Objective: Binge-eating disorder (BED) is the most prevalent eating disorder; however, few evidence-based treatments are available. The aim of this study was to evaluate the efficacy and safety of dasotraline, a novel dopamine and norepinephrine reuptake inhibitor, in adults with BED.
Methods: Patients with a DSM-5 diagnosis of BED (intent-to-treat sample, N = 315) were randomized to 12 weeks of double-blind treatment with once-daily, flexible doses (4, 6, or 8 mg/d) of dasotraline or placebo. Primary endpoint was change in diary-based assessment of number of binge-eating days per week at week 12. Key secondary endpoints included changes from baseline in Clinical Global Impressions-Severity of Illness scale (CGI-S) and Yale-Brown Obsessive Compulsive Scale Modified for Binge-Eating (YBOCS-BE) and percentage of subjects with cessation of binge eating in the final 4 weeks.
Results: Treatment with dasotraline was associated with a significantly greater reduction in binge-eating days per week at study endpoint (vs placebo; least squares mean [SE] difference score, −0.99 [0.17]; P < .0001; effect size [ES], 0.74). Significant endpoint improvement was observed for the 3 key secondary measures, CGI-S (P < .0001; ES, 0.95), YBOCS-BE (P < .0001; ES, 0.96), and 4-week cessation of binge eating (46.5% vs 20.6%; P < .0001). The most common adverse events in the dasotraline vs placebo groups were insomnia (44.6% vs 8.1%), dry mouth (27.4% vs 5.0%), decreased appetite (19.7% vs 6.9%), and anxiety (17.8% vs 2.5%). Discontinuation due to adverse events occurred in 11.3% of patients on dasotraline vs 2.5% on placebo.
Conclusions: The results of this placebo-controlled, double-blind study found dasotraline to be an efficacious, safe, and generally well-tolerated treatment for BED.
Trial Registration: ClinicalTrials.gov identifier: NCT02564588
Efficacy and Safety of Dasotraline in Adults With Binge-Eating Disorder:
A Randomized, Placebo-Controlled, Flexible-Dose Clinical Trial
ABSTRACT
Objective: Binge-eating disorder (BED) is the most prevalent eating disorder; however, few evidence-based treatments are available. The aim of this study was to evaluate the efficacy and safety of dasotraline, a novel dopamine and norepinephrine reuptake inhibitor, in adults with BED.
Methods: Patients with a DSM-5 diagnosis of BED (intent-to-treat sample, N = 315) were randomized to 12 weeks of double-blind treatment with once-daily, flexible doses (4, 6, or 8 mg/d) of dasotraline or placebo. Primary endpoint was change in diary-based assessment of number of binge-eating days per week at week 12. Key secondary endpoints included changes from baseline in Clinical Global Impressions-Severity of Illness scale (CGI-S) and Yale-Brown Obsessive Compulsive Scale Modified for Binge-Eating (YBOCS-BE) and percentage of subjects with cessation of binge eating in the final 4 weeks.
Results: Treatment with dasotraline was associated with a significantly greater reduction in binge-eating days per week at study endpoint (vs placebo; least squares mean [SE] difference score, −0.99 [0.17]; P < .0001; effect size [ES], 0.74). Significant endpoint improvement was observed for the 3 key secondary measures, CGI-S (P < .0001; ES, 0.95), YBOCS-BE (P < .0001; ES, 0.96), and 4-week cessation of binge eating (46.5% vs 20.6%; P < .0001). The most common adverse events in the dasotraline vs placebo groups were insomnia (44.6% vs 8.1%), dry mouth (27.4% vs 5.0%), decreased appetite (19.7% vs 6.9%), and anxiety (17.8% vs 2.5%). Discontinuation due to adverse events occurred in 11.3% of patients on dasotraline vs 2.5% on placebo.
Conclusions: The results of this placebo-controlled, double-blind study found dasotraline to be an efficacious, safe, and generally well-tolerated treatment for BED.
Trial Registration: ClinicalTrials.gov identifier: NCT02564588
J Clin Psychiatry 2020;81(5):19m13068
To cite: McElroy SL, Hudson JI, Grilo CM, et al. Efficacy and safety of dasotraline in adults with binge-eating disorder: a randomized, placebo-controlled, flexible-dose clinical trial. J Clin Psychiatry. 2020;81(5):19m13068.
To share: https://doi.org/10.4088/JCP.19m13068
© Copyright 2020 Physicians Postgraduate Press, Inc.
aLindner Center of HOPE, Mason, Ohio
bBiological Psychiatry Program, University of Cincinnati Medical College, Cincinnati, Ohio
cBiological Psychiatry Laboratory, McLean Hospital, Belmont, Massachusetts; and Department of Psychiatry, Harvard Medical School, Boston, Massachusetts
dDepartment of Psychiatry, Yale School of Medicine, New Haven, Connecticut
eSunovion Pharmaceuticals Inc., Marlborough, Massachusetts
*Corresponding author: Bradford Navia, MD, PhD, Sunovion Pharmaceuticals Inc., 84 Waterford Dr, Marlborough, MA 01752 ([email protected]).
Binge-eating disorder (BED) is the most common eating disorder, with a lifetime prevalence of 1.3%-3.5% in women and 0.4%-2.0% in men.1-3 BED is characterized by frequent episodes of excessive food intake accompanied by a sense of loss of control over eating, marked distress, and feelings of shame or guilt, but without inappropriate compensatory behaviors such as purging.4
BED is a chronic disorder with a median onset in the early 20s1-3; its course of illness is associated with a 2- to 3-fold increased risk for psychiatric and medical comorbidity, including depressive disorder, generalized anxiety or panic disorder, alcohol and substance abuse/dependence, and suicidal behavior. Commonly occurring medical comorbidity includes obesity, type 2 diabetes, hypertension, chronic pain conditions, metabolic syndrome, and overall poor health outcomes.1,3,5-9 Nonetheless, the majority of affected individuals never receive treatment specifically for BED.1,2
Mixed evidence for efficacy in the treatment of BED has been reported for various pharmacotherapies, including selective serotonin reuptake inhibitors (SSRIs) and anticonvulsants (topiramate, zonisamide).10-12 The amphetamine prodrug lisdexamfetamine dimesylate demonstrated efficacy in 4 randomized, placebo-controlled trials and is currently the only medication approved by the US Food and Drug Administration for the treatment of BED.13-15 Available evidence also supports use of specific psychological treatments, notably cognitive-behavioral and interpersonal therapies.16-19 Given the prevalence, medical comorbidity, and high burden of illness associated with BED, there is a significant unmet need for additional pharmacologic treatment options.
Dasotraline is a potent inhibitor of human dopamine and norepinephrine transporters, with a pharmacokinetic (PK) profile characterized by slow absorption and a long elimination half-life (t1/2, 47-77 hours) resulting in stable plasma concentrations over 24 hours with once-daily dosing.20,21
In a previously validated rat model of BED, dasotraline showed a significant dose-dependent reduction in binge consumption of chocolate compared to vehicle-treated controls.22 These findings, combined with its pharmacologic profile, suggest that dasotraline may be an effective treatment for individuals with BED. The aim of this study was to evaluate the efficacy and safety of flexibly dosed dasotraline in adults with moderate-to-severe BED.
