This work may not be copied, distributed, displayed, published, reproduced, transmitted, modified, posted, sold, licensed, or used for commercial purposes. By downloading this file, you are agreeing to the publisher’s Terms & Conditions.
Objective: Major depressive disorder (MDD) is one of the most common psychiatric disorders, conferring considerable individual, family, and community burden. To date, treatments for MDD have been derived from the monoamine hypothesis, and there is a paucity of emerging antidepressants, especially with novel mechanisms of action and treatment targets. N-acetylcysteine (NAC) is a redox-active glutathione precursor that decreases inflammatory cytokines, modulates glutamate, promotes neurogenesis, and decreases apoptosis, all of which contribute to the neurobiology of depression.
Method: Participants with a current episode of MDD diagnosed according to DSM-IV-TR criteria (N = 252) were treated with NAC or placebo in addition to treatment as usual for 12 weeks and were followed to 16 weeks. Data were collected between 2007 and 2011.
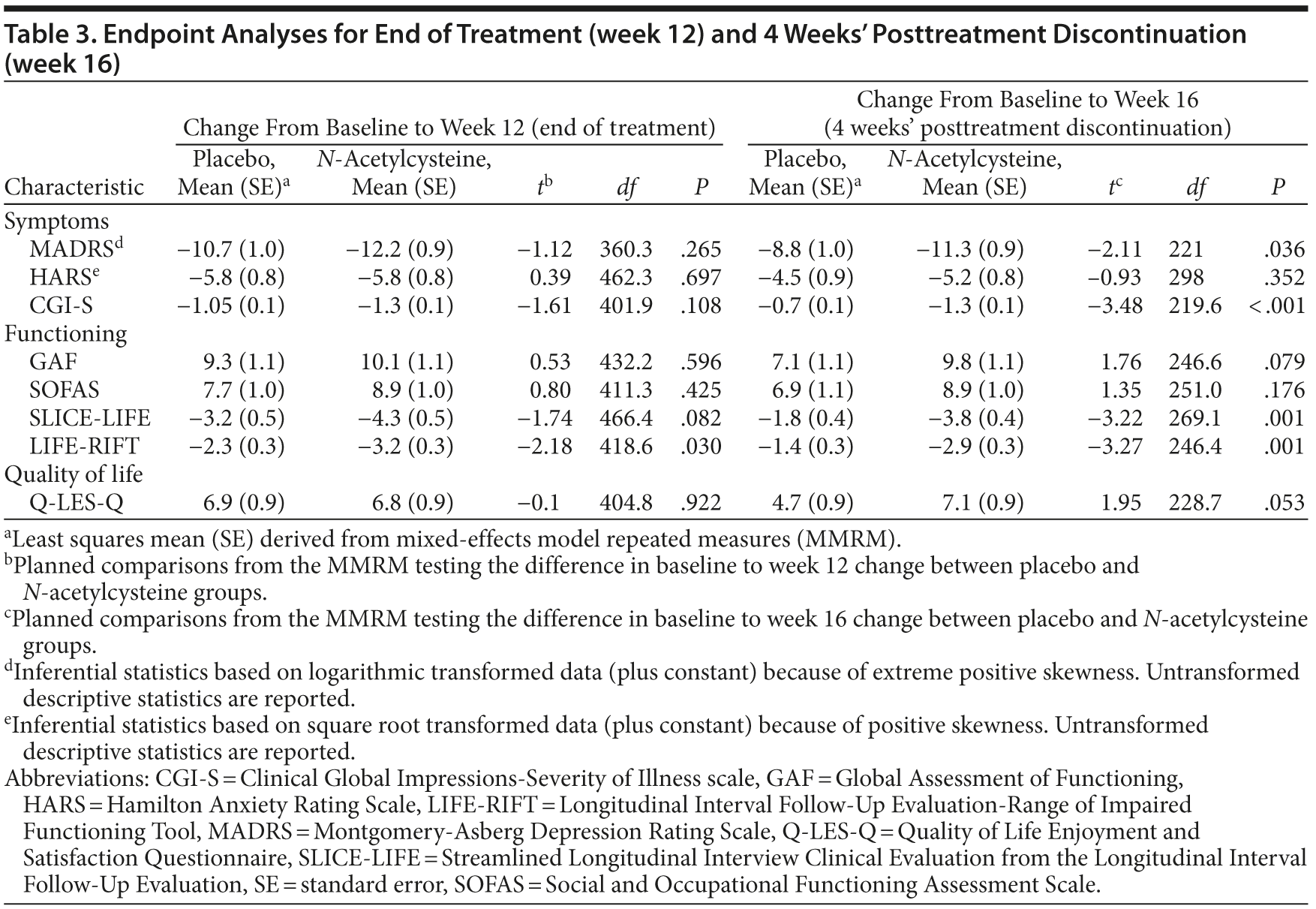
Results: The omnibus interaction between group and visit for the Montgomery-Asberg Depression Rating Scale (MADRS), the primary outcome measure, was not significant (F1,520.9 = 1.98, P = .067), and the groups did not separate at week 12 (t360.3 = −1.12, P = .265). However, at week 12, the scores on the Longitudinal Interval Follow-Up Evaluation-Range of Impaired Functioning Tool (LIFE-RIFT) differed from placebo (P = .03). Among participants with a MADRS score ≥ 25, NAC separated from placebo at weeks 6, 8, 12, and 16 (P < .05). Additionally, the rate of change between baseline and week 16 was significant (t221.03 = −2.11, P = .036). NAC treatment was superior to placebo at week 16 for secondary readouts of function and clinical impression. Remission and response were greater in the NAC group at week 16, but not at week 12. The NAC group had a greater rate of gastrointestinal and musculoskeletal adverse events.
Conclusions: Being negative at the week 12 end point, and with some positive secondary signals, the study provides only limited support for the role of NAC as a novel adjunctive therapy for MDD. These data implicate the pathways influenced by NAC in depression pathogenesis, principally oxidative and inflammatory stress and glutamate, although definitive confirmation remains necessary.
Trial Registration: www.anzctr.org.au Identifier: ACTRN12607000134426
The Efficacy of Adjunctive N-Acetylcysteine in Major Depressive Disorder: A Double-Blind, Randomized, Placebo-Controlled Trial

ABSTRACT
Objective: Major depressive disorder (MDD) is one of the most common psychiatric disorders, conferring considerable individual, family, and community burden. To date, treatments for MDD have been derived from the monoamine hypothesis, and there is a paucity of emerging antidepressants, especially with novel mechanisms of action and treatment targets. N-acetylcysteine (NAC) is a redox-active glutathione precursor that decreases inflammatory cytokines, modulates glutamate, promotes neurogenesis, and decreases apoptosis, all of which contribute to the neurobiology of depression.
Method: Participants with a current episode of MDD diagnosed according to DSM-IV-TR criteria (N = 252) were treated with NAC or placebo in addition to treatment as usual for 12 weeks and were followed to 16 weeks. Data were collected between 2007 and 2011.
Results: The omnibus interaction between group and visit for the Montgomery-Asberg Depression Rating Scale (MADRS), the primary outcome measure, was not significant (F1,520.9 = 1.98, P = .067), and the groups did not separate at week 12 (t360.3 = −1.12, P = .265). However, at week 12, the scores on the Longitudinal Interval Follow-Up Evaluation-Range of Impaired Functioning Tool (LIFE-RIFT) differed from placebo (P = .03). Among participants with a MADRS score ≥ 25, NAC separated from placebo at weeks 6, 8, 12, and 16 (P < .05). Additionally, the rate of change between baseline and week 16 was significant (t221.03 = −2.11, P = .036). NAC treatment was superior to placebo at week 16 for secondary readouts of function and clinical impression. Remission and response were greater in the NAC group at week 16, but not at week 12. The NAC group had a greater rate of gastrointestinal and musculoskeletal adverse events.
