Objective: The purpose of this study was to evaluate the effects of repetitive transcranial magnetic stimulation (rTMS) on suicidal ideation in patients with treatment-resistant major depression (TRD) (patients who failed to clinically respond to at least 2 medication trials).
Methods: We pooled data from 2 published prospective randomized controlled trials of rTMS applied to the dorsolateral prefrontal cortex in patients with TRD. We compared the effect of bilateral, left unilateral, and sham rTMS on suicidal ideation as measured by the suicide item of the 17-item Hamilton Depression Rating Scale (HDRS) (N = 156).
Results: Suicidal ideation resolved in 40.4%, 26.8%, and 18.8% of participants randomized to bilateral, left unilateral, and sham rTMS, respectively. The difference between bilateral and sham was significant (OR = 3.03; 95% CI, 1.19-7.71; P = .02), unlike the difference between left unilateral and sham (OR = 1.59; 95% CI, 0.61-4.12; P = .33). There was a modest correlation between change in suicidal ideation and change in depression severity (Pearson r = 0.38; P < .001) and no difference in change of HDRS-16 score between suicide remitters and nonremitters (P = .32).
Conclusions: Bilateral rTMS was superior to sham rTMS in reducing suicidal ideation in patients with TRD. Only a small portion of the reduction in suicidal ideation was attributable to the reduction in depressive symptoms. These data suggest that suicidal ideation could be a specific target symptom construct for rTMS.
Trial Registration: ClinicalTrials.gov identifiers: NCT01515215 and NCT00305045

Bilateral Repetitive Transcranial Magnetic Stimulation Decreases Suicidal Ideation in Depression

ABSTRACT
Objective: The purpose of this study was to evaluate the effects of repetitive transcranial magnetic stimulation (rTMS) on suicidal ideation in patients with treatment-resistant major depression (TRD) (patients who failed to clinically respond to at least 2 medication trials).
Methods: We pooled data from 2 published prospective randomized controlled trials of rTMS applied to the dorsolateral prefrontal cortex in patients with TRD. We compared the effect of bilateral, left unilateral, and sham rTMS on suicidal ideation as measured by the suicide item of the 17-item Hamilton Depression Rating Scale (HDRS) (N = 156).
Results: Suicidal ideation resolved in 40.4%, 26.8%, and 18.8% of participants randomized to bilateral, left unilateral, and sham rTMS, respectively. The difference between bilateral and sham was significant (OR = 3.03; 95% CI, 1.19-7.71; P = .02), unlike the difference between left unilateral and sham (OR = 1.59; 95% CI, 0.61-4.12; P = .33). There was a modest correlation between change in suicidal ideation and change in depression severity (Pearson r = 0.38; P < .001) and no difference in change of HDRS-16 score between suicide remitters and nonremitters (P = .32).
Conclusions: Bilateral rTMS was superior to sham rTMS in reducing suicidal ideation in patients with TRD. Only a small portion of the reduction in suicidal ideation was attributable to the reduction in depressive symptoms. These data suggest that suicidal ideation could be a specific target symptom construct for rTMS.
Trial Registration: ClinicalTrials.gov identifiers: NCT01515215 and NCT00305045
J Clin Psychiatry 2018;79(3):17m11692
To cite: Weissman CR, Blumberger DM, Brown PE, et al. Bilateral repetitive transcranial magnetic stimulation decreases suicidal ideation in depression. J Clin Psychiatry. 2018;79(3):17m11692.
To share: https://doi.org/10.4088/JCP.17m11692
© Copyright 2018 Physicians Postgraduate Press, Inc.
aTemerty Centre for Therapeutic Brain Intervention, Centre for Addiction and Mental Health, University of Toronto, Toronto, Ontario, Canada
bDepartment of Psychiatry, University of Toronto, Toronto, Ontario, Canada
cCampbell Family Mental Health Research Institute, Centre for Addiction and Mental Health, University of Toronto, Toronto, Ontario, Canada
dDepartment of Statistical Sciences, University of Toronto; Cancer Care Ontario, Toronto, Ontario, Canada
eInstitute of Medical Science, Faculty of Medicine, University of Toronto, Toronto, Ontario, Canada
fKrembil Research Institute, University Health Network, Toronto, Ontario, Canada
gMonash Alfred Psychiatry Research Centre, Alfred and Monash University Central Clinical School, Victoria, Australia
*Corresponding author: Zafiris J. Daskalakis, MD, PhD, FRCP(C), Centre for Addiction and Mental Health (CAMH), University of Toronto, 1001 Queen St West, Unit 4-1, Toronto, Ontario, Canada, M6J1H4 ([email protected]).