METHODS
Patients
Adults (18-55 years) eligible for enrollment met DSM-5 criteria for BED,4 confirmed with the Eating Disorders version of the Structured Clinical Interview for DSM Disorders,23-25 the Eating Disorder Examination Questionnaire (EDE-Q),24,26 and patient diaries. BED criteria required ≥ 2 binge-eating days per week for ≥ 6 months prior to screening and ≥ 3 binge-eating days per week for the 2 weeks prior to baseline.
Exclusion criteria included a body mass index (BMI) outside the range of 18-45 kg/m2; lifetime history of bulimia nervosa or anorexia nervosa; participation in a formal weight loss program in the 3 months prior to screening; a lifetime history of psychotic disorder, bipolar disorder, hypomania, or attention-deficit/hyperactivity disorder; history of moderate-to-severe depression within 6 months prior to screening; use of antidepressants, psychostimulants, or mood stabilizers within 3 months prior to screening; a history of substance abuse in the past 12 months; and a history of type 1 or type 2 diabetes or clinically significant hypertension or cardiovascular disease.
The study was approved by an institutional review board at each study site and conducted in accordance with the International Conference on Harmonization Guideline for Good Clinical Practice and the Declaration of Helsinki. Written informed consent was obtained from all patients prior to initiation of study procedures.
Study Design

- Dasotraline, a once-a-day medication that does not directly stimulate dopamine release, provides a potentially useful new treatment option for binge-eating disorder, a common disorder associated with poor health outcomes for which few evidence-based treatments are available.
- The results of this randomized, double-blind, placebo-controlled study indicate that dasotraline is an efficacious and generally well-tolerated treatment for binge-eating disorder.
This study (ClinicalTrials.gov identifier: NCT02564588) was conducted at 42 centers in the US, between October 28, 2015, and October 19, 2016. Following a screening period of up to 21 days, patients were randomized (1:1) to receive 12 weeks of double-blind, parallel-group treatment with once-daily, flexible doses of dasotraline (4 mg, 6 mg, and 8 mg) or placebo. Randomization was stratified based on number of binge-eating days per week determined from the BED diary over the 2 weeks prior to baseline (3-4 vs > 4 binge-eating days/week). Randomization was managed by a computer-based interactive voice/web response system.27
Dasotraline and placebo capsules were provided in identical blister packs. The allocation sequence was concealed from study patients and study personnel. Patients randomized to dasotraline were initially treated with a dose of 4 mg/d which was increased to 6 mg/d at week 2 (based on the investigator’s judgment), with all patients required to increase to a dose of 6 mg/d by week 4 or be discontinued from the study. Following the week 4 visit, the dose of dasotraline could be adjusted in increments or decrements of 2 mg/d for efficacy or tolerability reasons at the discretion of the investigator. The maximum permitted dose of dasotraline was 8 mg/d. No changes in dose were permitted during the final 2 weeks of study treatment (weeks 11-12).
Patients who did not enter the extension study had their medication discontinued (without taper) and participated in a 3-week medication discontinuation period designed to assess potential withdrawal effects.
Concomitant Medications
Hypnotics were permitted as needed. The following medications were prohibited: stimulants, antidepressants, medication for the treatment of eating disorders or obesity, and medications associated with weight gain or weight loss.
Efficacy Assessments
The primary efficacy endpoint was mean change from baseline to week 12 in number of binge-eating days per week, defined as a day with at least 1 binge-eating episode that meets DSM-5 criteria.4 Binge-eating days were determined at each study visit by investigator review and confirmation of patient daily diaries that was used to record the number of binge-eating episodes per day, per the methodology used in previous clinical trials in BED.13,14 Patient diaries were source material reviewed with the patient to determine whether a recorded episode fulfilled criteria for a binge-eating episode and to ascertain if there were other unrecorded binge-eating episodes during the previous interval.
Key secondary efficacy endpoints consisted of change from baseline in the Clinical Global Impressions-Severity of Illness scale (CGI-S) score, proportion of patients achieving remission, defined as 100% cessation of binge-eating episodes in the final 4 weeks of treatment, and change from baseline in the Yale-Brown Obsessive Compulsive Scale Modified for Binge-Eating (YBOCS-BE)28 total score. Other secondary endpoints included proportion of responders with ≥ 75% reduction in binge-eating episodes, change from baseline in number of binge-eating episodes per week, YBOCS-BE obsession and compulsion subscale scores, the 7-item version of the Eating Disorder Examination Questionnaire (EDE-Q7),29,30 and the Sheehan Disability Scale (SDS)31 total score.
Safety and Tolerability Assessments
Safety assessments included frequency of adverse events, serious adverse events, laboratory and electrocardiographic (ECG) assessments, vital signs, and weight. Suicidality was assessed by the Columbia—Suicide Severity Rating Scale.32 An abuse potential monitoring plan was implemented to detect any possible diversion or abuse of dasotraline or concurrent recreational use of non-study drugs.
For patients who did not enter the extension study and who discontinued study medication, potential withdrawal symptoms during the 3-week follow-up period were assessed with the Cocaine Selective Severity Assessment (CSSA),33 the Discontinuation-Emergent Signs and Symptoms (DESS) scale,34 the Hamilton Anxiety Rating Scale (HARS),35 and the Montgomery-Asberg Depression Rating Scale (MADRS).36
Statistical Analysis
Primary efficacy analyses were performed on the modified intent-to-treat (mITT) population, which was defined as all randomized patients who received at least 1 dose of study drug and had at least 1 postbaseline efficacy evaluation. The safety population was defined as all randomized patients who received at least 1 dose of study medication.
The primary outcome, 2 of the 3 key secondary efficacy outcomes (CGI-S; YBOCS-BE total score), and other outcomes (number of binge-eating episodes per week; EDE-Q7 global and subscale scores) were analyzed using a mixed model for repeated measures (MMRM) with fixed effects terms for treatment, visit (as a categorical variable), pooled center, baseline binge-eating days category (stratification factor), number of binge-eating days per week at baseline, and treatment-by-visit interaction. The third key secondary outcome (proportion of patients with 100% cessation of binge-eating episodes in the final 4 weeks of treatment) was analyzed using a logistic regression model with treatment, baseline binge days category (stratification factor), and baseline number of binge days per week as covariates. For other secondary efficacy variables (SDS total score), an analysis of covariance (ANCOVA) model was used.
For primary efficacy outcome, 4 sensitivity analyses were conducted to verify the missing at random assumption and/or model assumptions underlying the primary MMRM analysis of the primary efficacy endpoint at week 12 for the mITT population.
To address early dropouts under the assumption of missing not at random, a pattern-mixture model using a placebo-based multiple imputation method and a pattern mixture model using multiple imputations with penalties (ie, tipping point analysis by deflating the individually estimated treatment effect size by known factors) was performed to explore the robustness of the MMRM results for the primary analysis based on the mITT population.
