The Effect of Electroconvulsive Therapy on Neurocognitive Function in Treatment-Resistant Bipolar Disorder Depression
ABSTRACT
Objective: To compare the effects of right unilateral (RUL) electroconvulsive therapy (ECT) and algorithm-based pharmacologic treatment (APT) on neurocognitive function in treatment-resistant bipolar disorder depression.
Method: Inpatients with DSM-IV-TR–diagnosed, treatment-resistant bipolar depression, who were acutely admitted to 1 of the 7 clinical study centers in Norway, were recruited from May 2008 to April 2011 into a prospective, randomized controlled, 6-week acute treatment trial. General neurocognitive function was assessed with the MATRICS Consensus Cognitive Battery (MCCB), and retrograde memory for autobiographical events was assessed with the Autobiographical Memory Interview–Short Form (AMI-SF) before and shortly after (mean = 23.5 days) a trial with either RUL brief-pulse ECT (mean dose = 233.3 mC) or APT.
Results: Seventy-three patients entered, and 39 (nECT = 19, nAPT = 20) completed. Both groups showed improvements in all MCCB domain scores, with no significant differences between the study groups (no interaction effect: F1,37 = 1.52, P = NS). Improvements in neurocognitive performance were significantly correlated with reductions in depression ratings posttreatment. The AMI-SF score was significantly lower (based on consistent answers from pre- to posttreatment) in the ECT group (72.9%) than in the APT group (80.8%, P = .025), indicating reduced consistency in autobiographical memory after ECT.
Conclusions: General neurocognitive function was unaffected by RUL brief-pulse ECT treatment and positively related to improved mood in bipolar depression. Autobiographical memory consistency was reduced in patients treated with ECT. The results suggest that ECT can be used in treatment-resistant bipolar depression without compromising general neurocognitive function. The clinical relevance of reduced autobiographical memory consistency in
the ECT group requires further investigation.
Trial Registration: ClinicalTrials.gov identifier: NCT00664976
J Clin Psychiatry 2014;75(11):e1306–e1313
© Copyright 2014 Physicians Postgraduate Press, Inc.
Submitted: December 19, 2013; accepted April 4, 2014 (doi:10.4088/JCP.13m08960).
Corresponding author: Ute Kessler, MD, Division of Psychiatry,
Clinic for Psychosomatic Medicine, Haukeland University Hospital, 5021 Bergen, Norway ([email protected]).
Bipolar disorder is associated with modest neurocognitive impairments in all phases of the illness, across all neuropsychological domains, and with a moderate worsening of a subset of deficits in the acute states.1–3 Pharmacologic treatments exert various effects on cognitive function in bipolar disorder. Psychoactive drugs may improve cognition by targeting psychotic and mood symptoms or worsen it due to side effects mediated by anticholinergic, sedative, extrapyramidal, and blunting mechanisms.4 However, the present evidence is limited since no large and fully powered randomized controlled trials have been performed.4 Electroconvulsive therapy (ECT) is widely regarded as an effective treatment in bipolar depression, is shown to be equally effective in unipolar and bipolar depression,5 and has some documentation in the treatment of treatment-resistant patients.6 However, the clinical use of ECT is accompanied by safety concerns due to the potentially unfavorable long-lasting effects on memory and other neurocognitive functions.7,8
The literature on the severity, persistency, and pattern of neurocognitive deficits induced by ECT is inconsistent.9 This is mainly due to methodological factors related to distinguishing between the underlying illness (impact of depression on objective and subjective cognitive function), the treatment (different treatment techniques and parameters used when administering ECT), and the assessment (different nomenclature for various types of neurocognitive function, neurocognitive test battery, and timing of testing).8,10–13 Neurocognitive effects beyond the transient postictal disorientation14 include retrograde and anterograde memory dysfunctions.15 A meta-analysis of objective performance in various neurocognitive domains found that deficits associated with ECT are mainly limited to the first 3 days posttreatment and that they subsequently resolve or possibly even improve beyond baseline for some measures.9 However, that meta-analysis was limited by the lack of data on retrograde amnesia and autobiographical memory, which are the most persistent adverse effects of ECT.16,17 Autobiographical memory (memory for personal events and facts) is essential for self-definition,18 social interaction,19 and as a guide for present and future activities and problem-solving.19 Retrograde amnesia and loss of autobiographical memory are the most important complaints from the patients.7
MacQueen and colleagues20 found that memory impairment was greater in euthymic bipolar disorder patients who had previously received ECT than in those with an assumed equal past burden of illness but without prior ECT. However, only randomly allocating patients to different treatment conditions can ensure the absence of bias due to the illness burden differing between groups.
The aim of the present study was to compare the effects of right unilateral (RUL) brief-pulse ECT and algorithm-based pharmacologic treatment (APT) on general neurocognitive function and autobiographical memory consistency in treatment-resistant bipolar depression shortly after a randomized 6-week trial.
METHOD
Study Design
This prospective, randomized controlled, multicenter, 6-week acute treatment trial comparing the effects of ECT and APT on neurocognitive function in treatment-resistant bipolar depression was conducted from May 2008 to April 2011. The detailed protocol for the trial and the pretreatment neurocognitive function have been described previously.3,21
The baseline neurocognitive and autobiographical assessments were performed by blinded raters a mean of 4.8 days (SD = 4.6; range, −2 to 15 days) before treatment onset in the ECT group and 3.1 days (SD = 4.8; range, −6 to 15 days) in the APT group. One patient in the ECT group was tested after treatment onset. He was retained in the analyses since 1 single ECT session does not seem to give significant clinical cognitive decline.22 The posttreatment assessment was performed a mean of 56.5 days (SD = 11.3; range, 42–83 days) after the baseline assessment in the ECT group and 54.0 days (SD = 16.9; range, 41–106 days) in the APT group. In the ECT group, the assessment was performed at least 1 week (mean = 23.5 days, SD = 14.1 days) after the last ECT session.
