Objective: Evaluate efficacy and safety of a 2-month formulation of aripiprazole lauroxil (AL) with 1-day initiation during hospitalization for acute exacerbation of schizophrenia followed by transition to outpatient care.
Methods: The phase 3b double-blind Aripiprazole Lauroxil and Paliperidone palmitate: INitiation Effectiveness (ALPINE) study was conducted from November 2017 to March 2019. Adults with acute schizophrenia according to DSM-5 criteria were randomized (1:1) to AL (AL NanoCrystal Dispersion + oral aripiprazole 30 mg, day 1; AL 1,064 mg, day 8 and every 8 weeks [q8wk]) or paliperidone palmitate (PP 234 mg, day 1; PP 156 mg, day 8 and then q4wk) for 25 weeks. Patients remained hospitalized ≥ 2 weeks after randomization per protocol. Primary endpoint was within-group change in Positive and Negative Syndrome Scale total score (PANSST) from baseline to week 4. Secondary analyses included within- and between-group changes from baseline at various time points. Adverse events (AEs) and laboratory data were monitored.
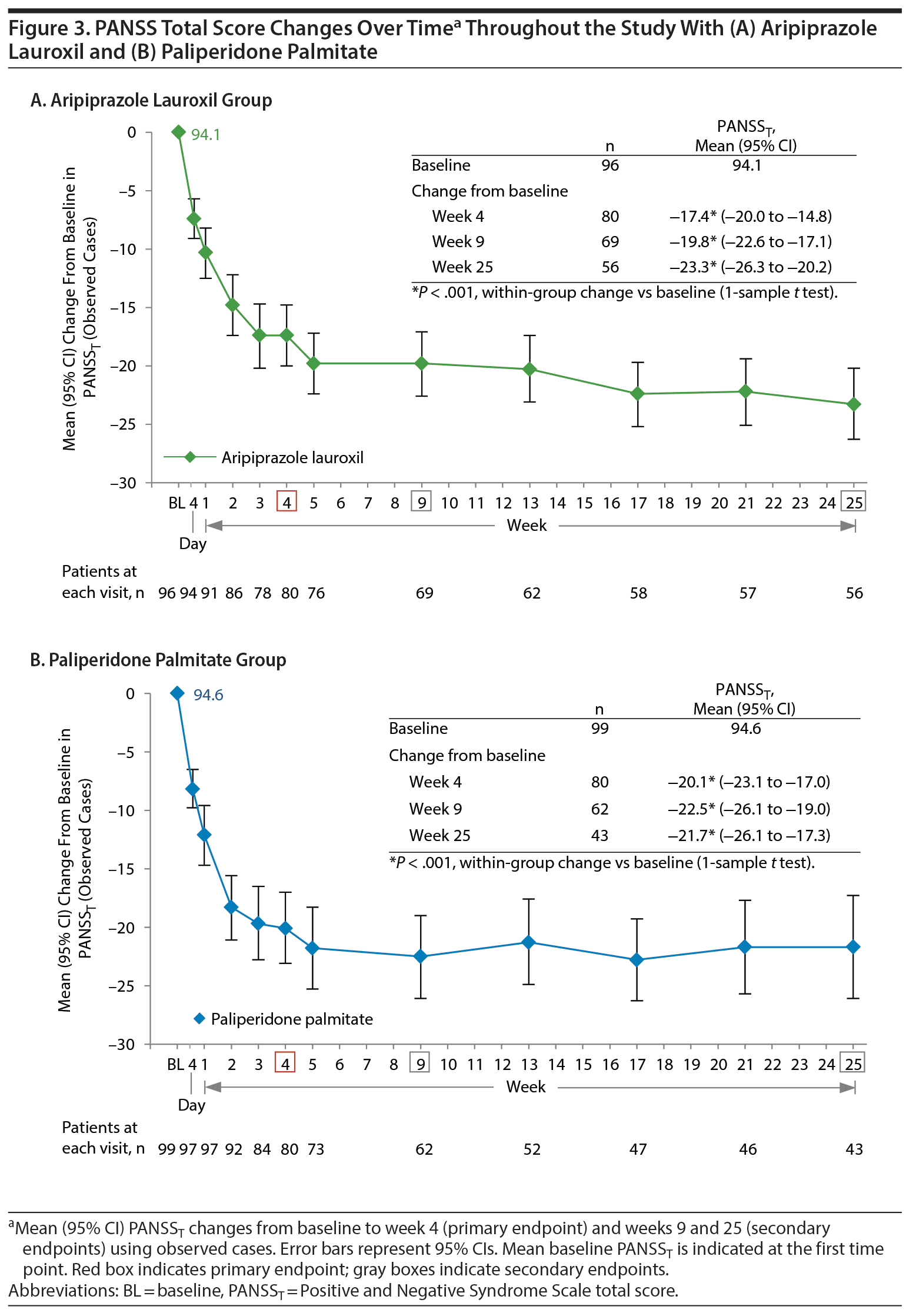
Results: A total of 200 patients were randomized (AL, n = 99; PP, n = 101); 56.6% and 42.6%, respectively, completed the study. For AL, the mean baseline PANSST was 94.1; scores were significantly reduced from baseline at week 4 (−17.4; P < .001) and were also reduced at weeks 9 (−19.8) and 25 (−23.3). With PP, PANSST also improved significantly from baseline (94.6) at week 4 (−20.1; P < .001) and also improved at weeks 9 (−22.5) and 25 (−21.7). The 3 most common AEs over 25 weeks in the AL group were injection site pain (17.2%), increased weight (9.1%), and akathisia (9.1%). The same AEs were the most common in the PP group (injection site pain [24.8%], increased weight [16.8%], and akathisia [10.9%]).
Conclusions: AL and PP were efficacious and well-tolerated for initiating treatment of schizophrenia in the hospital and continuing outpatient treatment.
Trial Registration: ClinicalTrials.gov identifier: NCT03345979
ABSTRACT
Objective: Evaluate efficacy and safety of a 2-month formulation of aripiprazole lauroxil (AL) with 1-day initiation during hospitalization for acute exacerbation of schizophrenia followed by transition to outpatient care.
Methods: The phase 3b double-blind Aripiprazole Lauroxil and Paliperidone palmitate: INitiation Effectiveness (ALPINE) study was conducted from November 2017 to March 2019. Adults with acute schizophrenia according to DSM-5 criteria were randomized (1:1) to AL (AL NanoCrystal Dispersion + oral aripiprazole 30 mg, day 1; AL 1,064 mg, day 8 and every 8 weeks [q8wk]) or paliperidone palmitate (PP 234 mg, day 1; PP 156 mg, day 8 and then q4wk) for 25 weeks. Patients remained hospitalized ≥ 2 weeks after randomization per protocol. Primary endpoint was within-group change in Positive and Negative Syndrome Scale total score (PANSST) from baseline to week 4. Secondary analyses included within- and between-group changes from baseline at various time points. Adverse events (AEs) and laboratory data were monitored.
Results: A total of 200 patients were randomized (AL, n = 99; PP, n = 101); 56.6% and 42.6%, respectively, completed the study. For AL, the mean baseline PANSST was 94.1; scores were significantly reduced from baseline at week 4 (−17.4; P < .001) and were also reduced at weeks 9 (−19.8) and 25 (−23.3). With PP, PANSST also improved significantly from baseline (94.6) at week 4 (−20.1; P < .001) and also improved at weeks 9 (−22.5) and 25 (−21.7). The 3 most common AEs over 25 weeks in the AL group were injection site pain (17.2%), increased weight (9.1%), and akathisia (9.1%). The same AEs were the most common in the PP group (injection site pain [24.8%], increased weight [16.8%], and akathisia [10.9%]).