In case of a deviation from the assumptions required for the primary analysis, 2 additional sensitivity analyses (permutation test and generalized linear mixed model analysis) were also performed to confirm the robustness of the primary analysis.
Descriptive statistics were used for safety variables. Rank ANCOVA was used to analyze changes in cholesterol, triglycerides, and glucose levels from baseline.
Assuming a mean difference of change from baseline to week 12 for dasotraline versus placebo of 0.8 (common SD = 1.75) in number of binge-eating days per week,10,37 a sample size of 102 patients per treatment group provided 90% power (α = .05) to reject the null hypothesis of no difference in the primary outcome. The sample size was adjusted to 150 patients per treatment group based on a projected dropout rate of 30%.
A hierarchical testing procedure was employed to maintain the overall type I error rate at the .05 level (2-sided) beginning with the primary measure and followed (in order) by key secondary endpoints: CGI-S, percentage of patients with 4-week cessation of binge eating, and YBOCS-BE total score. Other secondary endpoints, interim time points on all endpoints, and interaction terms were evaluated at a nominal .05 significance level (2-sided; not adjusted for multiplicity).
RESULTS
Patients and Disposition
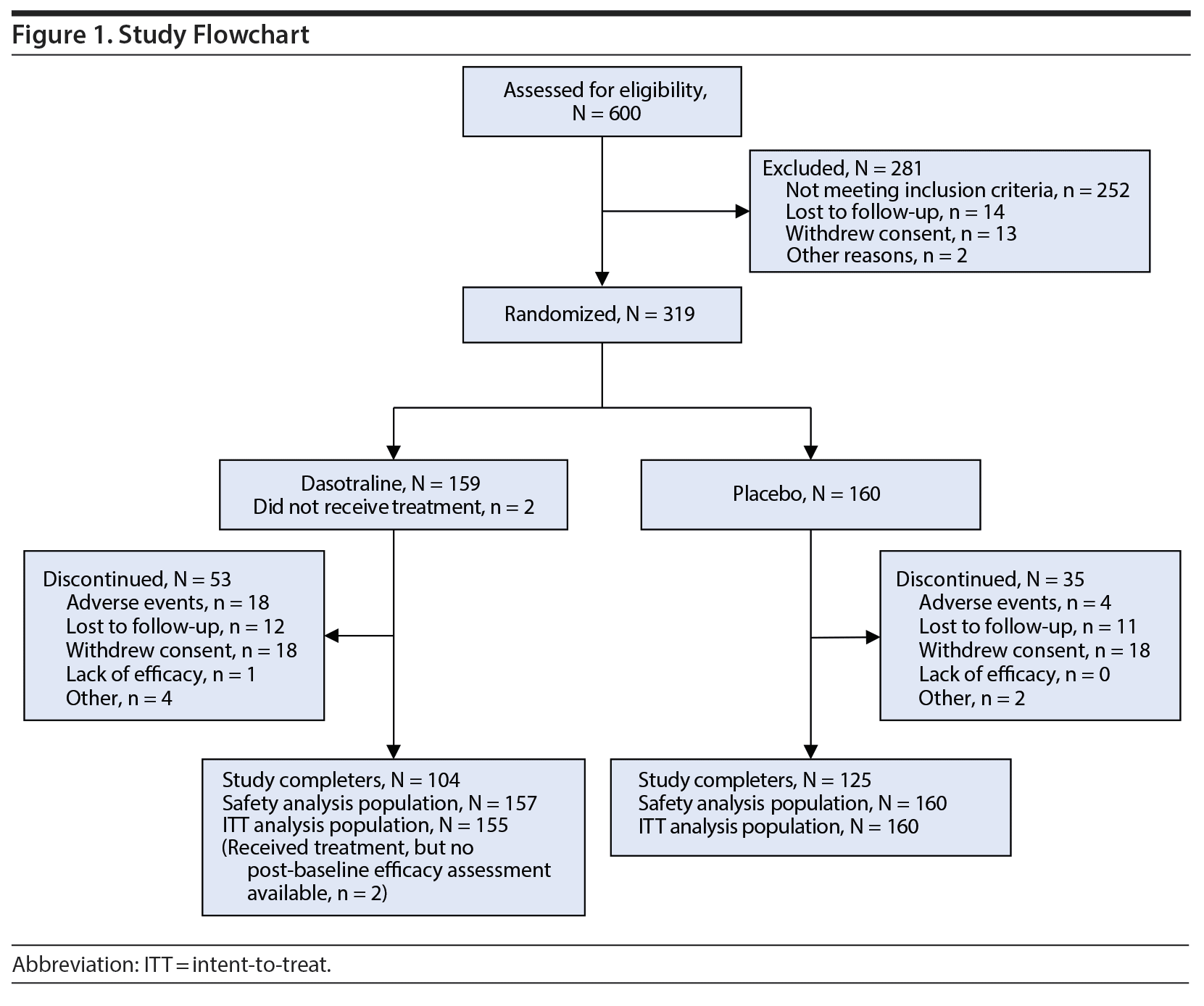
A total of 600 patients were screened, of whom 319 were randomized to study treatment (Figure 1); 317 patients received at least 1 dose of study drug and 315 had at least 1 postbaseline efficacy evaluation. Study completion rates were 66.7% for the dasotraline group and 72.4% for placebo group; reasons for study discontinuation are summarized in Figure 1.