Major depressive disorder (MDD) has an annual and lifetime prevalence of 4.7% and 11.3%, respectively, in Canada,1 and 6.6% and 16.2%, respectively, in the United States.2 MDD presents with several symptoms including suicidality, which encompasses suicidal ideation.3 The lifetime rate of completed suicide is 15% to 20% among patients with MDD or bipolar depression.4 About 90% of individuals who complete suicide suffer from a psychiatric illness.5 Depression appears to be the most significant risk factor for death by suicide, with a population attributable risk for suicide from depression of 28%.6
Fink and Kellner7,8 have advocated for the role of electroconvulsive therapy (ECT) in patients with mood disorders and suicidality. Lithium has also been shown to reduce suicidality in patients with mood disorders.9 However, both treatments are associated with adverse effects (eg, anterograde amnesia with ECT; renal disease with lithium). Experimental treatments such as ketamine10 have also been associated with antisuicidal effects. However, given that the number of completed suicides globally remains above 800,000 per year and that suicide is the second leading cause of death in individuals aged 15-29 years, other treatment options for suicidality are needed.11
Repetitive transcranial magnetic stimulation (rTMS) is an evidence-based treatment for patients with treatment-resistant major depression (TRD).12 While presumably not as efficacious as ECT, rTMS is more acceptable to many patients because it is less invasive, does not require anesthesia, carries less stigma, and is not associated with adverse cognitive effects.12 In a recent meta-analysis of 29 randomized controlled trials (RCTs) of rTMS applied to the left dorsolateral prefrontal cortex (DLPFC) for TRD (N = 1,371), the pooled response and remission odds ratios were both 3.3 for rTMS compared to sham.13 Considering that rTMS shows significant efficacy in TRD and that ECT is efficacious in treating suicidality,8 the question of whether rTMS can be used as a treatment specifically for suicidal ideation is consequential. The only sham-controlled trials that evaluated the effects of rTMS specifically on suicidal ideation to date are of small sample, with limited methodology and equivocal results.14,15,16
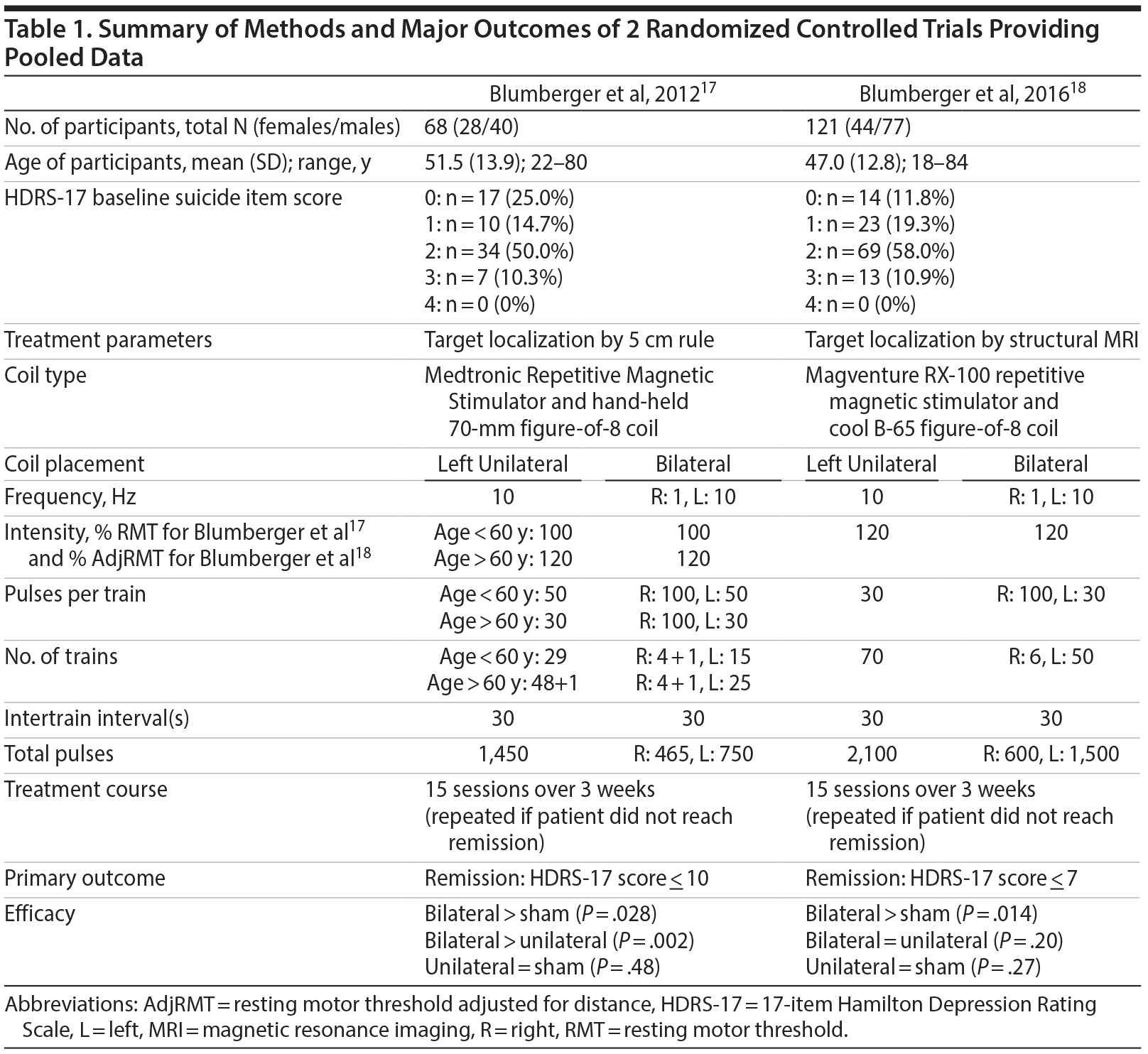