Conclusions: AL and PP were efficacious and well-tolerated for initiating treatment of schizophrenia in the hospital and continuing outpatient treatment.
Trial Registration: ClinicalTrials.gov identifier: NCT03345979
J Clin Psychiatry 2020;81(3):19m13207
To cite: Weiden PJ, Claxton A, Kunovac J, et al. Efficacy and safety of a 2-month formulation of aripiprazole lauroxil with 1-day initiation in patients hospitalized for acute schizophrenia transitioned to outpatient care: phase 3, randomized, double-blind, active-control ALPINE study. J Clin Psychiatry. 2020;81(3):19m13207.
To share: https://doi.org/10.4088/JCP.19m13207
© Copyright 2020 Physicians Postgraduate Press, Inc.
aAlkermes, Inc, Waltham, Massachusetts
bAltea Research, Las Vegas, Nevada
cCNS Network, LLC, Garden Grove, California
*Corresponding author: Peter J. Weiden, MD, Karuna Therapeutics, 33 Arch Street, Suite 3110, Boston, MA 02110 ([email protected]).
Long-acting injectable (LAI) antipsychotics provide continuous antipsychotic exposure over periods of weeks to months. It is believed that LAIs are an underused treatment option for schizophrenia.1-3 For inpatient services, starting an LAI rather than its oral counterpart may have several advantages for patients’ long-term treatment plans. Once efficacy and tolerability of the LAI are established during hospitalization, the discharge plan can include continuation of the LAI during transition to outpatient treatment.1,2,4,5 One barrier to more frequent use of LAIs could be fragmentation of psychiatric services, especially between inpatient and outpatient care.6-8 Although hospitalization is an opportune time to start an LAI, other competing priorities may preclude implementation.3 Inpatient services must prioritize stabilization of acute symptoms in a relatively short time frame. These immediate concerns might distract inpatient teams from recommending or starting LAIs during this time.3 Despite these challenges, placebo-controlled studies9-11 of LAIs initiated in the hospital have shown safety and efficacy of LAIs for treatment of acute symptoms of schizophrenia.
Another challenge facing inpatient services is whether there is adequate time to fully initiate the LAI before discharge, given that the duration of short-term hospitalizations for schizophrenia treatment averages 10.5 days in the United States.12 A common initiation strategy to achieve therapeutic plasma concentrations is to use the oral antipsychotic counterpart for a prescribed period of time after the first LAI injection. However, the time needed to complete oral regimens ranges from 2 to 3 weeks and therefore extends beyond most inpatient stays.9,13,14 As a result, using oral supplementation when starting an LAI during an acute hospital stay will often result in a need to continue an oral antipsychotic after discharge, which, in turn, poses a risk of premature cessation of the oral antipsychotic before the full initiation regimen is completed. Some LAI formulations can be initiated without continued oral supplementation. Aripiprazole lauroxil (AL; Aristada; Alkermes, Inc.; Waltham, Massachusetts) and paliperidone palmitate (PP; Invega Sustenna; Janssen Pharmaceuticals, Inc.; Titusville, New Jersey) are LAIs indicated for treatment of schizophrenia in adults and can be started without need for continued oral supplementation following the first LAI injection.15,16 Both LAIs may reduce the time required to fully complete LAI initiation before discharge.
The Aripiprazole Lauroxil and Paliperidone palmitate: INitiation Effectiveness (ALPINE) study was designed to examine efficacy and tolerability of starting either AL or PP in patients with acute exacerbation of schizophrenia during hospitalization for acute phase treatment (through week 4) as well as transition to outpatient care for ongoing continuation phase therapy (through week 25). The primary objective was to evaluate efficacy of starting a 2-month AL regimen using the 1-day initiation regimen in acutely symptomatic patients at 4 weeks after initiation, with secondary efficacy endpoints at 9 weeks (corresponding with a single dose interval of the initial 2-month AL injection) and at 25 weeks (corresponding with 3 AL injections at 8-week intervals). The study included a PP arm to provide an active control for AL while avoiding use of a placebo in patients with schizophrenia in need of active medication. Secondary efficacy objectives used the same time points (weeks 4, 9, and 25) for the PP arm.
METHODS
This phase 3b, randomized, double-blind, active-controlled study (Clinicaltrials.gov identifier: NCT03345979) was conducted from November 2017 to March 2019 at 16 US sites. The institutional review board/independent ethics committee for each study site approved the study protocol before patient enrollment. The study was conducted in accordance with Good Clinical Practice Guidelines and ethical principles derived from the Declaration of Helsinki. All study participants provided written informed consent before participating in study procedures.

- It is widely believed that long-acting injectable (LAI) antipsychotics remain underused for the treatment of schizophrenia. One potential barrier to treatment is that starting an LAI can be a challenge in acute inpatient settings given very short lengths of stay.
- The LAI aripiprazole lauroxil (AL) has a 2-month dose interval option with the 1-day initiation option. Patients started on this regimen can be discharged without a need for antipsychotic dosing for 2 months.
- In this study, efficacy and safety of using 1-day initiation to start and continue patients on the AL 2-month dose interval option into outpatient treatment (total treatment time, 25 weeks) were consistent with results from previous studies of starting other AL monthly regimens using 3-week oral aripiprazole supplementation. No additional safety issues were observed, and symptoms continued to improve over 25 weeks.
Patients
Eligible patients were adults (aged 18-65 years) diagnosed with schizophrenia according to Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5), criteria17 (confirmed by Mini-International Neuropsychiatric Interview 7.0.2 for Schizophrenia and Psychotic Disorder Studies18) with an acute exacerbation or relapse of symptoms that began ≤ 2 months before screening and warranted hospitalization. Additional key eligibility criteria included prior history of hospitalization for schizophrenia and Positive and Negative Syndrome Scale total score (PANSST)19 of 80′ ’120 (inclusive) with a score of ≥ 4 on ≥ 2 of any of the following for PANSS positive symptoms items: delusions (item 1), conceptual disorganization (item 2), hallucinations (item 3), and suspiciousness/persecution (item 6). Clinical Global Impression-Severity of Illness scale (CGI-S)20 scores ≥ 4 (moderately ill or worse) were also required.
Key exclusion criteria included current primary DSM-5 diagnosis other than schizophrenia; currently deemed to be at imminent risk of suicide; first antipsychotic treatment within the past 12 months; history of recent LAI treatment; history of treatment resistance; or history of hypersensitivity, intolerance, or inadequate response to aripiprazole, risperidone, or paliperidone.