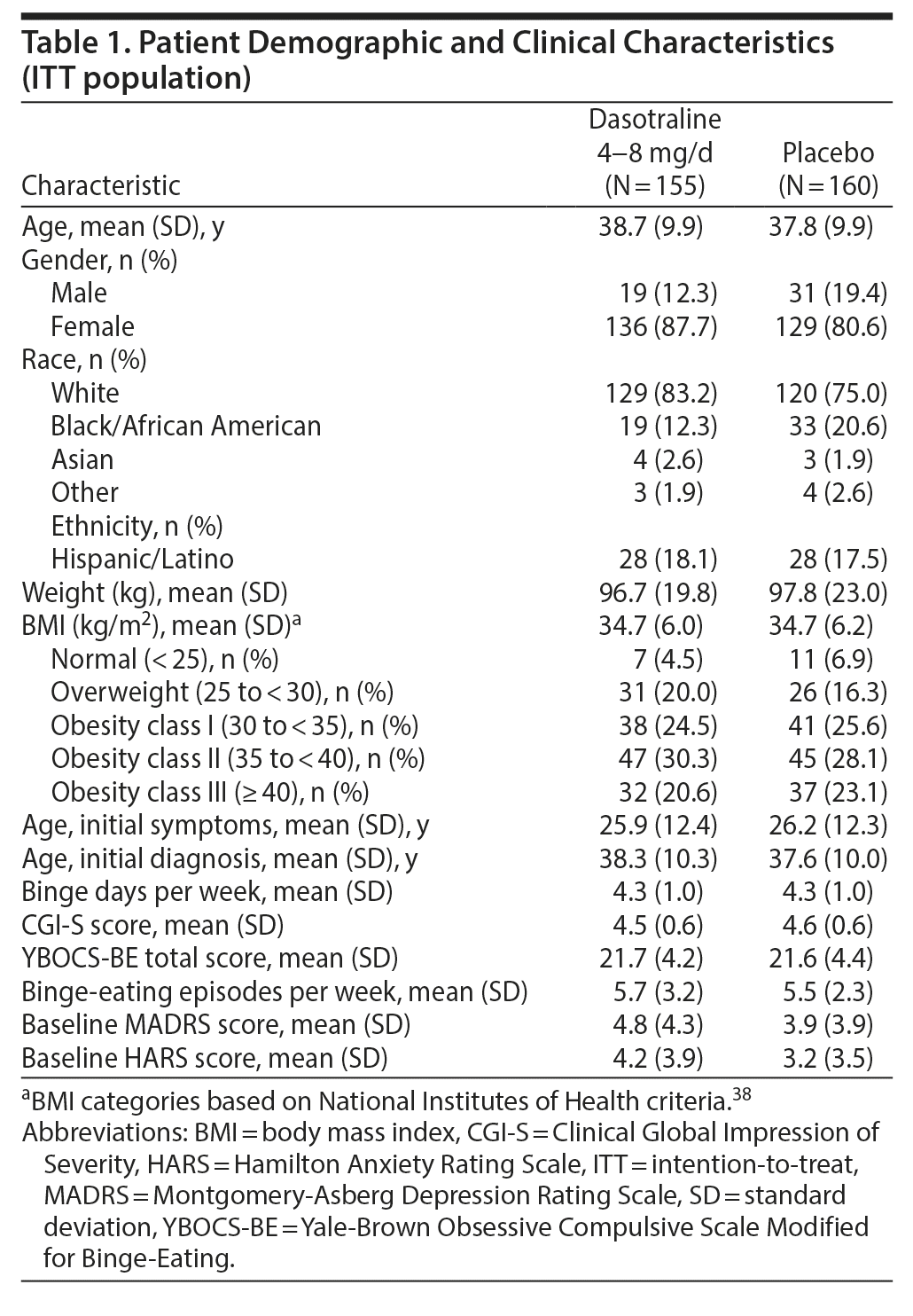
Clinical and demographic characteristics were comparable between the two groups (Table 1). The overall mean age was 38.3 years; the majority were white (79.0%) and female (84.1%), with a mean BMI of 34.7 kg/m2; and were obese, meeting National Institutes of Health (NIH) criteria (1998) for class I (25.1%), class II (29.2%), or class III (21.9%). The majority were not diagnosed with BED until screening despite a mean duration of nearly 10-12 years in binge-eating symptoms. The mean baseline number of binge-eating episodes per week was 5.6, with a mean of 4.25 binge-eating days per week. The mean daily dose of dasotraline was 5.5 mg/d, with a similar proportion of patients taking a modal daily dose of 4 mg (29.0%), 6 mg (27.7%), and 8 mg (27.1%).
Efficacy
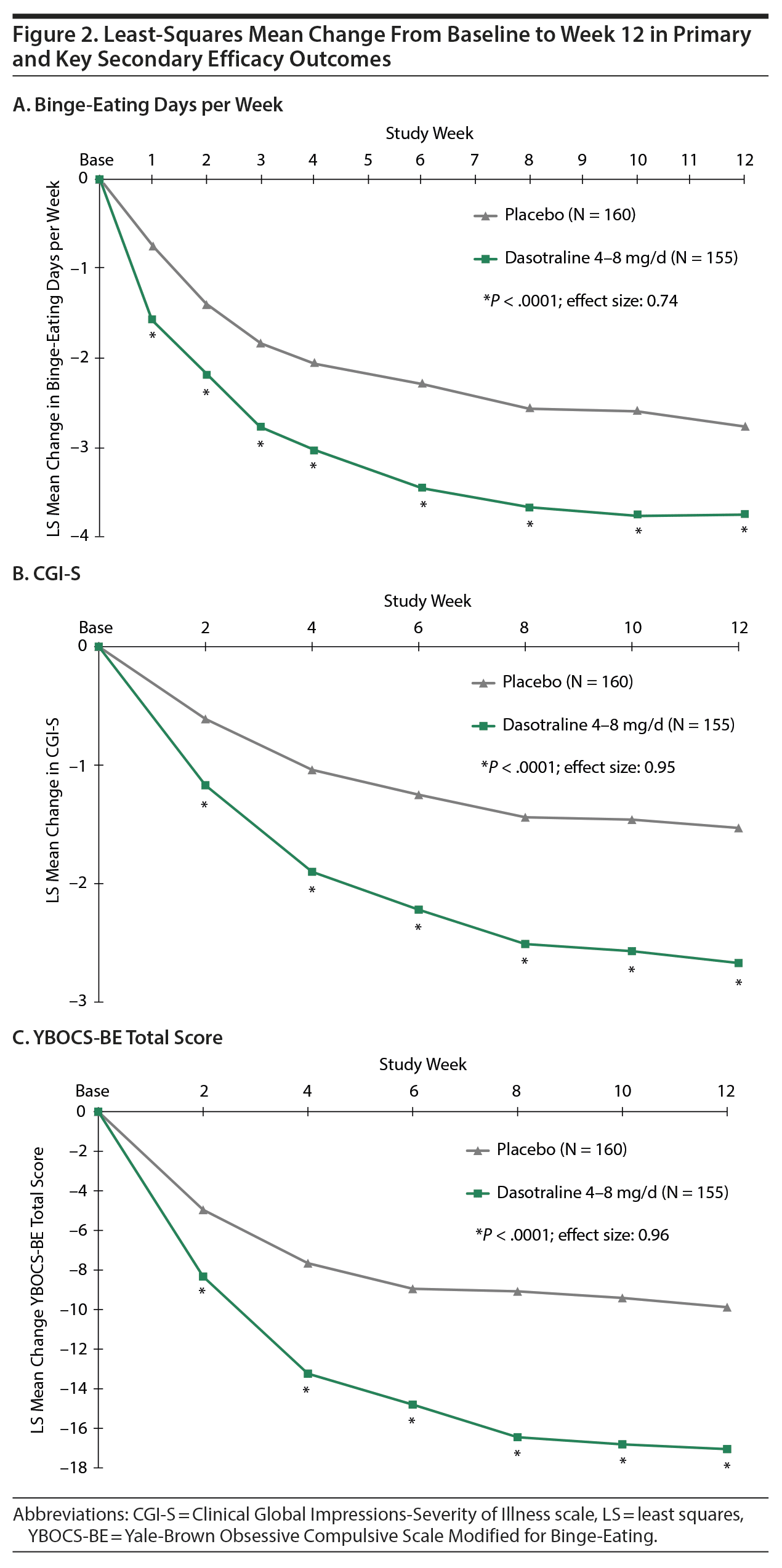
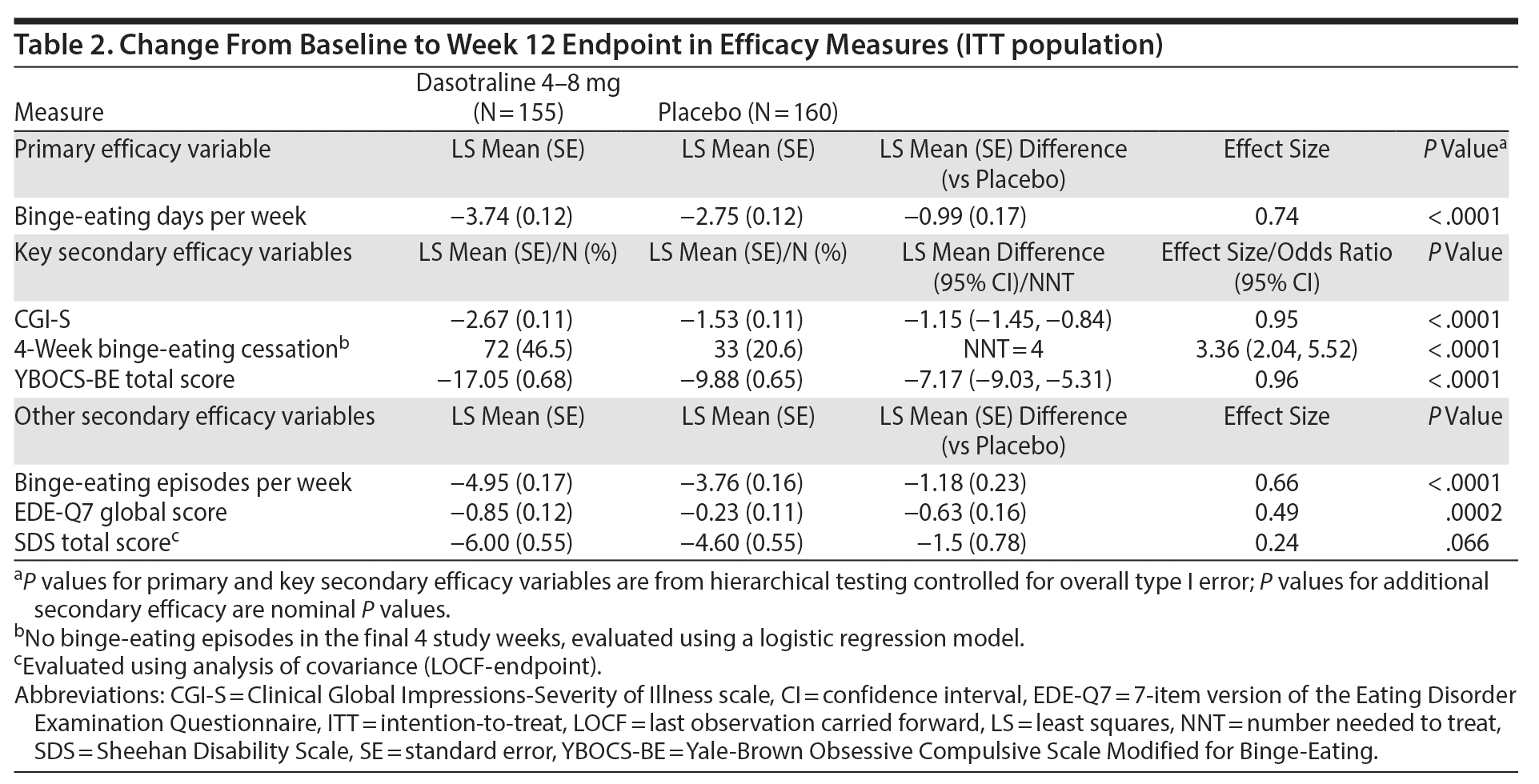
The primary analysis showed a significant reduction from baseline in the LS mean (SE) number of binge-eating days per week for dasotraline vs placebo at week 12 (−3.74 [0.12] vs −2.75 [0.12]; P < .0001; effect size = 0.74; Figure 2). Significant treatment group differences were evident at week 1 and at all subsequent assessment visits (Figure 2).
Four sensitivity analyses, conducted to verify the missing at random assumption and/or model assumptions underlying the primary efficacy MMRM analyses, confirmed the robustness of the primary efficacy outcome (see Supplementary Table 1 and Supplementary Methods).
Key secondary efficacy measures. On the 3 key secondary measures, treatment with dasotraline was associated with significantly greater reduction in the CGI-S score (−2.67 vs −1.53; P < .0001; effect size = 0.95; Figure 2B); a significantly higher proportion of patients achieving