To comprehensively address the question of whether rTMS reduces suicidal ideation in patients with TRD, we analyzed data from 2 published RCTs17,18 that compared efficacy of bilateral, unilateral, and sham rTMS on TRD, with study sizes of 6817 and 12118 subjects. In both trials, bilateral rTMS was shown to be significantly more efficacious than sham rTMS at inducing remission from TRD (remission rates of 34.6%17 and 20%18). The results for efficacy of unilateral rTMS compared to bilateral rTMS on TRD in these studies was less conclusive, with bilateral rTMS more efficacious than unilateral in one study (P = .002)17 but not in the second (P = .20).18 See Table 1 for details of these studies. Given the similarities in methods and results, we combined these data sets to examine the effects of rTMS specifically on suicidal ideation within this patient sample. With the viewpoint that suicidal ideation is a symptom construct that is related, but distinct from, other depression symptomatology, as indexed by the 17-item Hamilton Depression Rating Scale (HDRS-17), we hypothesized that, similar to its effect on other depressive symptoms in the original studies, bilateral rTMS would be significantly more effective at reducing suicidal ideation than sham, yet that this improvement would not be closely correlated with remission of other depressive symptoms (HDRS-16).

- There is a fundamental lack of understanding of the phenomenology of, and few evidence-based treatments for, suicidal ideation and suicidality in general.
- Bilateral repetitive transcranial magnetic stimulation is a viable treatment option for treatment-resistant depression, and early evidence suggests that it appears to be successful in treating comorbid suicidal ideation.
METHODS
We analyzed pooled data from 2 published RCTs.17,18 As summarized in Table 1, these 2 RCTs used similar methods and had similar results, with identical definitions of TRD: a failure to achieve clinical response with, or failure to tolerate, at least 2 antidepressant medications of different classes over the span of at least 6 weeks in the current depressive episode.17-19 Both original studies were approved by the Centre for Addiction and Mental Health research ethics board, and written informed consent was obtained from all patients prior to the studies’ onset.17,18 Suicidal ideation was measured with the HDRS-17 suicide item (item 3) throughout the treatment phase. This item consists of the following scoring system: 0 (absent), 1 (feels life is not worth living), 2 (wishes he were dead or any thoughts of possible death to self), 3 (suicide ideas or gestures), 4 (attempts at suicide—any serious attempts). Nearly all HDRS-17 assessments of both original studies were completed by 1 bachelor’s -level psychology graduate, trained by coauthor Z.J.D., therein removing concerns about interrater reliability.