Study Design and Treatments
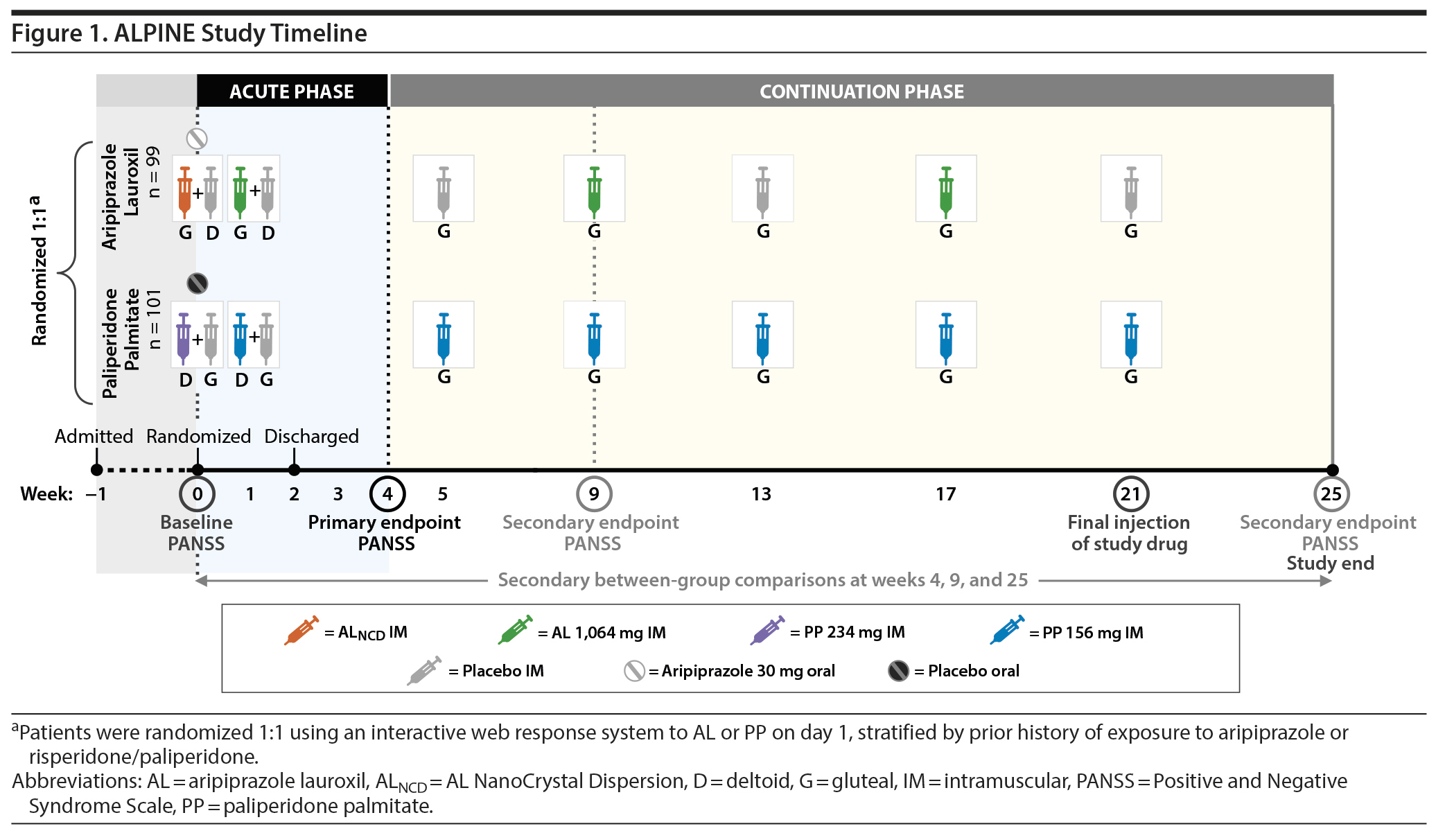
The ALPINE study duration was approximately 26 weeks, with a 1-week screening period and a 25-week double-blind LAI treatment period (Figure 1). The study included an initial inpatient stay during the screening period and at least the first 2 weeks of double-blind treatment. Patients were required to stay in the hospital for ≥ 2 weeks. If doing so was clinically appropriate, patients were discharged 2 weeks after randomization but could delay discharge 1 week if needed. Post-discharge follow-up schedule began with outpatient visits at weeks 3, 4, and 5 and then changed to an every-4-weeks visit schedule until the last visit at week 25 (weeks 9, 13, 17, 21, and 25).
Inpatient LAI Initiation
Patients were randomized 1:1 to AL or PP on day 1 by interactive web response system. Before randomization (at screening), patients were stratified by prior history of exposure to aripiprazole and/or risperidone/paliperidone (stratification level 1: prior exposure to both aripiprazole and risperidone/paliperidone or exposure to none of these medications; level 2: prior exposure to either aripiprazole or risperidone/paliperidone). Those patients who had reliable histories of having received (and tolerated) both did not have any further oral antipsychotic exposure before their randomization injection visit. Those with a history of only risperidone/paliperidone exposure received oral aripiprazole 5 mg as a test dose to establish tolerability during the first 2 days of inpatient screening. Those exposed to only aripiprazole received 2 days of oral risperidone 1 mg, and those with no exposure to either received the 2 agents, 8 hours apart over 2 days. Prior antipsychotic medications were discontinued upon inpatient admission, with a washout period of 2 to 5 days.
Patients randomized to AL received the 1-day AL initiation regimen (AL NanoCrystal Dispersion [ALNCD] gluteal injection + single 30-mg dose of oral aripiprazole) with placebo deltoid injection on study day 1. The first AL 1,064-mg injection (gluteal) was administered on day 8 with a placebo deltoid injection to reflect timing of the PP initiation regimen to preserve the blind (Figure 1). The 1,064-mg AL dose was chosen because it was approved after the pivotal efficacy study9 and has the longest dose interval available among AL regimens.
Patients randomized to PP received a single PP 234-mg deltoid injection with a placebo gluteal injection and oral placebo tablet on day 1 and a PP 156-mg injection (deltoid) with placebo injection (gluteal) on day 8. After day 1, no oral antipsychotic was permitted with either treatment arm.
Outpatient LAI Treatment
After the AL 1,064-mg (gluteal) injection on day 8, AL was administered every 8 weeks (q8wk; weeks 9 and 17), with placebo injections (gluteal) administered at weeks 5, 13, and 21 to match the timing of PP injections (Figure 1) to maintain the blind.
PP 156-mg injections (gluteal) were administered q4wk after the day 8 injection (weeks 5, 9, 13, 17, and 21).
Assessments
Efficacy was assessed using the PANSS at screening, randomization (day 1), day 4, weeks 1 and 2 (inpatient), and each outpatient visit. Baseline was defined as the last nonmissing assessment before the first dose of study drug on day 1. The primary efficacy endpoint was change in PANSST from baseline to week 4 within each treatment group. Secondary analyses included within-treatment group changes in PANSST from baseline to weeks 9 and 25 and between-group comparisons at weeks 4, 9, and 25. Safety and tolerability endpoints included incidence of adverse events (AEs), serious AEs (SAEs), and AEs leading to discontinuation. Additional standard safety assessments included laboratory parameters (eg, hematology, chemistry, metabolic parameters, prolactin, urinalysis), vital signs, body weight, injection site reactions, abnormal movement scales,21-23 Columbia-Suicide Severity Rating Scale,24 and side effects scales.25,26
Statistical Analysis
The safety population included all patients who received ≥ 1 dose of study drug. Efficacy was assessed for all patients in the safety population with ≥ 1 post baseline PANSS assessment. No formal sample size calculations were performed. Study sites were pooled to ensure ≥ 10 patients per treatment group within each pooled site to improve estimation precision; 7 pooled sites resulted.
