Objective: To assess the effects of a supratherapeutic dose of SPN-812, a drug currently under investigation as a treatment for attention-deficit/hyperactivity disorder, on cardiac repolarization (QTc) in healthy adults.
Methods: The study was conducted from June 27, 2018, to July 10, 2018. It had a double-blind, randomized, crossover design in which subjects received a 3-treatment sequence—placebo, 400 mg moxifloxacin, and 1,800 mg SPN-812 for 2 consecutive days (separated by at least a 3-day washout). The primary endpoint was the correlation between the change from baseline (CFB) in individual heart rate corrected QT interval (QTcI) (ΔQTcI) and viloxazine and 5-hydroxyviloxazine glucuronide (5-OH-VLX-gluc) plasma concentrations (Cps). The secondary endpoint was the time point placebo-adjusted CFB in QTcI (ΔΔQTcI) for viloxazine. For assay sensitivity, the correlations between moxifloxacin Cp and the ΔQTcI, and moxifloxacin and time point ΔΔQTcI were evaluated. Additional evaluations included Fridericia’s formula QT correction, heart rate, and the PR and QRS intervals. Changes in electrocardiogram (ECG) morphology along with other safety parameters were also analyzed and reported.
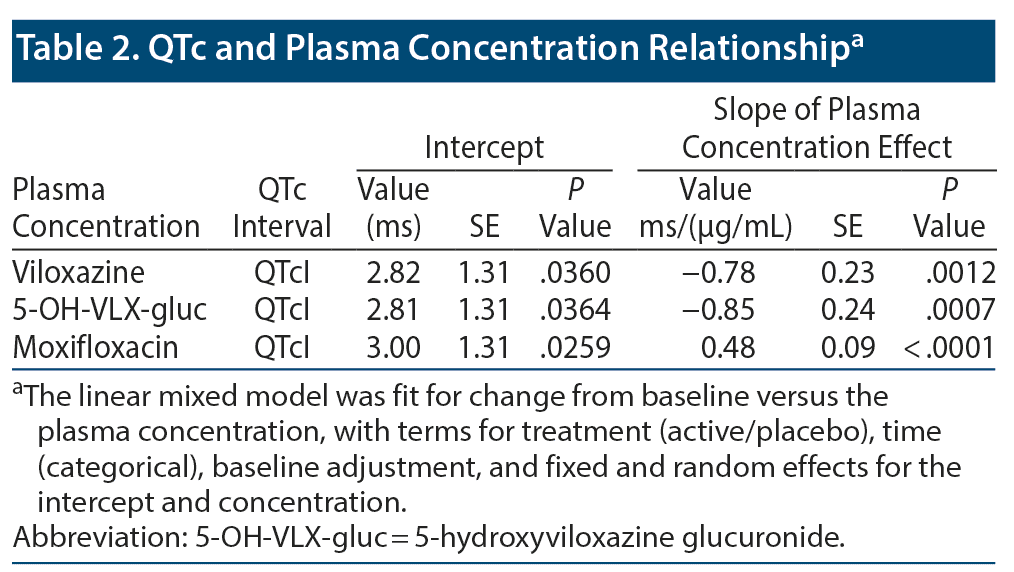
Results: The correlation between ΔQTcI and viloxazine Cp demonstrated a statistically significant negative slope (P = .0012). 5-OH-VLX-gluc Cp and ΔQTcI also demonstrated a statistically significant negative slope (P = .0007). Secondary time point analyses showed no effect of SPN-812 on QTcI. Assay sensitivity with moxifloxacin was confirmed. Safety parameters were acceptable.
Conclusions: This study demonstrated that SPN-812 had no effect on cardiac repolarization or other ECG parameters in healthy adults, suggesting that it is not associated with a risk for cardiac arrhythmias or other electrocardiographic parameters.
Objective: To assess the effects of a supratherapeutic dose of SPN-812, a drug currently under investigation as a treatment for attention-deficit/hyperactivity disorder, on cardiac repolarization (QTc) in healthy adults.
Methods: The study was conducted from June 27, 2018, to July 10, 2018. It had a double-blind, randomized, crossover design in which subjects received a 3-treatment sequence—placebo, 400 mg moxifloxacin, and 1,800 mg SPN-812 for 2 consecutive days (separated by at least a 3-day washout). The primary endpoint was the correlation between the change from baseline (CFB) in individual heart rate corrected QT interval (QTcI) (ΔQTcI) and viloxazine and 5-hydroxyviloxazine glucuronide (5-OH-VLX-gluc) plasma concentrations (Cps). The secondary endpoint was the time point placebo-adjusted CFB in QTcI (ΔΔQTcI) for viloxazine. For assay sensitivity, the correlations between moxifloxacin Cp and the ΔQTcI, and moxifloxacin and time point ΔΔQTcI were evaluated. Additional evaluations included Fridericia’s formula QT correction, heart rate, and the PR and QRS intervals. Changes in electrocardiogram (ECG) morphology along with other safety parameters were also analyzed and reported.
Results: The correlation between ΔQTcI and viloxazine Cp demonstrated a statistically significant negative slope (P = .0012). 5-OH-VLX-gluc Cp and ΔQTcI also demonstrated a statistically significant negative slope (P = .0007). Secondary time point analyses showed no effect of SPN-812 on QTcI. Assay sensitivity with moxifloxacin was confirmed. Safety parameters were acceptable.
Conclusions: This study demonstrated that SPN-812 had no effect on cardiac repolarization or other ECG parameters in healthy adults, suggesting that it is not associated with a risk for cardiac arrhythmias or other electrocardiographic parameters.
