Objective: To assess the efficacy, tolerability, and safety of brexpiprazole as adjunctive therapy to antidepressant treatments (ADTs) in adults with major depressive disorder (as defined by DSM-IV-TR criteria) and inadequate response to ADTs.
Method: Patients with historical inadequate response to 1-3 ADTs were enrolled. All patients entered a prospective 8-week phase on physician-determined, open-label ADT. Those with inadequate response were randomized to ADT + brexpiprazole 2 mg/d or ADT + placebo for 6 weeks. The study was conducted between July 2011 and May 2013. The primary efficacy end point was change from baseline to week 6 in Montgomery-Asberg Depression Rating Scale (MADRS) total score. The key secondary end point was change from baseline to week 6 in Sheehan Disability Scale (SDS) mean score. The efficacy population comprised all patients who had ≥ 1 dose of study drug in the double-blind phase and both baseline and ≥ 1 postrandomization MADRS scores. The efficacy population per final protocol included patients from the efficacy population who met amended randomization criteria of inadequate response throughout prospective treatment.
Results: Brexpiprazole (n = 175) reduced mean MADRS total score versus placebo (n = 178) at week 6 in the efficacy population per final protocol (−8.36 vs −5.15, P = .0002). Brexpiprazole improved SDS mean score versus placebo (−1.35 vs −0.89, P = .0349). The most common treatment-related adverse events were weight gain (brexpiprazole, 8.0%; placebo, 3.1%) and akathisia (7.4% vs 1.0%).
Conclusions: Adjunctive brexpiprazole therapy demonstrated efficacy and was well tolerated in patients with major depressive disorder and inadequate response to ADTs.
Trial Registration: ClinicalTrials.gov identifier: NCT01360645
This work may not be copied, distributed, displayed, published, reproduced, transmitted, modified, posted, sold, licensed, or used for commercial purposes. By downloading this file, you are agreeing to the publisher’s Terms & Conditions.
See article by Thase et al
ABSTRACT
Objective: To assess the efficacy, tolerability, and safety of brexpiprazole as adjunctive therapy to antidepressant treatments (ADTs) in adults with major depressive disorder (as defined by DSM-IV-TR criteria) and inadequate response to ADTs.
Method: Patients with historical inadequate response to 1-3 ADTs were enrolled. All patients entered a prospective 8-week phase on physician-determined, open-label ADT. Those with inadequate response were randomized to ADT + brexpiprazole 2 mg/d or ADT + placebo for 6 weeks. The study was conducted between July 2011 and May 2013. The primary efficacy end point was change from baseline to week 6 in Montgomery-Asberg Depression Rating Scale (MADRS) total score. The key secondary end point was change from baseline to week 6 in Sheehan Disability Scale (SDS) mean score. The efficacy population comprised all patients who had ≥ 1 dose of study drug in the double-blind phase and both baseline and ≥ 1 postrandomization MADRS scores. The efficacy population per final protocol included patients from the efficacy population who met amended randomization criteria of inadequate response throughout prospective treatment.
Results: Brexpiprazole (n = 175) reduced mean MADRS total score versus placebo (n = 178) at week 6 in the efficacy population per final protocol (−8.36 vs −5.15, P = .0002). Brexpiprazole improved SDS mean score versus placebo (−1.35 vs −0.89, P = .0349). The most common treatment-related adverse events were weight gain (brexpiprazole, 8.0%; placebo, 3.1%) and akathisia (7.4% vs 1.0%).
Conclusions: Adjunctive brexpiprazole therapy demonstrated efficacy and was well tolerated in patients with major depressive disorder and inadequate response to ADTs.
Trial Registration: ClinicalTrials.gov identifier: NCT01360645
J Clin Psychiatry 2015;76(9):1224-1231
dx.doi.org/10.4088/JCP.14m09688
© Copyright 2015 Physicians Postgraduate Press, Inc.
aPerelman School of Medicine of the University of Pennsylvania, Philadelphia, Pennsylvania
bOtsuka Pharmaceutical Development & Commercialization, Inc, Princeton, New Jersey
cH. Lundbeck A/S, Valby, Copenhagen, Denmark
*Corresponding author: Michael E. Thase, MD, Department of Psychiatry, Perelman School of Medicine of the University of Pennsylvania, 3535 Market St, Philadelphia, PA 19104 ([email protected]).
Effective treatment of patients with major depressive disorder (MDD) not responding adequately to first-line antidepressant treatment (ADT) remains an important unmet need.1,2 For inadequate response to an optimized trial of first-line ADT, current guidelines recommend switching ADT, adding a second ADT or adding adjunctive therapy with a non-ADT.1,3 Adjunctive second-generation antipsychotic therapies such as olanzapine,4 quetiapine,5,6 and aripiprazole7 are associated with significant improvements in treatment response and remission; however, their side effect profile may limit use in clinical practice.8,9 Prominent side effects vary from drug to drug,10,11 ie, weight gain with olanzapine,12 sedation with quetiapine,5 and akathisia with aripiprazole.7 Thus, there is ongoing interest in identifying adjunctive strategies that offer the rapid efficacy of antipsychotics while reducing frequency and burden of side effects.
Serotoninergic (5-HT), dopaminergic (D), and noradrenergic systems appear to play important roles in ADT mechanisms of action.13,14 Brexpiprazole is a rationally designed serotonin-dopamine activity modulator, with partial agonism at serotonin 5-HT1A and dopamine D2 receptors at similar potency, and potent antagonism at 5-HT2A and norepinephrine α1B and α2C receptors.15 The therapeutic potential of brexpiprazole as an adjunctive treatment for depression has been demonstrated in animal models.16 Brexpiprazole differs from aripiprazole in terms of its lower intrinsic activity at the D2 receptor.15 Brexpiprazole binds to 5-HT1A and 5-HT2A receptors, with around 10 times higher affinity than aripiprazole.15 In addition, brexpiprazole is equipotent at 5-HT1A, 5-HT2A, and D2 receptors, while the relative potencies of aripiprazole at these receptors differ.15 Brexpiprazole’s partial agonism with low intrinsic D2 receptor activity suggests a potential stabilizing effect on dopaminergic function and low potential to induce side effects (extrapyramidal symptoms, hyperprolactinemia, tardive dyskinesia) associated with blockade of dopamine transmission.17 Preclinical models have confirmed the low potential of brexpiprazole to induce antipsychotic-related side effects.15,18 In particular, we predicted that brexpiprazole’s 5-HT2A/D2 receptor binding profile, along with the low intrinsic D2 activity, may predict low rates of activation-like side effects (akathisia, insomnia, or restlessness).15 Furthermore, brexpiprazole has a moderate affinity, relative to D2/5-HT1A receptor affinity, for histamine H1,15 which may result in low levels of sedation.