Conclusions: Being negative at the week 12 end point, and with some positive secondary signals, the study provides only limited support for the role of NAC as a novel adjunctive therapy for MDD. These data implicate the pathways influenced by NAC in depression pathogenesis, principally oxidative and inflammatory stress and glutamate, although definitive confirmation remains necessary.
Trial Registration: www.anzctr.org.au Identifier: ACTRN12607000134426
J Clin Psychiatry 2014;75(6):628-636
© Copyright 2014 Physicians Postgraduate Press, Inc.
Submitted: March 3, 2013; accepted November 5, 2013(doi:10.4088/JCP.13m08454).
Corresponding author: Michael Berk, MD, PhD, Deakin University, PO Box 281, Geelong 3220, Australia ([email protected]).
Considerable attention has been paid recently to the weak pipeline of emerging agents in psychiatry, and in particular, the paucity of truly novel antidepressant agents.1 New insights into the putative biology of depression have indicated alternative mechanisms of action for the development of novel antidepressants, including inflammation and oxidative stress. Glutathione, a tripeptide consisting of glutamate, glycine, and cysteine, is the dominant free radical scavenger within the brain that buffers reactive oxidative species. N-acetylcysteine can reliably enhance the synthesis of glutathione by increasing the availability of cysteine, the rate-limiting synthetic step.2,3 N-acetylcysteine also has other actions germane to the known pathophysiology of depression, such as enhancing neurogenesis, blocking apoptosis, reducing inflammation, protecting against mitochondrial toxicity, and modulating glutamate,4,5 which make N-acetylcysteine a promising translational bridge between these pathways and the development of targeted therapies.
Clinically, N-acetylcysteine has demonstrated efficacy in the treatment of schizophrenia, mood symptoms in bipolar disorder, smoking and cannabis cessation, gambling, and autism.6-10 We therefore aimed to test its efficacy in the acute treatment of major depressive disorder (MDD) by conducting a randomized, placebo-controlled trial of N-acetylcysteine as adjunctive treatment. Specifically, we hypothesized that addition of N-acetylcysteine would reduce Montgomery-Asberg Depression Rating Scale (MADRS)11 scores (primary outcome) in comparison to placebo. We were also interested in determining the effectiveness of N-acetylcysteine in comparison to placebo with respect to secondary outcomes such as anxiety, functioning, and quality of life.
METHOD
Study Design
This was a double-blind, randomized, placebo-controlled trial, conducted over 12 weeks, to compare N-acetylcysteine with placebo, adjunctive to treatment as usual, in the acute treatment of moderate to severe MDD. Postdiscontinuation measurements were made approximately 4 weeks after trial completion (week 16), during which the blind was maintained. This trial was conducted at 4 sites: Geelong, Melbourne, and Bendigo in the state of Victoria, and Sydney in the state of New South Wales, Australia, in accordance with the Good Clinical Practice guidelines and institutional review board approval. The study was registered on the Australian and New Zealand Clinical Trials Registry (www.anzctr.org.au identifier: ACTRN12607000134426).
The inclusion criteria for this study were being aged 18 years or older; having the capacity to consent to the study and to follow its instructions and procedures; fulfilling the DSM-IV-TR diagnostic criteria for MDD,12 single episode or recurrent, as well as a score of ≥ 18 on the MADRS,11 at the time of entry into the study; being on stable treatment for at least 2 weeks prior to randomization if participants were on psychotherapy or antidepressant therapy; and utilizing effective contraception if females of child-bearing age were sexually active. Exclusion criteria were a concurrent diagnosis of bipolar I or II disorder or bipolar disorder not otherwise specified; a primary clinical diagnosis of a personality disorder; failure in 3 or more adequate trials of antidepressant therapy or ECT for the current major depressive episode; presence of a known or suspected clinically unstable systemic medical disorder, including recent gastrointestinal ulcers; pregnant or breastfeeding status; current users of greater than 500 mg/d of N-acetylcysteine, 200 μg/d of selenium, or 500 IU/d of vitamin E; and/or history of anaphylactic reaction to N-acetylcysteine or any component of the preparation. Adherence was assessed by pill counts of returned packs.

- N-acetylcysteine has shown efficacy in diverse syndromes, from depression in bipolar disorder to schizophrenia, autism, and addictions.
- This study provides limited support for adjunctive N-acetylcysteine, particularly in more severe depression.
Participant Recruitment and Allocation
Participants were recruited from 2007 to 2011 through local advertisement and contact with local psychiatric inpatient units, community mental health teams, general practitioners, and private psychiatrists. Diagnosis was confirmed using a structured interview, the Mini-International Neuropsychiatric Interview (MINI-plus).13 Written informed consent was obtained from study participants following a complete description of the study.
Participants were randomly allocated, in a double-blind fashion, to receive N-acetylcysteine (2 ×— 500 mg capsules twice daily) or placebo, in addition to existing treatments for their major depressive episode (treatment as usual). N-acetylcysteine was supplied by Zambon (Milan, Italy), and encapsulated by DFC-Pharmamed Pty Ltd (Sydney, Australia) in accordance with Good Manufacturing Practice guidelines. The choice of dose was based on that used in our previous trials of adjunctive N-acetylcysteine in schizophrenia14 and bipolar disorder,10,15 which have appeared to be efficacious and well tolerated in both trials, and is also distanced by a fair margin from the maximum dose of 5,000 mg/d used in published trials.16 To facilitate double-blinding, the trial medications (both N-acetylcysteine and placebo) were dispensed in identical numbers and capsule formulations in sealed containers by the trial pharmacist. Furthermore, to mask the distinct smell of the N-acetylcysteine preparation, the placebo capsules were dusted with a tiny amount of N-acetylcysteine so that all capsules had a similar odor.
Outcome Measures
A battery of validated outcome measures focusing on both depressive symptomatology and global clinical and functional status was used at baseline and at weeks 2, 4, 6, 8, 12, and 16 (postdiscontinuation). This included the MADRS (primary outcome), Clinical Global Impressions-Improvement (CGI-I) and -Severity of Illness (CGI-S) scales,17 Hamilton Anxiety Rating Scale (HARS),18 Global Assessment of Functioning (GAF) scale,12 Social and Occupational Functioning Assessment Scale (SOFAS),19 Streamlined Longitudinal Interview Clinical Evaluation from the Longitudinal Interval Follow-Up Evaluation (SLICE-LIFE),20 Longitudinal Interval Follow-Up Evaluation-Range of Impaired Functioning Tool (LIFE-RIFT),21 and Quality of Life Enjoyment and Satisfaction Questionnaire (Q-LES-Q), short form.22 Individuals were withdrawn from the trial under the following conditions: failure to take the trial medication for 7 consecutive days, cessation of effective contraception or confirmed pregnancy, withdrawal of consent, or emergence of serious adverse events suspected to be associated with the trial medication. Participant reports of adverse effects were recorded, appropriately managed according to medical assessment, and monitored.
Statistical Analyses
The trial was powered based on the results of the study conducted in bipolar disorder.23 Statistical analysis was conducted blind to treatment allocation. All analyses were conducted in accordance with the International Conference on Harmonization E9 statistical principles.24 Independent samples, t tests, and χ2 analyses were used to test for differences between the 2 treatment groups at baseline. These inferential statistics were also used to compare participants who completed or discontinued the intervention.
There were 2 end points for the trial: (1) at the end of 12 weeks of treatment and (2) at 4 weeks’ posttreatment (at 16 weeks). All randomized participants who had at least 1 postbaseline assessment were included in the intent-to-treat analysis. Analysis was performed by a consultant biostatistician (S.M.C.), using SPSS Statistics Version 20 (IBM Corp; Armonk, New York) on a cleaned and locked database.