Change in PANSST from baseline to weeks 4, 9, and 25 was tested for AL and PP separately using 1-sample t tests (observed cases). Changes in PANSST from baseline to weeks 4, 9, and 25 were compared between treatment groups using mixed model for repeated measures (MMRM). Models included treatment, visit, treatment-by-visit interaction, baseline PANSST, stratification factor, and pooled study site as covariates and an unstructured variance-covariance matrix for within-subject variability. AEs were summarized descriptively for the first 4 weeks of treatment and cumulatively for the full treatment period (from week 0 to week 25).
RESULTS
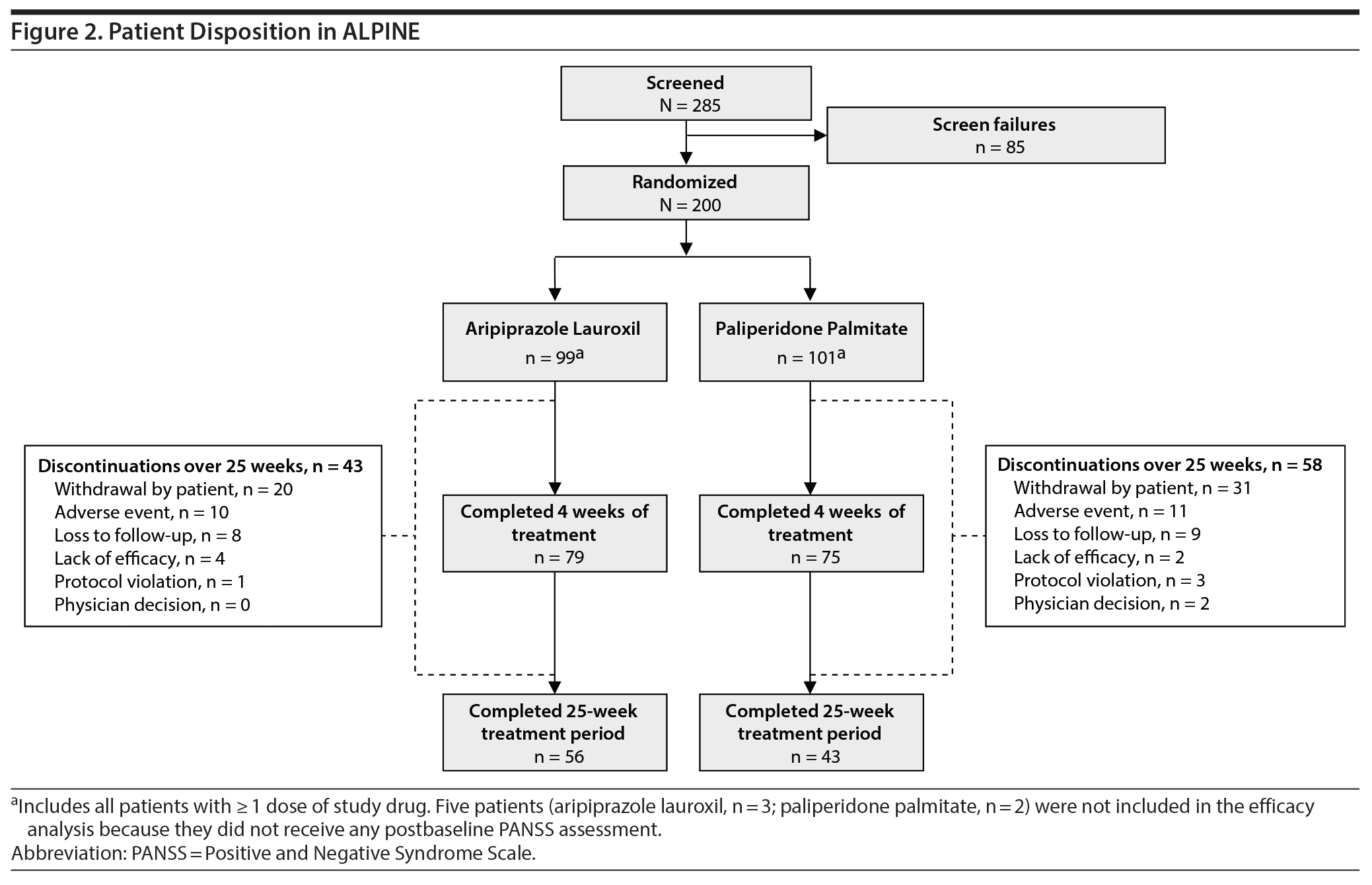
In total, 200 patients were randomized and received their respective LAI initiation regimen on day 1 (AL, n = 99; PP, n = 101; Figure 2). All 200 patients were included in the safety analyses, and 195 were included in the efficacy analysis (AL, n = 96; PP, n = 99). Seventy-seven percent of patients completed the 4-week acute phase (AL, 79.8%; PP, 74.3%); 25-week completion rates were 56.6% for AL and 42.6% for PP.
The most common reasons for discontinuation in both treatment groups were withdrawal by patient, AEs, and loss to follow-up, both throughout the study (Figure 2) and among patients who discontinued during the first 4 weeks of treatment (Supplementary Table 1).
Demographic and baseline clinical characteristics were similar in both groups (Supplementary Table 2). Of the 200 patients, 162 (81.0%) had a history of prior exposure to risperidone/paliperidone, 112 (56.0%) had prior exposure to aripiprazole, and 26 (13.0%) had no prior exposure to any of these antipsychotics. Of the 99 patients randomized to the AL treatment arm, 43 were given the test dose of oral aripiprazole for 2 days and 17 were given oral risperidone for 2 days, with 12 of these patients receiving both aripiprazole and risperidone test doses. Of the 101 patients randomized to the PP arm, 45 were given the test dose of oral aripiprazole for 2 days and 21 were given oral risperidone for 2 days, with 14 of these patients receiving both aripiprazole and risperidone test doses.
A total of 138 (69.0%) of the 200 patients reported having antipsychotic exposure in the 30 days prior to the screening visit; the last antipsychotic received prior to screening is listed by treatment group in Supplementary Table 3. Overall, quetiapine was reported most commonly (18.5%), followed by olanzapine (17.0%), risperidone/paliperidone (15.0%), and aripiprazole (10.5%), with the remaining patients split between another atypical antipsychotic (4.0%) or a conventional antipsychotic (also 4.0%). Patients without any identified antipsychotic (n = 62 [31.0%]) presumably had no known exposure in the 30 days prior to screening.
Efficacy
Patients in the AL group achieved statistically significant improvement in PANSST from baseline to week 4, with a mean change of −17.4 based on observed cases (P < .001; Figure 3A). Continued improvement in PANSST from baseline with AL treatment was observed at weeks 9 (−19.8) and 25 (−23.3; both P < .001).
With PP, improvement in PANSST from baseline was also statistically significant at weeks 4 (−20.1), 9 (−22.5), and 25 (−21.7; all P < .001; Figure 3B).
The study was not powered to formally test between-group differences. The 95% CIs for LS mean changes in PANSST over time from the MMRM analysis overlap at each time point for AL and PP (Supplementary Figure 1). LS mean (95% CI) difference in change from baseline in PANSST with AL versus PP was 2.0 (−1.5 to 5.5) at week 4, 2.7 (−1.0 to 6.4) at week 9, and −0.9 (−5.0 to 3.2) at week 25. The 95% CIs for differences with AL versus PP all included zero.