Subjects
The subjects eligible for inclusion in the study comprised 73 treatment-resistant (defined as nonresponse to 2 lifetime trials of antidepressants and/or mood stabilizer with documented efficacy in bipolar depression) acutely admitted patients ≥ 18 years of age who were referred for inpatient treatment at 1 of 7 participating clinical study centers in Norway. They fulfilled the DSM-IV-TR23 criteria for bipolar disorder type I or II depression with a cutoff score of ≥ 25 on the Montgomery-Asberg Depression Rating Scale (MADRS)24 and were evaluated to have clinical indications for ECT treatment.
Patients were excluded if they had received ECT within the previous 6 months; had a history of nonresponse to ECT treatment; had a rapid cycling course; were currently using medication, alcohol, or substances incompatible with the treatments in this study; or had conditions assumed to affect neurocognitive function such as Parkinson’s disease, multiple sclerosis, and stroke. Patients had to be sufficiently fluent in Norwegian to ensure valid responses in psychometric testing (ie, having Norwegian as their primary language or having received compulsory schooling in Norwegian), and they had to be able to cooperate under neurocognitive testing.
Patients were randomly assigned to receive either ECT or APT.
Intervention
Electroconvulsive therapy. The ECT procedure (0.5-ms pulse width, 900-mA pulse amplitude, with RUL electrode placement ad modem d’Elia25) was standardized as described in detail elsewhere.21 High-dose RUL ECT has been shown to be as effective as bilateral treatment with less severe and persistent cognitive side effects.26 Three sessions were administered per week for up to 6 weeks. The initial stimulus energy was determined by an age-based method27 that was adjusted for gender. The appropriateness of the dosage was determined at each treatment based on seizure duration, δ-waves, reorientation time, and clinical effect; and adjustments were made accordingly in subsequent treatments. Anesthetic medication consisted of thiopental (mean = 3.9 mg/kg) and succinylcholine chloride (mean = 0.8 mg/kg). If a patient randomized to ECT reached remission (defined as an MADRS score of ≤ 12) before the end of the 6-week trial, the ECT treatment was terminated, and the patient was switched to pharmacologic maintenance therapy.
Algorithm-based pharmacologic treatment. Patients in the APT group were treated according to the treatment algorithm for bipolar depression as reported by Goodwin and Jamison.28 The algorithm was to be followed step-by-step. Patients who had previously experienced either no effect or intolerable side effects on a medication listed in the algorithm could be switched to the next treatment option according to the algorithm.
Assessments
Symptom intensity was assessed by trained clinicians (psychiatrists, psychologists, and psychiatric nurses) using the MADRS24 and the PANSS-pos (Positive and Negative Syndrome Scale for Schizophrenia, positive subscale).29 Global functioning was assessed with the GAF-S (Global Assessment of Functioning–Split version, symptom subscale).30
Neurocognitive assessment was carried out by clinical neuropsychologists or test assistants trained in standardized neuropsychological testing. The premorbid IQ was estimated using a Norwegian research version31 of the NART (National Adult Reading Test).32 General neurocognitive function was assessed with the Norwegian version33 of the MATRICS Consensus Cognitive Battery (MCCB),34 which is designed for use in clinical trials assessing neurocognitive function in schizophrenia and related psychiatric disorders, as described in detail elsewhere.3 Raw scores from each of the 9 administered tests were converted into standardized T scores with a mean of 50 and an SD of 10, based on age- and gender-corrected norms from the MCCB manual.35 The T scores for the 6 assessed domains were used to compute a mean neurocognitive composite score. The MCCB task evaluating verbal learning, the Hopkins Verbal Learning Test–Revised (HVLT-R),36 measures the free immediate recall of words presented over multiple trials and quantifies episodic anterograde memory. Autobiographical memory was assessed using a version of the Columbia University Autobiographical Memory Interview–Short Form (AMI-SF)37 that had been translated into Norwegian by 2 of the authors (U.K. and H.K.S.). The AMI-SF score is based on answers to 30 questions about 6 autobiographical events. The patients were asked at both test occasions to generate details about presented topics, and consistency with pretreatment answers was measured.
Statistical Analysis
The characteristics of the patients in the 2 treatment groups were compared using t tests for normally distributed continuous variables, Mann-Whitney tests for nonnormally distributed continuous variables, and exact χ2 tests for categorical variables.
A multivariate repeated-measures analysis of variance (MANOVA) was performed for the 6 MCCB domain scores with treatment group (APT vs ECT) as the between-group variable and assessment time (pre- vs posttreatment) as the within-group variable. Follow-up mixed between–within repeated-measures analyses of variance (ANOVAs) were performed for each of the domain scores as well as for the composite score so as to control for chance findings due to multiple testing. Effect sizes (partial η2 values) for the effects of time and group and the interaction effect between time and group were computed. The AMI-SF pre- and posttreatment scores were analyzed by a mixed between–within ANOVA, whereas the AMI-SF–consistency scores in the 2 groups were compared using t tests. Correlational analyses were performed between neurocognitive measures and depressive symptoms (using MADRS). The level of statistical significance was set at P ≤ .05. All statistical analyses were performed using the Statistical Package for the Social Sciences (version 20.0, SPSS, Chicago, Illinois).
Ethical Considerations
The study was approved by the Regional Committee for Medical Research Ethics, Central Norway; the Norwegian Data Inspectorate; and the Norwegian Medicines Agency. All subjects were evaluated by the treating clinician as being capable of giving informed consent, and they provided informed written consent to participate after both treatment options and possible side effects had been fully explained. The study is registered in ClinicalTrials.gov: NCT00664976.