Differences between the 2 groups with respect to depressive symptomatology (primary outcome) and measures of anxiety, functioning, and quality of life (secondary outcomes) were assessed using the likelihood-based mixed-effects model repeated-measures (MMRM) approach. The MMRM model included the fixed, categorical effects of group, visit, and group-by-visit interaction. The Toeplitiz covariance structure was used to model the relations between observations on different occasions. Planned comparisons (t tests) using MMRM were conducted to examine group differences in mean change on the outcome measures from baseline (week 0) to the 2 end points (week 12 and week 16 [end point analysis]).
Response (≤ 50% reduction from baseline to end point) and remission (MADRS score ≤ 7) on the MADRS were also analyzed, and differences between the 2 groups were examined using Fisher exact test. All tests of treatment effects were conducted using a 2-sided α level of .05. Given the exploratory nature of the secondary study, α, or type I error, was also set at .05 for these analyses. No adjustments were made for multiple comparisons because they can result in a higher type II rate, reduced power, and increased likelihood of missing important findings.25
RESULTS
Sample Characteristics
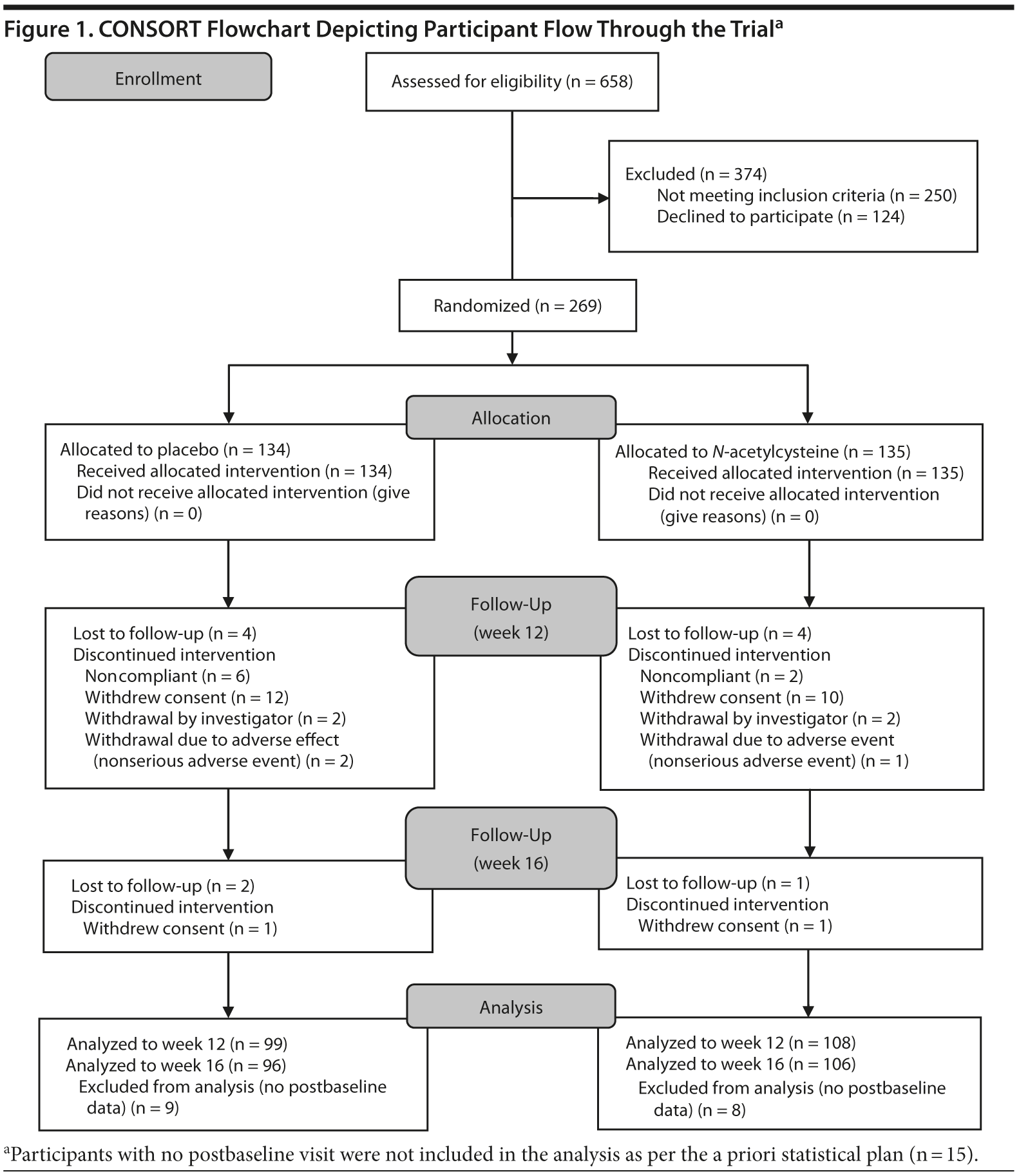
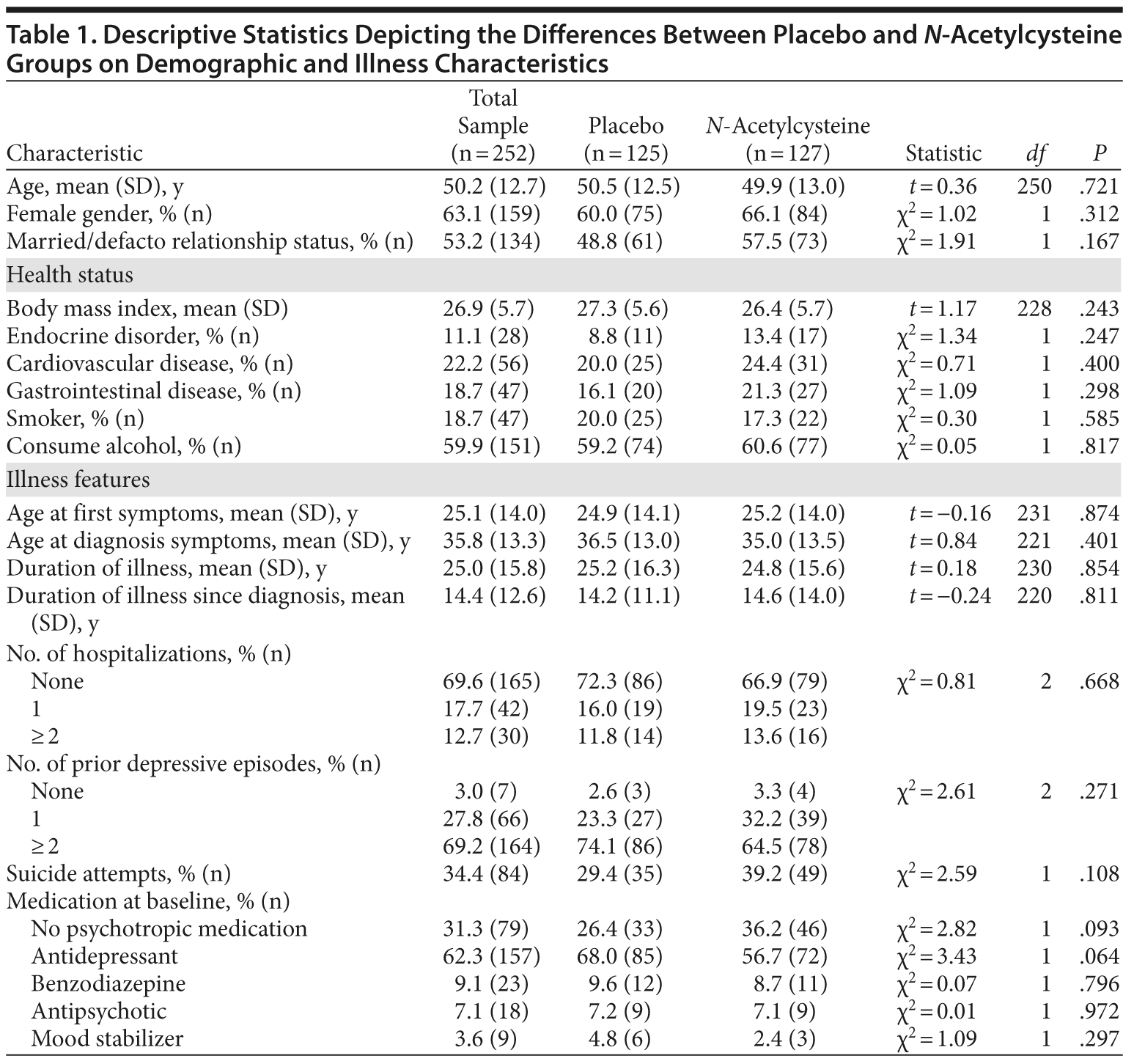
Individuals meeting DSM-IV-TR criteria for MDD and having a score of 18 or higher on the MADRS (N = 269) were randomized (Figure 1). Seventeen participants were excluded, as they had no postbaseline data. Of the remaining 252 participants, 159 were female, with a mean (SD) age of 50.2 (12.7) years. Nearly 60% of the sample consumed alcohol and nearly two-thirds were on antidepressant medication at baseline (Table 1).