A phase 2, placebo-controlled study (reference 19 and data on file, Otsuka, Princeton, New Jersey) of patients with MDD who had shown inadequate response to ADT suggested that adjunctive brexpiprazole 1.5 ± 0.5 mg/d was efficacious and well tolerated. Efficacy of adjunctive brexpiprazole (3 mg/d) was demonstrated in a phase 3 study (NCT01360632; the Polaris trial) in patients with MDD and inadequate ADT response.20 The present phase 3 study (331-10-228; NCT01360645, the Pyxis trial) objectives were to evaluate efficacy, safety, and tolerability of brexpiprazole at a fixed dose of 2 mg/d as adjunctive therapy in patients with MDD and inadequate response to ADTs.
METHOD
Patients
Patients were recruited at 59 study centers in the United States (74.9% of patients), Poland (9.7%), France (8.5%), Canada (4.8%), and Slovakia (2.1%). The study included outpatients aged 18−65 years who met Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, Text Revision (DSM-IV-TR) criteria for a single or recurrent nonpsychotic episode of MDD21 of at least 8 weeks’ duration. During the current episode, patients must have reported an inadequate response, defined as < 50% reduction in symptoms via patient self-reports on the Massachusetts General Hospital Antidepressant Treatment Response Questionnaire (ATRQ),22 to an adequate trial of between 1 and 3 ADTs, including their most recent drug treatment. Eligible patients had a 17-item Hamilton Depression Rating Scale (HDRS-17)23,24 total score ≥ 18 both at screening and on the first day of prospective treatment. Key exclusion criteria and concomitant medication regulations are provided (see /JCP/article/Pages/2015/v76n09/v76n0931.aspx&Type=Supplement” target=”_blank”>eAppendix 1 at Psychiatrist.com).
The study was conducted in compliance with the International Conference on Harmonisation Good Clinical Practice Consolidated Guideline. The protocol was approved by independent ethics committees, and all patients provided informed consent to participate.
Study Design
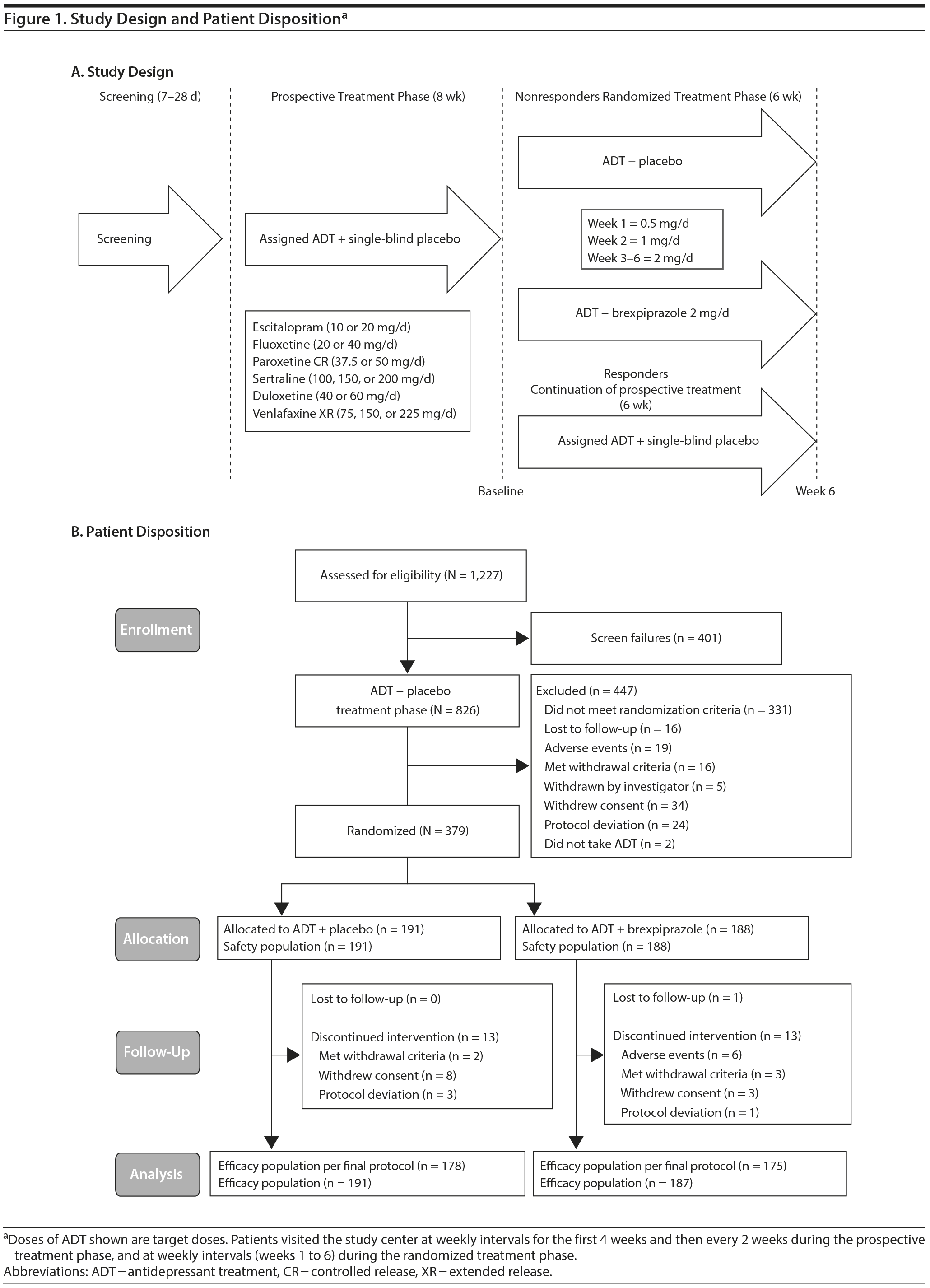
This randomized, double-blind, placebo-controlled, fixed-dose study was conducted between July 2011 and May 2013 and comprised a screening phase (7−28 days), an 8-week single-blind prospective treatment phase, and a 6-week double-blind randomized treatment phase (Figure 1A).