RESULTS
Inclusion of Patients
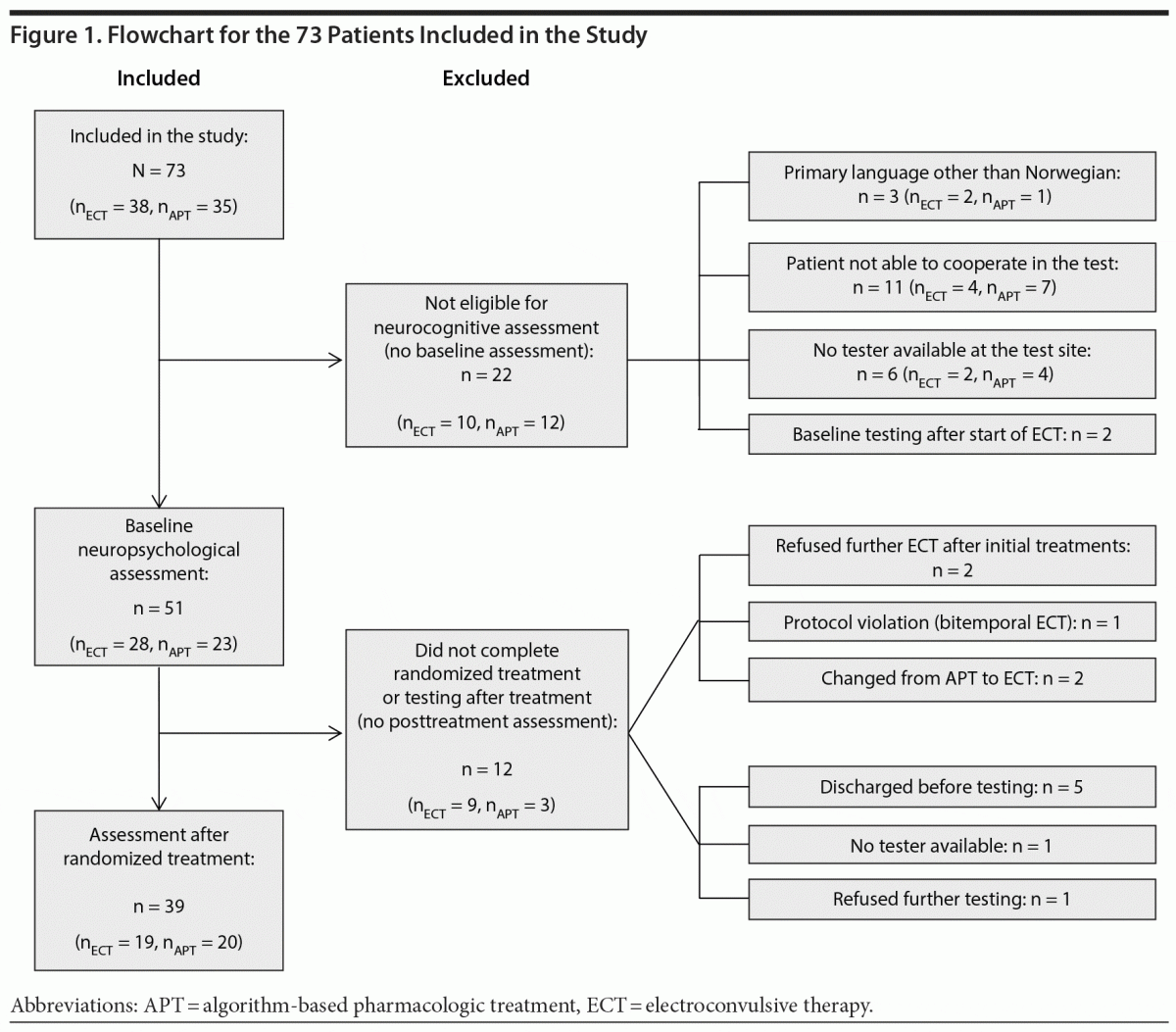
A flowchart for the inclusion of patients is shown in Figure 1.
Demographic Characteristics
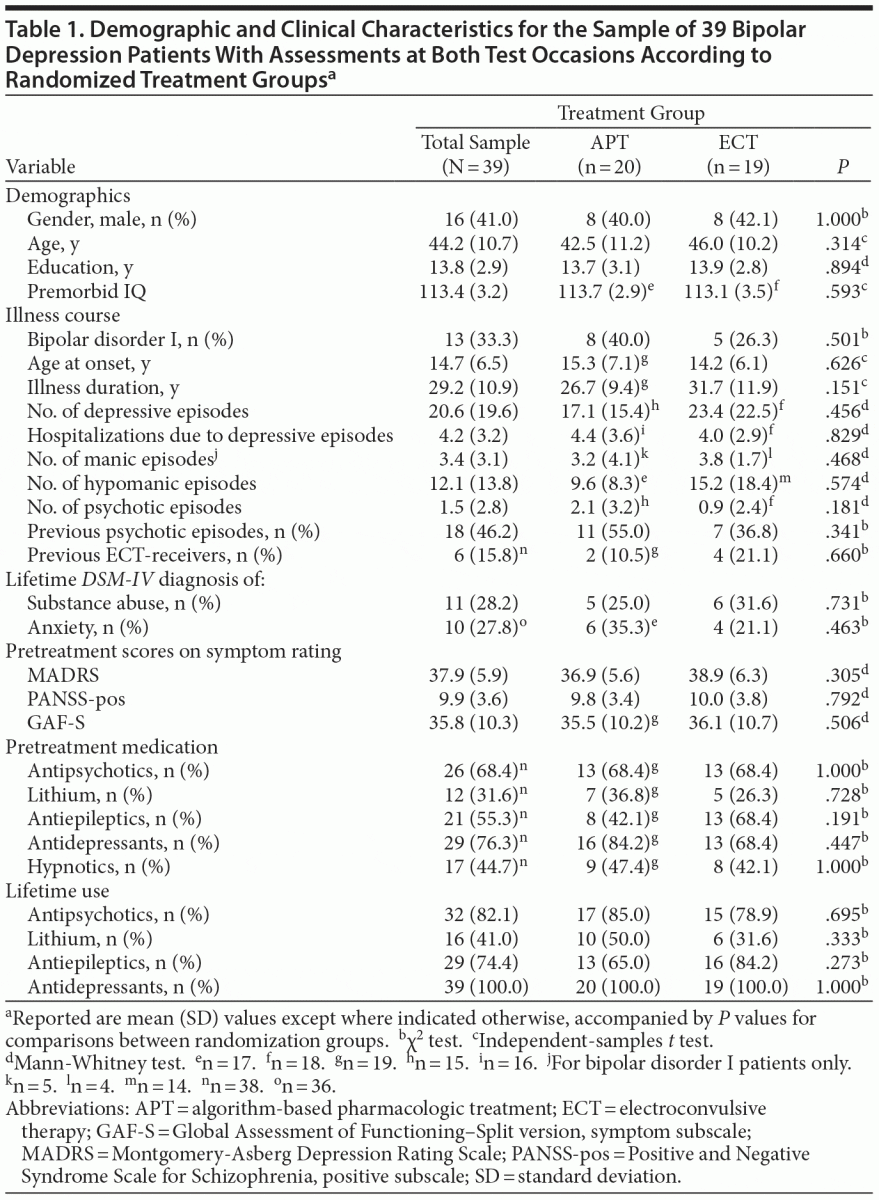
The demographic and clinical characteristics of patients who were assessed at both test occasions (n = 39) are listed in Table 1. The 34 patients who were not included in the analyses were older at inclusion (mean [SD] age = 52.8 [11.0] vs 44.2 [10.7] years, P = .001) and older