Baseline Characteristics
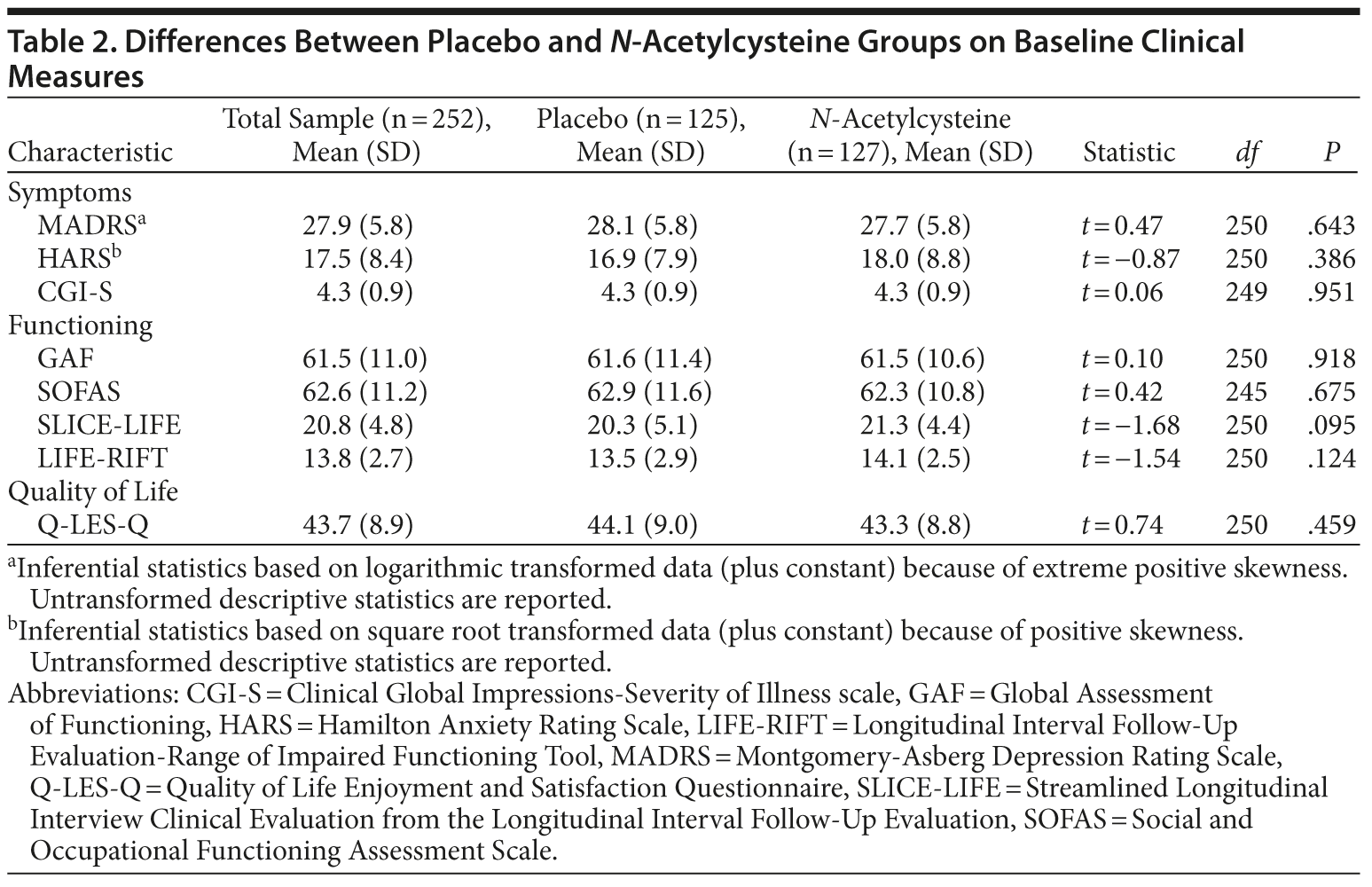
The 2 treatment groups were similar on all demographic (see Table 1) and baseline clinical and functioning measures (Table 2). Participant flow is illustrated in Figure 1.
Participant Flow
Of the 252 participants with postbaseline data, 207 (82.1%) completed week 12 (Figure 1), with 99 (73.9%) in the placebo and 108 (80.0%) in the N-acetylcysteine group (χ21 = 1.46, P = .226). At week 16, 202 participants (80.2%) completed the study, with 96 (71.6%) in the placebo group and 106 (78.5%) in N-acetylcysteine group (χ21 = 1.76, P = .185). There were no significant differences between completers and noncompleters at week 12 or at week 16 with respect to any of the baseline variables.