Safety and Tolerability
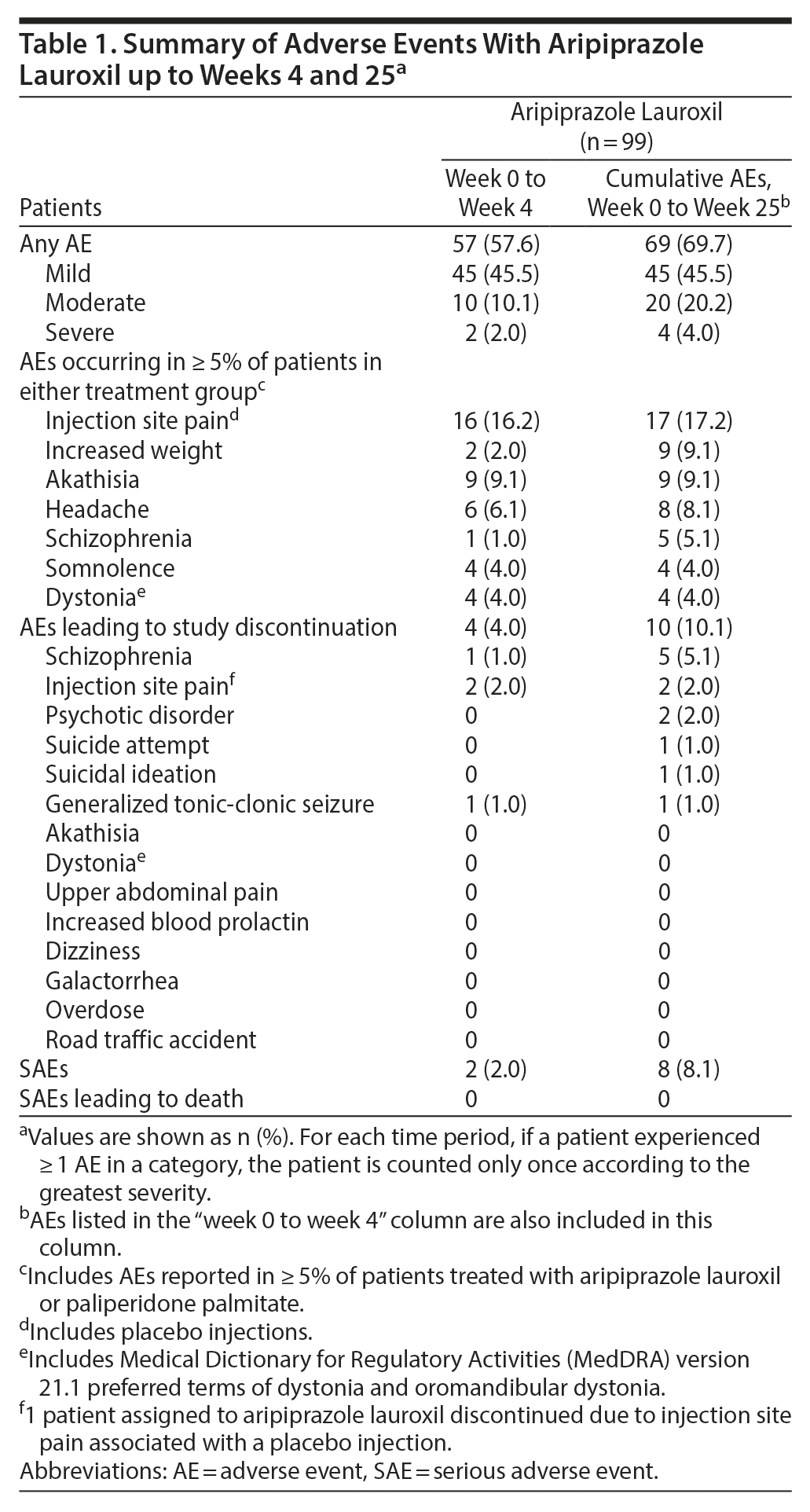
Aripiprazole lauroxil adverse events. During 25 weeks of treatment, 69 (69.7%) of 99 AL-treated patients experienced ≥ 1 AE; AEs were largely mild or moderate in severity (94.2%). Most patients (57/99 [57.6%]) experienced AEs during the first 4 weeks of treatment (Table 1). AEs reported by ≥ 5% of AL-treated patients through week 25 were injection site pain, increased weight, akathisia, headache, and schizophrenia. The 3 most frequently reported AEs with AL through week 25 included injection site pain (17/99 [17.2%]), which was predominantly mild (16/17) and occurred with active and placebo injections; akathisia (9/99 [9.1%]), which was also predominantly mild (8/9); and increased weight (9/99 [9.1%]), which was mild or moderate.
SAEs occurred in 8 AL-treated patients (8.1%) (Table 1 and Supplementary Table 4), and there was no trend in time on treatment to occurrence of SAEs. Ten AL-treated patients (10.1%) discontinued because of AEs. AEs leading to discontinuation in ≥ 2 patients receiving AL were schizophrenia, injection site pain (1 associated with placebo injection), and psychotic disorders.
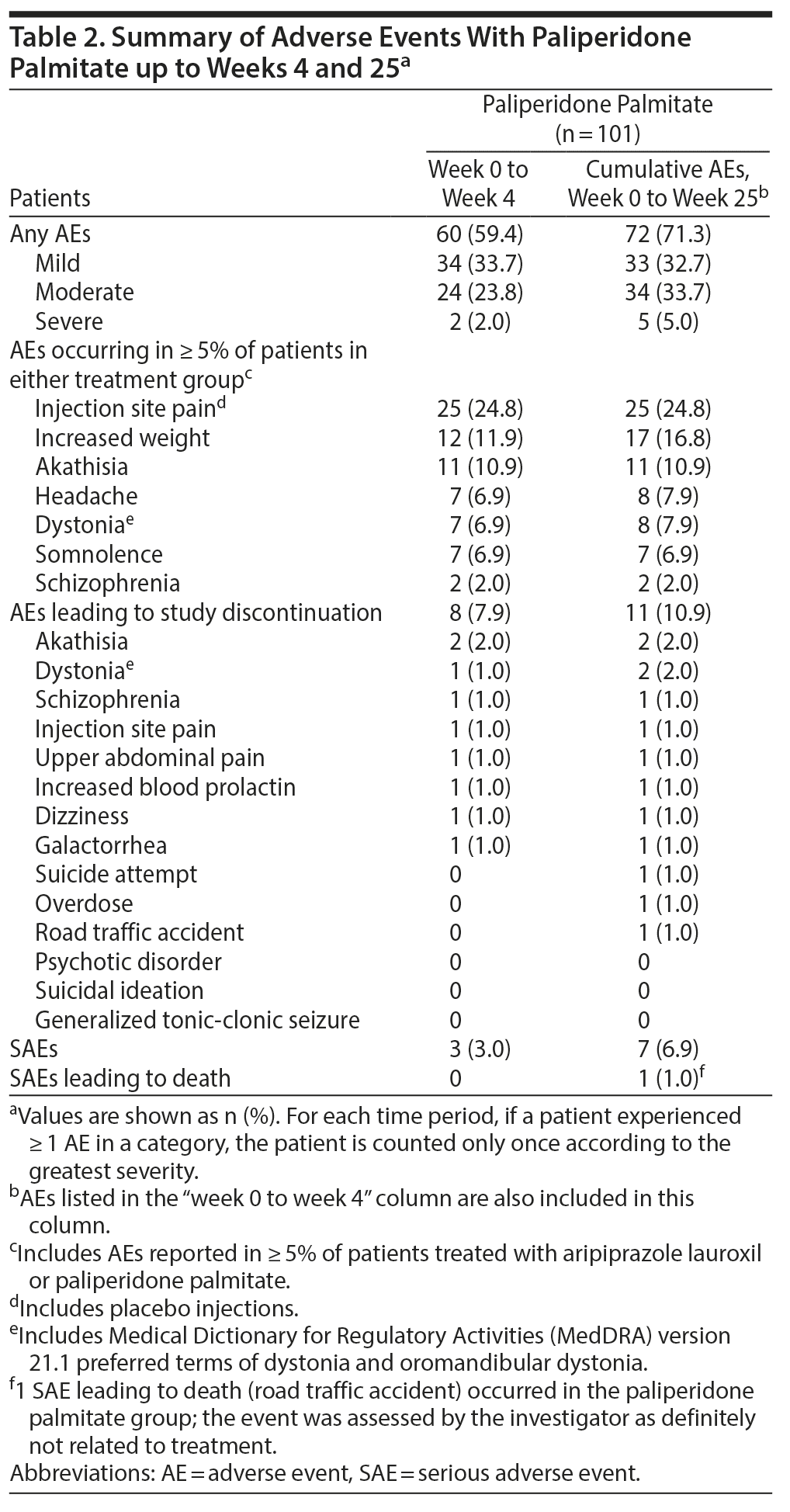
Paliperidone palmitate adverse events. During 25 weeks of treatment, 72 (71.3%) of 101 PP-treated patients experienced ≥ 1 AE; most AEs were mild or moderate in severity (93.1%). Most patients (60/101 [59.4%]) experienced AEs during the first 4 weeks of treatment (Table 2). AEs reported by ≥ 5% of patients through week 25 with PP were injection site pain, increased weight, akathisia, headache, dystonia, and somnolence. The 3 most frequently reported AEs with PP through week 25 included injection site pain (25/101 [24.8%]), which was predominantly mild (22/25) and occurred with active and placebo injections; akathisia (11/101 [10.9%]), which was also predominantly mild (8/11); and increased weight (17/101 [16.8%]), which was mild or moderate.