at the onset of affective symptoms (mean age = 20.1 [10.1] vs 14.7 [6.5] years, P = .011) relative to the 39 included patients. Patients with (n = 39) and without (n = 12) a posttreatment assessment were compared on demographic, clinical, and neuropsychological measures at baseline, without significant differences (data not shown).
Treatment Variables
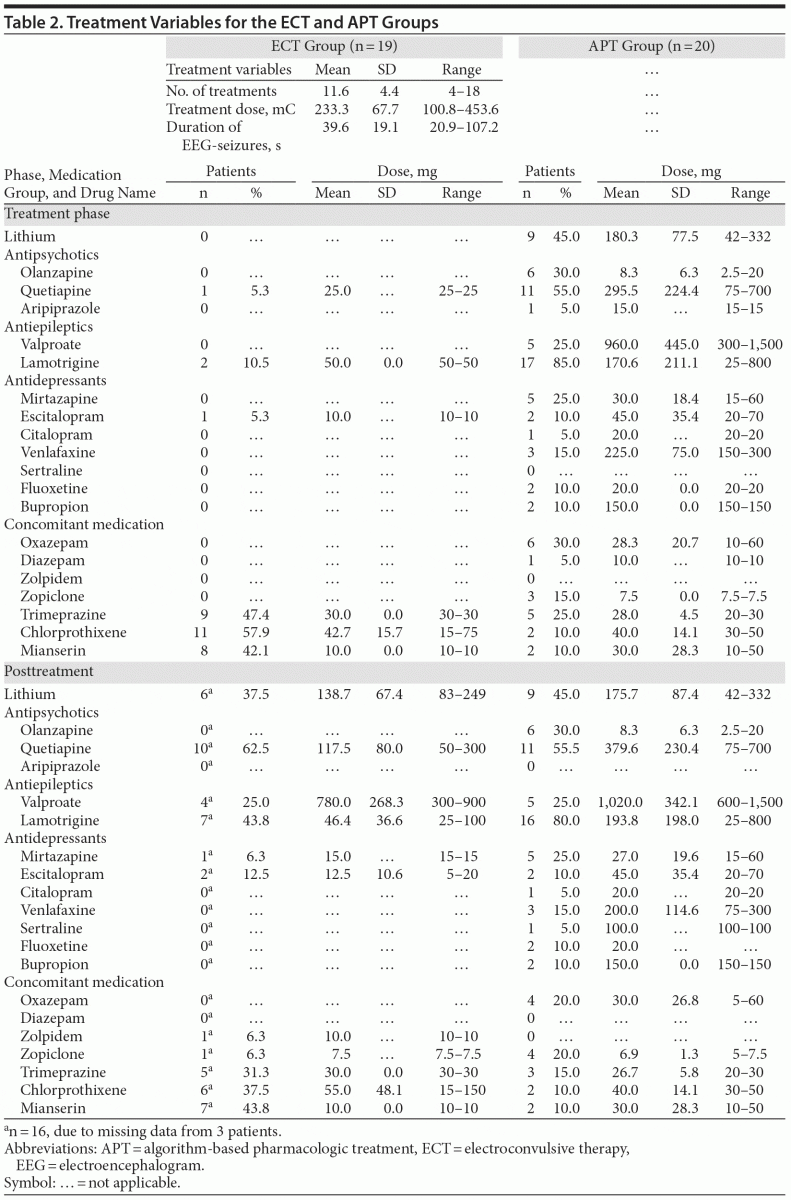
Details for the provided ECT-stimuli and the administered medication are given in Table 2.