Depressive Symptomatology
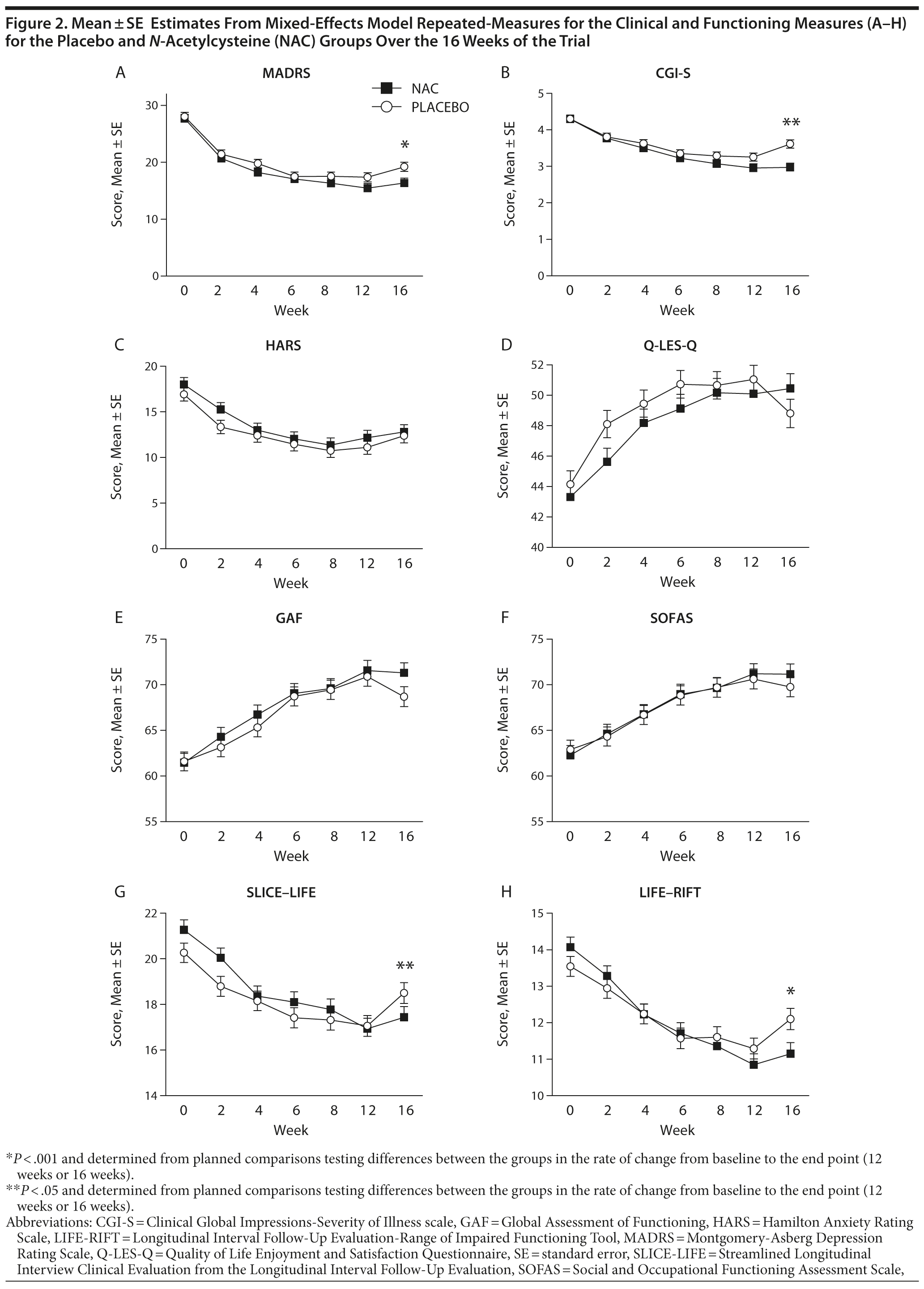
Over most time points, the 2 groups were similar in terms of MADRS assessed levels of depressive symptoms, with greater divergence between groups noted at weeks 12 and 16 (Figure 2). The omnibus interaction between group and visit for the MADRS rating scale was not significant (F1,520.9 = 1.98, P = .067); end point and the groups did not separate at week 12 (t360.3 = −1.12, P = .265). However, the rate of change in each group (N-acetylcysteine and placebo) was significant from baseline to week 16 (t221.03 = −2.11, P = .036) (Table 3), with the rate of change greater in the N-acetylcysteine group. When comparing postdiscontinuation of treatment effects, the group differences in the rate of change between weeks 12 (last treatment visit) and 16 (postdiscontinuation phase) were not significant (t1,152.9 = 1.536, P = .125).
At week 12, there were no significant differences between the groups with respect to response or remission criteria. However, at the 16-week end point, response was significantly greater in the N-acetylcysteine group compared to placebo (N-acetylcysteine, 36.6% [n = 42]; placebo, 25.0% [n = 24]; P = .027). Similarly, remission was more likely to be reached at 16 weeks in the N-acetylcysteine group (N-acetylcysteine, 17.9% [n = 19]; placebo, 6.2% [n = 6]; P = .017).
For week 12, the number needed to treat (NNT) for response on the MADRS was 17 and the NNT for remission was 18. For week 16, the NNT for response was 6.8, and, for remission, the NNT was 8.6.
In the N-acetylcysteine group, 41.9% (n = 52) had no antidepressants, 12.1% (n = 15) stopped their antidepressants, and 46% (n = 57) were on antidepressants for the duration of the study. Similar rates were found in the placebo group, with 30.9% (n = 38) having no antidepressants, 14.6% (n = 18) stopped their antidepressants, and 54.5% (n = 67) were on antidepressants for the 16 weeks. The differences between the groups were not significant (χ22 = 3.25, P = .197).
Secondary Outcomes: Symptoms
For the CGI-S, the omnibus interaction between group and time was significant (F6,442.7 = 2.50, P = .022) (see Figure 2B). End point analysis indicated no difference in rate of reduction from baseline to week 12. There was, however, a significant difference in rate of reduction of symptom severity from baseline to week 16, with the N-acetylcysteine group showing significantly more improvement (t219.6 = −3.48, P < .001). There was a significant group difference at week 16 on the CGI-S (t1189.6 = 2.64, P = .008), with the placebo group showing significant worsening of symptom severity (P < .001) and the N-acetylcysteine group showing no change in symptoms (P = .872).
Secondary Outcomes: Anxiety, Functioning, and Quality of Life
There were no significant between-group differences over time on the GAF or the SOFAS. For SLICE-LIFE, the interaction between group and visit was significant (F6,612.3 = 2.73, P = .013). End point analysis was not significant for week 12; however, there was a significant difference in rate of reduction of symptom severity from baseline to week 16, with the N-acetylcysteine group showing significantly more improvement (t269.1 = −3.22, P = .001). The rate of change in the postdiscontinuation phase did not differ between the groups on SLICE-LIFE (t1,050.6 = 1.67, P = .095).
For the LIFE-RIFT, the omnibus interaction was not significant (F6,489.9 = 29.95, P = .062); however, end point analyses revealed that the rate of change from baseline to week 12 (t418.6 = −2.18, P = .030) and baseline to week 16 was significant, (t246.4 = −3.27, P = .001), with the N-acetylcysteine group showing significantly greater improvements in functioning than the placebo group. The rate of change in the postdiscontinuation phase did not differ between the groups on the LIFE-RIFT (t1,090.1 = 1.39, P = .165).
On the Q-LES-Q, there was a significant interaction between visit and group (F6,461.8 = 2.35, P = .030). End point analysis, however, failed to find any significant differences between the 2 groups. The 2 groups differed significantly at week 16 (t1,071.5 = −2.52, P = .012), with the placebo group demonstrating significant worsening of quality of life from weeks 12 to 16 (P = .003), whereas the N-acetylcysteine group remained stable (P = .625).
Supplementary Analyses
Potential confounders were examined, including site, age, gender, metabolic disorder (yes/no), severity of illness at baseline (MADRS score < 25, or ≥ 25), duration of illness, antidepressant use (yes/no), benzodiazepine use (yes/no), and antipsychotic use (yes/no) as 3-way interactions between group, visit, and the confounding variable in separate MMRMs.
There was a significant 3-way interaction with the CGI for group by visit by severity of illness at baseline (F6,439.7 = 2.13, P = .049) (see Supplementary eFigure 1 at PSYCHIATRIST.COM). At weeks 6, 8, 12, and 16, participants with the more severe baseline depressive symptoms (MADRS score ≥ 25) in the N-acetylcysteine group had significantly lower CGI-S scores than those in the placebo group (all P values < .05).
We divided participants into tertiles on the basis of age (≤ 46 years, 47-56 years, and ≥ 57 years), where a significant 3-way interaction was found with group, visit, and age on the CGI-S (3-way interaction, F12,436.13 = 2.35, P = .006). There was a significant separation of N-acetylcysteine from placebo in the middle tertile, not in the younger and older tertiles (see Supplementary eFigure 2). Specifically, in the 47- to 56-year-old age range, the N-acetylcysteine group had significantly lower mean CGI-S scores from week 6 through to week 16 (all P values < .001).
There were no between-group changes in antidepressant use from baseline to week 12 (P = .231) or week 16 (P = .197). There were a total of 9 serious adverse events, 5 in the N-acetylcysteine group and 4 in the placebo group, with no significant differences in the groups observed (χ21 = 0.10, P = .753). In terms of adverse events, the N-acetylcysteine group had a significantly greater percentage of gastrointestinal problems (33.9%, n = 43) compared to placebo (18.4%, n = 23) (χ21 = 7.79, P = .005). Similarly, the N-acetylcysteine group (3.9%, n = 5) was more likely to have musculoskeletal complaints (back pain [n = 1], joint pain [n = 3], and muscle spasms [n = 1]) than the placebo group (0.00%, n = 0) (χ21 = 5.021, P = .025). Adherence was assessed by audit of returned capsules. There was an 88.7% compliance rate on available data; but 74% of data were missing.