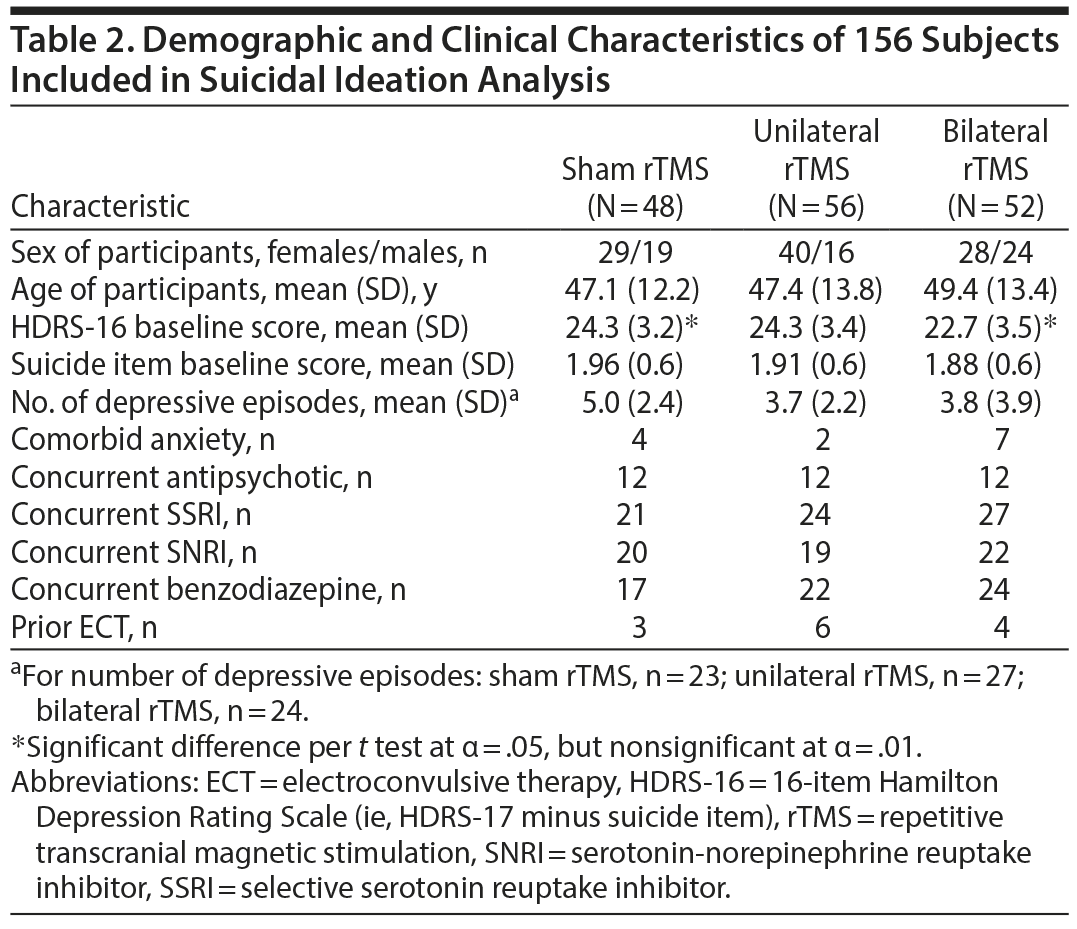
We analyzed measurements at baseline before initiation of the intervention and at primary endpoint, 3 or 6 weeks later depending on the participant’s response.17,18 Acute suicidality was an exclusion criterion in both studies. As such, there were no subjects with HDRS-17 suicide item score greater than 3 in either trial. Our primary outcome was resolution of suicidal ideation, defined as a decrease from any nonzero score at baseline to a score of zero at study endpoint on the HDRS-17 suicide item.8,20 After we removed 33 participants with baseline suicide scores of 0 (ie, no suicidal ideation), 156 participants remained in the analysis.
To test our primary hypothesis, we used odds ratios to compare resolution of suicidal ideation in bilateral rTMS and left unilateral rTMS versus sham rTMS. We used a Pearson correlation to assess the relationship between change in suicidal ideation (ie, suicide item score) and change in depression severity (ie, total score on the HDRS-17 after removing the suicide item: HDRS-16). To further assess this relationship, we performed a Welch 2-sample t test comparing average change in HDRS-16 from baseline to endpoint between suicide remitters and nonremitters for the 156 subjects in the remission analysis.
RESULTS
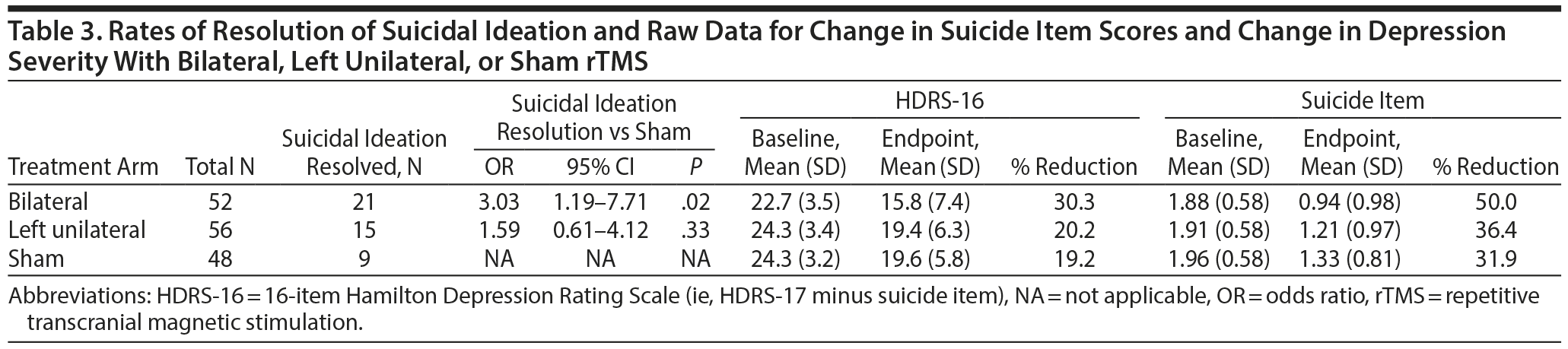
The demographic and clinical characteristics of the 156 participants included in these analyses are summarized in Table 2. The only significant difference between active groups compared to sham rTMS was a significantly lower baseline HDRS-16 score in the bilateral rTMS group compared to sham. Baseline and endpoint scores of the suicide item and HDRS-16 are shown in Table 3. Suicidal ideation resolved in 40.4%, 26.8%, and 18.8% of participants randomized to bilateral, left unilateral, and sham rTMS, respectively. The difference in resolution of suicidal ideation between bilateral and sham rTMS was significant (OR = 3.03; 95% CI, 1.19-7.71; P = .02), unlike the difference between left unilateral and sham rTMS (OR = 1.59; 95% CI, 0.61-4.12; P = .33) (Table 3). Of the 33 participants in the original studies with baseline suicide item scores of 0, 4 in the sham rTMS group (N = 13), 2 in the unilateral rTMS group (N = 6), and 1 in the bilateral rTMS group (N = 14) had suicidal ideation at the study’s end.
