Because this piece does not have an abstract, we have provided for your benefit the first 3 sentences of the full text.
To the Editor: Serum brain-derived neurotrophic factor (BDNF) may be a treatment efficacy marker in depression and may be altered in obsessive-compulsive disorder (OCD) and anxiety disorders. Rapastinel is an N-methyl-d-aspartate glutamate receptor modulator shown to affect BDNF levels in vitro. It exerts acute therapeutic effects on OCD symptoms without ketamine-like side effects.
This work may not be copied, distributed, displayed, published, reproduced, transmitted, modified, posted, sold, licensed, or used for commercial purposes. By downloading this file, you are agreeing to the publisher’s Terms & Conditions.
Effects of Rapastinel (Formerly GLYX-13) on Serum Brain-Derived Neurotrophic Factor in Obsessive-Compulsive Disorder
To the Editor: Serum brain-derived neurotrophic factor (BDNF) may be a treatment efficacy marker in depression1,2 and may be altered in obsessive-compulsive disorder (OCD) and anxiety disorders.3,4 Rapastinel is an N-methyl-d-aspartate glutamate receptor modulator shown to affect BDNF levels in vitro.5 It exerts acute therapeutic effects on OCD symptoms without ketamine-like side effects.6 We analyzed exploratory data regarding serum BDNF samples collected from OCD patients before and after rapastinel infusion. Our goal was to determine whether (1) rapastinel alters serum BDNF levels in the interval from 0 to 230 minutes postinfusion and (2) whether changes in BDNF levels correlated with changes in OCD symptoms during this period.
Methods. We received approval from an institutional review board and recruited 7 unmedicated outpatients with OCD (ages 18-55 years) between March 2014 to March 2015, and the patients provided written informed consent. Eligible patients met criteria for OCD (both DSM-IV7 and DSM-58) and were at least moderately symptomatic (Yale-Brown Obsessive Compulsive Scale9,10 score ≥ 16).11 Three (43%) of the 7 OCD subjects had no other psychiatric comorbidity. Two subjects (29%) met criteria for comorbid generalized anxiety disorder. Two others (29%) met criteria for comorbid major depression, with baseline 17-item Hamilton Depression Rating Scale (HDRS-17)12 scores of 11 (mild) and 14 (moderate), respectively. The median HDRS-17 score at baseline was 4 (normal), with a range of 1-14.
All participants (N = 7) received a single 3- to 5-minute intravenous infusion of rapastinel (10 mg/kg). Blood was drawn in the morning (after an overnight fast) at baseline and 40, 110, and 230 minutes postinfusion. Serum BDNF levels were determined using a Millipore ChemiKine Sandwich ELISA Kit.13 OCD severity was assessed at baseline and 230 minutes using the YBOCS Challenge Scale,14 a 10-item self-report form assessing obsessions and compulsions over the previous hour. Depression severity was assessed at identical time points using the HDRS-17.12
We assessed BDNF level changes over time using the nonparametric Wilcoxon signed rank test. A mixed-effects regression model assessed BDNF levels’ time trend. Spearman correlation tested whether changes in serum BDNF levels from baseline to 230 minutes postinfusion correlated with OCD symptom severity change. As a negative control, we compared BDNF levels of the current patient sample to those of an independent cohort of 7 OCD patients who received a ketamine infusion,15 with BDNF serum levels determined in identical intervals, at the same time of day (morning), after an overnight fast, per recommended protocols.13 Both sample sets were stored, processed, and analyzed concurrently using the same ELISA kit and identical methods.