DISCUSSION
The data from this clinical trial provide only limited support for the role of adjunctive N-acetylcysteine in reducing depressive symptoms in individuals with MDD. The N-acetylcysteine and placebo groups did not separate on the MADRS at week 12, with separation only evident at the postdiscontinuation visit, week 16. However, at week 12, the scores on the LIFE-RIFT differed from placebo, which is noteworthy in 2 contexts: functional recovery lags symptomatic recovery in many studies,26 and scores on the LIFE-RIFT had the highest effect size in the study of N-acetylcysteine in bipolar depression.15 The suggestion of a particular effect on functioning necessitates replication, given the burden of this symptom cluster. Remission and response were greater in the N-acetylcysteine group at week 16, but not at week 12. Further, there was an indication of greater effect in those with more severe depression (MADRS score of 25 or more) concordant with patterns evident in antidepressant treatment studies.27 It is hard to interpret the data beyond the 12-week end point, although the persistence of a pattern of benefit and the potentially greater between-group differences at week 16 are noteworthy, but it needs to be emphasized that this was not a primary end point. Whether this reflects a potentially delayed neuroprotective effect would be a hypothesis for further investigation.
The effects of N-acetylcysteine are indeed modest but may be more clinically meaningful among more severely ill individuals. Age influenced efficacy, with those on N-acetylcysteine in the midtertile separating from placebo, while treatment groups in older and younger participants failed to separate. Interestingly, this is in keeping with the general pattern seen in antidepressant studies.28 End point analyses of measures of global impression, the CGI-S and functioning, the SLICE-LIFE, and LIFE-RIFT identified a significant benefit of N-acetylcysteine treatment. With regard to adverse events, more gastrointestinal and musculoskeletal adverse events occurred in the N-acetylcysteine group.
These findings are therefore partially concordant with data from a 6-month double-blind, placebo-controlled trial15 of adjunctive N-acetylcysteine (1,000 mg twice a day) in 75 participants with bipolar disorder. In that study, end point ratings on both the MADRS and the Bipolar Depression Rating Scale (BDRS)29 revealed a significant decrease in depressive symptoms on N-acetylcysteine as compared to placebo, with large effect sizes on both measures and comparable changes in functioning and quality of life. Interestingly, 17 participants in that study met criteria for MDD, and of the 10 participants on N-acetylcysteine, 8 were responders at end point, contrasting with 1 responder of 7 participants allocated to placebo.9 Further, in the 2-month, open-label phase of a randomized, placebo-controlled, maintenance clinical trial of bipolar disorder, with an index polarity of depression, the mean BDRS score at baseline was 19.7 (standard error [SE] = 0.8), declining to 11.1 (SE = 0.8) after the 8-week open-label treatment phase (P < .001). Again, significant improvements in functioning and quality of life were seen.15
In all of these studies, it is noteworthy that the clinical benefits were slow to emerge, being evident near the end point of each trial. This reflects the putative mechanism of action of N-acetylcysteine. Glutamatergic agents such as ketamine and AZD6765 are distinguished by their rapid action, and the difference in speed of onset of N-acetylcysteine suggests a distinct mechanism,30 either involving or independent of glutamate. In this context, spectroscopy data suggest a role of N-acetylcysteine on glutamate-glutamine, N-acetylaspartate, and myo-inositol.31 Depression is extensively documented to be associated with oxidative stress; for comprehensive reviews of the topic, see Hardan et al32 and Garcia et al.33 N-acetylcysteine counters the effects of reactive oxidative species and gradually rectifies the abnormalities in oxidative biology, inflammation, apoptosis, and mitochondrial function found in depression.34-37 N-acetylcysteine may be addressing any of these multiple pathways to neuroprogression that are described in depression.38 N-acetylcysteine has been shown to be potentially efficacious in a bewildering array of divergent disorders, from bipolar disorder10,15,23 to schizophrenia,6,14 obsessive-compulsive disorder,39 nailbiting,40 autism,41 attention-deficit/hyperactivity disorder,42 cocaine and cannabis abuse,43 smoking, and blast traumatic brain injury,44 although there are negative studies, including those in bulimia45 and pediatric trichotillomania,46 and the methodological quality of trials is highly variable.4 As N-acetylcysteine has multiple mechanisms of action, it remains to be clarified if it works on common targets across disorders, such as oxidative stress or inflammation, or if some actions, such as glutamate-cysteine exchange, are more important to some disorders than others, such as addictions.47
Strengths of the study include its relatively large sample size and paucity of exclusion criteria, features designed to reflect real world clinical usage. It is generally harder to demonstrate efficacy in adjunctive as compared to monotherapy designs, where baseline therapy is an inevitable confound. Had the study utilized a higher cutoff for depression severity, more robust findings may have ensued. The prior schizophrenia and depression studies suggest the effects of N-acetylcysteine are slow to emerge and that the duration of the study, while long for an antidepressant trial, may be too short for this specific agent.12,13 While the inclusion criterion of 2 weeks of stable prior pharmacologic and psychological therapy was done to enhance feasibility and generalization, this may have compromised the ability of this trial to detect a difference between treatments because the placebo group is likely to gain significant benefits of continued therapy during the trial. There was a trend in the difference in the proportion of patients receiving antidepressant medication at baseline, with a higher proportion in the placebo group (68.0%) than N-acetylcysteine (56.7%). This might have compromised the ability of the trial to detect between-group differences. Independent remote assessments for eligibility, which may reduce placebo response rates, were not done.48 Lastly, dose may be an issue; a recent study42 in ADHD showed greater efficacy at 4.8 g than 2.4 g daily. Similarly, in a study of blast traumatic brain injury, there was reported efficacy of a 4.0 g loading dose.44 These are considerably higher than the dose used in this study and raises the question of optimal dosage.42
CONCLUSION
This study suggests a potential benefit for adjunctive N-acetylcysteine in MDD, particularly in more severe depression, although this study was not positive on the primary outcome. These data thus provide limited support for N-acetylcysteine but additionally implicate the pathways influenced by N-acetylcysteine in depression pathogenesis, principally oxidative and inflammatory stress, neurotrophins, and glutamate. These findings open the door to identifying additional pathways for novel drug development for the treatment of depression.49
Drug names: ketamine (Ketalar and others).
Author affiliations: IMPACT Strategic Research Centre, Deakin University, School of Medicine, Barwon Health, Geelong (Drs Berk, Dean, and Dodd); Orygen Youth Health Research Centre (Drs Berk and Cotton); Centre of Youth Mental Health (Drs Berk and Cotton), Florey Institute for Neuroscience and Mental Health (Drs Berk, Dean, Jeavons, Schapkaitz and Bush; and Mss Kohlmann, Hewitt, and Robbins), and Department of Psychiatry, Royal Melbourne Hospital (Drs Berk, Dean, and Dodd), University of Melbourne Parkville; Discipline of Psychiatry, University of Adelaide, Adelaide (Dr Ng); Discipline of Psychiatry, Sydney Medical School, University of Sydney, Sydney (Dr Malhi and Mss Tanious, Moss, and Cobb); Department of Psychiatry, CADE Clinic, Royal North Shore Hospital, St. Leonards (Dr Malhi and Ms Tanious), Australia; and Department of Psychosomatic Medicine and Psychotherapy, Klinikum rechts der Isar der TU, Munich, Germany (Dr Allwang).
