Objective: This study aimed to examine the degree of clinical and functional improvement after paliperidone long-acting injectable (LAI) administration according to the duration of illness.
Methods: Patients with schizophrenia diagnosed by ICD-10 criteria who were planned to start once-monthly paliperidone LAI were recruited from 2010 to 2017. Clinical and functional changes were measured every 4 weeks using the Clinical Global Impressions-Severity of Illness scale (CGI-S) and Personal and Social Performance scale (PSP), respectively, for 6 months after paliperidone LAI initiation. Improvements after starting paliperidone LAI were compared among patients with duration of illness < 3 years, ≥ 3 and < 10 years, and ≥ 10 years.
Results: A total of 1,166 participants (duration of illness < 3 years, n = 240; 3 ≤ duration of illness < 10 years, n = 442; duration of illness ≥ 10 years, n = 484) were enrolled. The total olanzapine-equivalent doses of antipsychotics and the LAI monotherapy proportion at the final visit were significantly different among the 3 duration of illness groups (dose: F2,1163 = 18.41, P < .001; monotherapy: χ22 = 11.73, P = .003). The changes in CGI-S score were significantly different according to the duration of illness, and those with duration of illness < 3 years showed the best improvement (group ×— week: χ212 = 25.33, P = .013). All 3 groups showed significantly improved PSP scores (week: χ26 = 294.2, P < .001).
Conclusions: Starting paliperidone LAI significantly improved clinical and functional outcomes in patients with schizophrenia, especially those with shorter duration of illness. These findings suggest that LAI antipsychotic administration may be considered in early-stage schizophrenia for improved outcomes.

ABSTRACT
Objective: This study aimed to examine the degree of clinical and functional improvement after paliperidone long-acting injectable (LAI) administration according to the duration of illness.
Methods: Patients with schizophrenia diagnosed by ICD-10 criteria who were planned to start once-monthly paliperidone LAI were recruited from 2010 to 2017. Clinical and functional changes were measured every 4 weeks using the Clinical Global Impressions-Severity of Illness scale (CGI-S) and Personal and Social Performance scale (PSP), respectively, for 6 months after paliperidone LAI initiation. Improvements after starting paliperidone LAI were compared among patients with duration of illness < 3 years, ≥ 3 and < 10 years, and ≥ 10 years.
Results: A total of 1,166 participants (duration of illness < 3 years, n = 240; 3 ≤ duration of illness < 10 years, n = 442; duration of illness ≥ 10 years, n = 484) were enrolled. The total olanzapine-equivalent doses of antipsychotics and the LAI monotherapy proportion at the final visit were significantly different among the 3 duration of illness groups (dose: F2,1163 = 18.41, P < .001; monotherapy: χ22 = 11.73, P = .003). The changes in CGI-S score were significantly different according to the duration of illness, and those with duration of illness < 3 years showed the best improvement (group ×— week: χ212 = 25.33, P = .013). All 3 groups showed significantly improved PSP scores (week: χ26 = 294.2, P < .001).
Conclusions: Starting paliperidone LAI significantly improved clinical and functional outcomes in patients with schizophrenia, especially those with shorter duration of illness. These findings suggest that LAI antipsychotic administration may be considered in early-stage schizophrenia for improved outcomes.
J Clin Psychiatry 2021;82(1):20m13446
To cite: Kim S, Kim S, Koh M, et al. Effects of long-acting injectable paliperidone palmitate on clinical and functional outcomes in patients with schizophrenia based on illness duration. J Clin Psychiatry. 2021;82(1):20m13446.
To share: https://doi.org/10.4088/JCP.20m13446
© Copyright 2021 Physicians Postgraduate Press, Inc.
aDepartment of Psychiatry, Seoul National University Bundang Hospital, Gyeonggi-do, Republic of Korea
bMedical Affairs, Janssen Korea, Seoul, Republic of Korea
cDepartment of Biostatistics and Computing, Yonsei University College of Medicine, Seoul, Republic of Korea
dDepartment of Psychiatry, The Catholic University of Korea, Seoul St Mary’s Hospital, Seoul, Republic of Korea
eDepartment of Psychiatry, Keyo Hospital, Gyeonggi-do, Republic of Korea
fDepartment of Psychiatry, Korea University Guro Hospital, Seoul, Republic of Korea
gDepartment of Psychiatry, Daesung Green Hospital, Gyeongsangbuk-do, Republic of Korea
hDepartment of Psychiatry, St John of God Hospital, Gwangju, Republic of Korea
iDepartment of Psychiatry, Saevit hospital, Gyeongsangbuk-do, Republic of Korea
jDepartment of Neuropsychiatry, Keio University School of Medicine, Tokyo, Japan
kDepartment of Psychiatry, Seoul National University College of Medicine, Seoul, Republic of Korea
lDepartment of Brain and Cognitive Sciences, Seoul National University College of Natural Sciences, Seoul, Republic of Korea
*Corresponding author: Euitae Kim, MD, PhD, Department of Psychiatry, Seoul National University College of Medicine, Seoul National University Bundang Hospital, 82, Gumi-ro 173 beon-gil, Bundang-gu, Seongnam-si, Gyeonggi-do, Republic of Korea 13620 ([email protected]).
Schizophrenia is a chronic and severe mental illness with psychotic symptoms that affect one’s ability to interpret reality. These symptoms typically appear in young adulthood and may have serious social consequences and even be associated with lifelong disability. Although clinical remission is not rare with adequate antipsychotic treatment,1 most patients experience recurring psychotic relapses along the disease course that result in clinical deterioration and functional impairments.2,3
Multiple epidemiologic studies4,5 have reported that antipsychotic medications effectively prevent psychotic relapses after symptomatic remission. Furthermore, a previous meta-analysis5 demonstrated that antipsychotic drugs significantly reduce the relapse rates at 1 year of treatment with a number of needed to treat of 3, which indicates relapse prevention in every 3 patients treated with antipsychotic medications. However, given that schizophrenia generally requires long-term maintenance treatment, poor treatment adherence is a serious concern since it increases the psychotic relapse risk.6
As a result, long-acting injectable (LAI) antipsychotic drugs, which ameliorate the daily burden of taking oral medication and prevent psychotic relapse from poor adherence, have been introduced. Various benefits of LAI antipsychotics have been reported, especially with respect to psychotic relapse prevention. Studies based on prospective nationwide databases in Sweden7,8 have reported an association of LAI antipsychotics with a substantially lower rehospitalization risk compared with equivalent oral formulations. These findings further indicate the superiority of LAI antipsychotics in relapse prevention in patients with schizophrenia. Furthermore, previous studies have reported functional improvements in patients with schizophrenia treated with LAI antipsychotics. Specifically, a previous systemic analysis9 of randomized controlled trials demonstrated the superiority of LAI antipsychotics over oral antipsychotics with respect to functioning and service engagement. Moreover, a prospective study10 reported that treatment with paliperidone LAI improved functioning, as measured using the Personal and Social Performance scale (PSP), in non-acutely ill, symptomatic patients with schizophrenia. Functional improvements after treatment with LAI antipsychotics are significant since they are associated with better prognosis and community adjustment, which are long-term treatment goals in patients with schizophrenia.11
Despite the aforementioned advantages, LAI antipsychotics have mainly been used to maintain treatment adherence with a focus on psychotic relapse; therefore, compared to oral medication, they have been underutilized in schizophrenia treatment. Specifically, LAI antipsychotics are mostly administered in the later stages of the illness upon identification of nonadherence with psychotic relapses. However, the use of LAI antipsychotics should not be limited to improving treatment adherence by patients in the later stage given the common suboptimal adherence at all illness stages. Suboptimal adherence to oral antipsychotics is associated with stable and nonacute active psychotic symptoms.12-14 Moreover, a strong correlation of persistent active psychotic symptoms with negative symptoms and functional impairments has been reported even after adjustment for the confounding effects of the duration of untreated period.15,16 These findings suggest that LAI antipsychotics could improve the clinical and functional outcomes of patients taking oral antipsychotics at all illness stages. This idea is supported by a previous prospective study17 that reported clinically significant improvements in symptoms, functioning, and treatment satisfaction in stable, non-acutely ill but symptomatic patients on oral antipsychotic monotherapy after switching to paliperidone LAI.