cessation of binge-eating episodes (46.5% vs 20.6%; P < .0001; Table 2); and a significantly greater reduction in the YBOCS-BE total score (−17.05 vs −9.88; P < .0001; effect size = 0.96; Figure 2C). For the CGI-S and YBOCS-BE total score, significant treatment group differences were evident at week 2 and at all subsequent assessment visits.
On additional secondary measures, dasotraline was associated with a higher proportion of patients showing at least 75% reduction from baseline in binge-eating episodes per week (78.1% vs 46.9%; nominal P < .0001) and significant improvement in the EDE-Q7 global scale score (nominal P = .0002; Table 2 and Supplementary Figure 1). Change in the SDS total score was numerically greater for the dasotraline group versus the placebo group, but the difference did not achieve statistical significance (nominal P = .066; Table 2).
Subgroup analysis of primary endpoint. In a pre-planned analysis, no significant treatment-by-subgroup interaction effects were observed in LS mean change at week 12 in binge-eating days per week for gender, race, age group, ethnicity, or baseline severity (assessed by number of binge-eating episodes per week). For the non-obese (< 30 kg/m2) vs obese (≥ 30 kg/m2) subgroups, a significant (nominal P = .0069) treatment interaction effect was observed (in favor of non-obese patients) for the week 12 dasotraline vs placebo comparison of change in mean binge-eating days per week (non-obese: −3.61 vs −1.85; obese: −3.77 vs −3.05); the nature of this significant interaction was evaluated using a post hoc Gail and Simon test which showed that significance was due to a difference in the magnitude of the treatment effect of dasotraline and not to a qualitative difference in the direction of the treatment effect.