During the 8-week prospective treatment phase, all patients received single-blind placebo adjunctive to standard ADT (Table 1). Patients who were switched from a previous ADT had a washout period of at least 24 hours before initiating treatment. Antidepressant treatment was titrated from the starting dose to the maximum-tolerated dose to optimize the potential for response.

- Availability of effective antidepressant treatments with better tolerability profiles remains a significant unmet need for patients with major depressive disorder (MDD); clinical use of adjunctive second-generation antipsychotics can be limited by their tolerability profiles.
- Adjunctive brexpiprazole 2 mg improved depressive symptoms compared with antidepressant monotherapy in patients with MDD and inadequate response to antidepressant treatment.
- Brexpiprazole was well tolerated in this population.
During prospective treatment, patients’ outcomes were assessed to determine eligibility to enter the randomized treatment phase. Patients were eligible for randomization if they had inadequate response to the prospective ADT, had a negative drug screen, and were considered suitable for adjunctive therapy by the investigator. Inadequate response was initially defined as < 50% reduction from the start of ADT in HDRS-17 total score, HDRS-17 total score ≥ 14, and Clinical Global Impressions-Improvement scale (CGI-I)25 score ≥ 3 at the end of prospective treatment. While this study was ongoing, additional analyses were performed on data from a completed phase 2 study of similar design (reference 19 and data on file, Otsuka, Princeton, New Jersey). It was found that a number of patients in that study had seemingly adequate improvement in Montgomery-Asberg Depression Rating Scale (MADRS) and CGI-I scores at various times during the prospective treatment period, but subsequent worse scores at time of randomization. These patients did not show a consistent lack of response and would have been considered adequate responders if evaluated at another time point during the prospective phase. A number of these patients showed significant improvement again during the randomized phase, even if they were continuing on ADT alone. In order to exclude patients with seemingly variable response to ADT, this study’s protocol was amended to specify that patients had to meet more refined inadequate response criteria throughout prospective treatment (HDRS-17 score ≥ 14; < 50% reduction from baseline in HDRS-17, as well as < 50% reduction in MADRS total score between start of prospective treatment and each scheduled visit, and CGI-I score ≥ 3 at each scheduled visit) to be eligible for randomization and also to blind the investigator to the revised criteria. Eligible patients were randomized to receive double-blind ADT + brexpiprazole or ADT + placebo (1:1) for 6 weeks. The ADT dose in the randomized phase was the same as the last dose from the prospective phase. Randomization was conducted via an interactive voice or web response system using a fixed-block, computer-generated randomization schedule with a block size of 4 and stratified by study center.
Patients who responded to ADT during prospective treatment continued to receive single-blind placebo plus the same ADT for an additional 6 weeks. Patients who completed the additional 6 weeks of ADT and eligible patients who completed the randomized treatment phase were invited to participate in an open-label rollover study (331-10-238; ClinicalTrials.gov identifier: NCT01360866).
Outcome Measures
Efficacy assessments were made at baseline (end of prospective treatment) and during randomized treatment. The MADRS26 was administered at each weekly visit using the Structured Interview Guide.27 Patients completed the Sheehan Disability Scale (SDS)28,29 at baseline and at weeks 3 and 6. The HDRS-17 and Hamilton Anxiety Rating Scale (HARS)25,30,31 were administered using Structured Interview Guides32,33 at baseline and at week 6. At each visit, the investigator rated patients with the Clinical Global Impressions-Severity of Illness scale (CGI-S)25 and CGI-I from start of prospective phase or from baseline. Patients completed the Inventory of Depressive Symptomatology-Self-Report (IDS-SR)34 at each visit.
Safety and tolerability were evaluated by recording treatment-emergent adverse events (TEAEs) and serious adverse events (SAEs). Extrapyramidal symptom scales were administered at each visit, including the Simpson-Angus Scale (SAS),35 Abnormal Involuntary Movement Scale (AIMS),25 and Barnes Akathisia Rating Scale (BARS).36 Clinical laboratory tests and a 12-lead electrocardiogram (ECG) were conducted every 2 weeks, and vital signs were measured at each visit. Suicidality was monitored at each visit using the Columbia-Suicide Severity Rating Scale.37,38 Patients completed the Massachusetts General Hospital Sexual Functioning Questionnaire39 at baseline and at week 6. Least squares (LS) mean change in body weight at week 6 was derived from an analysis of covariance model, with treatment as factors and baseline value as covariate, on observed case data.
Data Analysis
Sample size calculations were based on a predicted between-group difference of 3.0 points (SD = 8.5) in mean MADRS total score change from baseline to week 6.19 A sample size of 340 evaluable patients (170 in each treatment group) was projected to yield at least 90% power to detect treatment effects at a 2-tailed significance level of .05. It was planned to randomize 370 patients (185 in each treatment group) to allow for 5%-10% nonevaluable patients.
The safety population included all patients who received at least 1 dose of brexpiprazole or placebo during the randomized treatment phase. The efficacy population comprised all patients in the safety population who had MADRS scores at baseline and on at least 1 occasion after randomization. The efficacy population per final protocol included all patients from the efficacy population who met the amended randomization criteria for inadequate response. The statistical analysis plan prespecified the analyses of the efficacy population per final protocol as well as the efficacy population. Data reported here focus on the efficacy population per final protocol, since inadequate response was more reliably assessed and this population is more homogenous. Detailed outcomes for the efficacy population are provided in Supplementary eTable 1.