J Clin Psychiatry 2020;81(6):20m13395
To cite: Nasser A, Faison SL, Liranso T, et al. Evaluation of the effect of SPN-812 (viloxazine extended-release) on QTc interval in healthy adults. J Clin Psychiatry. 2020;81(6):20m13395.
To share: https://doi.org/10.4088/JCP.20m13395
© Copyright 2020 Physicians Postgraduate Press, Inc.
aSupernus Pharmaceuticals, Inc, Rockville, Maryland
bDepartment of Psychiatry, Massachusetts General Hospital, Boston, Massachusetts
ceResearch Technology, Inc, Philadelphia, Pennsylvania
*Corresponding author: Azmi Nasser, PhD, Supernus Pharmaceuticals, Inc., 9715 Key West Ave, Rockville, MD 20850 ([email protected]).
Attention-deficit/hyperactivity disorder (ADHD) is one of the most common neurodevelopmental disorders. Current pharmacologic treatments for ADHD include a number of US Food and Drug Administration (FDA)-approved stimulant (various formulations of methylphenidate and amphetamines) and nonstimulant (guanfacine, clonidine, and atomoxetine) medications.1 However, many of these medications are contraindicated or should be used with caution in certain populations of patients, for instance, patients with cardiac abnormalities or cardiovascular problems,2-6 agitation, Tourette syndrome, tics, sleep disturbances,2,3 suicidal ideation,4 sedation, or somnolence.5,6 High rates of comorbidities and complexity of ADHD management, evidenced by high rates of nonresponders or partial responders to current ADHD medications,7 indicate the need for additional effective therapies with favorable safety profiles.
SPN-812 (viloxazine extended-release) is currently under investigation as a novel treatment for ADHD. It is a multimodal serotonergic and noradrenergic modulating agent with demonstrated activity at serotonin receptors and the norepinephrine transporter. In vivo, viloxazine has been shown to increase serotonin (5-HT), norepinephrine, and dopamine levels in the prefrontal cortex,8 a region strongly implicated in ADHD pathophysiology. In vitro, viloxazine exhibits antagonistic activity at 5-HT2B receptors and agonistic activity at 5-HT2C receptors,8 although the downstream effects of this activity remain to be fully elucidated. The objective of this phase 1 study was to assess the potential effects of a supratherapeutic dose of SPN-812 (1,800 mg once daily) on cardiac repolarization relative to positive and negative controls in healthy adult subjects.
Prolongation of the QT interval is a well-established indicator for potential drug-induced cardiovascular risks. Prolongation of this interval can increase the risk for cardiac arrhythmias and may lead to a specific, potentially fatal, form of polymorphic ventricular tachycardia called torsades de pointes (TdP).9,10 Although rare with non-antiarrhythmic drugs, effects on cardiac repolarization by psychotropic agents have previously been observed; for example, tricyclic antidepressants, citalopram, chlorpromazine, haloperidol, ziprasidone, and lithium have all been associated with incidents of prolongation of the QT interval.11-14 Given the potentially severe consequences of drug-induced effects on cardiac repolarization, evaluating this safety parameter during the clinical development of novel agents is essential11,12 and required by the FDA. A thorough QT (TQT) study is an accepted and recommended design for this assessment.15
METHODS
Subjects
This study included healthy adult male and female subjects, 18-45 years of age, whose body mass index ranged from 18 to 28 kg/m2 (inclusive). Subjects were current nonsmokers who had not used any nicotine-containing products (chewed, smoked, or replacement) within 45 days prior to screening and had a negative urine drug test on screening and entry. All subjects were considered medically healthy by the sudy investigator, which included physical examination, medical history, clinical laboratory tests, vital signs, and electrocardiograms (ECGs). Subjects were excluded if they had clinically significant ECG abnormalities at screening, including QT interval corrected for heart rate (HR) using Fridericia’s formula (QTcF) that was > 450 ms for males and > 470 ms for females, QRS duration > 110 ms, PR interval > 200 ms, second or third-degree atrioventricular block, or any cardiac rhythm other than sinus rhythm that was interpreted by the study investigator to be clinically significant. Subjects were also excluded if they had a family history of QTc prolongation or unexplainable sudden death at < 50 years of age. Other prespecified exclusion criteria were the presence of any kind of cardiovascular disorder/condition known to increase the possibility of QT prolongation or history of additional risk factors for TdP (eg, heart failure, hypokalemia, family history of long QT syndrome or Brugada syndrome) or cardiac disorders.

- Given the complexity of ADHD management, it is critical to match patients with the right medicine from both safety and efficacy perspectives.
- SPN-812 (viloxazine extended-release) had no effect on cardiac repolarization or other electrocardiographic parameters in healthy adults, suggesting it is not associated with a risk for cardiac arrhythmias and torsades de pointes.
- These data suggest that administration of SPN-812 does not cause an additional risk to ADHD patients treated concurrently with QT-prolonging medications (eg, certain antibiotics) or others who might be at risk of QT interval prolongation.
Study Design
The study was conducted from June 27, 2018, to July 10, 2018. This was a phase 1, double-blind (except for moxifloxacin), randomized, 3-treatment, 3-period, 6-sequence crossover design study in healthy adult subjects evaluating the electrocardiographic effects of SPN-812. Moxifloxacin was used as a positive control for assay sensitivity, as it has been previously demonstrated to prolong the QTc interval.16,17 The study was conducted in compliance with the International Council for Harmonisation (ICH) Good Clinical Practice guidelines and the Declaration of Helsinki. Prior to initiation of any study-related procedure (including screening), all participants provided written informed consent.
Subjects were randomized to receive a treatment sequence that included all 3 treatments: placebo (negative control), 400 mg moxifloxacin (positive control), and 1,800 mg SPN-812 (supratherapeutic dose as determined by the maximum tolerated dose after repeated dosing; double Williams Latin square schema). Treatment included 3 dosing periods, with differing treatments given for 2 consecutive days. Each dosing period was separated by a washout of at least 3 days, allowing for 4 days between ECG and pharmacokinetic (PK) measurements (collected on the second day of dosing).