Safety
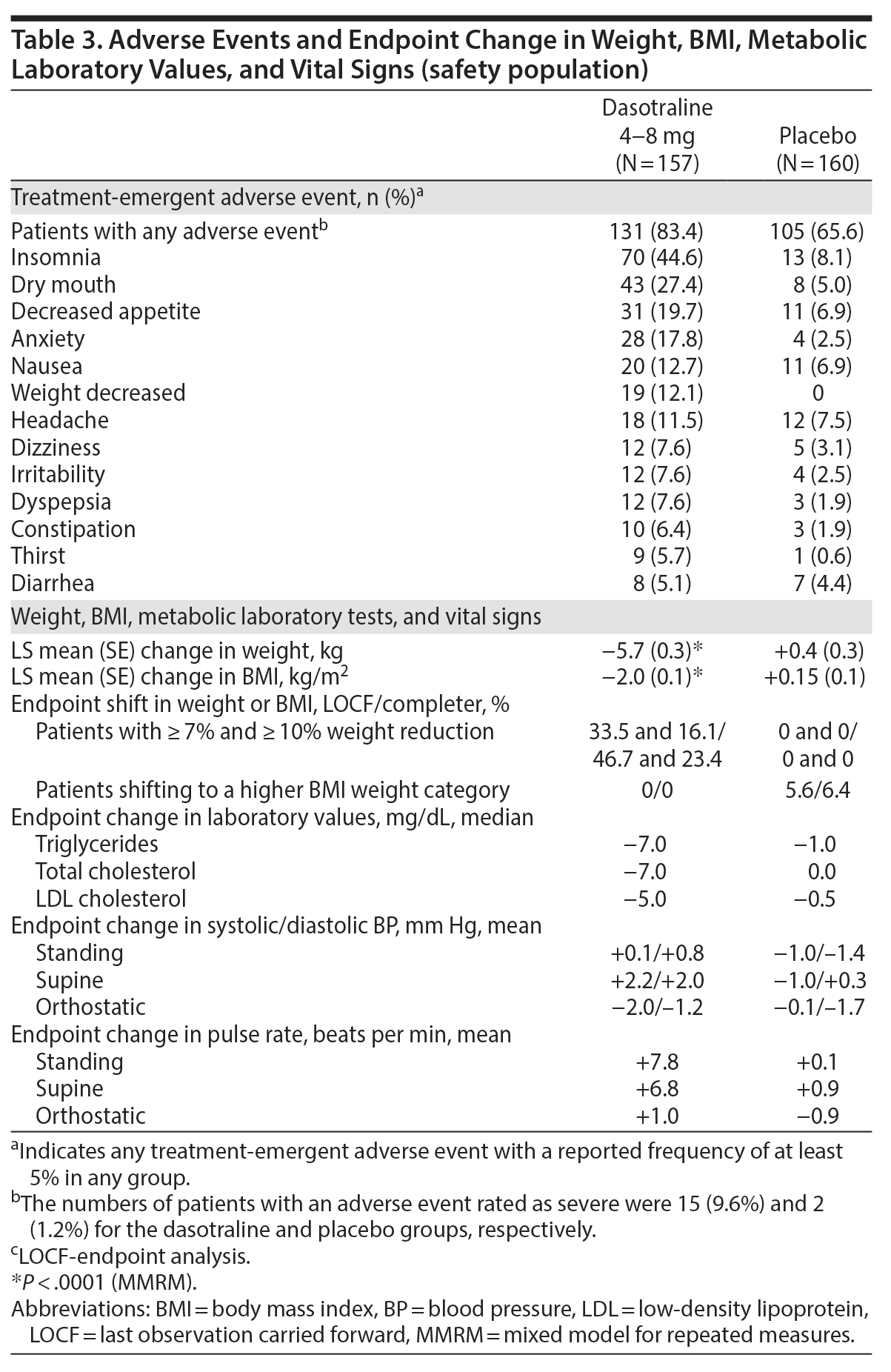
The most frequent treatment-emergent adverse events (≥ 10%) in the dasotraline group were insomnia, dry mouth, decreased appetite, anxiety, nausea, decreased weight, and headache (Table 3). Eighteen patients (11.5%) in the dasotraline group and 4 (2.5%) in the placebo group discontinued due to an adverse event (Figure 1). In the dasotraline group, 8 patients (5.1%) reported “severe” insomnia, and 2 patients (1.3%) discontinued due to insomnia.
Five patients in the dasotraline group reported psychosis-related events (formication, delusion, auditory hallucinations, paranoia, visual illusions or hallucinations); only 1 event (paranoia rated as severe in a 20-year-old female) resulted in study discontinuation (see Supplementary Table 2). Two patients in the dasotraline group reported obsessive-compulsive disorder spectrum-related behaviors (trichotillomania, dermatillomania, rated as moderate) that both resolved (see Supplementary Table 2). No psychosis or mania-related events occurred in the placebo group. No deaths were reported in the study. There was 1 serious adverse event, suicidal ideation in a 20-year-old female in the final week of treatment with dasotraline 6 mg/d; the patient was hospitalized, and the event resolved within 4 days.
In patients who discontinued study medication (dasotraline, N = 29; placebo, N = 22), no signs or symptoms of withdrawal were identified based on an increase in severity scores on the CSSA, DESS, MADRS, or HARS during the 3-week discontinuation period. No patients in either treatment group had suspected or known abuse or diversion of study drug. One patient in the placebo group had a suspected abuse of alcohol, illicit substances, over-the-counter drugs, or prescription drugs obtained outside the study protocol.
Treatment with dasotraline was associated with greater mean reduction in weight and BMI compared to placebo (see Table 3). Endpoint changes on dasotraline in serum chemistries and hematologies were generally small and not clinically meaningful; small but consistent reductions in lipid parameters were observed; and no clinically meaningful changes were noted in blood pressure, pulse rate, and ECG parameters (see Table 3).
DISCUSSION
This is the first study to evaluate the efficacy and safety of dasotraline for the treatment of adults with moderate-to-severe BED. Twelve weeks of double-blind treatment with dasotraline, in once-daily flexible doses of 4, 6, or 8 mg, provided statistically significant reduction in the number of binge-eating days per week, with onset of improvement notable as early as week 1 and with a large effect size. The efficacy findings for dasotraline in treating BED were robust, observed across key secondary outcome measures (CGI-S, YBOCS-BE), and confirmed by multiple sensitivity analyses. Dasotraline-treated patients also showed significant improvement on the EDE-Q7 global score (effect size, 0.49), which captures behaviors and cognitions related to weight and shape concerns and attempts to restrict food intake.29,30 Taken together, these results suggest that in addition to reducing binge-eating episodes, dasotraline treatment is associated with clinically meaningful improvement in behaviors that may constitute the core psychopathology of BED.
Evidence for efficacy in BED has been reported for cognitive-behavioral and interpersonal therapies16-19,39; however, these therapies are not widely available and may not be as effective in achieving weight loss, a potentially important limitation since obesity is a frequent complication of BED. Evidence for efficacy in BED has also been demonstrated for pharmacotherapies (SSRIs and selected anticonvulsants)10-12 and most notably lisdexamfetamine dimesylate,13-15 with treatment effects comparable to those observed in this trial. However, the pharmacology of dasotraline is characterized by potent inhibition of dopamine and norepinephrine transporters,20,21 and a PK profile is further characterized by relatively slow absorption and a long elimination half-life20 resulting in stable plasma concentrations over 24 hours when administered as once-daily dosing. These properties result in a sustained effect that may benefit individuals with BED who experience binge-eating episodes throughout the 24-hour dosing interval.
Substance abuse and substance dependence are common problems in individuals with BED.1,40 Results of a placebo and methylphenidate-comparator controlled abuse liability study in recreational stimulant users41 suggest that the pharmacologic and PK profile of dasotraline may be associated with a relatively low abuse potential. The study found that dasotraline, in single doses of 8 mg and 16 mg, was indistinguishable from placebo across all pharmacodynamic measures associated with abuse potential.41 In the current study, no known abuse or diversion of dasotraline occurred, and abrupt discontinuation of dasotraline was not associated with withdrawal-related symptomatology (as measured by the CSSA, DESS, HARS, or MADRS). Further study is needed to determine whether dasotraline may be a potentially useful medication in individuals with BED who have a higher risk of substance abuse.