Baseline was defined as the last available measurement prior to starting the randomized treatment phase. The primary efficacy end point was change from baseline to week 6 in MADRS total score. The primary analysis was conducted by fitting a mixed model repeated-measures analysis with an unstructured variance covariance structure. The model included fixed class effect terms for treatment, study center, visit week, and an interaction term of treatment by visit week. The model also included the interaction term of baseline MADRS total scores by visit week as covariates.
The key secondary efficacy end point was change from baseline to week 6 in SDS mean score. A hierarchical testing procedure was used in order to maintain overall experiment-wise type I error rate at .05. Other secondary efficacy end points were analyzed at a nominal .05 level (2-sided). MADRS response was defined as ≥ 50% reduction from baseline in MADRS total score. CGI-I response was defined as a score of 1 (very much improved) or 2 (much improved). MADRS remission was defined as a MADRS total score of ≤ 10 with ≥ 50% reduction from baseline. Further details are given in eAppendix 1.
RESULTS
Patients
Three hundred seventy-nine patients were randomized to brexpiprazole (n = 188) or placebo (n = 191) (Figure 1B). The randomized treatment phase was completed by 174/188 (92.6%) brexpiprazole and 178/191 (93.2%) placebo patients. The efficacy population included 187 and 191 patients in the brexpiprazole and placebo groups, respectively, while the efficacy population per final protocol included 175 and 178 patients, respectively.
Demographic and baseline psychiatric characteristics and baseline MADRS total and SDS mean scores were similar between treatment groups (Table 1). Investigator-assigned ADTs were relatively well balanced between the 2 groups. The mean baseline CGI-I score was 3.5 in both groups, indicating that the study population was moderately ill and showed minimal improvement despite 8 weeks of prospective ADT. During the current episode, 81.7%, 16.5%, and 1.9% of randomized patients had 1, 2, and 3 prior ADT failures, respectively.
Efficacy Assessments
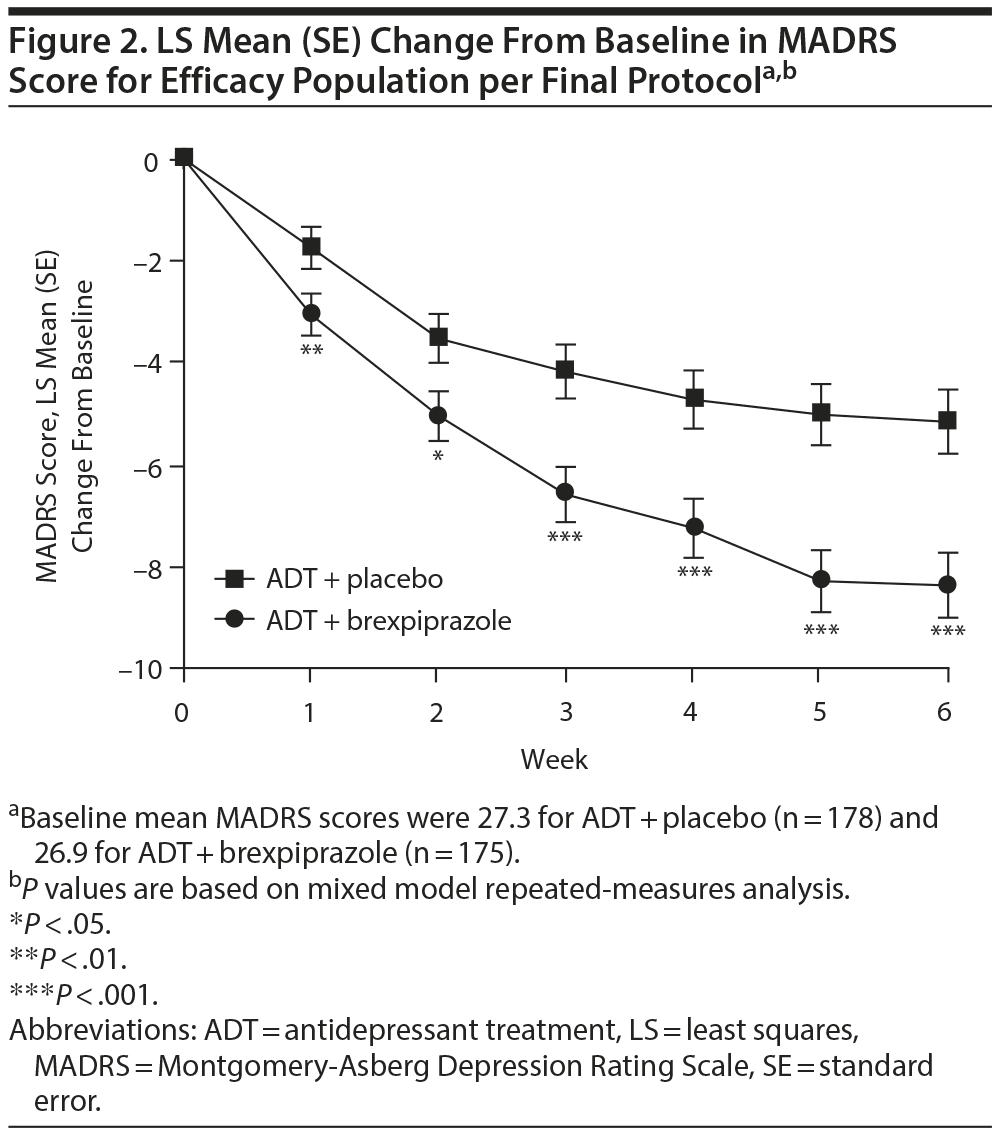
MADRS score (primary end point). Mean reduction from baseline to week 6 in MADRS total score was greater for brexpiprazole compared with placebo (LS mean = −8.36 vs −5.15; LS mean difference = −3.21 [95% CI, −4.87 to −1.54], P = .0002; efficacy population per final protocol) with difference between treatment groups apparent from the first week onward (Figure 2).