At baseline (day −1 of Period 1), eligible subjects were randomized to 1 of 6 treatment sequences and had baseline triplicate ECGs recorded for 24 hours. On day 1 of each period, subjects received study medication (according to their randomization scheme) following a light breakfast. On day 2 of each period (the steady-state is achieved by day 2 of once-daily dosing, based on multiple-ascending dose study), predose triplicate ECGs and PK plasma samples were collected. After this collection, subjects received study medication (the same study medication as day 1 of that period) after a light breakfast, followed by postdose triplicate ECGs and PK blood sampling for 24 hours at specified time points.
Dose Selection
According to the regulatory requirements, the QTc prolongation should be measured at the substantial multiples of highest clinically relevant exposure of the test drug, if feasible.15 When this is not feasible, it is instead appropriate to evaluate the QTc effects of the maximum tolerated dose of the compound with the inclusion of a positive control in order to demonstrate assay sensitivity.15,18 In a multiple-ascending dose study conducted in 56 subjects (S.L.F., data on file, 2015), the maximum tolerated daily multiple dose of SPN-812 was 1,800 mg and the maximum tolerated single dose was 2,100 mg (yielding Cmax values of 11.6 μg/mL and 10.7 μg/mL). The most common dose-limiting adverse reactions in this study were nausea, vomiting, dizziness, and headache, which led to discontinuations from the study in 2 subjects at the maximum dose. It is, therefore, not feasible to assess the ECG effects of SPN-812 at Cmax above approximately 10-11 μg/mL. Based on these data as well as the findings from a SPN-812 metabolism assessment study, which showed that renal clearance was a major elimination route for SPN-812 (approximately 90% cleared within 24 hours of the dose, 100% within 96 hours),19 the supratherapeutic dose of SPN-812 1,800 mg was selected and positive control (moxifloxacin)17 was used.
Assessments
QT assessment and pharmacokinetic sampling. Twelve-lead ECGs were obtained digitally (Mortara Instrument H-12, Milwaukee, Wisconsin), which recorded 10-second ECGs in triplicate at baseline (day −1 of Period 1) and on day 2 of the dosing period. Time points included the following: −0.75, −0.5, −0.25, 0, 0.5, 1, 2, 3, 4, 5, 7, 9, 12, 18, and 24 hours. Patients were positioned supine for 10-15 minutes before and 5 minutes after ECG recording. A centralized ECG reading laboratory (ERT, eResearch Technology, Inc., Philadelphia, Pennsylvania) was used to read the ECGs with interpretation by a high-resolution, manual, on-screen caliper method, with annotations to minimize interreader variability. The central ECG laboratory was blinded to both subjects and their treatment. To determine drug concentrations in plasma, blood samples (4 mL) were collected in blood collection tubes on day 2 of the dosing period at the following time points: 0 (predose), 0.5, 1, 2, 3, 4, 5, 7, 9, 12, 18, and 24 hours postdose.
Outcome measures. The primary endpoint was the correlation between the change from baseline (CFB) in individual heart rate corrected QT interval (QTcI) (ΔQTcI) and viloxazine and 5-OH-VLX-gluc plasma concentration (Cp). The secondary endpoint was the time point placebo adjusted CFB in QTcI (ΔΔQTcI) for viloxazine. For assay sensitivity, the correlations between moxifloxacin Cp and the ΔQTcI, and moxifloxacin and time point ΔΔQTcI were evaluated. Additionally, evaluations included QTcF, HR, the PR and QRS intervals, and changes in ECG morphology. The concentrations of viloxazine, 5-OH-VLX-gluc, and moxifloxacin in plasma were determined using validated achiral chromatographic tandem mass spectrometry methods.
Safety. Safety endpoints included monitoring adverse events (AEs), clinical laboratory tests, vital signs, physical examinations, and ECGs. AEs were identified by the study investigator and by subject report. The AEs were assessed for their level of severity and relatedness to the study drug. Any AEs occurring after administration of the first dose were considered treatment-emergent AEs (TEAEs).
Statistical analysis. The sample size for this trial was based on an assessment of the noninferiority of SPN-812 relative to placebo in the primary analysis. SPN-812 would be declared to have no influence on QTc if the null hypothesis, that the upper bound of the 2-sided 90% confidence interval (CI) of the predicted mean ΔΔQTcI was greater than 10 ms at the observed mean maximum Cp (Cmax) for the supratherapeutic dose of viloxazine, can be rejected. The sample size was also based on the need to demonstrate assay sensitivity with moxifloxacin.
These analyses used a linear mixed-effects modeling approach to explore the relationship between the CFB in QTc intervals (ΔQTcI and ΔQTcF) as well as other ECG parameters (HR, the PR and QRS intervals) and Cps of viloxazine and 5-OH-VLX-gluc.20,21 To establish assay sensitivity, (1) the slope of the relationship between the placebo-corrected CFB for QTcI and the Cp of moxifloxacin should be positive, and (2) the lower limit of the 2-sided 90% CI at the observed Cmax of moxifloxacin should be > 5 ms.
