggests that cariprazine may be beneficial in treating negative symptoms, mood dysphoria, and cognitive impairment associated with schizophrenia.33,36–41 Additionally, short-term clinical trials have suggested that cariprazine is generally well tolerated, with no clinically relevant adverse effects on metabolic parameters, prolactin, or electrocardiogram QT interval.42 How the unique pharmacology of cariprazine may affect schizophrenia beyond efficacy in positive symptoms is currently under investigation, and it is not known if its distinct receptor profile translates into specific antihostility effects in patients with schizophrenia.
Limitations of these analyses include their post hoc nature, pooled design, lack of active comparator, and use of conventional P values that did not control for multiple comparisons. Due to the short duration of the pooled studies, the full effect of cariprazine may not have been established. Subjects were not specifically selected for their history of aggressive and hostile behavior; hostility in this patient sample was largely determined by verbal expression, not overt physical assault. Given the etiologically heterogeneous nature of hostility in patients with schizophrenia, lack of information about the reasons for hostility in these patients is a limitation. Baseline levels of hostility were low, so it is not possible to generalize these results to patients with high levels of hostility. Of note, however, the effect size for cariprazine at week 6 increased with baseline hostility values, suggesting that it is reasonable to expect that treatment would be as effective for patients with higher levels of hostility. Although proxy outcomes were used to measure complex hostility behavior, the methodology used in these analyses was comparable to the methods used in other investigations measuring the putative effects of atypical antipsychotics on hostility.8–10,25,26,43 Ideally, clinical studies to assess hostility associated with schizophrenia should be conducted in patients prospectively selected for hostile and aggressive behaviors; however, this approach is problematic given that these behaviors are uncommon among patients with schizophrenia and inherent methodological issues (eg, large sample size, long baseline and trial periods, selection bias, special housing units) would be difficult to overcome in this challenging patient population.44
CONCLUSIONS
Aggressive behavior can be seen in some patients with schizophrenia, and incidents of associated violence can greatly increase the burden and stigma of the disease.12 In post hoc analyses, treatment with cariprazine had significantly greater effects on hostility than treatment with placebo in patients with an acute exacerbation of schizophrenia. The antihostility effects were partially independent of PANSS positive symptom items and independent of sedation. The greatest cariprazine effect was seen in patients with the highest level of baseline hostility. Although effective treatment for hostility in patients with schizophrenia is available, no treatment is suitable for every patient, and effective new options may improve comprehensive disease management. Results of these post hoc analyses suggest that cariprazine may be an effective treatment for hostility in patients with schizophrenia.
Submitted: June 20, 2015; accepted October 21, 2015.
Drug names: aripiprazole (Abilify), cariprazine (Vraylar), clozapine (Clozaril, FazaClo, and others), olanzapine (Zyprexa and others), quetiapine (Seroquel and others), risperidone (Risperdal and others), ziprasidone (Geodon and others).
Potential conflicts of interest: Dr Citrome has engaged in collaborative research with or received consulting/speaking fees from Actavis, Alexza, Alkermes, AstraZeneca, Avanir, Bristol-Myers Squibb, Eli Lilly, Forum, Genentech, Janssen, Lundbeck, Merck, Mylan, Novartis, Noven, Otsuka, Pfizer, Reckitt Benckiser, Reviva, Shire, Sunovion, Takeda, Teva, Jazz, Medivation, and Valeant. He owns small numbers of common-stock shares in Bristol-Myers Squibb, Eli Lilly, Johnson & Johnson, Merck, and Pfizer. Drs Durgam and Lu acknowledge a potential conflict of interest as employees of Forest Research Institute, an Allergan affiliate, and are stock shareholders of Actavis Plc. Mr Ferguson acknowledges a potential conflict of interest as an employee of Prescott Medical Communications Group, Chicago, Illinois, a contractor of Forest Research Institute, an Allergan affiliate. Dr Laszlovszky acknowledges a potential conflict of interest as an employee of Gedeon Richter Plc and patent owner of cariprazine.