Dasotraline was safe and generally well tolerated in BED patients. Common adverse events (≥ 10%) in the dasotraline group were insomnia, dry mouth, decreased appetite, anxiety, nausea, decreased weight, and headache. Most events were mild to moderate in severity, resulting in discontinuation in 11.5% of dasotraline-treated patients compared to 2.5% of placebo patients. One serious event (suicidal ideation) occurred in a dasotraline-treated patient in the final week of treatment. Adverse events rated as severe by more than 1 patient in the dasotraline group were insomnia and anxiety. However, despite its frequency, insomnia resulted in discontinuation in only 3 patients. Psychosis-related events were reported by 5 dasotraline-treated patients and typically were mild-to-moderate in severity, were transient, and did not lead to study discontinuation.
Modest increases were observed on dasotraline in supine heart rate (mean, +6.8 bpm), but no significant increase in systolic or diastolic blood pressure was observed. No clinically meaningful effects were observed on the QTc interval or other ECG parameters.
BED is associated with a high prevalence of obesity that contributes to the increased risk of medical comorbidity including metabolic syndrome and type 2 diabetes.1,2,5-9 In the current study, 76% of patients met NIH criteria for obesity based on BMI,38 with 22% meeting class III criteria (BMI ≥ 40 kg/m2). Of patients who completed 12 weeks of treatment, 46.7% in the dasotraline group, and none in the placebo group, experienced clinically meaningful (≥ 7%) weight reduction. Small reductions were also observed in the dasotraline group in triglycerides, total cholesterol, and LDL. Long-term studies are needed to determine the clinical significance of these findings.
Several limitations of the current study should be noted. Patients with clinically significant psychiatric or medical comorbidity were excluded. Assessment of binge-eating days was based on investigator review performed at each study visit of patient-reported daily diaries, which may be subject to recall bias; however, a number of trials support the validity of this instrument as an endpoint.12-15 The flexible-dose design of the current trial did not permit evaluation of dose-response effects; given this uncertainty, the lowest effective dose should be utilized to minimize adverse effects. Further investigation is needed to establish the longer-term efficacy and safety of dasotraline beyond 12 weeks of treatment.15
CONCLUSIONS
The results of this double-blind, 12-week, placebo-controlled study indicate that dasotraline is an efficacious, safe, and generally well-tolerated treatment for individuals with moderate-to-severe BED. Further investigation is needed to assess the long-term effects of dasotraline in this population.
Submitted: August 30, 2019; accepted April 29, 2020.
Published online: September 8, 2020.
Potential conflicts of interest: Drs Deng, Koblan, Goldman, Navia, Hopkins, and Loebel are employees of Sunovion Pharmaceuticals, Inc. Dr McElroy has been a consultant to or member of the scientific advisory board of Avanir, Bracket, F. Hoffmann-La Roche Ltd., Mitsubishi Tanabe Pharma Corporation, Myriad, Naurex, Novo Nordisk, Otsuka, Shire, Sunovion, and Takeda (Shire); has been a principal or co-investigator on studies sponsored by Alkermes, Allergan, Avanir, Azevan, Forest, Marriott Foundation, Medibio, Myriad, National Institute of Mental Health, Naurex, Neurocrine, Novo Nordisk, Shire, Sunovion, and Takeda Pharmaceutical Company Limited; and is an inventor on United States Patent No. 6,323,236 B2, Use of Sulfamate Derivatives for Treating Impulse Control Disorders, and along with the patent’s assignee, University of Cincinnati, Cincinnati, Ohio, has received payments from Johnson & Johnson, which has exclusive rights under the patent. Dr Hudson has received grant support from Shire and Sunovion and has received consulting fees from diaMentis, Idorsia, Shire, and Sunovion. Dr Grilo reports, in the past 12 months, receiving honoraria for lectures delivered for CME-related activities and plenaries and lectures at professional academic conferences and reports royalties from academic books published by Guilford Press and Taylor & Francis Publishers. Beyond 12 months, he reports having received consultant fees from Shire and Sunovion and honoraria for CME-related lectures and for lectures delivered at grand rounds and professional academic conferences nationally and internationally. Dr Guerdjikova is a consultant to Bracket.
Funding/support: The study was funded by Sunovion Pharmaceuticals Inc., Marlborough, Massachusetts.
Role of the sponsor: The sponsors were involved in the study design; collection, analysis, and interpretation of data; writing of the report; and decision to submit the article for publication.
Previous presentation: The results of the study were presented, in part, at the annual meeting of the American Psychiatric Association; May 20-24, 2017; San Diego, California.
Acknowledgment: Editorial and medical writing support was provided by Dr Edward Schweizer of Paladin Consulting Group and was funded by Sunovion Pharmaceuticals Inc.
Supplementary material: See accompanying pages.
REFERENCES
1.Hudson JI, Hiripi E, Pope HG Jr, et al. The prevalence and correlates of eating disorders in the National Comorbidity Survey Replication. Biol Psychiatry. 2007;61(3):348-358. PubMed CrossRef
2.Kessler RC, Berglund PA, Chiu WT, et al. The prevalence and correlates of binge eating disorder in the World Health Organization World Mental Health Surveys. Biol Psychiatry. 2013;73(9):904-914. PubMed CrossRef
3.Udo T, Grilo CM. Prevalence and correlates of DSM-5-defined eating disorders in a nationally representative sample of US adults. Biol Psychiatry. 2018;84(5):345-354. PubMed CrossRef
4.American Psychiatric Association. Diagnostic and Statistical Manual for Mental Disorders. Fifth Edition. Washington, DC: American Psychiatric Association; 2013.