RESULTS
Subjects
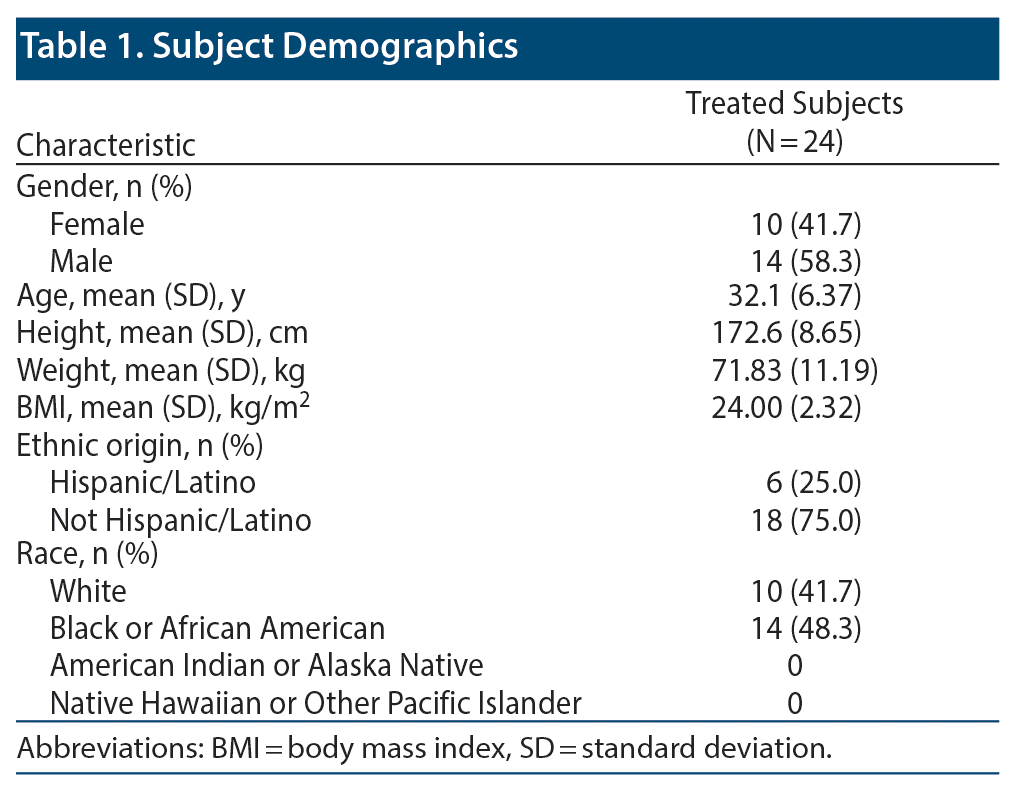
Baseline demographics of the study population are shown in Table 1. Twenty-two of 24 subjects completed all 3 treatment arms of the trial. Two subjects did not complete the study due to dermatologic AEs that were considered likely unrelated to the study drug. In both cases, the subjects withdrew after completing at least 1 treatment period. Safety and baseline demographic data were assessed for all 24 subjects. Cardiac repolarization was assessed in 23 of 24 subjects. One subject who withdrew completed the SPN-812 treatment period; therefore, they were included in the analysis for cardiac repolarization.
Effect of SPN-812 on Cardiac Repolarization
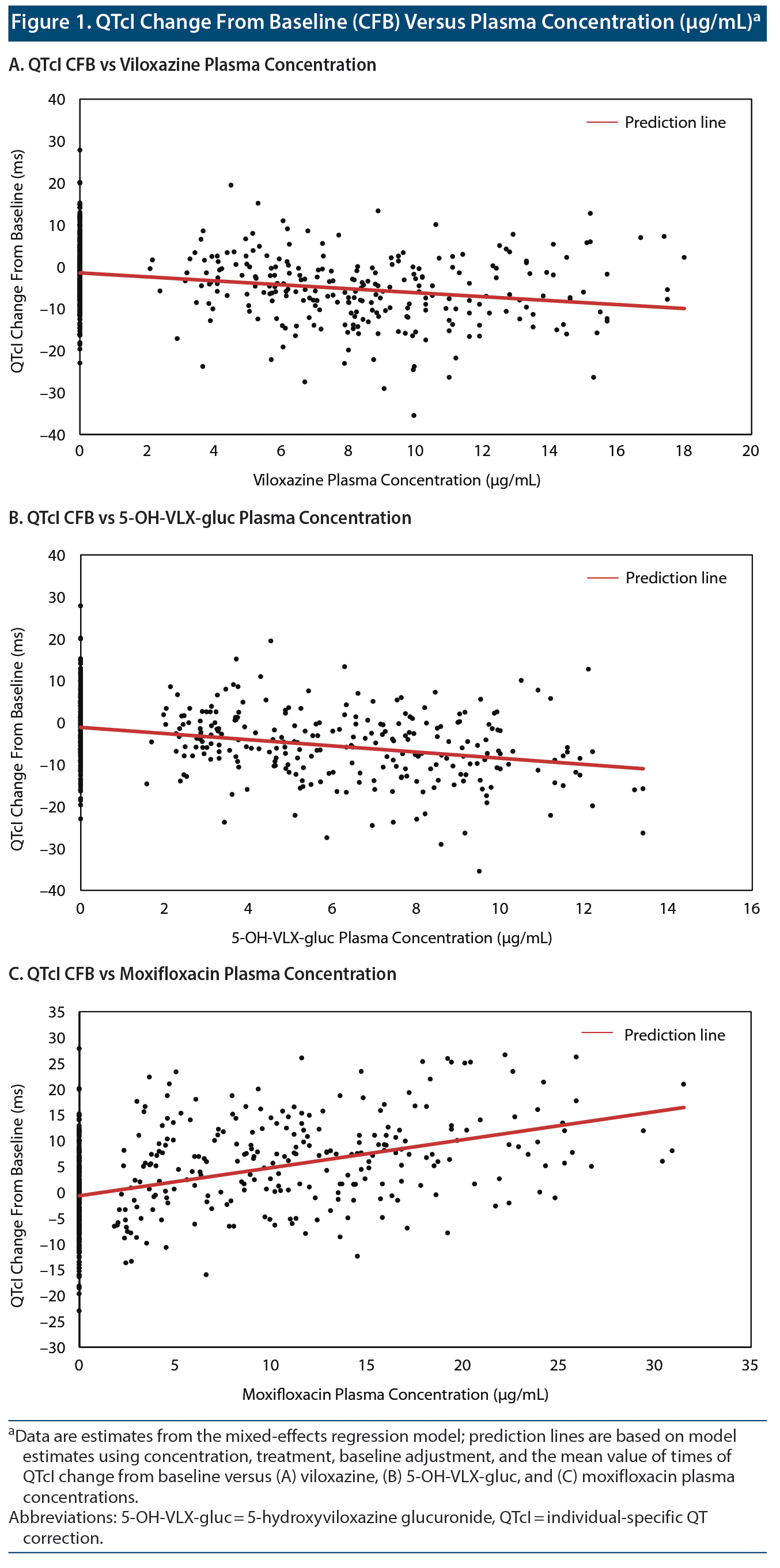
The relationship between ΔQTcI and viloxazine Cp demonstrated a statistically significant negative slope (P = .0012; Figure 1A and Table 2). The relationship of 5-OH-VLX-gluc and ΔQTcI also demonstrated a statistically significant negative slope (P = .0007; Figure 1B and Table 2).