General Neurocognitive Function
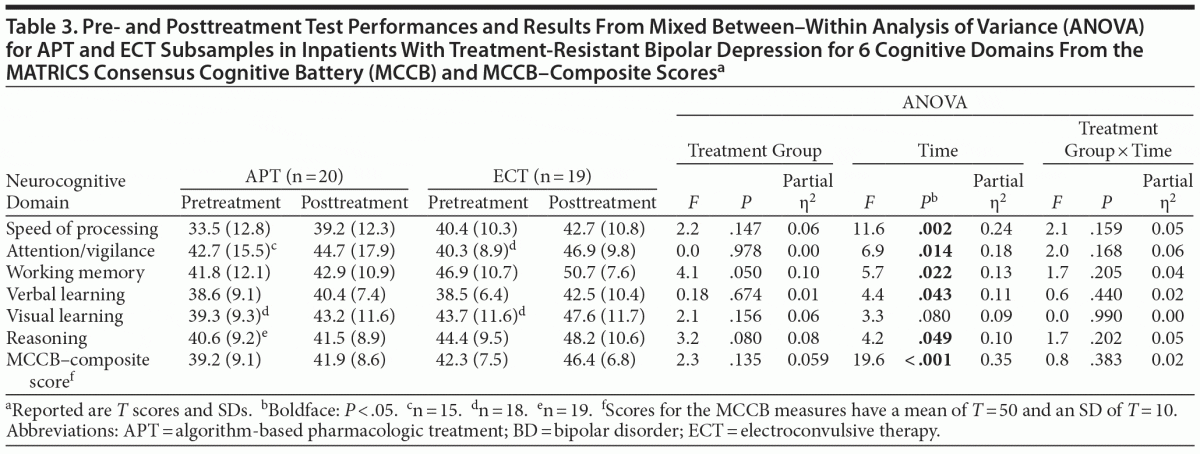
Age- and gender-corrected T scores for the 6 MCCB domains and the composite scores accompanied with results from a mixed between–within ANOVA are reported in Table 3. The initial MANOVA for the 6 domain scores identified a significant effect of time (F1,37 = 19.29, P < .001), but neither a group effect (F1,37 = 2.19, P = .147) nor an interaction effect (F1,37 = 1.52, P = .226) was identified. Overall, the analyses showed a significant change from pre- to posttreatment toward normalization of neurocognitive scores with no significant difference between the treatment groups. At the pretreatment assessment, both groups of patients showed reduced performance in most of the neurocognitive domains (1.0–1.5 SD below the normal mean). After treatment, the patients performed better on all measures. The effect sizes for these differences are classified as medium to large.
Autobiographical Memory
Pre- and posttreatment scores on the AMI-SF are reported in Table 4, along with the results from the repeated-measures ANOVA. There was a significant main effect for time, indicating a reduced autobiographical memory consistency in both groups from pre- to posttreatment, and a significant interaction between treatment group and time, indicating an additional reduction in autobiographical memory consistency in the ECT group compared to the APT group. The effect size for this interaction (η2 = 0.14) is classified as large.38 The AMI-SF–consistency score (based on consistency from pre- to posttreatment) was lower in the ECT group (72.9% [9.7%]) than in the APT group (80.8% [10.1%], P = .025), indicating a reduced autobiographical memory consistency in the ECT group.
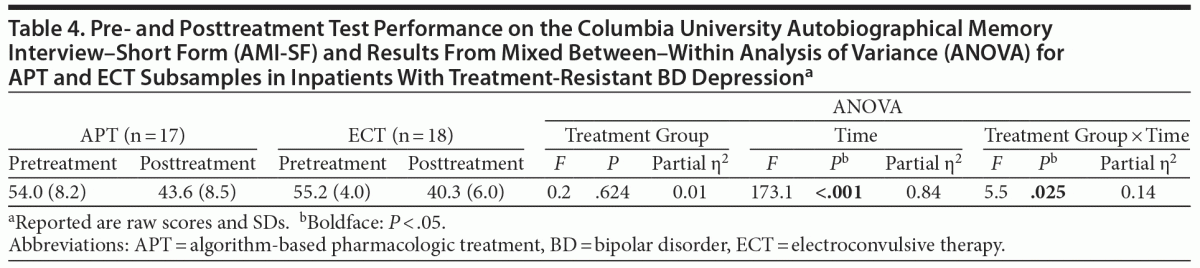
The changes in MCCB scores from pre- to posttreatment and in AMI-SF–consistency were not correlated (data not shown). This indicates that the change in performance of objective neurocognitive tasks is not related to retrograde amnesia. There was no correlation between time from the last ECT session to posttreatment assessment and any of the MCCB scores, change in MCCB scores, or AMI scores (data not shown).
Neurocognitive Function and Depressive Symptoms
The MADRS score was reduced from 37.9 (5.9) before treatment to 17.0 (10.1) after treatment. The changes in MADRS score and MCCB–composite score from pre- to posttreatment assessments were correlated (Spearman ρ = 0.335, P = .037), indicating that patients recovering from depression also exhibited improved neurocognitive function. The posttreatment MCCB–composite score was significantly higher for patients in remission (MADRS ≤ 12, n = 13) than for nonremitted patients (n = 26) (48.1 vs 42.0, P = .023), while the AMI-SF score did not differ between remitted and nonremitted patients (76.8 [10.2] vs 76.6 [11.6], P = .960), either in the APT group (82.3 vs 79.9, P = .660) or in the ECT group (70.9 vs 73.9, P = .551).
DISCUSSION
The main findings of the present study were that patients with treatment-resistant bipolar depression randomized to ECT had no reduction in neurocognitive performance but exhibited reduced autobiographical memory consistency compared to patients randomized to APT. This is the first randomized controlled trial of the effects on cognition of ECT compared with pharmacologic treatment in bipolar depression, and hence, the reported results are not directly comparable to previous findings.
The finding that ECT treatment was not associated with any reduction in general neurocognitive function compared to psychopharmacologic treatment of bipolar depression is consistent with the results of ECT studies of patients with mainly major depression that have documented a normalization of neurocognitive function shortly after ECT.9 Differences relative to studies documenting impaired processing speed and verbal and working memory after ECT,17,39,40 or more global cognitive impairment,41 might be due to the shorter interval between the last ECT session and posttreatment assessment in those studies. There is an active ongoing debate about the neurocognitive side effects of ECT, but they have not been investigated by randomized studies, with the exception of continuation ECT.42 Even if it is possible that the lack of effect in the present study was due to type II errors, we could exclude a deterioration of cognitive function on a group level.