Moreover, there has been increasing evidence for the effectiveness of LAI antipsychotics in the early disease stage18,19 when compared with oral antipsychotics. However, only a few of these studies have compared the effect of LAI antipsychotics among patients with schizophrenia with varying duration of illness,10,20,21 while there have been lines of evidence regarding oral antipsychotics.22-24 A post hoc analysis20 of two multicenter studies reported that paliperidone LAI was more effective in patients with recently diagnosed schizophrenia. Further, a randomized study on patients with a criminal history21 showed that paliperidone LAI had an advantage in reducing treatment failure risk in patients with recent-onset schizophrenia compared to those with more chronic illness. Although these findings suggest that the treatment benefits of LAI antipsychotics are more pronounced in patients with recently diagnosed schizophrenia,10,20 their generalization is limited by the study populations.
Thus, the current study was conducted to examine the differences in the effect of LAI antipsychotics in patients with schizophrenia according to illness stage. Specifically, we aimed to evaluate the degree of clinical and functional improvement after paliperidone LAI administration in patients with schizophrenia with a varying duration of illness by using postmarketing surveillance (PMS) data.

- Despite increasing evidence for the effectiveness of long-acting injectable (LAI) antipsychotics in the early disease stage of schizophrenia, LAI antipsychotics are mostly administered in the later stages of the illness.
- Only a few studies have compared the effect of LAI antipsychotics among patients with schizophrenia with varying duration of illness.
- These study results suggest that the administration of LAI antipsychotics may be considered in the earlier phases of schizophrenia for better outcomes.
METHODS
This study was approved by the Institutional Review Board of Seoul National University Bundang Hospital, Gyeonggi-do, Korea, and was conducted in accordance with the Helsinki Declaration of 1975, as revised in 2008.
Participants
This study enrolled patients who agreed to be treated with paliperidone LAI and to participate in PMS of paliperidone LAI. Specifically, we enrolled patients who were diagnosed with schizophrenia according to ICD-1025 criteria in 105 hospitals and clinics in South Korea between July 26, 2010, and July 25, 2017. Participants received a full explanation of the study and provided written informed consent.
Study Design
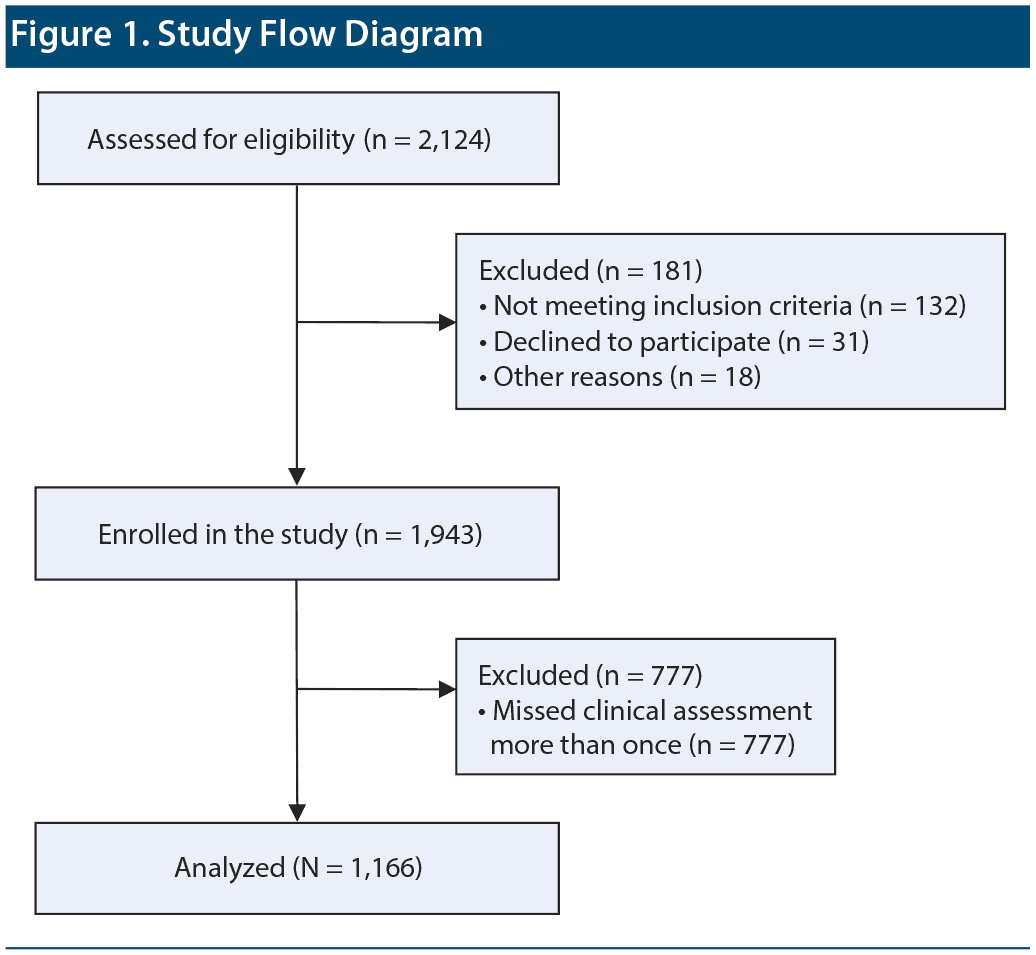
Figure 1 presents the study flow diagram. Clinical interviews were used to obtain the patients’ baseline information, including medical history, concomitant medication, and reason for prescribing paliperidone LAI. Subsequently, the patients received their first paliperidone LAI injection (baseline) and a second injection of loading dose after 1 week. The injections were administered intramuscularly at the deltoid or gluteal area. The total study duration was 25 weeks with follow-up scheduled injections at 4-week intervals after the second injection of paliperidone LAI. Information on concomitant medications was collected at every visit during the study period.
Assessments
The effectiveness of paliperidone LAI was measured at every visit during the surveillance. The patients’ overall clinical condition was assessed using the Clinical Global Impressions-Severity of Illness scale (CGI-S) (range, 1 [not ill] to 7 [most extremely ill]).26 The functional aspect of the patients was examined using the PSP, which assesses the domains of personal and social behaviors. The PSP total score ranges from 1 to 100 and is interpreted as follows: ≥ 71, mild difficulty; 31-70, varying degrees of difficulty; and ≤ 30, poor level of functioning requiring supervision.11
Throughout the follow-up period, adverse drug reactions were monitored, documented, and classified into the following 3 subcategories: (1) extrapyramidal symptoms (EPS)/movement disorder, (2) sleep disturbance, and (3) prolactinemia/sexual dysfunction.