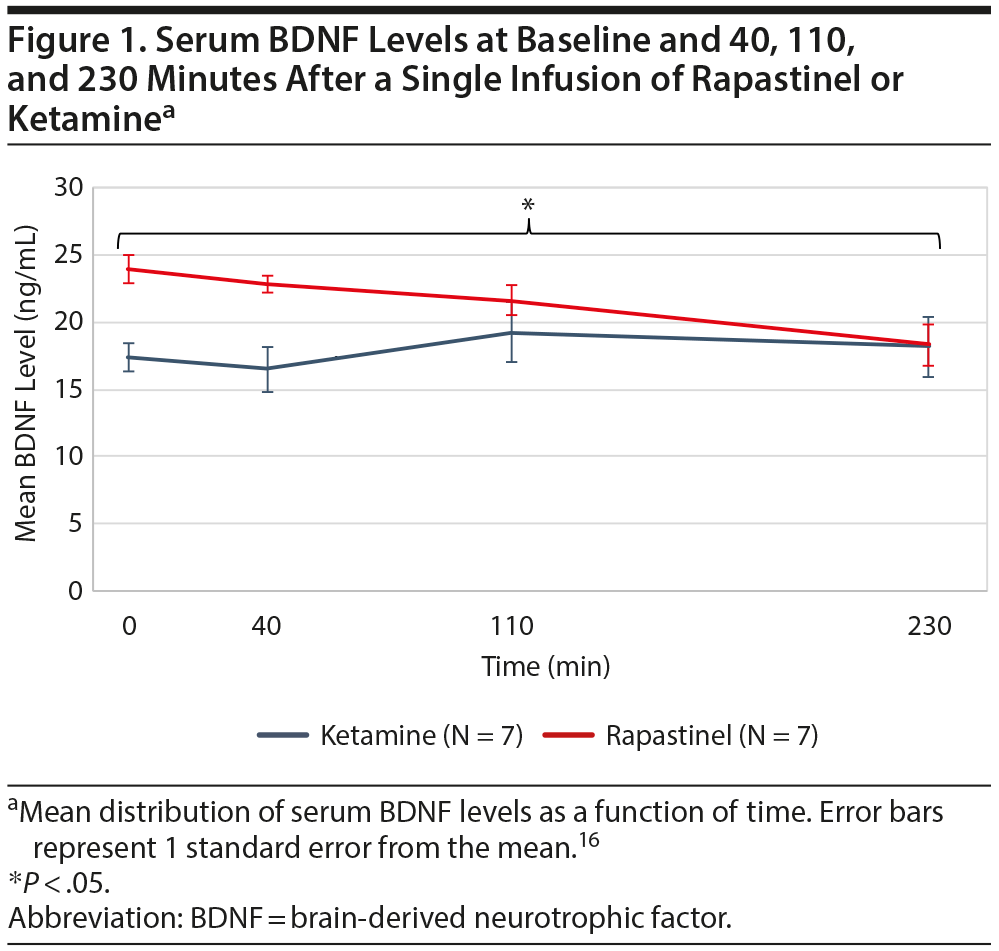
Results. In the rapastinel group, baseline median serum BDNF level was 22.3 ng/mL (range, 16.8-34.9 ng/mL). At 230 minutes, median BDNF level was 17.55 ng/mL (range, 12.6-26 ng/mL) and decreased in 6 out of 7 participants. This reduction was statistically significant (P = .03; Figure 1). BDNF levels exhibited a significant time-trend, decreasing from baseline to 230 minutes (β = -0.02, P = .00003). The median YBOCS Challenge Scale score at baseline was 28 (range, 24-39) and decreased in all participants (N = 7) at 230 minutes (median = 15; range, 3-20). From baseline to 230 minutes, changes in serum BDNF levels were highly correlated with changes in YBOCS Challenge Scale scores (r = 0.86, P = .01).
In contrast, in the ketamine group, the infusion did not significantly decrease serum BDNF levels over time. Baseline BDNF levels did not differ between the rapastinel and the ketamine OCD groups (P = .16).
In this small sample, rapastinel reduced OCD patients’ serum BDNF levels from baseline to 230 minutes, and these changes correlated with changes in OCD symptom severity. In a control sample, ketamine reduced OCD symptom severity but did not change serum BDNF levels.
In major depression, serum BDNF may be a biomarker of successful treatment,1,2 with reports of serum BDNF increasing with depression symptom reduction. In contrast, we found changes in the opposite direction: serum BDNF decreased with OCD symptom reduction. Increases in BDNF are consistent with the neurotrophic hypothesis of depression, in which antidepressants mediate therapeutic benefit by up-regulating brain BDNF, reversing stress-related decreases in brain BDNF, and reversing neuronal atrophy.17,18 The decreases in serum BDNF we observed with OCD symptom improvement suggest that BDNF may play a different role in OCD and stress-related mood disorders.