Potential conflicts of interest: Dr Berk has received grant support from National Institutes of Health, Simons Autism Foundation, Cancer Council of Victoria, CRC for Mental Health, Stanley Medical Research Foundation, Medical Benefits Fund, National Health and Medical Research Council (NHMRC), Beyond Blue, Geelong Medical Research Foundation, Bristol-Myers Squibb, Eli Lilly, GlaxoSmithKline, Organon, Meat and Livestock Board, Novartis, Mayne Pharma, and Servier; has been a speaker for AstraZeneca, Bristol-Myers Squibb, Eli Lilly, GlaxoSmithKline, Janssen-Cilag, Lundbeck, Merck, Pfizer, Sanofi Synthelabo, Servier, Solvay, and Wyeth; and has served as a consultant to AstraZeneca, Bristol-Myers Squibb, Eli Lilly, GlaxoSmithKline, Janssen-Cilag, Lundbeck, and Servier. Dr Bush is a shareholder in Prana Biotechnology, Mesoblast, Cogstate, and Eucalyptus Pty Ltd. Drs Berk and Bush are co-inventors on two provisional patents regarding the use of N-acetylcysteine and related compounds for psychiatric indications, assigned to the Mental Health Research Institute. Dr Dean has received grant support from the Brain and Behavior Foundation, Simons Autism Foundation, Stanley Medical Research Institute, Lilly, NHMRC, and Australasian Society for Bipolar and Depressive Disorders/Servier. Dr Dodd has received research support from the Stanley Medical Research Institute, NHMRC, Beyond Blue, Australian Rotary Health Research Fund, Simons Foundation, Geelong Medical Research Foundation, Eli Lilly, GlaxoSmithKline, Organon, Mayne Pharma, and Servier; has received speakers and advisory board fees from Eli Lilly; and has received conference travel support from Servier. Dr Malhi has received research support from AstraZeneca, Eli Lilly, Organon, Pfizer, Servier, and Wyeth; has been a speaker for AstraZeneca, Eli Lilly, Janssen-Cilag, Lundbeck, Pfizer, Ranbaxy, Servier, and Wyeth; and has been a consultant for AstraZeneca, Eli Lilly, Janssen-Cilag, Lundbeck, and, Servier. Drs Cotton, Jeavons, Allwang, Schapkaitz, and Ng and Mss Tanious, Kohlmann, Hewitt, Moss, Robbins, and Cobb have no conflicts of interest.
Funding/support: The authors would like to acknowledge financial support from the National Health and Medical Research Council (APP628395) and Australian Rotary Health. Dr Berk is supported by a NHMRC Senior Principal Research Fellowship (1059660). The authors would also like to acknowledge service support provided by Barwon Health, the Bendigo Health Care Group, the Cooperative Research Centre for Mental Health, and the Royal North Shore Hospital.
Role of the sponsors: The funding organizations had no role in the design or conduct of the study; the collection, management, analysis, or interpretation of data; or the preparation, review, or approval of the manuscript.
Supplementary material: Available at PSYCHIATRIST.COM.
REFERENCES
1. Ledford H. Depression drug disappoints. Nature. 2011;479(7373):278. PubMed doi:10.1038/479278a
2. Dean OM, van den Buuse M, Berk M, et al. N-acetyl cysteine restores brain glutathione loss in combined 2-cyclohexene-1-one and d-amphetamine-treated rats: relevance to schizophrenia and bipolar disorder. Neurosci Lett. 2011;499(3):149-153. PubMed doi:10.1016/j.neulet.2011.05.027
3. Lavoie S, Murray MM, Deppen P, et al. Glutathione precursor, N-acetyl-cysteine, improves mismatch negativity in schizophrenia patients. Neuropsychopharmacology. 2008;33(9):2187-2199. PubMed doi:10.1038/sj.npp.1301624
4. Dean O, Giorlando F, Berk M. N-acetylcysteine in psychiatry: current therapeutic evidence and potential mechanisms of action. J Psychiatry Neurosci. 2011;36(2):78-86. PubMed
5. Berk M, Malhi GS, Gray LJ, et al. The promise of N-acetylcysteine in neuropsychiatry. Trends Pharmacol Sci. 2013;34(3):167-177. PubMed doi:10.1016/j.tips.2013.01.001
6. Berk M, Munib A, Dean O, et al. Qualitative methods in early-phase drug trials: broadening the scope of data and methods from an RCT of N-acetylcysteine in schizophrenia. J Clin Psychiatry. 2011;72(7):909-913. PubMed doi:10.4088/JCP.09m05741yel
7. Magalhaes PV, Dean OM, Bush AI, et al. N-acetyl cysteine add-on treatment for bipolar II disorder: a subgroup analysis of a randomized placebo-controlled trial. J Affect Disord. 2011;129(1-3):317-320. PubMed doi:10.1016/j.jad.2010.08.001
8. Magalh×£es PV, Dean OM, Bush AI, et al. A preliminary investigation on the efficacy of N-acetyl cysteine for mania or hypomania. Aust N Z J Psychiatry. 2013;47(6):564-568. PubMed doi:10.1177/0004867413481631
9. Magalh×£es PV, Dean OM, Bush AI, et al. N-acetylcysteine for major depressive episodes in bipolar disorder. Rev Bras Psiquiatr. 2011;33(4):374-378. PubMed doi:10.1590/S1516-44462011000400011
10. Berk M, Dean O, Cotton SM, et al. The efficacy of N-acetylcysteine as an adjunctive treatment in bipolar depression: an open label trial. J Affect Disord. 2011;135(1-3):389-394. PubMed doi:10.1016/j.jad.2011.06.005
11. Montgomery SA, Asberg M. A new depression scale designed to be sensitive to change. Br J Psychiatry. 1979;134(4):382-389. PubMed doi:10.1192/bjp.134.4.382
12. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, Text Revision. Washington, DC: American Psychiatric Association; 2000.
13. Sheehan DV, Lecrubier Y, Sheehan KH, et al. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. 1998;59(suppl 20):22-33, quiz 34-57. PubMed
14. Berk M, Copolov D, Dean O, et al. N-acetyl cysteine as a glutathione precursor for schizophrenia—a double-blind, randomized, placebo-controlled trial. Biol Psychiatry. 2008;64(5):361-368. PubMed doi:10.1016/j.biopsych.2008.03.004
15. Berk M, Copolov DL, Dean O, et al. N-acetyl cysteine for depressive symptoms in bipolar disorder—a double-blind randomized placebo-controlled trial. Biol Psychiatry. 2008;64(6):468-475. PubMed doi:10.1016/j.biopsych.2008.04.022
16. Louwerse ES, Weverling GJ, Bossuyt PM, et al. Randomized, double-blind, controlled trial of acetylcysteine in amyotrophic lateral sclerosis. Arch Neurol. 1995;52(6):559-564. PubMed doi:10.1001/archneur.1995.00540300031009
17. Spearing MK, Post RM, Leverich GS, et al. Modification of the Clinical Global Impressions (CGI) Scale for use in bipolar illness (BP): the CGI-BP. Psychiatry Res. 1997;73(3):159-171. PubMed doi:10.1016/S0165-1781(97)00123-6
18. Hamilton M. The assessment of anxiety states by rating. Br J Med Psychol. 1959;32(1):50-55. PubMed doi:10.1111/j.2044-8341.1959.tb00467.x
19. Goldman HH, Skodol AE, Lave TR. Revising axis V for DSM-IV: a review of measures of social functioning. Am J Psychiatry. 1992;149(9):1148-1156. PubMed
20. Keller MB, Lavori PW, Friedman B, et al. The Longitudinal Interval Follow-up Evaluation: a comprehensive method for assessing outcome in prospective longitudinal studies. Arch Gen Psychiatry. 1987;44(6):540-548. PubMed doi:10.1001/archpsyc.1987.01800180050009
21. Leon AC, Solomon DA, Mueller TI, et al. The Range of Impaired Functioning Tool (LIFE-RIFT): a brief measure of functional impairment. Psychol Med. 1999;29(4):869-878. PubMed doi:10.1017/S0033291799008570
22. Endicott J, Nee J, Harrison W, et al. Quality of Life Enjoyment and Satisfaction Questionnaire: a new measure. Psychopharmacol Bull. 1993;29(2):321-326. PubMed
23. Berk M, Dean OM, Cotton SM, et al. Maintenance N-acetyl cysteine treatment for bipolar disorder: a double-blind randomized placebo controlled trial. BMC Med. 2012;10(1):91. PubMed doi:10.1186/1741-7015-10-91
24. International conference on harmonisation: guidance on statistical principles for clinical trials (ICH-E9). http://www.fda.gov/downloads/drugs/guidancecomplianceregulatoryinformation/guidances/ucm073137.pdf. Updated September 1998. Accessed March 31, 2014.