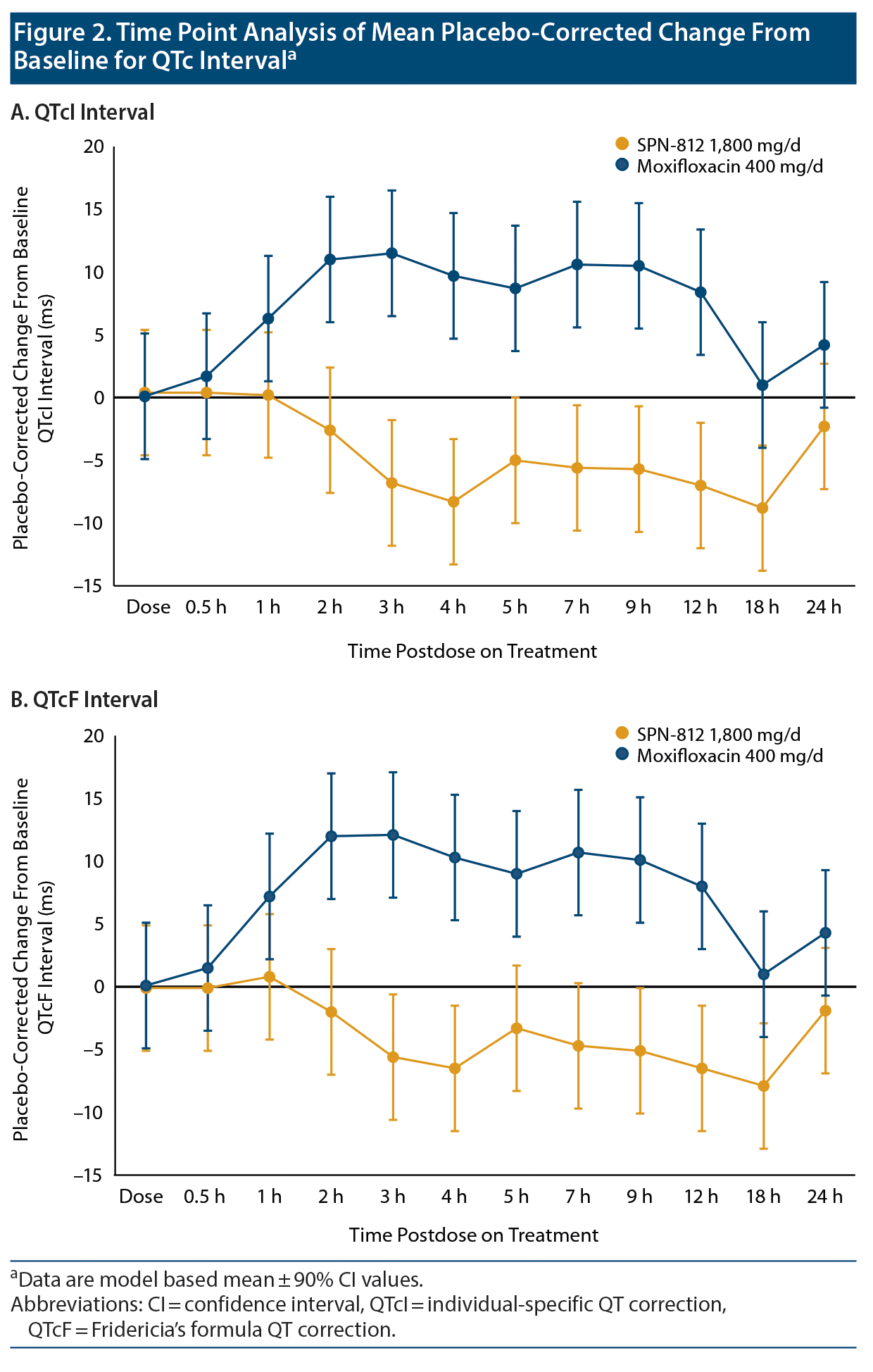
Time point analysis of QTcI and QTcF data, likewise, supported the fact that SPN-812 treatment did not induce an increase in QTc interval from baseline by either assessment over the 24-hour period (Figure 2A and Figure 2B). The peak placebo-corrected CFB for QTcI was 0.4 ms (upper 2-sided 90% CI 4.0 ms). Placebo-corrected, time-averaged, mean changes in QT, QTcI, and QTcF following SPN-812 treatment were all negative (−4.4 ms, −4.2 ms, and −3.6 ms, respectively). In addition, no subjects exhibited new abnormal U waves, new inverted T waves, new > 480 ms absolute QTc duration, new QTcF > 480 ms, a > 30 ms change in QTc from baseline, or > 30 ms in QTcF. There were also no new ST segment depression changes and no new second- or third-degree heart blocks.