A multiple logistic regression model with a treatment-by-study interaction term was not a significant improvement over the simpler model without this interaction term (likelihood ratio testing χ2 = 5.82, P = .12). Thus, there is no direct evidence that the 2 studies differed in terms of the treatment effect on suicidal ideation (see Supplementary eTable 1).
The correlation (Pearson r) between change in suicidal ideation and change in depression was 0.38 (P < .001). There was no difference between suicide remitters and nonremitters in change of HDRS-16 according to Welch 2-sample t test (P = .3198).
DISCUSSION
We pooled data from 2 published prospective randomized sham-controlled trials of rTMS in patients with TRD and compared the effects of bilateral, left unilateral, and sham rTMS on suicidal ideation. We found that suicidal ideation was more likely to resolve with bilateral rTMS than sham rTMS, but this was not the case for left unilateral rTMS. There was only a modest correlation between change in suicidal ideation and change in depression severity. Below, we discuss each of these findings.
The relationship between change in suicidal ideation and change in depression severity was significant in our study, but the correlation was modest: the change in depression severity accounted for approximately 15% of the change in suicidal ideation. Furthermore, the rate of resolution of suicidal ideation was higher than the rate of remission of depressive symptoms (40.4% vs 25.8% with bilateral rTMS). When patients were divided into suicide remitter and nonremitter groups, the change in HDRS-16 scores did not differ across groups. This supports the concept that the response of suicidal ideation to bilateral rTMS treatment does not depend on overall depressive symptom response, or vice versa. Furthermore, singling out suicidality through the suicide item of the HDRS-17 has been validated21 through a principal component analysis of a study on 281 suicide attempters, in which the suicide item was successfully isolated into one of 3 independent dimensional factors for the total HDRS-17. Similar results were found in a large outpatient study (N = 660) in which factor analysis found 3 factors for the HDRS-17, one being a “cognitive” factor consisting of suicide and guilt items.22 We therefore conclude that suicidal ideation is not simply related to improvement of depressive symptoms but can be treated as a separate entity, which is congruent with emerging evidence that suicidality may be its own transdiagnostic neuro-endophenotype.23 Specific prefrontal structures and serotonin neurotransmission are key mechanisms of dysfunction in suicidality.24 Our findings suggest that suicidal ideation could be a specific target symptom construct for rTMS delivered to the DLPFC bilaterally. Additional work is needed to determine whether rTMS is also effective in reducing suicidal ideation in patients with other psychiatric disorders and whether other target regions exert an effect on suicidal ideation.
The HDRS-17 suicide item has been used previously to measure treatment response, specifically by Kellner et al8 to assess resolution of suicidality with ECT treatment. In that study, suicidality resolved in 81% of 131 patients defined as “high suicide” (ie, with a score of 3 or 4 on the HDRS-17 suicide item).8 This number is greater than that demonstrated in our study (ie, 40.4% with bilateral rTMS) but baseline levels of suicidality were much higher in the ECT study, which limits the comparison of these 2 studies. In another pooled secondary analysis on treatment of late-life depression, the HDRS-17 suicide item was successfully used to stratify suicide risk at baseline and predict response of suicidality to antidepressant treatment.20 These studies support the approach of measuring response of suicidality to treatment through the HDRS-17 suicide item and emphasize the clinical utility of isolating suicidality or suicidal ideation as a symptom construct independent of depression.
There have been previous studies that assessed the efficacy of various forms of TMS on resolution of suicidal ideation. Three of these studies were sham-controlled TMS trials, and while results were promising, they failed to show significant clinical effect of TMS on suicidal ideation.14,15,16 Of note, 1 trial15 was a pilot study and administered only 9 sessions of high-frequency left DLPFC rTMS (3 times daily over 3 days), leaving open the possibility of underdosing compared to the number of sessions administered in the trials examined in this study (see Table 1). This trial also included only inpatients, which limits comparison to the outpatient sample in our study. Another trial14 only conducted intermittent theta burst stimulation over 4 days. Finally, all 3 of these studies used left unilateral DLPFC-TMS rather than the sequential bilateral technique that showed significant antisuicidal ideation effects in the present study, the implications of which are described below. Of the other research on TMS and suicidal ideation, 2 open-label trials found significant decreases in suicidal ideation with deep TMS25 and left prefrontal rTMS26 over time. In a direct comparison of rTMS to ECT in depressed patients, rTMS treatment significantly decreased suicidal ideation, although significantly less than ECT.27 Taken together, the existing evidence of various forms of TMS treatment on suicidal ideation in depression is patchwork, with mixed results, and nonexistent in terms of right DLPFC targeted treatment.