Seven PP-treated patients (6.9%) experienced SAEs (Table 2 and Supplementary Table 5), and there was no trend in time on treatment to occurrence of SAEs. There was 1 SAE with fatal outcome (road traffic accident) in the PP group that was assessed as unrelated to treatment. Eleven patients (10.9%) discontinued because of AEs. AEs leading to discontinuation in ≥ 2 patients receiving PP were akathisia and dystonia (including 1 event each of dystonia and oromandibular dystonia).
DISCUSSION
Transition from inpatient to outpatient care after acute hospitalization for schizophrenia is a critical time. One in 11 schizophrenia patients discharged from a short hospital stay in the United States is readmitted within 1 week.27 This 1-week readmission rate for schizophrenia is the highest among 20 medical and mental health conditions reported27 and speaks to the need to have prospective data evaluating interventions that start in the hospital and to assess outcomes during and after transitions of care for schizophrenia.
The ALPINE study was designed to provide prospective data on efficacy and safety of LAIs for treatment of an acute exacerbation of schizophrenia, started in the hospital and continued through transition of care, well into outpatient continuation treatment. Both LAIs used in ALPINE—aripiprazole lauroxil and paliperidone palmitate—have initiation regimens that can be completed within approximately 1 week or less of treatment initiation, well within the window of most short-term acute hospitalizations for schizophrenia.12 The 4-week retention rate of up to approximately 80% indicates that both AL and PP initiated in the acute setting were efficacious and well-tolerated for most ALPINE patients and provided support through the stressful experience of transition to outpatient care. Results from ALPINE can inform inpatient and outpatient services evaluating feasibility of starting LAI antipsychotics in hospitalized patients, where acute efficacy is required but relatively little time is available to fully initiate LAIs before transition to outpatient care.
The ALPINE study provided the first efficacy data for both the 1-day AL initiation regimen and the 2-month dose interval AL option, which were approved by the US Food and Drug Administration based on pharmacokinetic and safety studies.28-30 Acute symptom improvement in ALPINE patients who initiated AL with the 1-day initiation regimen was comparable to that previously observed in a pivotal study9 using 21-day oral aripiprazole supplementation. Turning to efficacy of the 2-month AL regimen, the trajectory of PANSST for the 1,064-mg dose continued every 8 weeks over the 25-week study was consistent with that reported with AL 441 mg and 882 mg q4wk in a previously published 12-week efficacy study9 followed by a 52-week safety study31 in which patients started on either of these AL q4wk regimens continued that regimen for up to 64 weeks. Similarly, the PP initiation regimen also effectively reduced symptoms of schizophrenia acutely, with durability of effect during continuation treatment consistent with that reported in previous studies.32-34
Concerns about safety of discharging patients on treatment with an LAI, or about potential safety issues developing after discharge, may be factors in clinicians’ hesitation to prescribe LAIs in acute hospital settings.3,35 In ALPINE, safety findings for AL and PP throughout the study were consistent with the known profiles of each of these LAIs and the respective oral formulations, indicating tolerability through transitions of care.9,11,36-40 The most frequent AEs (≥ 5%) in the AL group (injection site pain, increased weight, akathisia, headache, and schizophrenia) were among those previously reported for acute treatment with monthly AL 441-mg and 882-mg regimens.9,15,36 Similarly, the most common AEs (≥ 5%) for patients in the PP group (injection site pain, increased weight, akathisia, headache, dystonia, and somnolence) were in line with other published PP trials.32-34 For both treatment arms, most AEs (including injection site pain and first instances of akathisia) occurred during the first 4 weeks after LAI initiation. These results support published findings41-43 that tolerability of atypical LAIs is consistent with that of the respective oral formulations and indicate that starting an LAI in the hospital does not seem to pose any additional safety risks after discharge for either AL or PP above and beyond their oral counterparts.
There are several important limitations of the ALPINE study. The study was not designed as or powered for a direct comparison between AL and PP. The purpose of the blinded PP arm was to provide an active control with known efficacy; the primary efficacy outcome was therefore within-group changes from baseline. The absence of a placebo arm limits interpretation of efficacy findings. The nature of a phase 3b clinical trial limits generalizability of the findings to the broader hospitalized schizophrenia population starting LAIs; enrolling patients who consent to participate in research and excluding those with major comorbidities may introduce bias. Also, rates of successful transition and continuation of LAI after discharge in ALPINE may be higher than that observed in routine clinical care and might have been driven in part by clinical trial incentives or characteristics of enrolled patients.
As is common for long-term clinical trials in schizophrenia, there was a substantial rate of early withdrawal from ALPINE: approximately 50% of the patients discontinued before the end of the study at week 25. Despite limitations of comparing across studies, we note that the discontinuation rate of about 25% at week 4 and about 50% at week 25 in ALPINE is within the expected range for LAI antipsychotic studies.38,44-47 The nature of clinical trials is such that the persistence of treatment in clinical practice might differ—in either direction—from these retention rates. The study design was limited to only 2 LAI treatments, and the particular LAIs used are alike in not requiring oral antipsychotic supplementation beyond the first day of treatment initiation. Therefore, post-discharge results may not generalize to use of the AL 21-day oral aripiprazole supplementation option or the initiation of other LAIs requiring oral supplementation.