Similar results were seen for brexpiprazole versus placebo in the efficacy population (LS mean = −8.27 vs −5.15; LS mean difference = −3.12 [95% CI, −4.70 to −1.54], P = .0001) (Supplementary eFigure 1).
Secondary end points. In the efficacy population per final protocol, brexpiprazole produced a greater reduction from baseline to week 6 than placebo in mean SDS score (P = .0349; Table 2). Brexpiprazole produced numerical improvements on work/school, social life, and family life subscales. Greater improvements from baseline to week 6 in the brexpiprazole group compared with placebo were also seen in physician-rated HDRS-17 and CGI-S (P < .001; Table 2). Greater improvement in the brexpiprazole group compared with placebo was seen in CGI-I score at week 6 (P = .0003) and change from baseline to week 6 in HARS total score (P = .0376; Table 2). There was a higher proportion of responders at week 6, whether defined by MADRS score (P = .0429) or CGI-I (P = .0002), in the brexpiprazole group compared with placebo (Table 2). Secondary end point outcomes for the efficacy population were similar to those for the efficacy population per final protocol (Supplementary eTable 1).
Safety and tolerability assessments. The most frequent TEAEs (≥ 5%) in patients receiving brexpiprazole were weight gain (8.0%) and akathisia (7.4%) (Table 3), which were generally considered by investigators to be mild to moderate. Activating side effects such as restlessness, insomnia, and anxiety were reported by only a few patients (restlessness, 6/188 [3.2%] vs 0%; insomnia, 4/188 [2.1%] vs 4/191 [2.1%]; anxiety, 7/188 [3.7%] vs 3/191 [1.6%], for brexpiprazole vs placebo, respectively). Somnolence, fatigue, and sedation were also uncommon (somnolence, 8/188 [4.3%] vs 1/191 [0.5%]; fatigue, 3/188 [1.6%] vs 3/191 [1.6%]; and sedation, 2/188 [1.1%] vs 0%). No suicide, attempted suicide, or deaths were reported during the study.
Mean body weight change at week 6 (observed cases) was 1.64 kg for brexpiprazole vs 0.36 kg for placebo (LS mean difference = 1.28 kg, P < .0001). An increase in body weight of ≥ 7% from baseline at any visit was seen in 9/187 (4.8%) brexpiprazole patients versus 5/190 (2.6%) placebo patients.
Mean prolactin concentrations in the brexpiprazole group increased from baseline to last visit by 8.3 ng/mL in female patients and 2.2 ng/mL in male patients (baseline = 10.0 and 7.5 ng/mL, respectively); smaller mean changes were seen in the placebo group (female = +0.3 ng/mL, male = +0.3 ng/mL; baseline = 9.9 and 7.1 ng/mL, respectively). There were no reports of amenorrhea or gynecomastia, and only 2 patients receiving brexpiprazole (compared with 1 patient in the placebo group) reported decreased libido. One hyperprolactinemia TEAE was reported for a female patient in the brexpiprazole group. An increase in prolactin concentration > 3 times the upper limit of normal range was recorded at week 2 for 1 male patient receiving brexpiprazole; the value returned to baseline level by the last visit.
Mean changes from baseline to last visit (fasting values) for brexpiprazole versus placebo groups were −0.83 versus −3.38 mg/dL for triglycerides (baseline = 135.22 vs 135.53 mg/dL), −0.40 vs −0.30 mg/dL for glucose (baseline = 93.62 vs 94.22 mg/dL), +1.21 vs +0.82 mg/dL for high-density lipoprotein cholesterol (baseline = 61.09 vs 61.08 mg/dL), +2.60 vs +0.84 mg/dL for total cholesterol (baseline = 205.88 vs 209.69 mg/dL), and +1.37 vs −0.02 mg/dL for low-density lipoprotein cholesterol (baseline = 118.21 vs 122.61 mg/dL). Metabolic-related TEAEs were reported by 2 brexpiprazole patients (dyslipidemia; hypercholesterolemia) and 1 placebo patient (increased triglycerides). A change in fasting triglycerides from normal (< 150 mg/dL) to high (200 to < 500 mg/dL) levels occurred during randomized treatment in 15/115 (13.0%) and 5/119 (4.2%) brexpiprazole and placebo patients, respectively. Among patients who did not meet any criteria for metabolic syndrome at baseline, 3 or more criteria were met during randomized treatment by 3/133 (2.3%) brexpiprazole versus 2/148 (1.4%) placebo patients.
There were no other consistent differences between treatment groups in clinical laboratory results, vital signs, and ECGs.
Two of the 3 extrapyramidal symptom rating scales used showed small increases in mean scores for the brexpiprazole group over the randomized treatment phase. Least squares mean changes from baseline to last visit for brexpiprazole versus placebo were 0.18 versus −0.02 for SAS total score (LS mean difference = 0.20, P = .0038), 0.03 versus 0.04 for AIMS total score (LS mean difference = −0.01, P = .8663), and 0.14 versus −0.04 for BARS global score (LS mean difference = 0.18, P = .0005). One patient from the brexpiprazole group discontinued treatment due to akathisia.
No suicidal behavior was reported on the Columbia-Suicide Severity Rating Scale. During randomized treatment, a similarly low proportion of patients in the brexpiprazole and placebo groups reported emergent (9/188 [4.8%] vs 12/191 [6.3%]) or worsening (3/188 [1.6%] vs 4/191 [2.1%]) suicidal ideation.
No emergent sexual dysfunction was observed on the Massachusetts General Hospital Sexual Functioning Questionnaire; total and individual item scores were similar between treatment groups. Change from baseline to last visit in overall sexual satisfaction score for brexpiprazole and placebo groups indicated comparable improvement (LS mean = −0.27 vs −0.20; LS mean difference = −0.07, P = .5421).