Statistical Analysis
The per-protocol population, defined as participants who were followed up for over 25 weeks as scheduled with assessments done at every visit, was included for analysis. The eligible participants for the analysis were allocated to the following 3 groups based on their duration of illness: (1) Group I: patients with duration of illness < 3 years; (2) Group II: patients with 3 ≤ duration of illness < 10 years; and (3) Group III: patients with duration of illness ≥ 10 years. One-way analysis of variance and Pearson χ2 tests were used for between-group comparisons of demographic variables. Changes in CGI-S and PSP total scores, the predefined endpoints in the study, were compared between the groups by using generalized linear models and generalized estimating equation for repeated-measures analysis, taking the between-group differences at baseline into account. For an exploratory analysis to investigate the change in antipsychotic dose, the antipsychotic medication dosage was converted to the olanzapine-equivalent dose, and the proportion of the paliperidone LAI dose in the total dose of all prescribed antipsychotic medications was calculated for each patient.27-29 The proportion was analyzed using generalized linear models. All statistical analyses were performed using SAS software version 9.4 (2013; SAS Institute; Cary, North Carolina). Statistical significance was set at P < .05.
RESULTS
Baseline Demographic and Clinical Characteristics of the Participants
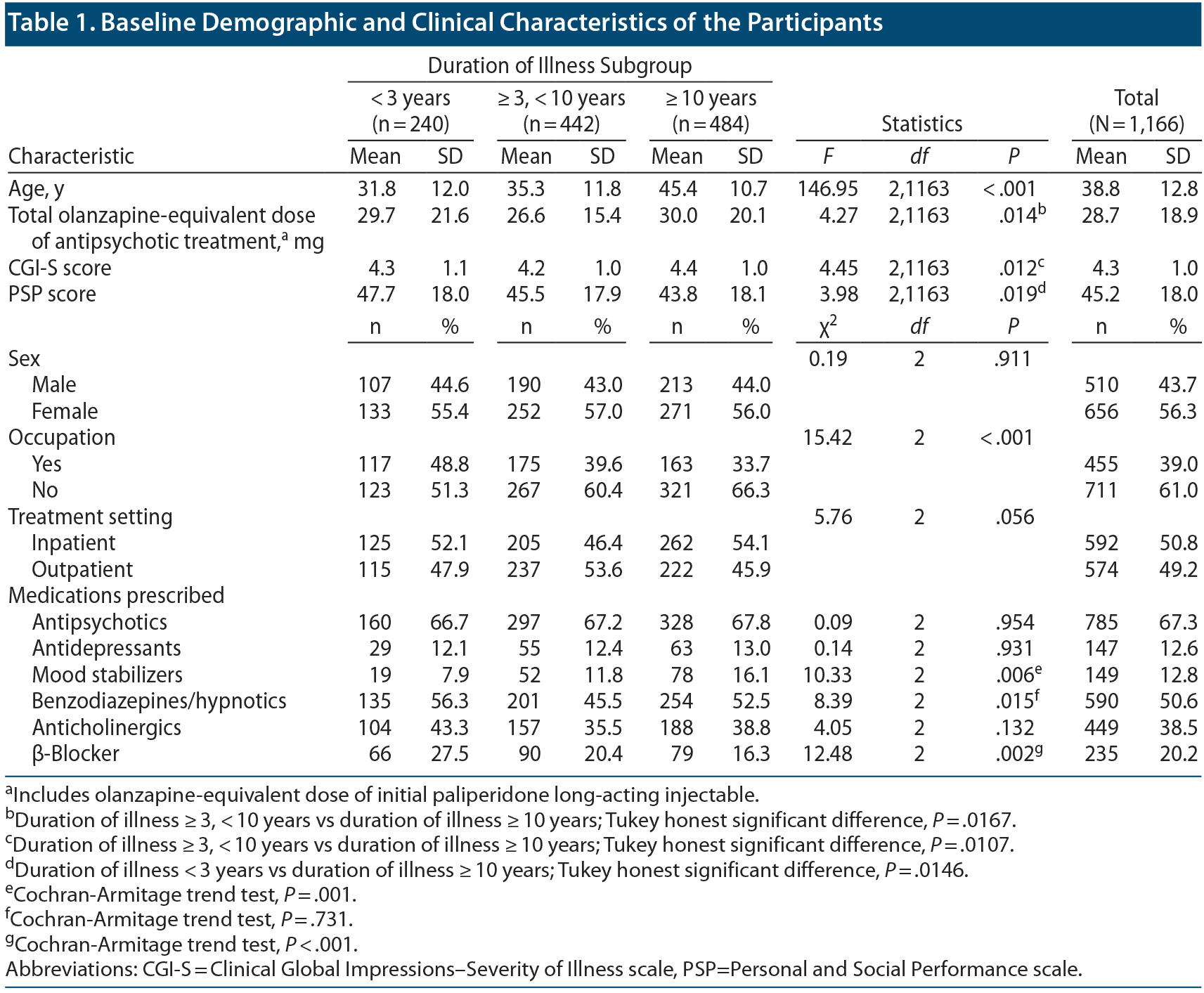
Among 1,943 patients who participated in the PMS of paliperidone LAI, 1,166 patients with schizophrenia were included for analysis (Group I, n = 240; Group II, n = 442; Group III, n = 484). Table 1 presents the demographic and clinical characteristics of the participants. The mean ± SD age of the patients was 38.8 ± 12.8 years, while the percentage of female patients was 56.0%. The mean ± SD baseline CGI-S and PSP scores were 4.3 ± 1.0 and 45.2 ± 18.0, respectively. The mean ± SD total olanzapine-equivalent dose of the antipsychotics at baseline was 28.7 ± 18.9 mg.
There were significant between-group differences in age (F2,1163 = 146.95, P < .001) but not in sex (χ22 = 0.19, P = .911). There were significant between-group differences in the olanzapine-equivalent doses of the antipsychotics at baseline (F2,1163 = 4.27, P = .014). There were no significant between-group differences in the percentage of patients taking antipsychotics, antidepressants, or anticholinergics (antipsychotics: χ22 = 0.09, P = .954; antidepressants: χ22 = 0.14, P = .931; anticholinergics: χ22 = 4.05, P = .132). However, there were some between-group differences in the percentage of patients taking mood stabilizers, benzodiazepines, and β-blockers (mood stabilizers: χ22 = 10.33, P = .006; benzodiazepines: χ22 = 8.39, P = .015; β-blockers: χ22 = 12.48, P = .002). There were significant between-group differences in baseline CGI-S and PSP scores as well as in occupation status (CGI-S: F2,1163 = 4.45, P = .012; PSP: F2,1163 = 3.98, P = .019; occupation: χ22 = 15.42, P < .001). There were no significant between-group differences in baseline percentage of hospitalizations (χ22 = 5.76, P = .056).