Limitations include small sample size and differences that may exist in typical community patients with comorbid depressive symptoms. In addition, peripheral BDNF levels have been studied as a readily accessible biomarker, under the assumption that serum levels reflect brain levels, given that BDNF has been reported to cross the blood-brain barrier.19 However, peripheral changes in BDNF cannot be exclusively attributed to changes in brain BDNF since the exact source and function of peripheral serum BDNF are still being elucidated. Platelets bind, store, and release upon agonist stimulation; megakaryocytes, platelet progenitors, also can release BDNF proteins into serum.20 Finally, we are unable to compare serum BDNF levels at baseline to those reported in OCD4 and other disorders3 due to the variety of sample collection and BDNF analysis methods used; indeed, when 6 different BDNF commercial assays were compared, interassay variation ranged from 5% to 20%.13 To avoid this problem, we used identical optimized sample collection (morning, under fasting conditions), storage, processing, and analysis methods to compare rapastinel samples with the control ketamine samples.
In sum, while both rapastinel and ketamine acutely relieved OCD symptoms, they acted differently on serum BDNF levels. Elucidating their mechanisms of action21 requires further investigation.
References
1. Sen S, Duman R, Sanacora G. Serum brain-derived neurotrophic factor, depression, and antidepressant medications: meta-analyses and implications. Biol Psychiatry. 2008;64(6):527-532. PubMed CrossRef
2. Polyakova M, Stuke K, Schuemberg K, et al. BDNF as a biomarker for successful treatment of mood disorders: a systematic & quantitative meta-analysis. J Affect Disord. 2015;174:432-440. PubMed CrossRef
3. Bandelow B, Baldwin D, Abelli M, et al. Biological markers for anxiety disorders, OCD and PTSD: a consensus statement, part II: neurochemistry, neurophysiology and neurocognition. World J Biol Psychiatry. 2017;18(3):162-214. PubMed CrossRef
4. Maina G, Rosso G, Zanardini R, et al. Serum levels of brain-derived neurotrophic factor in drug-naive obsessive-compulsive patients: a case-control study. J Affect Disord. 2010;122(1-2):174-178. PubMed CrossRef
5. Lepack AE, Bang E, Lee B, et al. Fast-acting antidepressants rapidly stimulate ERK signaling and BDNF release in primary neuronal cultures. Neuropharmacology. 2016;111:242-252. PubMed CrossRef
6. Rodriguez CI, Zwerling J, Kalanthroff E, et al. Effect of a novel NMDA receptor modulator, rapastinel (formerly GLYX-13) in OCD: a pilot study. Am J Psychiatry. 2016;173(12):1239-1241. PubMed CrossRef
7. American Psychiatric Association. Diagnostic and Statistical Manual for Mental Disorders. Fourth Edition, Text Revision. Washington, DC: American Psychiatric Association; 2000.
8. American Psychiatric Association. Diagnostic and Statistical Manual for Mental Disorders. Fifth Edition. Washington, DC: American Psychiatric Association; 2013.
9. Goodman WK, Price LH, Rasmussen G, et al. The Yale-Brown Obsessive Compulsive Scale, I: development, use, and reliability. Arch Gen Psychiatry. 1989;46(11):1006-1011. PubMed CrossRef
10. Goodman WK, Price LH, Rasmussen G, et al. The Yale-Brown Obsessive Compulsive Scale, II: validity. Arch Gen Psychiatry. 1989;46(11):1012-1016. PubMed CrossRef
11. Tolin DF, Abramowitz JS, Diefenbach GJ. Defining response in clinical trials for obsessive-compulsive disorder: a signal detection analysis of the Yale-Brown Obsessive Compulsive Scale. J Clin Psychiatry. 2005;66(12):1549-1557. PubMed CrossRef
12. Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23(1):56-62. PubMed CrossRef
13. Polacchini A, Metelli G, Francavilla R, et al. A method for reproducible measurements of serum BDNF: comparison of the performance of six commercial assays. Sci Rep. 2015;5(1):17989. PubMed CrossRef
14. Goodman W, Price LH, Woods SW, et al. Pharmacologic challenges in obsessive-compulsive disorder. In: Zohar J, Insel T, eds. Psychobiology of OCD. New York, NY: Springer; 1989:183.