In addition, tolerability to oral aripiprazole and/or risperidone for those patients without prior exposure was assessed using oral test doses consisting of 5 mg/d of aripiprazole and/or 1 mg/d of risperidone during the first 2 days before randomization. This method is consistent with methods used in previous phase 3 studies with LAIs9,32; however, while this approach will allow for assessment of immediate hypersensitivity, full evaluation of tolerability can take up to 2 weeks.15,16 Further, because the fixed-dose design did not allow for dose adjustments, results may not reflect safety or efficacy results for optimized dosing. Finally, the q4wk visits and LAI/placebo injection schedule was driven by considerations regarding blinding; therefore, ALPINE results do not address potential impact of starting hospitalized patients on a 2-month AL dose interval.
CONCLUSIONS
In the phase 3b, randomized, double-blind ALPINE study, starting acutely symptomatic patients with schizophrenia on a 2-month dose regimen of AL with the 1-day initiation regimen demonstrated safety and efficacy consistent with that seen in prior AL studies. These findings support clinical effectiveness of the 1-day initiation regimen. Also, this study demonstrated AL efficacy and tolerability in patients discharged on the AL 2-month regimen for continuation treatment. The inclusion of PP provided an active control with known safety and efficacy that can also be rapidly initiated. Both regimens can be effectively used in patients with schizophrenia through the often challenging transition from acute hospitalization to outpatient continuation treatment.
Submitted: December 10, 2019; accepted April 3, 2020
Published online: May 19, 2020.
Potential conflicts of interest: Dr Weiden is a former employee of Alkermes, Inc., and may own stock/options in the company. Drs Du, Yao, Yagoda, Bidollari, and Claxton; Ms Keane; and Mr Cash are employees of Alkermes, Inc., and may own stock/options in the company. Dr Kunovac reports research support received from Acadia; Alder; Alkermes, Inc.; Allergan; Amgen; AOBiome; Aptinyx; Astellas; Avanir; Axsome; AZTherapies; Bayer; Biohaven; Bionomics; Boehringer Ingelheim; Braeburn; Braintree Laboratories; Forest; Lundbeck; Indivior; Intra-Cellular; Janssen; Marinus; Myovant; Naurex; Ogeda; Pfizer; ROVI; Sage; SyneuRx; Takeda; Teva; Tolmar; and Tonix and reports consulting arrangements with Alkermes, Inc.; Janssen; Neurocrine; Otsuka; Shire; and Sunovion. Dr Walling reports research support received from Novartis; Johnson & Johnson PRD; Sunovion; Janssen; Alkermes, Inc.; Allergan; Astellas; Biohaven Takeda; Otsuka; Noven; CoMentis; Intra-Cellular; Lupin; Avanir; Lundbeck; Roche; ROVI; Boehringer Ingelheim; Acadia; and Sage and consulting arrangements with Otsuka and Janssen.
Funding/support: This study was sponsored by Alkermes, Inc (Waltham, Massachusetts). Medical writing and editorial support was provided by Gina Daniel, PhD, Kathleen Dorries, PhD, and Jane Phillips, PhD, at Peloton Advantage, LLC (Parsippany, NJ), an OPEN Health company, and funded by Alkermes, Inc.
Role of the sponsor: The clinical trial was conducted by Alkermes, Inc. Employees of Alkermes, Inc., reviewed the design and analysis of this study. The authors interpreted the evidence and made the decision to submit the manuscript.
Previous presentation: SIRS Annual Meeting; April 10−14, 2019; Orlando, Florida ▪ ASCP Annual Meeting; May 28−31, 2019; Scottsdale, Arizona ▪ Psych Congress; October 3−6, 2019; San Diego, California ▪ NEI Congress; November 7−10, 2019; Colorado Springs, Colorado.
Acknowledgments: The authors thank the patients who participated in the ALPINE study and all clinical personnel. We thank Kendra Mangan of Alkermes, Inc, clinical trial manager, for her substantial contribution. Kendra Mangan is a former employee of Alkermes, Inc.
Supplementary material: See accompanying pages.
REFERENCES
1.Heres S. Long-acting injectable antipsychotics: an underutilized treatment option. J Clin Psychiatry. 2014;75(11):1263-1265. PubMed CrossRef
2.Citrome L. Aripiprazole long-acting injectable formulations for schizophrenia: aripiprazole monohydrate and aripiprazole lauroxil. Expert Rev Clin Pharmacol. 2016;9(2):169-186. PubMed CrossRef
3.Kishimoto T, Sanghani S, Russ MJ, et al. Indications for and use of long-acting injectable antipsychotics: consideration from an inpatient setting. Int Clin Psychopharmacol. 2017;32(3):161-168. PubMed CrossRef
4.Weiden PJ, Kozma C, Grogg A, et al. Partial compliance and risk of rehospitalization among California Medicaid patients with schizophrenia. Psychiatr Serv. 2004;55(8):886-891. PubMed CrossRef
5.Buchanan RW, Kreyenbuhl J, Kelly DL, et al; Schizophrenia Patient Outcomes Research Team (PORT). The 2009 schizophrenia PORT psychopharmacological treatment recommendations and summary statements. Schizophr Bull. 2010;36(1):71-93. PubMed CrossRef
6.Okumura Y, Sugiyama N, Noda T. Timely follow-up visits after psychiatric hospitalization and readmission in schizophrenia and bipolar disorder in Japan. Psychiatry Res. 2018;270:490-495. PubMed CrossRef
7.Marcus SC, Chuang CC, Ng-Mak DS, et al. Outpatient follow-up care and risk of hospital readmission in schizophrenia and bipolar disorder. Psychiatr Serv. 2017;68(12):1239-1246. PubMed CrossRef
8.Kurdyak P, Vigod SN, Newman A, et al. Impact of physician follow-up care on psychiatric readmission rates in a population-based sample of patients with schizophrenia. Psychiatr Serv. 2018;69(1):61-68. PubMed CrossRef
9.Meltzer HY, Risinger R, Nasrallah HA, et al. A randomized, double-blind, placebo-controlled trial of aripiprazole lauroxil in acute exacerbation of schizophrenia. J Clin Psychiatry. 2015;76(8):1085-1090. PubMed CrossRef
10.Kane JM, Peters-Strickland T, Baker RA, et al. Aripiprazole once-monthly in the acute treatment of schizophrenia: findings from a 12-week, randomized, double-blind, placebo-controlled study. J Clin Psychiatry. 2014;75(11):1254-1260. PubMed CrossRef
11.Pandina GJ, Lindenmayer JP, Lull J, et al. A randomized, placebo-controlled study to assess the efficacy and safety of 3 doses of paliperidone palmitate in adults with acutely exacerbated schizophrenia. J Clin Psychopharmacol. 2010;30(3):235-244. PubMed CrossRef
12.Owens PL, Fingar KR, McDermott KW, et al. Inpatient Stays Involving Mental and Substance Use Disorders, 2016: Statistical Brief #249. March 2019. Healthcare Cost and Utilization Project (HCUP) Statistical Briefs. Rockville, MD: Agency for Healthcare Research and Quality; 2019.
13.Brissos S, Veguilla MR, Taylor D, et al. The role of long-acting injectable antipsychotics in schizophrenia: a critical appraisal. Ther Adv Psychopharmacol. 2014;4(5):198-219. PubMed CrossRef
14.Gilday E, Nasrallah HA. Clinical pharmacology of paliperidone palmitate a parenteral long-acting formulation for the treatment of schizophrenia. Rev Recent Clin Trials. 2012;7(1):2-9. PubMed CrossRef
15.Aristada [package insert]. Waltham, MA: Alkermes, Inc.; 2018.