Funding/support: Supported by funding from Forest Laboratories, LLC, an Allergan affiliate (Jersey City, New Jersey), and Gedeon Richter Plc (Budapest, Hungary).
Role of the sponsor: Forest Laboratories, LLC, an Allergan affiliate, and Gedeon Richter Plc were involved in the study design, collection (via contracted clinical investigator sites), analysis and interpretation of data, and the decision to present these results.
Acknowledgment: Writing assistance and editorial support for the preparation of this manuscript were provided by Carol Brown, MS, of Prescott Medical Communications Group, Chicago, Illinois, a contractor of Forest Research Institute, an Allergan affiliate.
REFERENCES
1. Volavka J, Czobor P, Derks EM, et al; EUFEST Study Group. Efficacy of antipsychotic drugs against hostility in the European First-Episode Schizophrenia Trial (EUFEST). J Clin Psychiatry. 2011;72(7):955–961. doi:10.4088/JCP.10m06529 PubMed
2. Walsh E, Buchanan A, Fahy T. Violence and schizophrenia: examining the evidence. Br J Psychiatry. 2002;180(6):490–495. doi:10.1192/bjp.180.6.490 PubMed
3. Citrome L, Volavka J. The psychopharmacology of violence: making sensible decisions. CNS Spectr. 2014;19(5):411–418. doi:10.1017/S1092852914000054 PubMed
4. Volavka J, Citrome L. Pathways to aggression in schizophrenia affect results of treatment. Schizophr Bull. 2011;37(5):921–929. doi:10.1093/schbul/sbr041 PubMed
5. Volavka J, Czobor P, Citrome L, et al. Effectiveness of antipsychotic drugs against hostility in patients with schizophrenia in the Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) study. CNS Spectr. 2014;19(5):374–381. doi:10.1017/S1092852913000849 PubMed
6. Witt K, van Dorn R, Fazel S. Risk factors for violence in psychosis: systematic review and meta-regression analysis of 110 studies. PLoS ONE. 2013;8(2):e55942. doi:10.1371/journal.pone.0055942 PubMed
7. Kay SR, Fiszbein A, Opler LA. The Positive and Negative Syndrome Scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13(2):261–276. doi:10.1093/schbul/13.2.261 PubMed
8. Citrome L, Volavka J, Czobor P, et al. Effects of clozapine, olanzapine, risperidone, and haloperidol on hostility among patients with schizophrenia. Psychiatr Serv. 2001;52(11):1510–1514. doi:10.1176/appi.ps.52.11.1510 PubMed
9. Czobor P, Volavka J, Meibach RC. Effect of risperidone on hostility in schizophrenia. J Clin Psychopharmacol. 1995;15(4):243–249. doi:10.1097/00004714-199508000-00002 PubMed
10. Volavka J, Czobor P, Citrome L, et al. Efficacy of aripiprazole against hostility in schizophrenia and schizoaffective disorder: data from 5 double-blind studies. J Clin Psychiatry. 2005;66(11):1362–1366. doi:10.4088/JCP.v66n1103 PubMed
11. Ahmed AO, Hunter KM, Van Houten EG, et al. Cognition and other targets for the treatment of aggression in people with schizophrenia. Ann Psychiatry Ment Health. 2014;2(1):1004.
12. Krakowski MI, Czobor P, Citrome L, et al. Atypical antipsychotic agents in the treatment of violent patients with schizophrenia and schizoaffective disorder. Arch Gen Psychiatry. 2006;63(6):622–629. doi:10.1001/archpsyc.63.6.622 PubMed
13. Durgam S, Starace A, Li D, et al. An evaluation of the safety and efficacy of cariprazine in patients with acute exacerbation of schizophrenia: a phase II, randomized clinical trial. Schizophr Res. 2014;152(2–3):450–457. doi:10.1016/j.schres.2013.11.041 PubMed
14. Kane JM, Zukin S, Wang Y, et al. Efficacy and safety of cariprazine in acute exacerbation of schizophrenia: results from an international, phase III clinical trial. J Clin Psychopharmacol. 2015;35(4):367–373. PubMed
15. Durgam S, Cutler AJ, Lu K, et al. Cariprazine in acute exacerbation of schizophrenia: a fixed-dose, phase 3, randomized, double-blind, placebo- and active-controlled trial. J Clin Psychiatry. 2015;76(12):e1574–e1582.
16. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, Text Revision. Washington, DC: American Psychiatric Association; 2000.
17. Guy W. Clinical Global Impressions. In: Guy W, ed. ECDEU Assessment Manual for Psychopharmacology: Publication ADM 76-338. Rockville, MD: National Institute of Mental Health, Psychopharmacology Research Branch; 1976:217–222.
18. Kay SR, Opler LA, Fiszbein A. Positive and Negative Syndrome Scale Manual. North Tonawanda, NY: Multi-Health Systems; 2000.
19. Kenward MG, Roger JH. Small sample inference for fixed effects from restricted maximum likelihood. Biometrics. 1997;53(3):983–997. doi:10.2307/2533558 PubMed
20. Topiwala A, Fazel S. The pharmacological management of violence in schizophrenia: a structured review. Expert Rev Neurother. 2011;11(1):53–63. doi:10.1586/ern.10.180 PubMed
21. Volavka J, Citrome L. Heterogeneity of violence in schizophrenia and implications for long-term treatment. Int J Clin Pract. 2008;62(8):1237–1245. doi:10.1111/j.1742-1241.2008.01797.x PubMed
22. Volavka J, Czobor P, Nolan K, et al. Overt aggression and psychotic symptoms in patients with schizophrenia treated with clozapine, olanzapine, risperidone, or haloperidol. J Clin Psychopharmacol. 2004;24(2):225–228. doi:10.1097/01.jcp.0000117424.05703.29 PubMed
23. Citrome L. Clozapine for schizophrenia: life-threatening or life-saving treatment? clozapine, despite its side effect burden, may be the most effective and have the lowest mortality risk among all available antipsychotics. Curr Psychiatr. 2009;8(12):56-63.
24. Chengappa KN, Goldstein JM, Greenwood M, et al. A post hoc analysis of the impact on hostility and agitation of quetiapine and haloperidol among patients with schizophrenia. Clin Ther. 2003;25(2):530–541. doi:10.1016/S0149-2918(03)80094-2 PubMed
25. Citrome L, Casey DE, Daniel DG, et al. Adjunctive divalproex and hostility among patients with schizophrenia receiving olanzapine or risperidone. Psychiatr Serv. 2004;55(3):290–294. doi:10.1176/appi.ps.55.3.290 PubMed
26. Citrome L, Volavka J, Czobor P, et al. Efficacy of ziprasidone against hostility in schizophrenia: post hoc analysis of randomized, open-label study data. J Clin Psychiatry. 2006;67(4):638–642. doi:10.4088/JCP.v67n0415 PubMed
27. Swanson JW, Swartz MS, Van Dorn RA, et al. A national study of violent behavior in persons with schizophrenia. Arch Gen Psychiatry. 2006;63(5):490–499. doi:10.1001/archpsyc.63.5.490 PubMed
28. Kiss B, Horváth A, Némethy Z, et al. Cariprazine (RGH-188), a dopamine D(3) receptor-preferring, D(3)/D(2) dopamine receptor antagonist-partial agonist antipsychotic candidate: in vitro and neurochemical profile. J Pharmacol Exp Ther. 2010;333(1):328–340. doi:10.1124/jpet.109.160432 PubMed
29. Kiss B, Horti F, Bobok A. Cariprazine, a D3/D2 dopamine receptor partial agonist antipsychotic, displays greater D3 receptor occupancy in vivo compared with other antipsychotics. Schizophr Res. 2012;136(suppl 1):S190. doi:10.1016/S0920-9964(12)70588-1
30. Slifstein M, Abi-Dargham A, D’Souza DC, et al. cariprazine demonstrates high dopamine D3 and D2 receptor occupancy in patients with schizophrenia: a clinical PET study with [11C]- (+) -PHNO. Neuropsychopharmacology. 2013;38(S2):S520.