DISCUSSION
In this trial, adjunctive brexpiprazole 2 mg/d improved depressive symptoms, measured by MADRS, compared with ADT monotherapy in patients with MDD and inadequate response to standard ADTs. Changes from baseline in other physician-rated depression scales (HDRS-17 and CGI-S) confirmed this effect. Brexpiprazole also improved social functioning, based on SDS mean score, compared with ADT monotherapy; the effect of brexpiprazole was largest on the disruptive effects of symptoms on social life, family life, and home responsibilities. It is perhaps unsurprising that brexpiprazole did not significantly improve work and school life compared with placebo. The length of time these patients have continued to experience depressive symptoms despite treatment will most likely have had considerable impacts on employment and education that cannot be resolved within the 6-week timeframe of this study.
A clinically relevant change in depression has been defined as a difference of at least 2 points over placebo in MADRS total score40,41; in this study, the difference between brexpiprazole and placebo was more than 3 points. The absolute reduction in MADRS total score observed with adjunctive brexpiprazole in this study (8.4; placebo, 5.2) was at the lower end of the range reported in pivotal studies of adjunctive aripiprazole versus placebo (8.8 vs 5.8; 8.5 vs 5.7; 10.1 vs 6.4) in patients with MDD and inadequate response to ADT.42-44 However, as the placebo response was lower in this brexpiprazole study than the aripiprazole studies, the difference over placebo was comparable. The difference over placebo in reduction of MADRS total score observed with brexpiprazole was also similar to that reported with adjunctive quetiapine extended release (XR) in similar studies (300 mg: 15.0 vs 12.2; 14.7 vs 11.7).45,46 It should be noted that direct comparisons between studies are limited by methodological differences. For example, fixed doses of brexpiprazole and quetiapine XR were evaluated, while aripiprazole was dosed flexibly. Furthermore, the studies of quetiapine XR did not include a prospective ADT phase, which may have resulted in differences between studies in the patient population.
Brexpiprazole was well tolerated in this study, with few patients discontinuing due to TEAEs or reporting SAEs. The most frequently reported TEAEs in the brexpiprazole group were weight gain and akathisia. Incidence of akathisia with brexpiprazole was lower than that reported in a meta-analysis47 of randomized studies of adjunctive aripiprazole. Mean changes from baseline in SAS total score and BARS global score in the brexpiprazole group were small and lower than those reported in pivotal studies of adjunctive aripiprazole.42,44 Incidences of insomnia and fatigue were low and comparable in adjunctive brexpiprazole and ADT monotherapy groups; restlessness, somnolence, and sedation were infrequently reported in the adjunctive brexpiprazole group. Brexpiprazole did not appear to have clinically relevant adverse effects on prolactin or metabolic parameters. Overall, the tolerability profile of brexpiprazole reflected its receptor pharmacology—low intrinsic activity at D2 receptors and moderately low affinity for receptors associated with sedation and weight gain.15 Adjunctive brexpiprazole did not induce or worsen suicidal ideation and, as compared with ADT monotherapy, did not have any adverse effect on sexual function.
Defining a coherent group of patients with true inadequate response in a prospective trial (as well as responders) has proven difficult using standardized rating scales.48 Here, our trial protocol was amended during the study to capture more refined criteria for inadequate response, in a blinded fashion, and reflect all visits during the prospective ADT trial period. The statistical analysis plan prespecified analyses of both the efficacy population and efficacy population per final protocol, and results were consistent across the 2 analyses.
Limitations of this study include lack of active comparator group and relatively short duration of the randomized treatment phase. If short-term efficacy of adjunctive brexpiprazole is confirmed in subsequent studies, documented maintenance of efficacy and safety of adjunctive brexpiprazole will be necessary with prolonged continuous therapy.
In conclusion, in this phase 3, randomized, placebo-controlled study, adjunctive brexpiprazole demonstrated efficacy and was well tolerated in patients with MDD and inadequate response to standard ADTs.
Submitted: November 26, 2014; accepted May 14, 2015.
Online first: August 4, 2015.
Drug names: aripiprazole (Abilify and others), duloxetine (Cymbalta and others), escitalopram (Lexapro and others), fluoxetine (Prozac and others), olanzapine (Zyprexa and others), paroxetine (Paxil, Pexeva, and others), quetiapine (Seroquel and others), sertraline (Zoloft and others), venlafaxine (Effexor XR and others).
Potential conflicts of interest: Dr Thase has received grants from Agency for Healthcare Research and Quality, Alkermes, Forest, National Institute of Mental Health, Otsuka, PharmaNeuroboost, and Roche; has acted as an advisor or consultant for Alkermes, AstraZeneca, Bristol-Myers Squibb, Cerecor, Eli Lilly, Forest Laboratories, Gerson Lehman Group, GlaxoSmithKline, Guidepoint Global, Lundbeck, MedAvante, Merck, Neuronetics, Novartis, Ortho-McNeil Pharmaceuticals, Otsuka, Pamlab, Pfizer, Shire, Sunovion, and Takeda; has received royalties from American Psychiatric Association, Guilford Publications, Herald House, and W. W. Norton & Company; and holds equity in MedAvante Inc. Dr Thase’s spouse is an employee of Peloton Advantage. Drs Youakim, Skuban, Hobart, Zhang, McQuade, Carson, Nyilas, and Sanchez and Ms Augustine are employees of Otsuka Pharmaceutical Development & Commercialization. Dr Eriksson is an employee of H. Lundbeck A/S.
Funding/support: Funding for this study was provided by Otsuka Pharmaceutical Development & Commercialization, Inc. (Princeton, New Jersey) and H. Lundbeck A/S (Valby, Denmark).
Role of the sponsor: The sponsors were responsible for the study design and conduct and the collection, management, analysis, and interpretation of the data. The authors, some of whom are employed by the sponsors, were also responsible for writing and reviewing this article. All authors approved the final version.
Previous presentation: Presented at the 22nd European Congress of Psychiatry; March 1-4, 2014; Munich, Germany ▪ and the American Psychiatric Association Annual Meeting; May 3-7, 2014; New York, New York.