16.Invega Sustenna [package insert]. Titusville, NJ: Janssen Pharmaceuticals; 2019.
17.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, DSM-5. Fifth Edition. Washington, DC: American Psychiatric Publishing; 2013.
18.Sheehan DV, Lecrubier Y, Sheehan KH, et al. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. 1998;59(Suppl 20):22-33, quiz 34-57. PubMed
19.Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13(2):261-276. PubMed CrossRef
20.Guy W. CGI Clinical Global Impressions. ECDEU Assessment Manual for Psychopharmacology. Rockville, MD: US Department of Health, Education, and Welfare, National Institute of Mental Health; 1976:217-222.
21.Guy W. Abnormal involuntary movement scale (AIMS). ECDEU Assessment Manual for Psychopharmacology. Rockville, MD: National Institute of Mental Health, National Institutes of Health; 1976:534-537.
22.Barnes TR. A rating scale for drug-induced akathisia. Br J Psychiatry. 1989;154(5):672-676. PubMed CrossRef
23.Simpson GM, Angus JW. A rating scale for extrapyramidal side effects. Acta Psychiatr Scand suppl. 1970;212(S212):11-19. PubMed CrossRef
24.Posner K, Brown GK, Stanley B, et al. The Columbia-Suicide Severity Rating Scale: initial validity and internal consistency findings from three multisite studies with adolescents and adults. Am J Psychiatry. 2011;168(12):1266-1277. PubMed CrossRef
25.Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. 1991;14(6):540-545. PubMed CrossRef
26.Lindström E, Lewander T, Malm U, et al. Patient-rated versus clinician-rated side effects of drug treatment in schizophrenia: clinical validation of a self-rating version of the UKU Side Effect Rating Scale (UKU-SERS-Pat). Nord J Psychiatry. 2001;55(suppl 44):5-69. PubMed
27.Fingar KR, Barrett ML, Jiang HJ. A Comparison of All-Cause 7-Day and 30-Day Readmissions, 2014: Statistical Brief #230. October 2017. Healthcare Cost and Utilization Project (HCUP) Statistical Briefs. Rockville, MD: Agency for Healthcare Research and Quality; 2017.
28.Hard ML, Mills RJ, Sadler BM, et al. Pharmacokinetic profile of a 2-month dose regimen of aripiprazole lauroxil: a phase I study and a population pharmacokinetic model. CNS Drugs. 2017;31(7):617-624. PubMed CrossRef
29.Hard ML, Wehr AY, Du Y, et al. Pharmacokinetic evaluation of a 1-day treatment initiation option for starting long-acting aripiprazole lauroxil for schizophrenia. J Clin Psychopharmacol. 2018;38(5):435-441. PubMed CrossRef
30.Hard ML, Wehr AY, Sadler BM, et al. Population pharmacokinetic analysis and model-based simulations of aripiprazole for a 1-day initiation regimen for the long-acting antipsychotic aripiprazole lauroxil. Eur J Drug Metab Pharmacokinet. 2018;43(4):461-469. PubMed CrossRef
31.McEvoy JP, Risinger R, Mykhnyak S, et al. Durability of therapeutic response with long-term aripiprazole lauroxil treatment following successful resolution of an acute episode of schizophrenia. J Clin Psychiatry. 2017;78(8):1103-1109. PubMed CrossRef
32.Pandina G, Lane R, Gopal S, et al. A double-blind study of paliperidone palmitate and risperidone long-acting injectable in adults with schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2011;35(1):218-226. PubMed CrossRef
33.Bossie CA, Sliwa JK, Ma YW, et al. Onset of efficacy and tolerability following the initiation dosing of long-acting paliperidone palmitate: post-hoc analyses of a randomized, double-blind clinical trial. BMC Psychiatry. 2011;11(1):79. PubMed CrossRef
34.Fu DJ, Bossie CA, Kern Sliwa J, et al. Paliperidone palmitate versus risperidone long-acting injection in markedly-to-severely ill schizophrenia subjects: onset of efficacy with recommended initiation regimens. Clin Schizophr Relat Psychoses. 2014;8(2):101-109, 109A. PubMed CrossRef
35.Geerts P, Martinez G, Schreiner A. Attitudes towards the administration of long-acting antipsychotics: a survey of physicians and nurses. BMC Psychiatry. 2013;13(1):58. PubMed CrossRef
36.Nasrallah HA, Aquila R, Du Y, et al. Long-term safety and tolerability of aripiprazole lauroxil in patients with schizophrenia. CNS Spectr. 2019;24(4):395-403. PubMed CrossRef
37.Gopal S, Hough DW, Xu H, et al. Efficacy and safety of paliperidone palmitate in adult patients with acutely symptomatic schizophrenia: a randomized, double-blind, placebo-controlled, dose-response study. Int Clin Psychopharmacol. 2010;25(5):247-256. PubMed CrossRef
38.Kramer M, Litman R, Hough D, et al. Paliperidone palmitate, a potential long-acting treatment for patients with schizophrenia: results of a randomized, double-blind, placebo-controlled efficacy and safety study. Int J Neuropsychopharmacol. 2010;13(5):635-647. PubMed CrossRef
39.Invega [package insert]. Titusville, NJ: Janssen, L.P.; 2019.
40.Abilify [package insert]. Tokyo, Japan: Otsuka Pharmaceutical Co, Ltd; 2019.
41.Correll CU, Citrome L, Haddad PM, et al. The use of long-acting injectable antipsychotics in schizophrenia: evaluating the evidence. J Clin Psychiatry. 2016;77(suppl 3):1-24. PubMed CrossRef
42.Misawa F, Kishimoto T, Hagi K, et al. Safety and tolerability of long-acting injectable versus oral antipsychotics: A meta-analysis of randomized controlled studies comparing the same antipsychotics. Schizophr Res. 2016;176(2-3):220-230. PubMed CrossRef
43.Leucht C, Heres S, Kane JM, et al. Oral versus depot antipsychotic drugs for schizophrenia-a critical systematic review and meta-analysis of randomised long-term trials. Schizophr Res. 2011;127(1-3):83-92. PubMed CrossRef
44.Nasser AF, Henderson DC, Fava M, et al. Efficacy, safety, and tolerability of RBP-7000 once-monthly risperidone for the treatment of acute schizophrenia: an 8-week, randomized, double-blind, placebo-controlled, multicenter phase 3 study. J Clin Psychopharmacol. 2016;36(2):130-140. PubMed CrossRef
45.Savitz AJ, Xu H, Gopal S, et al. Paliperidone palmitate 3-month treatment results in symptomatic remission in patients with schizophrenia: a randomized, multicenter, double-blind, and noninferiority study. Int Clin Psychopharmacol. 2017;32(6):329-336. PubMed CrossRef
46.Hough D, Gopal S, Vijapurkar U, et al. Paliperidone palmitate maintenance treatment in delaying the time-to-relapse in patients with schizophrenia: a randomized, double-blind, placebo-controlled study. Schizophr Res. 2010;116(2-3):107-117. PubMed CrossRef
47.Naber D, Hansen K, Forray C, et al. Qualify: a randomized head-to-head study of aripiprazole once-monthly and paliperidone palmitate in the treatment of schizophrenia. Schizophr Res. 2015;168(1-2):498-504. PubMed CrossRef
This PDF is free for all visitors!
Save
Cite