There is some early neurophysiological and neuroimaging evidence suggesting that targeting the right DLPFC may address neural substrates of suicidality specifically. For example, a study of near infrared spectroscopy measured blood flow in brain regions during a verbal fluency task and showed that patients with depression and suicidality had significant decreased mean oxy-hemoglobin in the right DLPFC, right orbitofrontal cortex (OFC), and right fronto-polar cortex regions when compared to patients without suicidality.28 Another study measuring metabolic rates of glucose with fluorodeoxyglucose positron emission tomography found significantly lower regional metabolism in the right DLPFC in depressed suicide attempters than in depressed nonattempters.29 Most recently, Sun et al30 demonstrated that greater baseline levels of cortical inhibition in the right DLPFC, measured through TMS-EEG, predict resolution of suicidal ideation by magnetic seizure therapy in patients with TRD. Collectively, these findings suggest that rTMS targeting the right DLPFC may have a more potent and specific effect on suicidal ideation, compared to the more commonly used left DLPFC-rTMS, which agrees with the results of our study.
There are some limitations to our study. Neither of the RCTs used in our study were designed or powered to test our specific hypothesis of the effect of rTMS on suicidal ideation. Also, the left unilateral rTMS did not do better than placebo for depression in both trials, which is not consistent with most studies of unilateral rTMS on TRD; this may have contributed to why bilateral rTMS alone was efficacious. However, emerging evidence suggests that bilateral rTMS may be a more efficacious treatment for TRD than unilateral rTMS.31 Bilateral treatment may cover the inherent biological and clinical phenomenological heterogeneity of depression including suicidality. In both trials, suicidal ideation was measured with the suicide item of the HDRS-17, as opposed to a more comprehensive suicide scale such as the Beck Suicidal Ideation Scale. This prevented a more nuanced assessment of the effect of rTMS on different aspects of suicidal ideation, such as active versus passive thoughts of death, as previously done.32 Also, this study did not include patients with severe, emergent suicidality, as measured by a score of 4 on the HDRS-17 suicide item, which limits the generalizability to emergent forms of suicidality or suicidal ideation. It is common to exclude patients with severe suicidality in rTMS studies, as these patients are often hospitalized on an involuntary basis due to the very high risk to themselves, precluding them from research participation. Our findings, therefore, do not generalize to this severely ill patient population. We are considering future rTMS studies on suicidality in inpatients that can potentially address this issue by including committed patients with high suicidality. Additionally, while there was no difference in baseline suicidal ideation between groups, the baseline HDRS-16 scores were statistically different between sham and bilateral rTMS groups (at α = .05 but not at α = .01), which suggests that response to bilateral rTMS for suicidal ideation may be confounded to some degree by the bilateral group having marginally lower baseline HDRS-16 scores; this should be controlled in future studies. Finally, both RCTs were from 1 research center, reducing the external validity of our findings.
Notwithstanding these limitations, our findings suggest that bilateral rTMS of the DLPFC may be an effective treatment for suicidal ideation in patients with TRD independent of its effect on overall depression severity. Future RCTs of rTMS should be designed with suicidal ideation as one of the primary outcomes. These trials need to use outcome scales specific to suicidal ideation and to enroll participants with high suicidality. They should also explore the effect of unilateral rTMS targeting the right DLPFC, not only in patients with MDD, but also in patients with other mental disorders associated with a high rate of suicidal ideation, such as borderline personality disorder and posttraumatic stress disorder. Finally, cortical targets outside the right DLPFC (eg, the right OFC) should also be assessed.
In summary, the present findings suggest that DLPFC-rTMS, when given bilaterally, may have therapeutic effects on suicidal ideation specifically, in addition to its antidepressant effects. This finding suggests that there could be a role for rTMS prior to ECT for treatment of suicidal ideation in TRD, similar to established depression treatment pathways.33 Given that only 1% of patients with TRD are currently treated with ECT33 and that neither treatment capacity nor patient acceptability for ECT is likely to improve several-fold in the foreseeable future, additional forms of intervention for TRD and suicidal ideation are urgently needed. The present study suggests that bilateral DLPFC-rTMS may offer an alternative intervention for suicidal ideation in cases where ECT is declined, not tolerated, or difficult to access.
Submitted: May 11, 2017; accepted September 29, 2017.
Published online: April 17, 2018.