15. Rodriguez CI, Wheaton M, Zwerling J, et al. Can exposure-based CBT extend the effects of intravenous ketamine in obsessive-compulsive disorder? an open-label trial. J Clin Psychiatry. 2016;77:408-409. PubMed CrossRef
16. Cousineau D. Confidence intervals in within-subject designs: a simpler solution to Loftus and Masson’s method. Tutor Quant Methods Psychol. 2005;1(1):42-45. CrossRef
17. Duman RS, Monteggia LM. A neurotrophic model for stress-related mood disorders. Biol Psychiatry. 2006;59(12):1116-1127. PubMed CrossRef
18. Duman RS, Heninger MD, Nestler EJ. A molecular and cellular theory of depression. Arch Gen Psychiatry. 1997;54(7):597-606. PubMed CrossRef
19. Pan W, Banks WA, Fasold MB, et al. Transport of brain-derived neurotrophic factor across the blood-brain barrier. Neuropharmacology. 1998;37(12):1553-1561. PubMed CrossRef
20. Chacon-Fernandez P, Sפuberli K, Colzani M, et al. Brain-derived neurotrophic factor in megakaryocytes. J Biol Chem. 2016;291(19):9872-9881. PubMed CrossRef
21. Liu RJ, Duman C, Kato T, et al. GLYX-13 produces rapid antidepressant responses with key synaptic and behavioral effects distinct from ketamine. Neuropsychopharmacology. 2017;42(6):1231-1242. PubMed CrossRef
aDepartment of Psychiatry and Behavioral Sciences, Stanford University, Stanford, California
bAnalytical Psychopharmacology Laboratory, Nathan Kline Institute, Research Foundation for Mental Hygiene of New York State, Orangeburg, New York
cDepartment of Psychiatry, College of Physicians and Surgeons, Columbia University, New York, New York
dNew York State Psychiatric Institute, New York, New York
eAptinyx, Inc, Evanston, Illinois
fThe Falk Center for Molecular Therapeutics, Department of Biomedical Engineering, McCormick School of Engineering and Applied Sciences, Northwestern University, Evanston, Illinois
gDepartment of Psychiatry, Weill Cornell Medical College, New York, New York
hVeterans Affairs Palo Alto Health Care System, Palo Alto, California
Potential conflicts of interest: Dr Simpson has received royalties from Cambridge University Press and UpToDate. Dr Moskal was founder of and owned stock in Naurex, and Dr Burch was an employee at Naurex when rapastinel was donated for the current study. Dr Rodriguez received rapastinel for the current study at no cost and is a consultant for Allergan, BlackThorn Therapeutics, and Rugen Therapeutics. Drs Linkovski, Filippou-Frye, Jo, and Lee; Mss Shen, Zwerling, and Cordell; and Mr Cooper report no additional financial or other relationships relevant to the subject of this letter.
Funding/support: This study was supported by the Brain and Behavior Research Foundation/NARSAD Ellen Schapiro and Gerald Axelbaum Investigator Award (Dr Rodriguez), Robert Wood Johnson Foundation Harold Amos Medical Faculty Development Program (Dr Rodriguez), the National Institutes of Mental Health (K23MH092434 and R01MH105461 [Dr Rodriguez], K24MH09155 [Dr Simpson]), the New York Presbyterian Youth Anxiety Center, and the New York State Psychiatric Institute. Rapastinel (formerly GLYX-13) study drug was supplied by Naurex (since drug donation, Naurex has been acquired by Allergan).
Acknowledgment: The authors thank the individuals who generously donated their time to participate in this research study.
Role of the sponsors: Naurex supplied study materials (rapastinel at no cost), and Naurex staff participated in the outline of the study but had no role in study selection or interpretation of the data. Although staff at Allergan reviewed the manuscript, final approval for the decision to submit the manuscript was the sole decision of the authors. The remaining providers of funding/support had no role in the design, analysis, interpretation, or publication of this study.
Trial registration: ClinicalTrials.gov identifiers: NCT02267629, NCT02062658
Supplementary material: See accompanying pages.
J Clin Psychiatry 2018;79(1):17l11824
To cite: Linkovski O, Shen H, Zwerling J, et al. Effects of rapastinel (formerly GLYX-13) on serum brain-derived neurotrophic factor in obsessive-compulsive disorder. J Clin Psychiatry. 2018;79(1):17l11824.
To share: https://doi.org/10.4088/JCP.17l11824
© Copyright 2018 Physicians Postgraduate Press, Inc.
This PDF is free for all visitors!
Save
Cite