Acknowledgment: Jennifer Stewart, MSc (QXV Communications, Macclesfield, United Kingdom), provided writing support, which was funded by Otsuka Pharmaceutical Development & Commercialization, Inc. (Princeton, New Jersey) and H. Lundbeck A/S (Valby, Denmark).
Supplementary material: Available at Psychiatrist.com.
REFERENCES
1. Connolly KR, Thase ME. If at first you don’ t succeed: a review of the evidence for antidepressant augmentation, combination and switching strategies. Drugs. 2011;71(1):43-64. PubMed doi:10.2165/11587620-000000000-00000
2. Han C, Yeh TL, Kato M, et al. Management of chronic depressive patients with residual symptoms. CNS Drugs. 2013;27(suppl 1):S53-S57. PubMed doi:10.1007/s40263-012-0034-x
3. Patkar AA, Pae CU. Atypical antipsychotic augmentation strategies in the context of guideline-based care for the treatment of major depressive disorder. CNS Drugs. 2013;27(suppl 1):S29-S37. PubMed doi:10.1007/s40263-012-0031-0
4. Croxtall JD, Scott LJ. Olanzapine/fluoxetine: a review of its use in patients with treatment-resistant major depressive disorder. CNS Drugs. 2010;24(3):245-262. PubMed doi:10.2165/11203830-000000000-00000
5. Pae CU, Sohi MS, Seo HJ, et al. Quetiapine XR: current status for the treatment of major depressive disorder. Prog Neuropsychopharmacol Biol Psychiatry. 2010;34(7):1165-1173. PubMed doi:10.1016/j.pnpbp.2010.03.023
6. Sanford M. Quetiapine extended release: adjunctive treatment in major depressive disorder. CNS Drugs. 2011;25(9):803-813. PubMed doi:10.2165/11207280-000000000-00000
7. Pae CU, Forbes A, Patkar AA. Aripiprazole as adjunctive therapy for patients with major depressive disorder: overview and implications of clinical trial data. CNS Drugs. 2011;25(2):109-127. PubMed doi:10.2165/11538980-000000000-00000
8. Wright BM, Eiland EH 3rd, Lorenz R. Augmentation with atypical antipsychotics for depression: a review of evidence-based support from the medical literature. Pharmacotherapy. 2013;33(3):344-359. PubMed doi:10.1002/phar.1204
9. Nelson JC, Papakostas GI. Atypical antipsychotic augmentation in major depressive disorder: a meta-analysis of placebo-controlled randomized trials. Am J Psychiatry. 2009;166(9):980-991. PubMed doi:10.1176/appi.ajp.2009.09030312
10. Citrome L. Adjunctive aripiprazole, olanzapine, or quetiapine for major depressive disorder: an analysis of number needed to treat, number needed to harm, and likelihood to be helped or harmed. Postgrad Med. 2010;122(4):39-48. PubMed doi:10.3810/pgm.2010.07.2174
11. Spielmans GI, Berman MI, Linardatos E, et al. Adjunctive atypical antipsychotic treatment for major depressive disorder: a meta-analysis of depression, quality of life, and safety outcomes. PLoS Med. 2013;10(3):e1001403. PubMed doi:10.1371/journal.pmed.1001403
12. Millen BA, Campbell GM, Beasley CM. Weight changes over time in adults treated with the oral or depot formulations of olanzapine: a pooled analysis of 86 clinical trials. J Psychopharmacol. 2011;25(5):639-645. PubMed doi:10.1177/0269881110370505
13. Blier P. Neurotransmitter targeting in the treatment of depression. J Clin Psychiatry. 2013;74(suppl 2):19-24. PubMed doi:10.4088/JCP.12084su1c.04
14. Moret C, Briley M. The importance of norepinephrine in depression. Neuropsychiatr Dis Treat. 2011;7(suppl 1):9-13. PubMed doi:10.2147/ndt.s19619
15. Maeda K, Sugino H, Akazawa H, et al. Brexpiprazole I: in vitro and in vivo characterization of a novel serotonin-dopamine activity modulator. J Pharmacol Exp Ther. 2014;350(3):589-604. PubMed doi:10.1124/jpet.114.213793
16. Hirose T, Maeda K, Stensb׸l TB, et al. Synergistic effects of brexpiprazole with SSRI/SNRI/diazepam on forced swim test and marble burying behaviour in mice. Biol Psychiatry. 2014;75(suppl 1):132S.
17. Casey DE. Side effect profiles of new antipsychotic agents. J Clin Psychiatry. 1996;57(suppl 11):40-45, discussion 46-52. PubMed
18. Maeda K, Lerdrup L, Sugino H, et al. Brexpiprazole II: antipsychotic-like and procognitive effects of a novel serotonin-dopamine activity modulator. J Pharmacol Exp Ther. 2014;350(3):605-614. PubMed doi:10.1124/jpet.114.213819
19. Thase ME, Fava M, Hobart M, et al. Efficacy of adjunctive OPC-34712 across multiple outcome measures in major depressive disorder: a phase II, randomized, placebo-controlled study. Neuropsychopharmacology. 2011;36:S302-S304.
20. Thase ME, Youakim JM, Skuban A, et al. Adjunctive brexpiprazole 1 and 3 mg for patients with major depressive disorder following inadequate response to antidepressants: a phase 3, randomized, double-blind study. J Clin Psychiatry. 2015;76(00):000-000.
21. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders.Fourth Edition, Text Revision. Washington, DC: American Psychiatric Association; 2000.
22. Chandler GM, Iosifescu DV, Pollack MH, et al. RESEARCH: Validation of the Massachusetts General Hospital Antidepressant Treatment History Questionnaire (ATRQ). CNS Neurosci Ther. 2010;16(5):322-325. PubMed doi:10.1111/j.1755-5949.2009.00102.x
23. Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23(1):56-62. PubMed doi:10.1136/jnnp.23.1.56
24. Hamilton M. Development of a rating scale for primary depressive illness. Br J Soc Clin Psychol. 1967;6(4):278-296. PubMed doi:10.1111/j.2044-8260.1967.tb00530.x
25. Guy W. ECDEU Assessment Manual for Psychopharmacology. US Department of Health, Education, and Welfare Publication (ADM) 76-338. Rockville, MD: National Institute of Mental Health; 1976:218-222.