5.Grilo CM, White MA, Masheb RM. DSM-IV psychiatric disorder comorbidity and its correlates in binge eating disorder. Int J Eat Disord. 2009;42(3):228-234. PubMed CrossRef
6.Swanson SA, Crow SJ, Le Grange D, et al. Prevalence and correlates of eating disorders in adolescents: results from the National Comorbidity Survey Replication Adolescent Supplement. Arch Gen Psychiatry. 2011;68(7):714-723. PubMed CrossRef
7.×gh T, Kovács G, Pawaskar M, et al. Epidemiology, health-related quality of life and economic burden of binge eating disorder: a systematic literature review. Eat Weight Disord. 2015;20(1):1-12. PubMed CrossRef
8.Mitchell JE. Medical comorbidity and medical complications associated with binge-eating disorder. Int J Eat Disord. 2016;49(3):319-323. PubMed CrossRef
9.Olguin P, Fuentes M, Gabler G, et al. Medical comorbidity of binge eating disorder. Eat Weight Disord. 2017;22(1):13-26. PubMed CrossRef
10.Reas DL, Grilo CM. Pharmacological treatment of binge eating disorder: update review and synthesis. Expert Opin Pharmacother. 2015;16(10):1463-1478. PubMed CrossRef
11.Brownley KA, Berkman ND, Peat CM, et al. Binge-eating disorder in adults: a systematic review and meta-analysis. Ann Intern Med. 2016;165(6):409-420. PubMed CrossRef
12.McElroy SL. Pharmacologic treatments for binge-eating disorder. J Clin Psychiatry. 2017;78(suppl 1):14-19. PubMed CrossRef
13.McElroy SL, Hudson JI, Mitchell JE, et al. Efficacy and safety of lisdexamfetamine for treatment of adults with moderate to severe binge-eating disorder: a randomized clinical trial. JAMA Psychiatry. 2015;72(3):235-246. PubMed CrossRef
14.McElroy SL, Hudson J, Ferreira-Cornwell MC, et al. Lisdexamfetamine dimesylate for adults with moderate to severe binge eating disorder: results of two pivotal phase 3 randomized controlled trials. Neuropsychopharmacology. 2016;41(5):1251-1260. PubMed CrossRef
15.Hudson JI, McElroy SL, Ferreira-Cornwell MC, et al. Efficacy of lisdexamfetamine in adults with moderate to severe binge-eating disorder: a randomized clinical trial. JAMA Psychiatry. 2017;74(9):903-910. PubMed CrossRef
16.Iacovino JM, Gredysa DM, Altman M, et al. Psychological treatments for binge eating disorder. Curr Psychiatry Rep. 2012;14(4):432-446. PubMed CrossRef
17.Peat CM, Berkman ND, Lohr KN, et al. Comparative effectiveness of treatments for binge-eating disorder: systematic review and network meta-analysis. Eur Eat Disord Rev. 2017;25(5):317-328. PubMed CrossRef
18.Grilo CM. Psychological and behavioral treatments for binge-eating disorder. J Clin Psychiatry. 2017;78(suppl 1):20-24. PubMed CrossRef
19.Linardon J, Messer M, Fuller-Tyszkiewicz M. Meta-analysis of the effects of cognitive-behavioral therapy for binge-eating-type disorders on abstinence rates in nonrandomized effectiveness studies: comparable outcomes to randomized, controlled trials? Int J Eat Disord. 2018;51(12):1303-1311. PubMed CrossRef
20.Hopkins SC, Sunkaraneni S, Skende E, et al. Pharmacokinetics and exposure-response relationships of dasotraline in the treatment of ADHD in adults. Clin Drug Investig. 2016;36(2):137-146. PubMed CrossRef
21.Koblan KS, Hopkins SC, Sarma K, et al. Dasotraline for the treatment of attention-deficit/hyperactivity disorder: a randomized, double-blind, placebo-controlled, proof-of-concept trial in adults. Neuropsychopharmacology. 2015;40(12):2745-2752. PubMed CrossRef
22.Heal DJ, Vickers SP, Hopkins SC, Koblan KS. Investigation of the effects of dasotraline in a validated rat model of binge-eating disorder. Neuropsychopharmacol. 2018;S34-S35.
23.First MB, Williams JBW, Spitzer RL, et al. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Clinical Trials Version (SCID-CT). New York, NY: Biometrics Research, New York State Psychiatric Institute; 2007.
24.Reas DL, Grilo CM, Masheb RM. Reliability of the Eating Disorder Examination-Questionnaire in patients with binge eating disorder. Behav Res Ther. 2006;44(1):43-51. PubMed CrossRef
25.Grilo CM, Masheb RM, Wilson GT. A comparison of different methods for assessing the features of eating disorders in patients with binge eating disorder. J Consult Clin Psychol. 2001;69(2):317-322. PubMed CrossRef
26.Fairburn CG, Beglin SJ. Assessment of eating disorders: interview or self-report questionnaire? Int J Eat Disord. 1994;16(4):363-370. PubMed
27.Ruikar V. Interactive Voice/Web Response System in clinical research. Perspect Clin Res. 2016;7(1):15-20. PubMed CrossRef
28.Deal LS, Wirth RJ, Gasior M, et al. Validation of the Yale-Brown Obsessive Compulsive Scale Modified for Binge Eating. Int J Eat Disord. 2015;48(7):994-1004. PubMed CrossRef
29.Grilo CM, Crosby RD, Peterson CB, et al. Factor structure of the Eating Disorder Examination interview in patients with binge-eating disorder. Obesity (Silver Spring). 2010;18(5):977-981. PubMed CrossRef
30.Grilo CM, Reas DL, Hopwood CJ, et al. Factor structure and construct validity of the Eating Disorder Examination-Questionnaire in college students: further support for a modified brief version. Int J Eat Disord. 2015;48(3):284-289. PubMed CrossRef
31.Sheehan D. The Anxiety Disease. New York, NY: Bantam Books; 1983.
32.Posner K, Brown GK, Stanley B, et al. The Columbia-Suicide Severity Rating Scale: initial validity and internal consistency findings from three multisite studies with adolescents and adults. Am J Psychiatry. 2011;168(12):1266-1277. PubMed CrossRef
33.Kampman KM, Volpicelli JR, McGinnis DE, et al. Reliability and validity of the Cocaine Selective Severity Assessment. Addict Behav. 1998;23(4):449-461. PubMed CrossRef
34.Rosenbaum JF, Fava M, Hoog SL, et al. Selective serotonin reuptake inhibitor discontinuation syndrome: a randomized clinical trial. Biol Psychiatry. 1998;44(2):77-87. PubMed CrossRef
35.Hamilton M. The assessment of anxiety states by rating. Br J Med Psychol. 1959;32(1):50-55. PubMed CrossRef
36.Montgomery SA, Åsberg M. A new depression scale designed to be sensitive to change. Br J Psychiatry. 1979;134(4):382-389. PubMed CrossRef
37.McElroy SL, Hudson JI, Capece JA, et al; Topiramate Binge Eating Disorder Research Group. Topiramate for the treatment of binge eating disorder associated with obesity: a placebo-controlled study. Biol Psychiatry. 2007;61(9):1039-1048. PubMed CrossRef
38.Clinical guidelines on the identification, evaluation, and treatment of overweight and obesity in adults: the evidence report. Obes Res. 1998;6(suppl 2):51S-209S. PubMed
39.Grilo CM, Masheb RM, Wilson GT, et al. Cognitive-behavioral therapy, behavioral weight loss, and sequential treatment for obese patients with binge-eating disorder: a randomized controlled trial. J Consult Clin Psychol. 2011;79(5):675-685. PubMed CrossRef
40.Becker DF, Grilo CM. Comorbidity of mood and substance use disorders in patients with binge-eating disorder: associations with personality disorder and eating disorder pathology. J Psychosom Res. 2015;79(2):159-164. PubMed CrossRef
41.Koblan KS, Hopkins SC, Sarma K, et al. Assessment of human abuse potential of dasotraline compared to methylphenidate and placebo in recreational stimulant users. Drug Alcohol Depend. 2016;159:26-34. PubMed CrossRef
Please sign in or purchase this PDF for $40.00.
Save
Cite