Potential conflicts of interest: Dr Blumberger has received research support from Canadian Institutes of Health Research (CIHR), US National Institutes of Health (NIH), Brain Canada, and the Temerty Family through the Centre for Addiction and Mental Health (CAMH) Foundation and the Campbell Research Institute; receives research support and in-kind equipment support for an investigator-initiated study from Brainsway; is the site principal investigator for three sponsor-initiated studies for Brainsway; receives in-kind equipment support from Magventure for an investigator-initiated study; and receives medication supplies for an investigator-initiated trial from Indivior. Dr Rajji received, during the past 5 years, research support from Brain Canada, Brain and Behavior Research Foundation, Canada Foundation for Innovation, Canada Research Chair, CIHR, Ontario Ministry of Health and Long-Term Care, Ontario Ministry of Research and Innovation, NIH, and the W. Garfield Weston Foundation and reports no competing interests. Dr Downar has received research funding from CIHR, Brain Canada, the Ontario Brain Institute, the Klarman Family Foundation, and the Edgestone Foundation, as well as in-kind equipment support from MagVenture for an investigator-initiated trial and a travel stipend from Lundbeck. Dr Mulsant currently receives research support from Brain Canada, CIHR, NIH, the CAMH Foundation, Eli Lilly (medications for a NIH-funded clinical trial), and Pfizer (medications for an NIH-funded clinical trial); has within the past 5 years received research support from Bristol-Myers Squibb (medications for an NIH-funded clinical trial), Pfizer/Wyeth (medications for an NIH-funded clinical trial), Capital Solution Design LLC (software used in a study founded by CAMH Foundation), and HAPPYneuron (software used in a study founded by Brain Canada) and directly owns stocks in General Electric (less than $5,000). Dr Fitzgerald is supported by a National Health and Medical Research Council Practitioner Fellowship (1078567); has received equipment for research from MagVenture A/S, Medtronic, Cervel Neurotech, and Brainsway and funding for research from Neuronetics and Cervel Neurotech; and is on the scientific advisory board for Bionomics. Dr Daskalakis has, within the last 5 years, received research and equipment in-kind support for an investigator-initiated study through Brainsway; has received speaker fees from Eli Lilly; and has served on the advisory boards for Hoffmann-La Roche and Merck. Drs Weissman, Brown, and Isserles have no disclosures.
Funding/support: No funding specific to this article was received.
Previous presentation: Data presented at Harvey Stancer Research Day, University of Toronto, Toronto, Ontario, Canada, June 16, 2016; and Society of Biological Psychiatry; May 18-20, 2017; San Diego, California.
Supplementary material: See accompanying pages.
REFERENCES
1. Patten SB, Williams JV, Lavorato DH, et al. Descriptive epidemiology of major depressive disorder in Canada in 2012. Can J Psychiatry. 2015;60(1):23-30. PubMed CrossRef
2. Kessler RC, Berglund P, Demler O, et al. The epidemiology of major depressive disorder: results from the National Comorbidity Survey Replication (NCS-R). JAMA. 2003;289(23):3095-3105. PubMed CrossRef
3. American Psychiatric Association. Diagnostic and Statistical Manual for Mental Disorders. Fifth Edition. Washington, DC: American Psychiatric Association; 2013.
4. Miret M, Ayuso-Mateos JL, Sanchez-Moreno J, et al. Depressive disorders and suicide: epidemiology, risk factors, and burden. Neurosci Biobehav Rev. 2013;37(10 pt 1):2372-2374. PubMed CrossRef
5. Beautrais AL, Joyce PR, Mulder RT, et al. Prevalence and comorbidity of mental disorders in persons making serious suicide attempts: a case-control study. Am J Psychiatry. 1996;153(8):1009-1014. PubMed CrossRef
6. Bernal M, Haro JM, Bernert S, et al. Risk factors for suicidality in Europe: results from the ESEMED study. J Affect Disord. 2007;101(1-3):27-34. PubMed CrossRef
7. Fink M, Kellner CH, McCall WV. The role of ECT in suicide prevention. J ECT. 2014;30(1):5-9. PubMed CrossRef
8. Kellner CH, Fink M, Knapp R, et al. Relief of expressed suicidal intent by ECT: a consortium for research in ECT study. Am J Psychiatry. 2005;162(5):977-982. PubMed CrossRef
9. Cipriani A, Hawton K, Stockton S, et al. Lithium in the prevention of suicide in mood disorders: updated systematic review and meta-analysis. BMJ. 2013;346:f3646. PubMed CrossRef
10. Larkin GL, Beautrais AL. A preliminary naturalistic study of low-dose ketamine for depression and suicide ideation in the emergency department. Int J Neuropsychopharmacol. 2011;14(8):1127-1131. PubMed CrossRef
11. Preventing suicide: a global imperative. WHO website. http://www.who.int/mental_health/suicide-prevention/world_report_2014/en/. 2014.