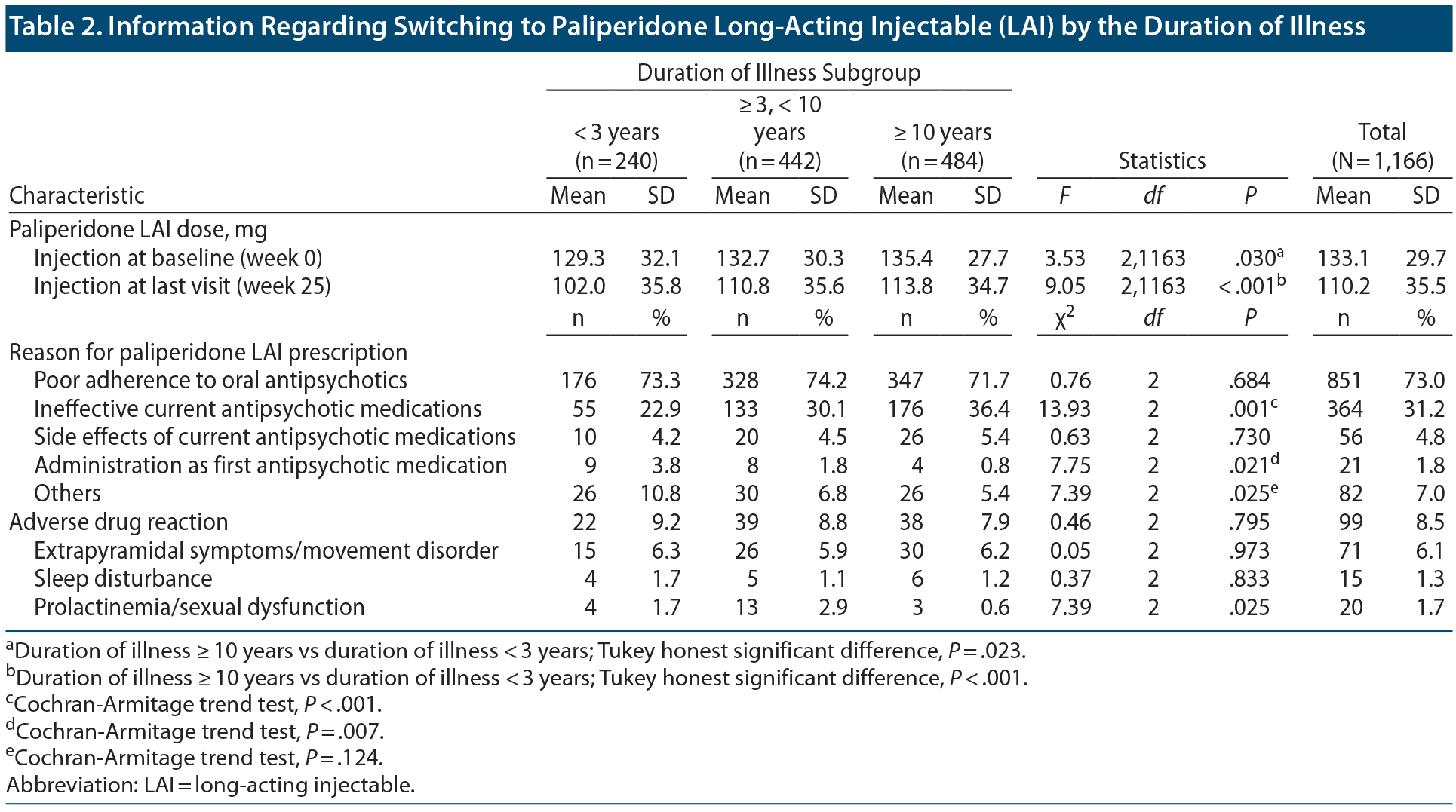
Information Regarding the Switch to Paliperidone LAI
Table 2 presents information regarding paliperidone LAI. The mean ± SD doses of paliperidone LAI at baseline and last visit (week 25) were 133.1 ± 29.7 mg and 110.2 ± 35.5 mg, respectively. There were significant between-group differences in the mean doses of paliperidone LAI at baseline and last visit (baseline: F2,1163 = 3.53, P = .030; last visit: F2,1163 = 9.05, P < .001). The most common reason for paliperidone LAI prescription was poor adherence to oral antipsychotics (73.0%), followed by an insufficient treatment effect of current antipsychotics (31.2%). There were significant between-group differences in the proportion of patients who reported ineffective current treatment as the reason for paliperidone LAI prescription (poor adherence: χ22 = 0.76, P = .684; ineffective current treatment: χ22 = 13.93, P < .001; side effects: χ22 = 0.63, P = .730; first antipsychotic medication: χ22 = 7.75, P = .021; others: χ22 = 7.39, P = .025).
Among the 1,166 patients, 99 patients (8.5%) exhibited adverse drug reactions after switching to paliperidone LAI; however, there were no significant between-group differences in the frequency of adverse drug reactions (χ22 = 0.46, P = .795) Among the 3 subcategories of adverse drug reactions, only prolactinemia/sexual dysfunction showed significant between-group differences (EPS/movement disorder: χ22 = 0.05, P = .973; sleep disturbance: χ22 = 0.37, P = .833; prolactinemia/sexual dysfunction: χ22 = 7.39, P = .025).
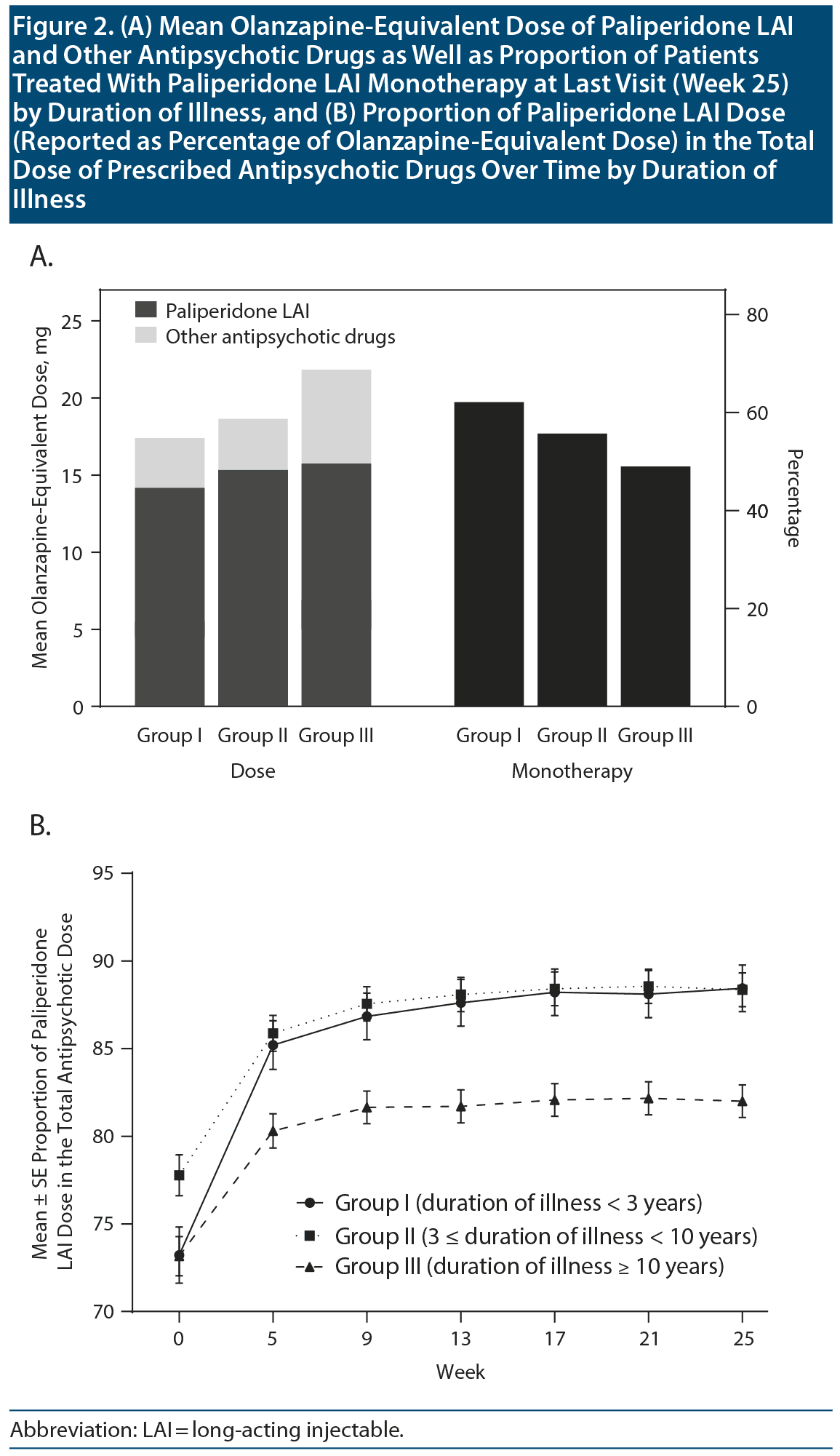
Changes in Antipsychotic Treatment After Switching to Paliperidone LAI
Figure 2A presents the mean ± SD olanzapine-equivalent dose of paliperidone LAI and other antipsychotic drugs at the final visit in the 3 groups (17.4 ± 8.8, 18.7 ± 8.7, and 21.8 ± 12.3 mg/d, respectively, for Groups I, II, and III) and the proportion of patients with paliperidone LAI as monotherapy in the 3 groups (62.1%, 55.7%, and 49.0%, respectively). There were between-group differences in the olanzapine-equivalent doses of the antipsychotics at the last visit (total antipsychotic drugs: F2,1163 = 18.41, P < .001; paliperidone LAI: F2,1163 = 9.05, P < .001). There were also significant between-group differences in the proportion of patients treated with paliperidone LAI monotherapy (χ22 = 11.73, P = .003). As shown in Figure 2B, there were significant between-group differences in the proportion of the paliperidone LAI dose in the total dose of all prescribed antipsychotic medications (group: F2,1155 = 12.17, P < .001; week: F6,1150 = 52.18, P < .001; group ×— week: F12,2300 = 1.41, P = .153). Group III had the highest total olanzapine-equivalent dose of antipsychotics (21.8 mg/d) and proportion of other antipsychotic drugs (17.9%) and also had the lowest proportion of paliperidone LAI monotherapy (49.0%) at final visit (Figure 2).