Other Secondary Measures
No clinically significant changes in HR, PR interval, and QRS duration as a result of SPN-812 administration were observed. Mean placebo-corrected changes from baseline were 0.3 bpm (HR), 1.4 ms (PR interval), and 0.7 ms (QRS duration) following SPN-812 treatment.
Assay Sensitivity
Assay sensitivity was successfully demonstrated with 400 mg/d of moxifloxacin. QTcI and QTcF were similar for 400 mg/d of moxifloxacin (Figure 2A and Figure 2B), with mean CFBs of 5.6 ms and 5.5 ms. Mean QTcI and lower bound of the 2-sided 90% CI values were > 5 ms at 2 hours postdose and reached a peak value of 11.5 ms (2-sided 90% CI, 6.8, 16.3) at 3 hours postdose. The resulting slope of the CFB for QTcI and moxifloxacin Cp was positive (Figure 1C and Table 2).
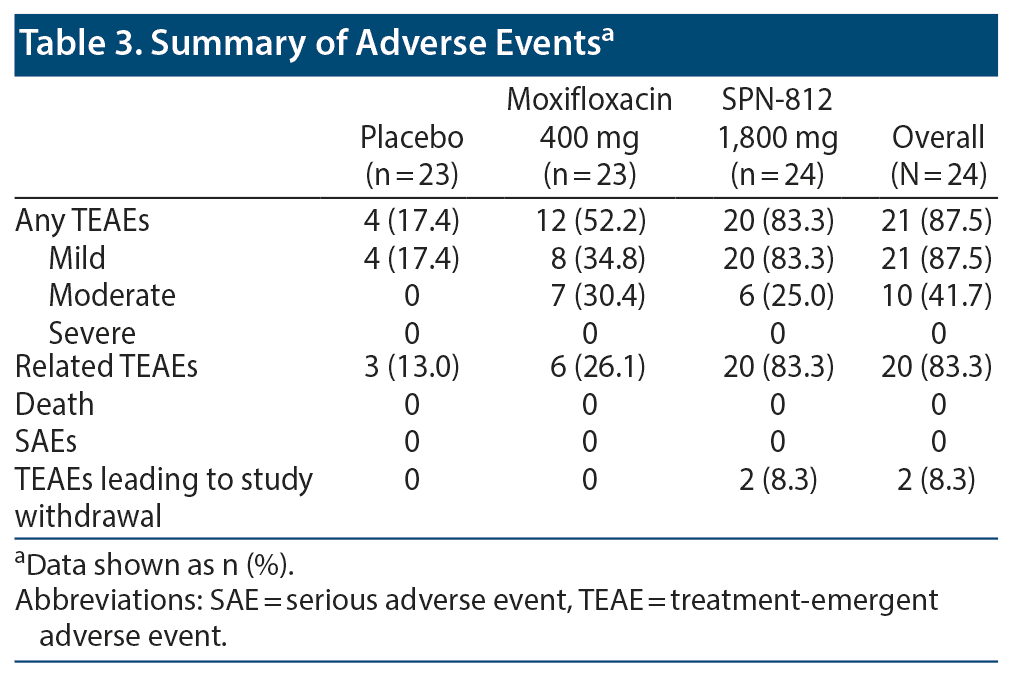
Safety
No deaths or serious AEs occurred in this study (Table 3). Overall, 21 subjects (87.5%) experienced TEAEs, and 20 (83.3%) experienced TEAEs considered related to SPN-812. TEAEs leading to discontinuation occurred in 2 subjects; however, they were not considered related to study medication. All TEAEs resolved by the end of the study.
DISCUSSION
ICH-E14 Guidance recommends evaluation of the ECG effects of a supratherapeutic exposure of all new drugs to identify those that may have significant risk of producing TdP.15 Since TdP is an infrequent, but potentially lethal, arrhythmia, evaluation of the risk of TdP for drugs has relied on the surrogate marker of drug-induced QTc prolongation. This phase 1 TQT study supports the cardiovascular safety of SPN-812 in accordance with this Guidance. The primary endpoint for this trial was based on the use of concentration-QTc effect modeling, which demonstrated a negative relationship between Cps of viloxazine (and its metabolite) and ΔQTcI. Using a linear mixed-effects model, the predicted mean change from baseline in QTcI at a supratherapeutic Cmax of 12.38 µg/mL was −9.7 ms for viloxazine, and supratherapeutic Cmax of 9.95 µg/mL was −9.3 ms for its metabolite 5-OH-VLX-gluc, with 90% 2-sided upper confidence bounds below zero. In contrast, drugs that are capable of producing TdP all have positive relationships between exposure and QTc increase. The secondary analysis, based on the time point analysis of ΔΔQTcI, demonstrated that the largest value for ΔΔQTcI occurred 0.5 hours after dosing, with a mean ΔΔQTcI of 0.4 ms and 90% upper confidence bound of 4.0 ms. These data demonstrate that a supratherapeutic dose of SPN-812 does not result in any clinically significant QTc prolongation. In addition, an outlier analysis used categorical cut points to determine if any subject showed a signal of a potential effect on cardiac repolarization not manifested in the central tendency data. The specific outlier criteria were new abnormal U waves, new > 500 ms absolute QTc duration, and a > 60 ms change in QTc from baseline. No subjects treated with SPN-812 met any of the QTc criteria, and no subject met nonspecific QTc outlier change from baseline of 30-60 ms. The data also showed no clinically meaningful effect of a supratherapeutic dose of SPN-812 on other ECG parameters.