26. Montgomery SA, Asberg M. A new depression scale designed to be sensitive to change. Br J Psychiatry. 1979;134(4):382-389. PubMed doi:10.1192/bjp.134.4.382
27. Williams JBW, Kobak KA. Development and reliability of a structured interview guide for the Montgomery Asberg Depression Rating Scale (SIGMA). Br J Psychiatry. 2008;192(1):52-58. PubMed doi:10.1192/bjp.bp.106.032532
28. Sheehan DV. The Anxiety Disease. New York, NY: Charles Scribner & Sons; 1983.
29. Sheehan DV, Harnett-Sheehan K, Raj BA. The measurement of disability. Int Clin Psychopharmacol. 1996;11(suppl 3):89-95. PubMed doi:10.1097/00004850-199606003-00015
30. Hamilton M. The assessment of anxiety states by rating. Br J Med Psychol. 1959;32(1):50-55. PubMed doi:10.1111/j.2044-8341.1959.tb00467.x
31. Hamilton M. The diagnosis and rating of anxiety. In: Lader MH, ed. Studies of Anxiety. Kent: Br J Psychiatry Spec Publ; 1969:76-79.
32. Shear MK, Vander Bilt J, Rucci P, et al. Reliability and validity of a structured interview guide for the Hamilton Anxiety Rating Scale (SIGH-A). Depress Anxiety. 2001;13(4):166-178. PubMed doi:10.1002/da.1033
33. Williams JB. A structured interview guide for the Hamilton Depression Rating Scale. Arch Gen Psychiatry. 1988;45(8):742-747. PubMed doi:10.1001/archpsyc.1988.01800320058007
34. Rush AJ, Gullion CM, Basco MR, et al. The Inventory of Depressive Symptomatology (IDS): psychometric properties. Psychol Med. 1996;26(3):477-486. PubMed doi:10.1017/S0033291700035558
35. Simpson GM, Angus JW. A rating scale for extrapyramidal side effects. Acta Psychiatr Scand suppl. 1970;45(S212):11-19. PubMed doi:10.1111/j.1600-0447.1970.tb02066.x
36. Barnes TR. A rating scale for drug-induced akathisia. Br J Psychiatry. 1989;154(5):672-676. PubMed doi:10.1192/bjp.154.5.672
37. Chappell P, Feltner DE, Makumi C, et al. Initial validity and reliability data on the Columbia-Suicide Severity Rating Scale. Am J Psychiatry. 2012;169(6):662-663, author reply 663. PubMed doi:10.1176/appi.ajp.2012.12010123
38. Posner K, Brown GK, Stanley B, et al. The Columbia-Suicide Severity Rating Scale: initial validity and internal consistency findings from three multisite studies with adolescents and adults. Am J Psychiatry. 2011;168(12):1266-1277. PubMed doi:10.1176/appi.ajp.2011.10111704
39. Labbate LA, Lare SB. Sexual dysfunction in male psychiatric outpatients: validity of the Massachusetts General Hospital Sexual Functioning Questionnaire. Psychother Psychosom. 2001;70(4):221-225. PubMed doi:10.1159/000056257
40. Duru G, Fantino B. The clinical relevance of changes in the Montgomery-Asberg Depression Rating Scale using the minimum clinically important difference approach. Curr Med Res Opin. 2008;24(5):1329-1335. PubMed doi:10.1185/030079908X291958
41. Montgomery SA, Möller HJ. Is the significant superiority of escitalopram compared with other antidepressants clinically relevant? Int Clin Psychopharmacol. 2009;24(3):111-118. PubMed doi:10.1097/YIC.0b013e32832a8eb2
42. Berman RM, Marcus RN, Swanink R, et al. The efficacy and safety of aripiprazole as adjunctive therapy in major depressive disorder: a multicenter, randomized, double-blind, placebo-controlled study. J Clin Psychiatry. 2007;68(6):843-853. PubMed doi:10.4088/JCP.v68n0604
43. Berman RM, Fava M, Thase ME, et al. Aripiprazole augmentation in major depressive disorder: a double-blind, placebo-controlled study in patients with inadequate response to antidepressants. CNS Spectr. 2009;14(4):197-206. PubMed
44. Marcus RN, McQuade RD, Carson WH, et al. The efficacy and safety of aripiprazole as adjunctive therapy in major depressive disorder: a second multicenter, randomized, double-blind, placebo-controlled study. J Clin Psychopharmacol. 2008;28(2):156-165. PubMed doi:10.1097/JCP.0b013e31816774f9
45. Bauer M, Pretorius HW, Constant EL, et al. Extended-release quetiapine as adjunct to an antidepressant in patients with major depressive disorder: results of a randomized, placebo-controlled, double-blind study. J Clin Psychiatry. 2009;70(4):540-549. PubMed doi:10.4088/JCP.08m04629
46. El-Khalili N, Joyce M, Atkinson S, et al. Extended-release quetiapine fumarate (quetiapine XR) as adjunctive therapy in major depressive disorder (MDD) in patients with an inadequate response to ongoing antidepressant treatment: a multicentre, randomized, double-blind, placebo-controlled study. Int J Neuropsychopharmacol. 2010;13(7):917-932. PubMed doi:10.1017/S1461145710000015
47. Thase ME, Trivedi MH, Nelson JC, et al. Examining the efficacy of adjunctive aripiprazole in major depressive disorder: a pooled analysis of 2 studies. Prim Care Companion J Clin Psychiatry. 2008;10(6):440-447. PubMed doi:10.4088/PCC.v10n0603
48. Lakoff A. The mousetrap: managing the placebo effect in antidepressant trials. Mol Interv. 2002;2(2):72-76. PubMed doi:10.1124/mi.2.2.72
Save
Cite
Advertisement
GAM ID: sidebar-top