Clinical and Functional Outcomes After Switching to Paliperidone LAI
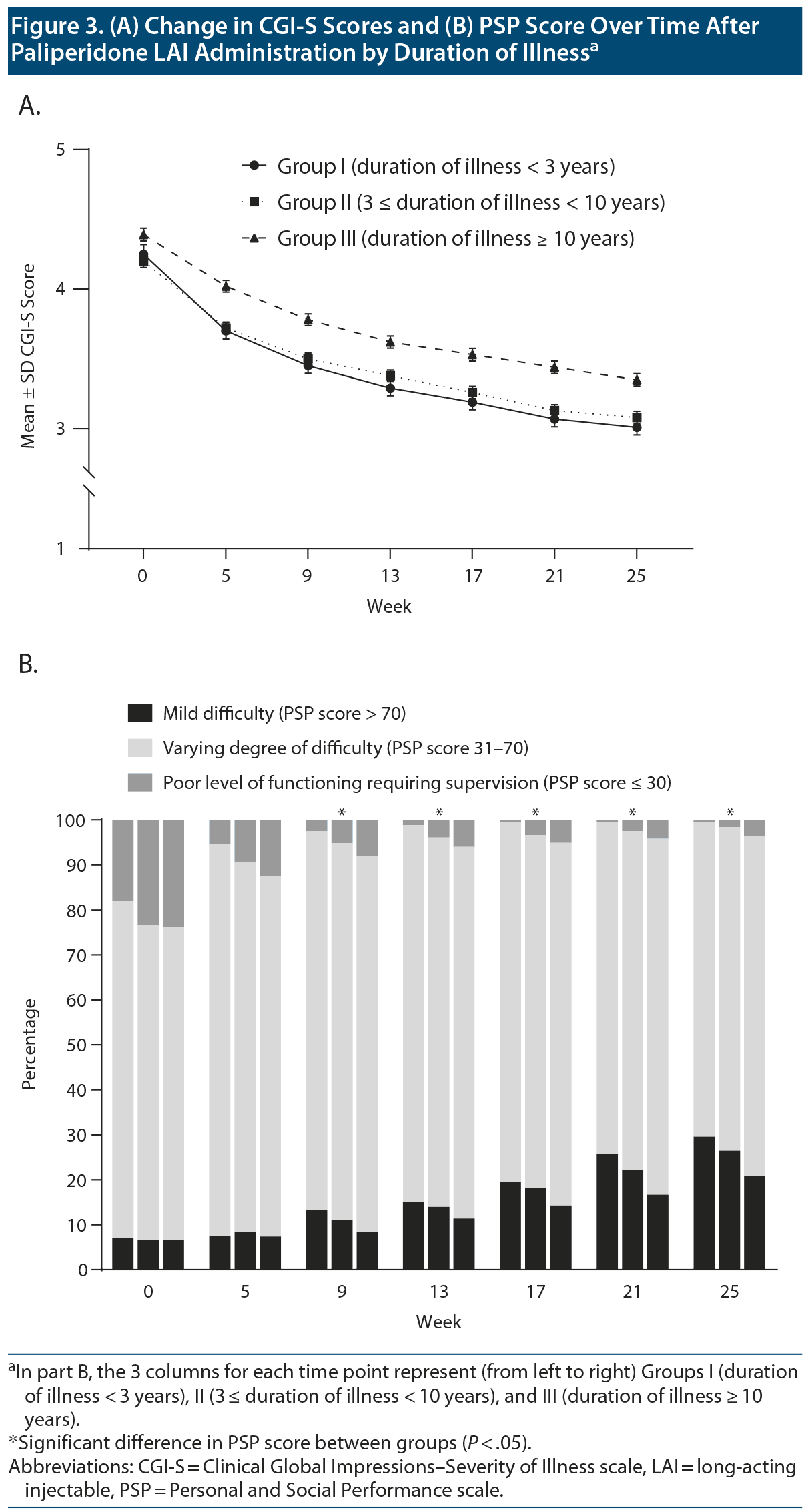
The mean ± SD CGI-S score at final visit was 3.0 ± 0.9, 3.1 ± 1.0, and 3.4 ± 1.0, respectively, for Groups I, II, and III. There were significant between-group differences in the mean± SD changes in CGI-S score (Group I: −1.2 ± 1.1; Group II: −1.1 ± 1.1; Group III: −1.0 ± 1.0), with Group I showing the greatest decrease in the mean ± SD CGI-S score (group: χ22 = 35.26, P < .001; week: χ26 = 1,238.17, P < .001; group ×— week: χ212 = 25.33, P = .013) (Figure 3A). The between-group differences in CGI-S score changes were still significant after adjusting for age and PSP total score (group: χ22 = 1.02, P = .602; week: χ26 = 1,238.17, P < .001; group ×— week: χ212 = 25.33, P = .013). All 3 groups showed a significant mean± SD increase in PSP scores at the final visit (Group I: 18.8 ± 16.8; Group II: 18.3 ± 16.2; Group III: 16.6 ± 15.7), with Group I showing the greatest increase in PSP score at the final visit (group: F2,1163 = 20.23, P < .001; group ×— week: F12,2316 = 1.01, P = .436). The between-group differences in PSP score changes were significant after adjusting for age and CGI-S score as well (group: F2,1161 = 16.11, P < .001; week: F6,1156 = 46.06, P < .001; group ×— week: F12,2312 = 5.94, P < .001). Figure 3B shows changes in the frequency distribution by PSP severity over time in each group. There were significant between-group differences in the proportion of mild or no functional impairment at 9 weeks and after (group: χ22 = 13.22, P = .001; week: χ26 = 294.2, P < .001; group ×— week: χ212 = 11.77, P = .465). There were significant differences in changes for each PSP domain after paliperidone LAI treatment as well (socially useful activities: group: χ22 = 43.24, P < .001; week: χ26 = 425.82, P < .001; group ×— week: χ212 = 24.63, P = .017; personal and social relationship: group: χ22 = 30.91, P < .001; week: χ26 = 433.03, P < .001; group ×— week: χ212 = 18.17, P = .111; self-care: group: χ22 = 44.72, P < .001; week: χ26 = 381.77, P < .001; group ×— week: χ212 = 44.47, P < .001; disturbing and aggressive behavior: group: χ22 = 32.80, P < .001; week: χ26 = 339.33, P < .001; group ×— week: χ212 = 23.13, P = .027).