The present study found a numerical (and, for most variables, significant) gain in all of the MCCB domain scores from pre- to posttreatment. The improvement in neurocognitive performance was positively correlated with the decrease in depressive symptoms.
We found a reduced consistency in autobiographical memory in the ECT group, which is consistent with previous findings of impairment of autobiographical memory after applying RUL brief-pulse ECT to patients with major depression.26,43 There was no healthy control group in the current study, which made it impossible to differentiate between normal and mood-associated loss of autobiographical memory consistency over time. Further, there are no normative data from healthy controls for the AMI-SF that could be compared directly with our results or used to estimate the normal change in autobiographical memory consistency. Thus, the clinical implications of the current finding of reduced autobiographical memory consistency remain uncertain. Previous studies have shown that the extent of autobiographical memory impairment depends on treatment parameters such as the electrode placement and pulse width.44 The treatment parameters in the present study represent a cognitively favorable choice, exceeded only by the use of ultrabrief pulses.17
The present study obtained contrasting results for autobiographical memory and general neurocognitive function measured with the MCCB task evaluating verbal learning: the HVLT-R. The AMI-SF and HVLT-R assess different memory functions. Memory is not unitary, but rather is divided into different memory systems based on somewhat different neural substrates.45 While the HVLT-R measures the free immediate recall of words presented over multiple trials and quantifies episodic anterograde memory, the AMI-SF assesses retrograde (both semantic and episodic) memory. In contrast to the item-focused HVLT-R, the AMI-SF is focused on personal events with affective valence. Another important difference between the memory tests from MCCB and the AMI-SF is the use of standardized objective tests versus personal memories.
The present study was subject to several limitations. As in other studies, the possible confounding effect of medications could not be evaluated. Our study was subject to a high dropout rate with only 53% fulfilling the final neuropsychological assessment. This probably reflects the illness burden of the acutely admitted, in-patient sample. The 12 patients who dropped out after baseline assessment did not differ from those who continued in demographic and baseline variables. They left the study for practical reasons (as shown in Figure 1). Assessing autobiographical memory constitutes a methodological challenge. The AMI-SF has several weaknesses, especially regarding the lack of normative data in both healthy and depressed samples, the inability to differentiate between different memory components, and the inability to capture an eventual improvement of autobiographical memory.46,47 The MCCB lacks measures for retrograde memory related to impersonal (public) events, which has been found to be more profound than for autobiographical events.43 The extent of retrograde memory loss thus remains uncertain. Also, the interval between the last ECT session and posttreatment assessment differed between patients. We found no correlation between any of the MCCB scores and time from the last ECT session to posttreatment assessment, which might have been due to the small sample. Previous research has found a time dependence in the resolution of ECT-induced neurocognitive impairment.48
In the current study, the neurocognitive impairment associated with ECT was limited to reduced autobiographical memory. The risk of this side effect has to be evaluated against the benefits of the possible symptomatic and functional recovery, but also against the alternative risk of the cognitive decline due to untreated depression, medications, and the consequences of treatment delay.49 The clinical relevance of our finding of reduced autobiographical memory consistency will also depend on the persistency of the impairment.
CONCLUSION
General neurocognitive function was unaffected by RUL ECT treatment and positively related to improved mood in bipolar depression. Autobiographical memory consistency was reduced in patients treated with ECT. The results suggest that ECT can be used in treatment-resistant bipolar depression without compromising general neurocognitive function. The clinical implication of reduced autobiographical memory consistency in the ECT group requires further investigation.
Drug names: aripiprazole (Abilify), bupropion (Wellbutrin, Aplenzin, and others), citalopram (Celexa and others), diazepam (Diastat, Valium, and others), escitalopram (Lexapro and others), fluoxetine (Prozac and others), lamotrigine (Lamictal and others), lithium (Lithobid and others), mirtazapine (Remeron and others), olanzapine (Zyprexa), quetiapine (Seroquel), sertraline (Zoloft and others), succinylcholine chloride (Anectine, Quelicin), venlafaxine (Effexor and others), zolpidem (Ambien, Edluar, and others).
Author affiliations: Moodnet Research Group, Division of Psychiatry (Drs Kessler and Oedegaard) and Centre for Clinical Research (Dr Eide), Haukeland University Hospital, Bergen; Department of Clinical Medicine, Section of Psychiatry (Drs Kessler and Oedegaard) and Department of Global Public Health and Primary Care (Dr Eide), University of Bergen, Bergen; Moodnet Research Group, Division of Psychiatry, Stavanger University Hospital, Stavanger (Dr Schoeyen); NORMENT, KG Jebsen Centre for Psychosis Research, Division of Mental Health and Addiction (Dr Andreassen) and Department of Psychosomatic Medicine (Dr Malt), Oslo University Hospital, Oslo; Institute of Clinical Medicine, Faculty of Medicine (Drs Andreassen, Malt, and Sundet) and Department of Psychology (Dr Sundet), University of Oslo, Oslo; and Department of Neuroscience, Faculty of Medicine, NTNU, Trondheim and Division of Psychiatry, St. Olav’s University Hospital, Trondheim (Drs Morken and Vaaler), Norway.
Potential conflicts of interest: Dr Andreassen has received speaker’s honorarium from Lundbeck, Osuka, and GSK. Dr Malt has received speaker’s honorarium for lecturing about mood disorders from Astra Zeneca, GSK, Lundbeck, The Norwegian Association for Hospitals, and The Norwegian Psychiatric Association and received payment from the Norwegian Directorate of Health for serving as an expert providing national guidelines for the treatment of bipolar and non-bipolar depression. Drs Kessler, Schoeyen, Eide, Oedegaard, Morken, Sundet, and Vaaler report that they have no conflicts of interest.