25. Rothman KJ. No adjustments are needed for multiple comparisons. Epidemiology. 1990;1(1):43-46. PubMed doi:10.1097/00001648-199001000-00010
26. Furukawa TA, Takeuchi H, Hiroe T, et al. Symptomatic recovery and social functioning in major depression. Acta Psychiatr Scand. 2001;103(4):257-261. PubMed doi:10.1034/j.1600-0447.2001.00140.x
27. Kirsch I, Deacon BJ, Huedo-Medina TB, et al. Initial severity and antidepressant benefits: a meta-analysis of data submitted to the Food and Drug Administration. PLoS Med. 2008;5(2):e45. PubMed doi:10.1371/journal.pmed.0050045
28. Anderson IM. Selective serotonin reuptake inhibitors versus tricyclic antidepressants: a meta-analysis of efficacy and tolerability. J Affect Disord. 2000;58(1):19-36. PubMed doi:10.1016/S0165-0327(99)00092-0
29. Berk M, Malhi GS, Cahill C, et al. The Bipolar Depression Rating Scale (BDRS): its development, validation and utility. Bipolar Disord. 2007;9(6):571-579. PubMed doi:10.1111/j.1399-5618.2007.00536.x
30. Zarate CA Jr, Mathews D, Ibrahim L, et al. A randomized trial of a low-trapping nonselective N-methyl-D-aspartate channel blocker in major depression. Biol Psychiatry. 2013;74(4):257-264. PubMed doi:10.1016/j.biopsych.2012.10.019
31. Das P, Tanious M, Fritz K, et al. Metabolite profiles in the anterior cingulate cortex of depressed patients differentiate those taking N-acetyl-cysteine versus placebo. Aust N Z J Psychiatry. 2013;47(4):347-354. PubMed doi:10.1177/0004867412474074
32. Leonard B, Maes M. Mechanistic explanations how cell-mediated immune activation, inflammation and oxidative and nitrosative stress pathways and their sequels and concomitants play a role in the pathophysiology of unipolar depression. Neurosci Biobehav Rev. 2012;36(2):764-785. PubMed doi:10.1016/j.neubiorev.2011.12.005
33. Maes M, FiÅ¡ar Z, Medina M, et al. New drug targets in depression: inflammatory, cell-mediated immune, oxidative and nitrosative stress, mitochondrial, antioxidant, and neuroprogressive pathways. And new drug candidates—Nrf2 activators and GSK-3 inhibitors. Inflammopharmacology. 2012;20(3):127-150. PubMed doi:10.1007/s10787-011-0111-7
34. Andreazza AC, Kauer-Sant’ anna M, Frey BN, et al. Oxidative stress markers in bipolar disorder: a meta-analysis. J Affect Disord. 2008;111(2-3):135-144. PubMed doi:10.1016/j.jad.2008.04.013
35. Zafir A, Banu N. Modulation of in vivo oxidative status by exogenous corticosterone and restraint stress in rats. Stress. 2009;12(2):167-177. PubMed doi:10.1080/10253890802234168
36. Gibson SA, Korade Z, Shelton RC. Oxidative stress and glutathione response in tissue cultures from persons with major depression. J Psychiatr Res. 2012;46(10):1326-1332. PubMed doi:10.1016/j.jpsychires.2012.06.008
37. Dean OM, Data-Franco J, Giorlando F, et al. Minocycline: therapeutic potential in psychiatry. CNS Drugs. 2012;26(5):391-401. PubMed doi:10.2165/11632000-000000000-00000
38. Moylan S, Maes M, Wray NR, et al. The neuroprogressive nature of major depressive disorder: pathways to disease evolution and resistance, and therapeutic implications. Mol Psychiatry. 2013;18(5):595-606. PubMed doi: 10.1038/mp.2012.33
39. Afshar H, Roohafza H, Mohammad-Beigi H, et al. N-acetylcysteine add-on treatment in refractory obsessive-compulsive disorder: a randomized, double-blind, placebo-controlled trial. J Clin Psychopharmacol. 2012;32(6):797-803. PubMed doi:10.1097/JCP.0b013e318272677d
40. Ghanizadeh A, Derakhshan N, Berk M. N-acetylcysteine versus placebo for treating nail biting: a double blind randomized placebo controlled clinical trial. Antiinflamm Antiallergy Agents Med Chem; 2013;12(3):223-228. PubMed
41. Hardan AY, Fung LK, Libove RA, et al. A randomized controlled pilot trial of oral N-acetylcysteine in children with autism. Biol Psychiatry. 2012;71(11):956-961. PubMed doi:10.1016/j.biopsych.2012.01.014
42. Garcia RJ, Francis L, Dawood M, et al. Attention deficit and hyperactivity disorder scores are elevated and respond to N-acetylcysteine treatment in patients with systemic lupus erythematosus. Arthritis Rheum. 2013;65(5):1313-1318. PubMed doi:10.1002/art.37893 .
43. Gray KM, Carpenter MJ, Baker NL, et al. A double-blind randomized controlled trial of N-acetylcysteine in cannabis-dependent adolescents. Am J Psychiatry. 2012;169(8):805-812. PubMed doi:10.1176/appi.ajp.2012.12010055
44. Hoffer ME, Balaban C, Slade MD, et al. Amelioration of acute sequelae of blast induced mild traumatic brain injury by N-acetyl cysteine: a double-blind, placebo controlled study. PLoS ONE. 2013;8(1):e54163. PubMed doi:10.1371/journal.pone.0054163
45. Guerdjikova AI, Blom TJ, Mori N, et al. N-acetylcysteine in bulimia nervosa—open-label trial. Eat Behav. 2013;14(1):87-89. PubMed doi:10.1016/j.eatbeh.2012.11.001
46. Bloch MH, Panza KE, Grant JE, et al. N-acetylcysteine in the treatment of pediatric trichotillomania: a randomized, double-blind, placebo-controlled add-on trial. J Am Acad Child Adolesc Psychiatry. 2013;52(3):231-240. PubMed doi:10.1016/j.jaac.2012.12.020
47. Schmaal L, Veltman DJ, Nederveen A, et al. N-acetylcysteine normalizes glutamate levels in cocaine-dependent patients: a randomized crossover magnetic resonance spectroscopy study. Neuropsychopharmacology. 2012;37(9):2143-2152. PubMed doi:10.1038/npp.2012.66
48. Fava M, Mischoulon D, Iosifescu D, et al. A double-blind, placebo-controlled study of aripiprazole adjunctive to antidepressant therapy among depressed outpatients with inadequate response to prior antidepressant therapy (ADAPT-A Study). Psychother Psychosom. 2012;81(2):87-97. PubMed doi:10.1159/000332050
49. Dean OM, Bush AI, Berk M. Translating the rosetta stone of N-acetylcysteine. Biol Psychiatry. 2012;71(11):935-936. PubMed doi:10.1016/j.biopsych.2012.04.001
This PDF is free for all visitors!
Save
Cite