The anticipated clinical dose of SPN-812 in children and adolescents produces a mean Cmax of approximately 7.5 µg/mL (4.4 µg/mL for 5-OH-VLX-gluc), well below that of the supratherapeutic exposure evaluated in this trial. The small but consistent decrease in QTc by SPN-812 observed in this trial was similar to that seen with bupropion and may suggest a protective effect of the drug.13 These changes are typically thought to be of no clinical concern. These results are consistent with previously published safety findings for SPN-812,22 as well as the cardiovascular safety data from the phase 3 studies of SPN-812.23-25
The finding that administration of a supratherapeutic dose of SPN-812 (ie, 1,800 mg) did not result in significant cardiovascular events suggests that SPN-812 has a large cardiovascular safety margin relative to the proposed therapeutic doses studied in its pivotal phase 3 trials.23-25 The highest doses of SPN-812 evaluated in the phase 3 trials were 400 mg in children (6-11 years old) and 600 mg in adolescents (12-17 years old). These doses correspond to approximately 700 mg following multiple dose administrations in healthy adults, based on overall exposure extrapolation. Given this margin, there should be minimal concern that the proposed therapeutic doses would result in significant cardiovascular risk to patients.
Within the context of other nonstimulants and stimulants used for the treatment of ADHD, nonstimulant treatments such as atomoxetine, guanfacine, and clonidine have been associated with various cardiac effects. For instance, although the risk for QT prolongation with atomoxetine at therapeutic doses has been characterized as small, the risk still necessitates a label warning for possible potentiation of cardiovascular risks associated with other QT-prolonging agents.4,26,27 Additionally, guanfacine has mixed effects on cardiac response. Guanfacine has been associated with QT/QTc prolongation and dose-dependent decreases in blood pressure and heart rate, as well as stabilizing effects on cardiac restitution, consistent with its lack of arrhythmia liability.5,28,29 Further, clonidine can cause dose-related decreases in blood pressure and heart rate and may worsen sinus node dysfunction and atrioventricular block.6 The cardiovascular effects of stimulant use for the treatment of ADHD have also been well characterized. Daily use of methylphenidate has been associated with increased blood pressure or heart rate.2 Stimulants carry a Warning and Precaution in their label for serious cardiovascular events, including sudden death, in children and adolescents with cardiac issues or structural abnormalities.3 This TQT study suggests that SPN-812 lacks these cardiovascular effects, supporting the suggestion that the cardiovascular safety profile for SPN-812 may be different from that of other ADHD treatments, nonstimulant and stimulant alike.
The present TQT study evaluated the cardiovascular effects of SPN-812, and the results support the cardiovascular safety of SPN-812 in a healthy adult population. Given the emergent importance of precision medicine,30 it is critical to match patients with the right medicine from both safety and efficacy perspectives. The cardiovascular safety of SPN-812 can be an important factor in the selection of treatment options for patients with ADHD. The obvious limitation of this study is the 2-day dosing tested in healthy adult volunteers, and more ECGs need to be collected long-term in the targeted populations.
This phase 1 study evaluated the effect of a supratherapeutic dose SPN-812 (viloxazine extended-release) on the QT interval in healthy adults. The data indicate there was no clinically significant effect of SPN-812 on cardiac repolarization or other ECG parameters. Assay sensitivity was confirmed by moxifloxacin. Overall, this evidence suggests that SPN-812 did not pose a risk for drug-induced arrhythmias in healthy adults.
Submitted: April 8, 2020; accepted August 10, 2020.
Published online: October 13, 2020.
Potential conflicts of interest: Drs Nasser, Faison, Liranso, Adewole, Busse, and Schwabe are employees of Supernus Pharmaceuticals, Inc. Dr Kleiman is an employee of eResearch Technology, Inc. For a list of Dr Fava’s lifetime disclosures, please visit Psychiatrist.com.
Funding/support: The study was funded and conducted by Supernus Pharmaceuticals, Inc.
Role of the sponsor: Drs Nasser, Faison, Liranso, Adewole, and Schwabe, employees of Supernus Pharmaceuticals, Inc., were involved in the study design, bioanalytical testing, safety monitoring, and reporting of the study results. Dr Busse, an employee of Supernus Pharmaceuticals, Inc., provided data interpretation support, preparation of the manuscript drafts, and publication management.
Acknowledgments: Editorial support was provided by IMPRINT Science, New York, New York, with funding from Supernus Pharmaceuticals, Inc.
REFERENCES
1. Wolraich ML, Hagan JF Jr, Allan C, et al; Subcommittee on Children and Adolescents With Attention-Deficit/Hyperactive Disorder. Clinical practice guideline for the diagnosis, evaluation, and treatment of attention-deficit/hyperactivity disorder in children and adolescents. Pediatrics. 2019;144(4):e20192528. PubMed CrossRef