Funding/support: This work was supported by grants from the Western Norway Health Authority and the participating hospitals.
Role of the sponsor: The sponsor had no role in this study.
Previous presentations: Poster presented at the DGPPN Congress 2013; November 29, 2013; Berlin, Germany; and at the 21st European Congress of Psychiatry (EPA 2013); April 8, 2013; Nice, France.
REFERENCES
1. Martínez-Arán A, Vieta E, Reinares M, et al. Cognitive function across manic or hypomanic, depressed, and euthymic states in bipolar disorder. Am J Psychiatry. 2004;161(2):262–270. doi:10.1176/appi.ajp.161.2.262 PubMed
2. Kurtz MM, Gerraty RT. A meta-analytic investigation of neurocognitive deficits in bipolar illness: profile and effects of clinical state. Neuropsychology. 2009;23(5):551–562. doi:10.1037/a0016277 PubMed
3. Kessler U, Schoeyen HK, Andreassen OA, et al. Neurocognitive profiles in treatment-resistant bipolar I and bipolar II disorder depression. BMC Psychiatry. 2013;13(1):105. doi:10.1186/1471-244X-13-105 PubMed
4. Vieta E. The influence of medications on neurocognition in bipolar disorder. Acta Psychiatr Scand. 2009;120(6):414–415. doi:10.1111/j.1600-0447.2009.01503.x PubMed
5. Bailine S, Fink M, Knapp R, et al. Electroconvulsive therapy is equally effective in unipolar and bipolar depression. Acta Psychiatr Scand. 2010;121(6):431–436. doi:10.1111/j.1600-0447.2009.01493.x PubMed
6. Medda P, Perugi G, Zanello S, et al. Response to ECT in bipolar I, bipolar II and unipolar depression. J Affect Disord. 2009;118(1–3):55–59. doi:10.1016/j.jad.2009.01.014 PubMed
7. Rose D, Fleischmann P, Wykes T, et al. Patients’ perspectives on electroconvulsive therapy: systematic review. BMJ. 2003;326(7403):1363. doi:10.1136/bmj.326.7403.1363 PubMed
8. Food and Drug Administration. Meeting to discuss the classification of electroconvulsive therapy devices (ECT). FDA Executive Summary. January 27–28, 2011. http://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/MedicalDevicesAdvisoryCommittee/neurologicalDevicesPanel/UCM240933.pdf. Accessed April 11, 2014.
9. Semkovska M, McLoughlin DM. Objective cognitive performance associated with electroconvulsive therapy for depression: a systematic review and meta-analysis. Biol Psychiatry. 2010;68(6):568–577. doi:10.1016/j.biopsych.2010.06.009 PubMed
10. Ingram A, Saling MM, Schweitzer I. Cognitive side effects of brief pulse electroconvulsive therapy: a review. J ECT. 2008;24(1):3–9. doi:10.1097/YCT.0b013e31815ef24a PubMed
11. Sackeim HA, Prudic J, Devanand DP, et al. Effects of stimulus intensity and electrode placement on the efficacy and cognitive effects of electroconvulsive therapy. N Engl J Med. 1993;328(12):839–846. doi:10.1056/NEJM199303253281204 PubMed
12. Sackeim HA, Prudic J, Fuller R, et al. The cognitive effects of electroconvulsive therapy in community settings. Neuropsychopharmacology. 2007;32(1):244–254. doi:10.1038/sj.npp.1301180 PubMed
13. Prudic J. Strategies to minimize cognitive side effects with ECT: aspects of ECT technique. J ECT. 2008;24(1):46–51. doi:10.1097/YCT.0b013e31815ef238 PubMed
14. Daniel WF, Crovitz HF. Disorientation during electroconvulsive therapy: technical, theoretical, and neuropsychological issues. Ann N Y Acad Sci. 1986;462(1):293–306. doi:10.1111/j.1749-6632.1986.tb51264.x PubMed
15. Kellner CH. Brain Stimulation in Psychiatry. Cambridge, UK: Cambridge University Press; 2012. doi:10.1017/CBO9780511736216
16. Sackeim HA. The cognitive effects of electroconvulsive therapy. In: Thal LJ, Moos WH, Gamzu ER, eds. Cognitive disorders: Pathophysiology and Treatment. New York: NY Marcel Decker; 1992:183–228.
17. Verwijk E, Comijs HC, Kok RM, et al. Neurocognitive effects after brief pulse and ultrabrief pulse unilateral electroconvulsive therapy for major depression: a review. J Affect Disord. 2012;140(3):233–243. doi:10.1016/j.jad.2012.02.024 PubMed
18. Welzer H, Markowitsch HJ. Towards a bio-psycho-social model of autobiographical memory. Memory. 2005;13(1):63–78. doi:10.1080/09658210344000576 PubMed
19. Bluck S. Autobiographical memory: exploring its functions in everyday life. Memory. 2003;11(2):113–123. doi:10.1080/741938206 PubMed
20. MacQueen G, Parkin C, Marriott M, et al. The long-term impact of treatment with electroconvulsive therapy on discrete memory systems in patients with bipolar disorder. J Psychiatry Neurosci. 2007;32(4):241–249. PubMed
21. Kessler U, Vaaler AE, Schøyen H, et al. The study protocol of the Norwegian randomized controlled trial of electroconvulsive therapy in treatment resistant depression in bipolar disorder. BMC Psychiatry. 2010;10(1):16. doi:10.1186/1471-244X-10-16 PubMed
22. Rami L, Goti J, Ferrer J, et al. Cognitive functions after only one ECT session: a controlled study. Psychiatry Res. 2008;158(3):389–394. doi:10.1016/j.psychres.2007.01.005 PubMed
23. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, Text Revision. Washington, DC: American Psychiatric Association; 2000.