DISCUSSION
To our knowledge, this prospective study is the first on outcome after the administration of paliperidone LAI in patients with schizophrenia with varying duration of illness. We observed clinical and functional improvements after administration of paliperidone LAI in all 3 groups, which is consistent with previous findings on the efficacy of LAI antipsychotics compared to that of oral medications in schizophrenia treatment. Furthermore, patients with shorter duration of illness showed better improvements, with a majority being on paliperidone LAI monotherapy at the final visit. This finding is indicative of the clinical utility of paliperidone LAI in the early phase of schizophrenia treatment.
In our study, patients with schizophrenia showed clinical improvements, as measured by the CGI-S, after 25-week administration of paliperidone LAI. Insufficient adherence to oral antipsychotics was the most common reason for the administration of paliperidone LAI (Table 2); moreover, none of the patients missed injections during the study period. Therefore, the observed clinical improvement could be attributed to the consistent antipsychotic drug delivery allowed by this formulation. Improving adherence is among the major challenges of schizophrenia treatment, and LAI antipsychotics address many of the problems associated with adherence to oral antipsychotics.30-32 Our findings regarding clinical improvements after switching to paliperidone LAI are consistent with previous findings regarding the superior efficacy of LAI antipsychotics compared with that of oral antipsychotics except for clozapine.7,33
Moreover, we observed functional improvements, as indicated by increased PSP scores after switching to paliperidone LAI. Specifically, there was a significant increase in the proportion of patients with mild functional difficulty (Figure 3B). There has been increasing emphasis on the importance of functioning outcomes in patients with schizophrenia with both clinical remission and social function being considered in recovery.34 Functional recovery in patients with schizophrenia is difficult, as demonstrated by a 20-year prospective study35 in which functional impairment in individuals with psychotic disorders was evident at the early illness stage and remained unchanged after illness onset. Therefore, the observed improvement in functional aspects after the administration of paliperidone LAI is an encouraging finding in schizophrenia treatment.
The degree of clinical and functional improvement with paliperidone LAI administration was dependent on duration of illness. Specifically, Group I showed the greatest improvement in CGI-S and PSP scores, although these scores did not differ significantly from those for Group II (Figure 3). This finding suggests that paliperidone LAI administration at an earlier disease stage has a better benefit. Nonadherence to treatment is more prominent in the earlier phase of schizophrenia36 and contributes to an increased risk of persistent psychotic symptoms and relapse.37 Therefore, the treatment adherence benefits of LAI antipsychotics should be greater in patients at an earlier disease stage.
On the other hand, suboptimal adherence is common in patients with longer duration of illness38,39 and could lead to a longer duration of active psychosis.13,40 Group III had the most unfavorable CGI-S and PSP scores and the highest antipsychotic drug doses at baseline (Table 1). Moreover, compared with Groups I and II, Group III had a significantly higher proportion of the patients who reported ineffectiveness of current antipsychotic treatment as a reason for paliperidone LAI administration (Table 2). This finding suggests that Group III may have presented with sustained psychotic symptoms that probably did not respond to antipsychotic treatment before the switch to paliperidone LAI. Prolonged or recurrent psychotic episodes may be related to disease progression.22,41,42 Furthermore, disease progression in patients with schizophrenia has been reported to accompany functional and structural changes in the brain that are associated with antipsychotic responsiveness,43-46 which leads to a poor outcome. For example, several imaging studies3,47,48 have reported an association of decreased gray matter volume with psychotic relapse and disease progression in patients with schizophrenia. Moreover, a prospective observational study on the use of paliperidone LAI in patients with schizophrenia49 reported that individuals with a greater total gray matter volume showed significantly greater psychotic symptom improvement. Taken together, the observed between-group differences in the clinical and functional outcomes after switching to paliperidone LAI may be related to disease progression due to suboptimal adherence and differences in antipsychotic responsiveness.24,50,51 Therefore, LAI antipsychotics should be considered in early illness stages to improve long-term prognosis in patients with schizophrenia by decreasing treatment nonadherence.
Notably, there were significant between-group differences in the total dose of antipsychotic medications, as well as in the dose of paliperidone LAI, at the final visit (Figure 2A). Specifically, Groups I and III presented the lowest and highest doses of total antipsychotic drugs and paliperidone LAI, respectively. This finding indicates that compared with patients with longer duration of illness, those with shorter duration of illness could be treated with a lower dose of antipsychotic drugs. Furthermore, Group I had the highest proportion of paliperidone LAI monotherapy at the final visit (Figure 2A). Most evidence-based guidelines52-56 recommend that the lowest effective dose of antipsychotic drugs and monotherapy are the favorable choice for schizophrenia treatment. Contrastingly, polypharmacy is a major contributor to a high total dose and an increased side effect burden; moreover, there is an association of cumulative antipsychotic dose with antipsychotic-associated side effects and mortality.57,58 Overall, the between-group differences in the antipsychotic doses and proportion of patients on monotherapy indicate that the benefits of LAI antipsychotics are more pronounced in the early disease phase.
Limitations
This study has several limitations to be considered when interpreting the results. First, we excluded some patients from the final analysis, which limits the generalizability of the results. Among those excluded patients, there might have been some who were nonadherent to paliperidone LAI. A previous retrospective study59 reported that less than half of patients treated with LAI antipsychotics after psychiatric hospitalization adhered to LAI treatment for over 6 months. Therefore, our findings might not represent the real-world population with respect to the adherence to LAI antipsychotics among patients with schizophrenia. Second, the effect of concurrent use of oral antipsychotics in some patients cannot be ignored. However, the olanzapine-equivalent dose of paliperidone LAI was substantially greater than that of other antipsychotic medications. Moreover, during the follow-up period, there was a decrease in the dose of most of the concurrent oral antipsychotics. These findings suggest that the effect of concurrent antipsychotic medications on clinical and functional outcome may be minimal. In the aspects of study design, a proper control group might have been helpful. Still, the current design seems adequate for the purpose of the study, which was to evaluate the differences in the effect of LAI antipsychotics in schizophrenia with varying illness duration. Lastly, we did not obtain information regarding the duration of the untreated period and the history of antipsychotic treatment before paliperidone LAI administration, which could have affected our results.60
CONCLUSION
Starting paliperidone LAI resulted in significantly improved clinical and functional outcomes in patients with schizophrenia, especially in those with shorter duration of illness. These results suggest that the administration of LAI antipsychotics may be considered in the earlier phases of schizophrenia for better outcomes.
Submitted: May 4, 2020; accepted December 1, 2020.
Published online: January 12, 2021.