2. Concerta [prescribing information]. Titusville, NJ: Janssen Pharmaceuticals, Inc; 2017.
3. Vyvanse [prescribing information]. Lexington, MA: Shire US Inc; 2018.
4. Strattera [prescribing information].Indianapolis, IN: Lilly USA, LLC; 2017.
5. Intuniv [prescribing information]. Lexington, MA: Shire US Inc; 2019.
6. Kapvay [prescribing information].St. Michael, Barbados: Concordia Pharmaceuticals Inc; 2016.
7. Biederman J, Mick E, Faraone SV. Age-dependent decline of symptoms of attention deficit hyperactivity disorder: impact of remission definition and symptom type. Am J Psychiatry. 2000;157(5):816-818. PubMed CrossRef
8. Yu C, Garcia-Olivares J, Candler S, et al. New insights into the mechanism of action of viloxazine: serotonin and norepinephrine modulating properties. J Exp Pharmacol. 2020;12:285-300. PubMed CrossRef
9. Cubeddu LX. QT prolongation and fatal arrhythmias: a review of clinical implications and effects of drugs. Am J Ther. 2003;10(6):452-457. PubMed CrossRef
10.Redfern WS, Carlsson L, Davis AS, et al. Relationships between preclinical cardiac electrophysiology, clinical QT interval prolongation and torsade de pointes for a broad range of drugs: evidence for a provisional safety margin in drug development. Cardiovasc Res. 2003;58(1):32-45. PubMed CrossRef
11.Anderson ME, Al-Khatib SM, Roden DM, et al; Duke Clinical Research Institute/American Heart Journal Expert Meeting on Repolarization Changes. Cardiac repolarization: current knowledge, critical gaps, and new approaches to drug development and patient management. Am Heart J. 2002;144(5):769-781. PubMed CrossRef
12.Haverkamp W, Breithardt G, Camm AJ, et al. The potential for QT prolongation and pro-arrhythmia by non-anti-arrhythmic drugs, clinical and regulatory implications: report on a policy conference of the European Society of Cardiology. Cardiovasc Res. 2000;47(2):219-233. PubMed CrossRef
13.Castro VM, Clements CC, Murphy SN, et al. QT interval and antidepressant use: a cross sectional study of electronic health records. BMJ. 2013;346:f288. PubMed CrossRef
14.Mischoulon D, Shelton RC, Baer L, et al. Ziprasidone augmentation of escitalopram for major depressive disorder: cardiac, endocrine, metabolic, and motoric effects in a randomized, double-blind, placebo-controlled study. J Clin Psychiatry. 2017;78(4):449-455. PubMed CrossRef
15.Food and Drug Administration, HHS. International Conference on Harmonisation; guidance on E14 clinical evaluation of QT/QTc interval prolongation and proarrhythmic potential for non-antiarrhythmic drugs: availability, notice. Fed Regist. 2005;70(202):61134-61135. PubMed
16.Bloomfield DM, Kost JT, Ghosh K, et al. The effect of moxifloxacin on QTc and implications for the design of thorough QT studies. Clin Pharmacol Ther. 2008;84(4):475-480. PubMed CrossRef
17.Darpo B, Benson C, Dota C, et al. Results from the IQ-CSRC prospective study support replacement of the thorough QT study by QT assessment in the early clinical phase. Clin Pharmacol Ther. 2015;97(4):326-335. PubMed CrossRef
18.E14 Clinical Evaluation of QT/QTc Interval Prolongation and Proarrhythmic Potential for Non-Antiarrhythmic Drugs—Questions and Answers (R3), Guidance for Industry. Revision 2. US Food and Drug Administration website. June 2017. https://www.fda.gov/media/71379/download.
19.Yu C. Metabolism and in vitro drug-drug interaction assessment of viloxazine. Xenobiotica. 2020;50(11):1285-1300. PubMed CrossRef
20.Garnett C, Bonate PL, Dang Q, et al. Scientific white paper on concentration-QTc modeling. J Pharmacokinet Pharmacodyn. 2018;45(3):383-397. PubMed CrossRef
21.Garnett C, Bonate PL, Dang Q, et al. Correction to: scientific white paper on concentration-QTc modeling. J Pharmacokinet Pharmacodyn. 2018;45(3):399. PubMed CrossRef
22.Johnson JK, Liranso T, Saylor K, et al. A phase II double-blind, placebo-controlled, efficacy and safety study of SPN-812 (extended-release viloxazine) in children with ADHD. J Atten Disord. 2020;24(2):348-358. PubMed CrossRef
23.Nasser A, Hull JT, Chowdhry FA, et al. 111. Phase 3, randomized, double-blind, placebo-controlled study (P301) assessing efficacy and safety of extended-release viloxazine in children with ADHD. CNS Spectr. 2020;25(2):272. CrossRef
24.Nasser A, Hull JT, Chowdhry FA, et al. 112. A phase 3, randomized, double-blind, placebo-controlled study (P302): efficacy and safety of extended-release viloxazine in adolescents with ADHD. CNS Spectr. 2020;25(2):272-273. CrossRef
25.Nasser A, Hull JT, Chowdhry FA, et al. 113. Phase 3, randomized, double-blind, placebo-controlled study (P303) assessing efficacy and safety of extended-release viloxazine in children with ADHD. CNS Spectr. 2020;25(2):273-274. CrossRef
26.Strattera 10mg hard capsules: summary of product characteristics. EMC website. Accessed February 20, 2020. https://www.medicines.org.uk/emc/product/5531/smpc/print.
27.Reed VA, Buitelaar JK, Anand E, et al. The safety of atomoxetine for the treatment of children and adolescents with attention-deficit/hyperactivity disorder: a comprehensive review of over a decade of research. CNS Drugs. 2016;30(7):603-628. PubMed CrossRef
28.Huss M, Chen W, Ludolph AG. Guanfacine extended release: a new pharmacological treatment option in Europe. Clin Drug Investig. 2016;36(1):1-25. PubMed CrossRef
29.Fossa AA, Zhou M, Robinson A, et al. Use of ECG restitution (beat-to-beat QT-TQ interval analysis) to assess arrhythmogenic risk of QTc prolongation with guanfacine. Ann Noninvasive Electrocardiol. 2014;19(6):582-594. PubMed CrossRef
30.Lobitz G, Armstrong K, Concato J, et al. The biological and biographical basis of precision medicine. Psychother Psychosom. 2019;88(6):333-340. PubMed CrossRef
Save
Cite
Advertisement
GAM ID: sidebar-top