24. Montgomery SA, Asberg M. A new depression scale designed to be sensitive to change. Br J Psychiatry. 1979;134(4):382–389. doi:10.1192/bjp.134.4.382 PubMed
25. d’Elia G. Unilateral electroconvulsive therapy. Acta Psychiatr Scand suppl. 1970;215:1–98. PubMed
26. Sackeim HA, Prudic J, Devanand DP, et al. A prospective, randomized, double-blind comparison of bilateral and right unilateral electroconvulsive therapy at different stimulus intensities. Arch Gen Psychiatry. 2000;57(5):425–434. doi:10.1001/archpsyc.57.5.425 PubMed
27. Abrams R. Electroconvulsive Therapy. 4th ed. New York, NY: Oxford University Press; 2002.
28. Goodwin FK, Jamison KR. Manic-Depressive Illness: Bipolar Disorders and Recurrent Depression. 2nd edition. Cary, NC: Oxford University Press; 2007.
29. Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13(2):261–276. doi:10.1093/schbul/13.2.261 PubMed
30. Pedersen G, Hagtvet KA, Karterud S. Generalizability studies of the Global Assessment of Functioning-Split version. Compr Psychiatry. 2007;48(1):88–94. doi:10.1016/j.comppsych.2006.03.008 PubMed
31. Sundet K, Vaskinn A. Estimating premorbid IQ (in Norwegian with English abstract). J Norwegian Psycholal Assoc. 2008;45(9):1108–1115.
32. Nelson HE, Willison JR. The National Adult Reading Test-Test Manual. 2nd edition. Windsor, UK: NFER-Nelson; 1991.
33. Mohn C, Sundet K, Rund BR. The Norwegian standardization of the MATRICS (Measurement and Treatment Research to Improve Cognition in Schizophrenia) Consensus Cognitive Battery. J Clin Exp Neuropsychol. 2012;34(6):667–677. doi:10.1080/13803395.2012.667792 PubMed
34. Nuechterlein KH, Green MF, Kern RS, et al. The MATRICS Consensus Cognitive Battery, part 1: test selection, reliability, and validity. Am J Psychiatry. 2008;165(2):203–213. doi:10.1176/appi.ajp.2007.07010042 PubMed
35. Nuechterlein KH, Green MF. MATRICS Consensus Cognitive Battery Manual [computer program]. Los Angeles, CA: Matrics Assessment Inc; 2006.
36. Brandt J, Benedict RHB. The Hopkins Verbal Learning Test-Revised. Odessa, FL: Psychological Assessment Resources, Inc; 2001.
37. McElhiney MC, Moody BJ, Sackeim HA. The Autobiographical Memory Interview–Short Form. New York, NY: Department of Biological Psychiatry: New York State Psychiatric Institute; 2001.
38. Cohen J. Statistical Power Analysis for the Behavioral Sciences. 2nd ed. Hillsdale, NJ: Lawrence Erlbaum Associates; 1988.
39. Ng C, Schweitzer I, Alexopoulos P, et al. Efficacy and cognitive effects of right unilateral electroconvulsive therapy. J ECT. 2000;16(4):370–379. doi:10.1097/00124509-200012000-00007 PubMed
40. O’Connor M, Brenninkmeyer C, Morgan A, et al. Relative effects of repetitive transcranial magnetic stimulation and electroconvulsive therapy on mood and memory: a neurocognitive risk-benefit analysis. Cogn Behav Neurol. 2003;16(2):118–127. doi:10.1097/00146965-200306000-00005 PubMed
41. Kellner CH, Knapp R, Husain MM, et al. Bifrontal, bitemporal and right unilateral electrode placement in ECT: randomised trial. Br J Psychiatry. 2010;196(3):226–234. doi:10.1192/bjp.bp.109.066183 PubMed
42. Smith GE, Rasmussen KG Jr, Cullum CM, et al; CORE Investigators. A randomized controlled trial comparing the memory effects of continuation electroconvulsive therapy versus continuation pharmacotherapy: results from the Consortium for Research in ECT (CORE) study. J Clin Psychiatry. 2010;71(2):185–193. doi:10.4088/JCP.08m04797gre PubMed
43. Lisanby SH, Maddox JH, Prudic J, et al. The effects of electroconvulsive therapy on memory of autobiographical and public events. Arch Gen Psychiatry. 2000;57(6):581–590. doi:10.1001/archpsyc.57.6.581 PubMed
44. Sackeim HA, Prudic J, Nobler MS, et al. Effects of pulse width and electrode placement on the efficacy and cognitive effects of electroconvulsive therapy. Brain Stimulat. 2008;1(2):71–83. doi:10.1016/j.brs.2008.03.001 PubMed
45. Markowitsch HJ. Autobiographical memory: a biocultural relais between subject and environment. Eur Arch Psychiatry Clin Neurosci. 2008;258(suppl 5):98–103. doi:10.1007/s00406-008-5021-3 PubMed
46. Semkovska M, McLoughlin DM. Measuring retrograde autobiographical amnesia following electroconvulsive therapy: historical perspective and current issues. J ECT. 2013;29(2):127–133. doi:10.1097/YCT.0b013e318279c2c9 PubMed
47. Semkovska M, Noone M, Carton M, et al. Measuring consistency of autobiographical memory recall in depression. Psychiatry Res. 2012;197(1–2):41–48. doi:10.1016/j.psychres.2011.12.010 PubMed
48. Semkovska M, Keane D, Babalola O, et al. Unilateral brief-pulse electroconvulsive therapy and cognition: effects of electrode placement, stimulus dosage and time. J Psychiatr Res. 2011;45(6):770–780. doi:10.1016/j.jpsychires.2010.11.001 PubMed
49. Swartz CM. Electroconvulsive and Neuromodulation Therapies. New York, NY: Cambridge University Press; 2009. doi:10.1017/CBO9780511576393