Potential conflicts of interest: Dr Euitae Kim has participated in advisory/speaker meetings organized by Janssen Korea, Otsuka Korea, and Bukwang Pharm Co. Ms SuYoun Kim, Dr Koh, and Ms GumJee Choi are current employees of Janssen Korea. Dr Uchida has received grants from Eisai, Otsuka, Dainippon-Sumitomo, and Meiji-Seika; speaker’s honoraria from Otsuka, Dainippon-Sumitomo, Eisai, and Meiji Seika; and advisory panel payments from Dainippon-Sumitomo within the past 3 years. Dr Takeuchi has received speaker’s fees from Kyowa, Janssen, Meiji Seika, Mochida, Otsuka, Sumitomo Dainippon, and Yoshitomiyakuhin.
Funding/support: This research was supported by Janssen Korea.
Role of the sponsor: The study sponsor was responsible for the collection and management of data and contributed to the conduct of the study, data analysis, and development and review of the manuscript.
REFERENCES
1. Lally J, Ajnakina O, Stubbs B, et al. Remission and recovery from first-episode psychosis in adults: systematic review and meta-analysis of long-term outcome studies. Br J Psychiatry. 2017;211(6):350-358. PubMed CrossRef
2. Schennach R, Obermeier M, Meyer S, et al. Predictors of relapse in the year after hospital discharge among patients with schizophrenia. Psychiatr Serv. 2012;63(1):87-90. PubMed CrossRef
3. Andreasen NC, Liu D, Ziebell S, et al. Relapse duration, treatment intensity, and brain tissue loss in schizophrenia: a prospective longitudinal MRI study. Am J Psychiatry. 2013;170(6):609-615. PubMed CrossRef
4. Leucht S, Tardy M, Komossa K, et al. Maintenance treatment with antipsychotic drugs for schizophrenia. Cochrane Database Syst Rev. 2012;(5):CD008016. PubMed
5. Leucht S, Tardy M, Komossa K, et al. Antipsychotic drugs versus placebo for relapse prevention in schizophrenia: a systematic review and meta-analysis. Lancet. 2012;379(9831):2063-2071. PubMed CrossRef
6. Goff DC, Falkai P, Fleischhacker WW, et al. The long-term effects of antipsychotic medication on clinical course in schizophrenia. Am J Psychiatry. 2017;174(9):840-849. PubMed CrossRef
7. Tiihonen J, Mittendorfer-Rutz E, Majak M, et al. Real-world effectiveness of antipsychotic treatments in a nationwide cohort of 29,823 patients with schizophrenia. JAMA Psychiatry. 2017;74(7):686-693. PubMed CrossRef
8. Taipale H, Mittendorfer-Rutz E, Alexanderson K, et al. Antipsychotics and mortality in a nationwide cohort of 29,823 patients with schizophrenia. Schizophr Res. 2018;197:274-280. PubMed CrossRef
9. Peters L, Krogmann A, von Hardenberg L, et al. Long-acting injections in schizophrenia: a 3-year update on randomized controlled trials published January 2016-March 2019. Curr Psychiatry Rep. 2019;21(12):124. PubMed CrossRef
10.Hargarter L, Bergmans P, Cherubin P, et al. Once-monthly paliperidone palmitate in recently diagnosed and chronic non-acute patients with schizophrenia. Expert Opin Pharmacother. 2016;17(8):1043-1053. PubMed CrossRef
11.Nasrallah H, Morosini P, Gagnon DD. Reliability, validity and ability to detect change of the Personal and Social Performance Scale in patients with stable schizophrenia. Psychiatry Res. 2008;161(2):213-224. PubMed CrossRef
12.Steger KA, Cassidy C, Rabinovitch M, et al. Impact of symptom resolution on medication adherence in first episode psychosis. Psychiatry Res. 2012;196(1):45-51. PubMed CrossRef
13.Bernardo M, Ca×±as F, Herrera B, et al. Adherence predicts symptomatic and psychosocial remission in schizophrenia: naturalistic study of patient integration in the community [in Spanish]. Rev Psiquiatr Salud Ment. 2017;10(3):149-159. PubMed CrossRef
14.Kane JM, Kishimoto T, Correll CU. Non-adherence to medication in patients with psychotic disorders: epidemiology, contributing factors and management strategies. World Psychiatry. 2013;12(3):216-226. PubMed CrossRef
15.Lyne J, Joober R, Schmitz N, et al. Duration of active psychosis and first-episode psychosis negative symptoms. Early Interv Psychiatry. 2017;11(1):63-71. PubMed CrossRef
16.Pelayo-Terán JM, Gajardo-Galán V, Gómez-Revuelta M, et al. Duration of active psychosis and functional outcomes in first-episode non-affective psychosis. Eur Psychiatry. 2018;52:29-37. PubMed CrossRef
17.Schreiner A, Caspi A, Bergmans P, et al. Switching from oral atypical antipsychotic monotherapy to paliperidone palmitate once-monthly in non-acute patients with schizophrenia: a prospective, open-label, interventional study. Psychopharmacology (Berl). 2017;234(1):3-13. PubMed CrossRef
18.Schreiner A, Aadamsoo K, Altamura AC, et al. Paliperidone palmitate versus oral antipsychotics in recently diagnosed schizophrenia. Schizophr Res. 2015;169(1-3):393-399. PubMed CrossRef
19.Huang M, Yu L, Pan F, et al. A randomized, 13-week study assessing the efficacy and metabolic effects of paliperidone palmitate injection and olanzapine in first-episode schizophrenia patients. Prog Neuropsychopharmacol Biol Psychiatry. 2018;81:122-130. PubMed CrossRef
20.Si T, Zhuo J, Turkoz I, et al. Once-monthly injection of paliperidone palmitate in patients with recently diagnosed and chronic schizophrenia: a post-hoc comparison of efficacy and safety. Expert Opin Pharmacother. 2017;18(17):1799-1809. PubMed CrossRef
21.Alphs L, Bossie C, Mao L, et al. Treatment effect with paliperidone palmitate compared with oral antipsychotics in patients with recent-onset versus more chronic schizophrenia and a history of criminal justice system involvement. Early Interv Psychiatry. 2018;12(1):55-65. PubMed CrossRef
22.Wyatt RJ. Neuroleptics and the natural course of schizophrenia. Schizophr Bull. 1991;17(2):325-351. PubMed CrossRef
23.Penttilפ M, Jפפskelפinen E, Hirvonen N, et al. Duration of untreated psychosis as predictor of long-term outcome in schizophrenia: systematic review and meta-analysis. Br J Psychiatry. 2014;205(2):88-94. PubMed CrossRef
24.Takeuchi H, Siu C, Remington G, et al. Does relapse contribute to treatment resistance? antipsychotic response in first- vs second-episode schizophrenia. Neuropsychopharmacology. 2019;44(6):1036-1042. PubMed CrossRef
25.Zivetz L. The ICD-10 Classification of Mental and Behavioural Disorders: Clinical Descriptions and Diagnostic Guidelines. World Health Organization; 1992.