12. Chung SW, Hoy KE, Fitzgerald PB. Theta-burst stimulation: a new form of TMS treatment for depression? Depress Anxiety. 2015;32(3):182-192. PubMed CrossRef
13. Berlim MT, van den Eynde F, Tovar-Perdomo S, et al. Response, remission and drop-out rates following high-frequency repetitive transcranial magnetic stimulation (rTMS) for treating major depression: a systematic review and meta-analysis of randomized, double-blind and sham-controlled trials. Psychol Med. 2014;44(2):225-239. PubMed CrossRef
14. Desmyter S, Duprat R, Baeken C, et al. The acute effects of accelerated repetitive transcranial magnetic stimulation on suicide risk in unipolar depression: preliminary results. Psychiatr Danub. 2014;26(suppl 1):48-52. PubMed
15. George MS, Raman R, Benedek DM, et al. A two-site pilot randomized 3 day trial of high dose left prefrontal repetitive transcranial magnetic stimulation (rTMS) for suicidal inpatients. Brain Stimul. 2014;7(3):421-431. PubMed CrossRef
16. Desmyter S, Duprat R, Baeken C, et al. Accelerated intermittent theta burst stimulation for suicide risk in therapy-resistant depressed patients: a randomized, sham-controlled trial. Front Hum Neurosci. 2016;10:480. PubMed CrossRef
17. Blumberger DM, Mulsant BH, Fitzgerald PB, et al. A randomized double-blind sham-controlled comparison of unilateral and bilateral repetitive transcranial magnetic stimulation for treatment-resistant major depression. World J Biol Psychiatry. 2012;13(6):423-435. PubMed CrossRef
18. Blumberger DM, Maller JJ, Thomson L, et al. Unilateral and bilateral MRI-targeted repetitive transcranial magnetic stimulation for treatment-resistant depression: a randomized controlled study. J Psychiatry Neurosci. 2016;41(4):E58-E66. PubMed CrossRef
19. Thase ME, Rush AJ. Treatment resistant depression. In: Bloom FE, Kupfer DJ, eds. Pharmacology: The Fourth Generation of Progress. New York, NY: Raven Press Ltd; 1995.
20. Szanto K, Mulsant BH, Houck P, et al. Occurrence and course of suicidality during short-term treatment of late-life depression. Arch Gen Psychiatry. 2003;60(6):610-617. PubMed CrossRef
21. Desseilles M, Perroud N, Guillaume S, et al. Is it valid to measure suicidal ideation by depression rating scales? J Affect Disord. 2012;136(3):398-404. PubMed CrossRef
22. Uher R, Farmer A, Maier W, et al. Measuring depression: comparison and integration of three scales in the GENDEP study. Psychol Med. 2008;38(2):289-300. PubMed CrossRef
23. Oquendo MA, Sullivan GM, Sudol K, et al. Toward a biosignature for suicide. Am J Psychiatry. 2014;171(12):1259-1277. PubMed CrossRef
24. Kaschka WP, Rujescu D. Biological Aspects of Suicidal Behavior. Basel, Switzerland: Karger Publishers; 2016.
25. Berlim MT, Van den Eynde F, Tovar-Perdomo S, et al. Augmenting antidepressants with deep transcranial magnetic stimulation (DTMS) in treatment-resistant major depression. World J Biol Psychiatry. 2014;15(7):570-578. PubMed CrossRef
26. Hadley D, Anderson BS, Borckardt JJ, et al. Safety, tolerability, and effectiveness of high doses of adjunctive daily left prefrontal repetitive transcranial magnetic stimulation for treatment-resistant depression in a clinical setting. J ECT. 2011;27(1):18-25. PubMed CrossRef
27. Keshtkar M, Ghanizadeh A, Firoozabadi A. Repetitive transcranial magnetic stimulation versus electroconvulsive therapy for the treatment of major depressive disorder, a randomized controlled clinical trial. J ECT. 2011;27(4):310-314. PubMed CrossRef
28. Pu S, Nakagome K, Yamada T, et al. Suicidal ideation is associated with reduced prefrontal activation during a verbal fluency task in patients with major depressive disorder. J Affect Disord. 2015;181:9-17. PubMed CrossRef
29. Sublette ME, Milak MS, Galfalvy HC, et al. Regional brain glucose uptake distinguishes suicide attempters from non-attempters in major depression. Arch Suicide Res. 2013;17(4):434-447. PubMed CrossRef
30. Sun Y, Farzan F, Mulsant BH, et al. Indicators for remission of suicidal ideation following magnetic seizure therapy in patients with treatment-resistant depression. JAMA Psychiatry. 2016;73(4):337-345. PubMed CrossRef
31. Brunoni AR, Chaimani A, Moffa AH, et al. Repetitive transcranial magnetic stimulation for the acute treatment of major depressive episodes: a systematic review with network meta-analysis. JAMA Psychiatry. 2017;74(2):143-152. PubMed CrossRef
32. Szanto K, Reynolds CF 3rd, Frank E, et al. Suicide in elderly depressed patients. Am J Geriatr Psychiatry. 1996;4(3):197-207. PubMed CrossRef
33. Blumberger DM, Mulsant BH, Daskalakis ZJ. What is the role of brain stimulation therapies in the treatment of depression? Curr Psychiatry Rep. 2013;15(7):368. PubMed CrossRef
Editor’s Note: We encourage authors to submit papers for consideration as a part of our Focus on Suicide section. Please contact Philippe Courtet, MD, PhD, at [email protected].
Please sign in or purchase this PDF for $40.00.
Save
Cite