31. Cho DI, Zheng M, Kim KM. Current perspectives on the selective regulation of dopamine D₂ and D₃ receptors. Arch Pharm Res. 2010;33(10):1521–1538. doi:10.1007/s12272-010-1005-8 PubMed
32. Gross G, Wicke K, Drescher KU. Dopamine D₃ receptor antagonism—still a therapeutic option for the treatment of schizophrenia. Naunyn Schmiedebergs Arch Pharmacol. 2013;386(2):155–166. doi:10.1007/s00210-012-0806-3 PubMed
33. Laszy J, Laszlovszky I, Gyertyán I. Dopamine D3 receptor antagonists improve the learning performance in memory-impaired rats. Psychopharmacology (Berl). 2005;179(3):567–575. doi:10.1007/s00213-004-2096-z PubMed
34. Millan MJ, Buccafusco JJ, Loiseau F, et al. The dopamine D3 receptor antagonist, S33138, counters cognitive impairment in a range of rodent and primate procedures. Int J Neuropsychopharmacol. 2010;13(8):1035–1051. doi:10.1017/S1461145710000775 PubMed
35. Sokoloff P, Diaz J, Le Foll B, et al. The dopamine D3 receptor: a therapeutic target for the treatment of neuropsychiatric disorders. CNS Neurol Disord Drug Targets. 2006;5(1):25–43. doi:10.2174/187152706784111551 PubMed
36. Joyce JN, Millan MJ. Dopamine D3 receptor antagonists as therapeutic agents. Drug Discov Today. 2005;10(13):917–925. doi:10.1016/S1359-6446(05)03491-4 PubMed
37. Kiss B, Laszlovszky I, Horváth A, et al. Subnanomolar dopamine D3 receptor antagonism coupled to moderate D2 affinity results in favourable antipsychotic-like activity in rodent models: I. neurochemical characterisation of RG-15. Naunyn Schmiedebergs Arch Pharmacol. 2008;378(5):515–528. doi:10.1007/s00210-008-0308-5 PubMed
38. Schwartz JC, Diaz J, Pilon C, et al. Possible implications of the dopamine D(3) receptor in schizophrenia and in antipsychotic drug actions. Brain Res Brain Res Rev. 2000;31(2–3):277–287. doi:10.1016/S0165-0173(99)00043-0 PubMed
39. Gyertyán I, Sághy K, Laszy J, et al. Subnanomolar dopamine D3 receptor antagonism coupled to moderate D2 affinity results in favourable antipsychotic-like activity in rodent models: II. behavioural characterisation of RG-15. Naunyn Schmiedebergs Arch Pharmacol. 2008;378(5):529–539. doi:10.1007/s00210-008-0311-x PubMed
40. Duman RS, Duric V, Banasr M, et al. Cariprazine exhibits dopamine D3 receptordependent antidepressant-like activity in the chronic unpredictable stress model of anhedonia. Neuropsychopharmacology. 2012;38(suppl 1):S84.
41. Zimnisky R, Chang G, Gyertyán I, et al. Cariprazine, a dopamine D(3)-receptor-preferring partial agonist, blocks phencyclidine-induced impairments of working memory, attention set-shifting, and recognition memory in the mouse. Psychopharmacology (Berl). 2013;226(1):91–100. doi:10.1007/s00213-012-2896-5 PubMed
42. Citrome L. Cariprazine in schizophrenia: clinical efficacy, tolerability, and place in therapy. Adv Ther. 2013;30(2):114–126. doi:10.1007/s12325-013-0006-7 PubMed
43. Volavka J, Zito JM, Vitrai J, et al. Clozapine effects on hostility and aggression in schizophrenia. J Clin Psychopharmacol. 1993;13(4):287–289. doi:10.1097/00004714-199308000-00012 PubMed
44. Volavka J, Citrome L. Atypical antipsychotics in the treatment of the persistently aggressive psychotic patient: methodological concerns. Schizophr Res. 1999;35(suppl):S23–S33. doi:10.1016/S0920-9964(98)00163-7 PubMed
This PDF is free for all visitors!
Save
Cite