26.Busner J, Targum SD. The Clinical Global Impressions Scale: applying a research tool in clinical practice. Psychiatry (Edgmont). 2007;4(7):28-37. PubMed
27.Inada T, Inagaki A. Psychotropic dose equivalence in Japan. Psychiatry Clin Neurosci. 2015;69(8):440-447. PubMed CrossRef
28.Leucht S, Samara M, Heres S, et al. Dose equivalents for antipsychotic drugs: the DDD method. Schizophr Bull. 2016;42(suppl 1):S90-S94. PubMed CrossRef
29.Rothe PH, Heres S, Leucht S. Dose equivalents for second generation long-acting injectable antipsychotics: the minimum effective dose method. Schizophr Res. 2018;193:23-28. PubMed CrossRef
30.Emsley R, Oosthuizen P, Koen L, et al. Oral versus injectable antipsychotic treatment in early psychosis: post hoc comparison of two studies. Clin Ther. 2008;30(12):2378-2386. PubMed CrossRef
31.Greene M, Yan T, Chang E, et al. Medication adherence and discontinuation of long-acting injectable versus oral antipsychotics in patients with schizophrenia or bipolar disorder. J Med Econ. 2018;21(2):127-134. PubMed CrossRef
32.Titus-Lay EN, Ansara ED, Isaacs AN, et al. Evaluation of adherence and persistence with oral versus long-acting injectable antipsychotics in patients with early psychosis. Ment Health Clin. 2018;8(2):56-62. PubMed CrossRef
33.Taipale H, Mehtפlפ J, Tanskanen A, et al. Comparative effectiveness of antipsychotic drugs for rehospitalization in schizophrenia-a nationwide study with 20-year follow-up. Schizophr Bull. 2018;44(6):1381-1387. PubMed CrossRef
34.Vita A, Barlati S. Recovery from schizophrenia: is it possible? Curr Opin Psychiatry. 2018;31(3):246-255. PubMed CrossRef
35.Velthorst E, Fett AJ, Reichenberg A, et al. The 20-year longitudinal trajectories of social functioning in individuals with psychotic disorders. Am J Psychiatry. 2017;174(11):1075-1085. PubMed CrossRef
36.Lang K, Meyers JL, Korn JR, et al. Medication adherence and hospitalization among patients with schizophrenia treated with antipsychotics. Psychiatr Serv. 2010;61(12):1239-1247. PubMed CrossRef
37.Morken G, Widen JH, Grawe RW. Non-adherence to antipsychotic medication, relapse and rehospitalisation in recent-onset schizophrenia. BMC Psychiatry. 2008;8(1):32. PubMed CrossRef
38.Kane JM. Improving patient outcomes in schizophrenia: achieving remission, preventing relapse, and measuring success. J Clin Psychiatry. 2013;74(9):e18. PubMed CrossRef
39.Marder SR. Overview of partial compliance. J Clin Psychiatry. 2003;64(suppl 16):3-9. PubMed
40.Alptekin K, Aydin H, Gucer KM, et al. Patient adherence and efficacy of quetiapine treatment in schizophrenia: results of a multicentre, naturalistic 6-month follow-up study. Int Clin Psychopharmacol. 2010;25(6):342-348. PubMed CrossRef
41.Perkins DO, Gu H, Boteva K, et al. Relationship between duration of untreated psychosis and outcome in first-episode schizophrenia: a critical review and meta-analysis. Am J Psychiatry. 2005;162(10):1785-1804. PubMed CrossRef
42.Anderson KK, Voineskos A, Mulsant BH, et al. The role of untreated psychosis in neurodegeneration: a review of hypothesized mechanisms of neurotoxicity in first-episode psychosis. Can J Psychiatry. 2014;59(10):513-517. PubMed CrossRef
43.Chou PH, Koike S, Nishimura Y, et al. Distinct effects of duration of untreated psychosis on brain cortical activities in different treatment phases of schizophrenia: a multi-channel near-infrared spectroscopy study. Prog Neuropsychopharmacol Biol Psychiatry. 2014;49:63-69. PubMed CrossRef
44.Rund BR. Does active psychosis cause neurobiological pathology? a critical review of the neurotoxicity hypothesis. Psychol Med. 2014;44(8):1577-1590. PubMed CrossRef
45.Jonak K, Krukow P, Jonak KE, et al. Quantitative and qualitative comparison of EEG-based neural network organization in two schizophrenia groups differing in the duration of illness and disease burden: graph analysis with application of the minimum spanning tree. Clin EEG Neurosci. 2019;50(4):231-241. PubMed CrossRef
46.Liemburg E, Sibeijn-Kuiper A, Bais L, et al. Prefrontal NAA and Glx levels in different stages of psychotic disorders: a 3T 1H-MRS Study. Sci Rep. 2016;6(1):21873. PubMed CrossRef
47.Lin Y, Li M, Zhou Y, et al. Age-related reduction in cortical thickness in first-episode treatment-naׯve patients with schizophrenia. Neurosci Bull. 2019;35(4):688-696. PubMed CrossRef
48.Van Haren NE, Cahn W, Hulshoff Pol HE, et al. Confounders of excessive brain volume loss in schizophrenia. Neurosci Biobehav Rev. 2013;37(10 pt 1):2418-2423. PubMed CrossRef
49.Altamura AC, Delvecchio G, Paletta S, et al. Gray matter volumes may predict the clinical response to paliperidone palmitate long-acting in acute psychosis: a pilot longitudinal neuroimaging study. Psychiatry Res Neuroimaging. 2017;261:80-84. PubMed CrossRef
50.Catts SV, O’ Toole BI. The treatment of schizophrenia: can we raise the standard of care? Aust N Z J Psychiatry. 2016;50(12):1128-1138. PubMed CrossRef
51.Tishler TA, Bartzokis G, Lu PH, et al. Abnormal trajectory of intracortical myelination in schizophrenia implicates white matter in disease pathophysiology and the therapeutic mechanism of action of antipsychotics. Biol Psychiatry Cogn Neurosci Neuroimaging. 2018;3(5):454-462. PubMed
52.Lehman AF, Lieberman JA, Dixon LB, et al; American Psychiatric Association; Steering Committee on Practice Guidelines. Practice guideline for the treatment of patients with schizophrenia, second edition. Am J Psychiatry. 2004;161(2 suppl):1-56. PubMed
53.National Collaborating Centre for Mental Health (UK). Psychosis and Schizophrenia in Adults: Treatment and Management: Updated. Edition 2014. UK: National Institute for Health and Care Excellence; 2014.
54.Gaebel W, Weinmann S, Sartorius N, et al. Schizophrenia practice guidelines: international survey and comparison. Br J Psychiatry. 2005;187(3):248-255. PubMed CrossRef
55.Galletly C, Castle D, Dark F, et al. Royal Australian and New Zealand College of Psychiatrists clinical practice guidelines for the management of schizophrenia and related disorders. Aust N Z J Psychiatry. 2016;50(5):410-472. PubMed CrossRef
56.Buchanan RW, Kreyenbuhl J, Kelly DL, et al; Schizophrenia Patient Outcomes Research Team (PORT). The 2009 schizophrenia PORT psychopharmacological treatment recommendations and summary statements. Schizophr Bull. 2010;36(1):71-93. PubMed CrossRef
57.Barnes TR, Paton C. Antipsychotic polypharmacy in schizophrenia: benefits and risks. CNS Drugs. 2011;25(5):383-399. PubMed CrossRef
58.Iversen TSJ, Steen NE, Dieset I, et al. Side effect burden of antipsychotic drugs in real life—impact of gender and polypharmacy. Prog Neuropsychopharmacol Biol Psychiatry. 2018;82:263-271. PubMed CrossRef
59.Gilbert JL, Nelson LA, Kriz CR, et al. Identifying predictors of primary adherence to second generation long-acting injectable antipsychotics following discharge from an acute inpatient psychiatry unit. Psychopharmacol Bull. 2019;49(2):8-16. PubMed
60.Marshall M, Lewis S, Lockwood A, et al. Association between duration of untreated psychosis and outcome in cohorts of first-episode patients: a systematic review. Arch Gen Psychiatry. 2005;62(9):975-983. PubMed CrossRef
Editor’s Note: We encourage authors to submit papers for consideration as a part of our Focus on Psychosis section. Please contact Ann K. Shinn, MD, MPH, at [email protected].
This PDF is free for all visitors!
Save
Cite