ABSTRACT
Objective: To determine if iloperidone, a second-generation antipsychotic, reduces symptoms of bipolar mania.
Methods: This phase 3, randomized, double-blind, placebo-controlled study was conducted in adults with bipolar mania at 27 US and international sites between April 2021 and September 2022. Participants were randomized 1:1 to iloperidone (up to 24 mg/d given twice daily) or placebo for 4 weeks. The primary efficacy endpoint was change from baseline to week 4 in Young Mania Rating Scale (YMRS) total score versus placebo. Secondary efficacy endpoints included change from baseline in the Clinical Global Impressions-Severity and Clinical Global Impression of Change scales.
Results: Altogether, 414 participants were randomized and administered at least 1 dose of study medication (iloperidone, n = 206; placebo, n = 208). Overall, 139 (67.1%) iloperidone patients and 153 (72.9%) placebo patients completed the study. Iloperidone demonstrated significant improvement versus placebo at week 4 for the primary and secondary endpoints. Differences in the least-squares mean (95% CI; P value) of change from baseline for YMRS total scores were −4.0 (−5.70 to −2.25; adjusted P = .000008). The most encountered adverse events with iloperidone were tachycardia, dizziness, dry mouth, alanine aminotransferase increased, nasal congestion, increased weight, and somnolence. The incidence of akathisia and extrapyramidal symptom–related treatment-emergent adverse events was low.
Conclusions: Iloperidone is effective in treating patients with bipolar mania. The tolerability and safety profile of iloperidone in bipolar mania is consistent with previous clinical studies of patients with schizophrenia, and no new safety concerns were identified.
Trial Registration: ClinicalTrials.gov identifier: NCT04819776; EudraCT: 2020–000405-83
J Clin Psychiatry 2024;85(1):23m14966
Author affiliations are listed at the end of this article.
Bipolar I disorder is a mood disorder characterized by at least 1 manic episode, presenting as increased energy, decreased need for sleep, increased psychomotor agitation, and racing thoughts or distractibility.1–3 Bipolar disorder is heritable,4–6 with an estimated lifetime prevalence for bipolar I disorder of 0.6%–1% in the general population,7–9 and has one of the highest rates of serious impairment among mood disorders.10,11 Patients can suffer from a plethora of associated comorbidities, including increased propensity for suicide and self-harm,12 substance abuse,13 obesity, and cardiovascular and metabolic disease,14 and have a severely decreased lifespan compared to the general population.15
Pharmacologic interventions for patients with bipolar disorder include second-generation antipsychotics (SGAs) and mood stabilizers as first-line treatment options,16,17 but tolerability and effectiveness can vary greatly from patient to patient. In 2011, a meta-analysis concluded that SGAs were more effective for treating bipolar mania than mood stabilizers (eg, lithium, divalproex, carbamazepine, and lamotrigine).18 This finding was later supported by a 2022 meta-analysis suggesting that most SGAs improve symptoms faster than mood stabilizers.17 This work also supported that SGAs improve psychotic symptoms in patients with bipolar mania, whereas all mood stabilizers analyzed did not.17 SGAs differ in their pharmacodynamic profiles, resulting in unique clinical profiles, especially on aspects of their safety and tolerability.19
Iloperidone is a second-generation antipsychotic approved by the US Food and Drug Administration (FDA) in 2009 for the treatment of schizophrenia in adults.20–24 Iloperidone and its primary metabolite, P88, possess high (nM) binding affinity for serotonin 5-HT2A and dopamine D2 and D3 receptors,25 and inhibition at these monoaminergic receptors is thought to contribute to the antimanic effects of iloperidone and other atypical antipsychotics, whether the bipolar mania is psychotic or nonpsychotic.25–27 Iloperidone and P88 are also potent norepinephrine NEα receptor antagonists, which has been proposed to account for the unique tolerability profile of iloperidone, including its reduced propensity for akathisia and extrapyramidal side effects (EPS) in comparison to other second-generation antipsychotics.28–31
Here, we report results from a phase 3, randomized, placebo-controlled study designed to assess the efficacy and safety of iloperidone for the acute treatment of manic or mixed episodes associated with bipolar I disorder (bipolar mania).
METHODS
Study Design and Patients
This phase 3, multicenter, randomized, double-blind, placebo-controlled, parallel-group study (ClinicalTrials.gov identifier NCT04819776; EudraCT identifier 2020–000405-83) was composed of 2 phases: a pre-randomization phase including a screening period (up to 7 days) and baseline evaluation period (1 day), and a double-blind, short-term treatment phase (28 days) designed to evaluate the efficacy and safety of iloperidone in the treatment of manic episodes (diagnosed using Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition [DSM-5] criteria).32 The study was conducted from April 4, 2021, through September 7, 2022.
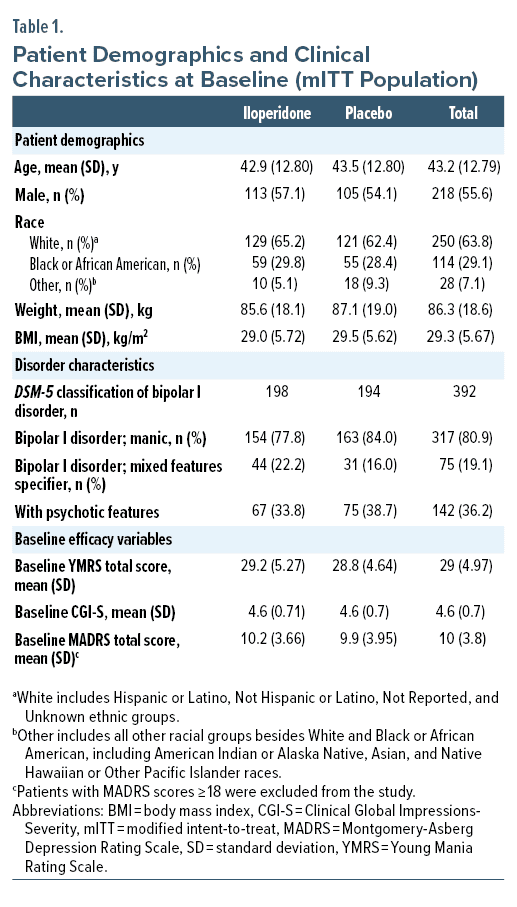
Male and female patients between 18–65 years of age, who had a diagnosis of bipolar I disorder, with or without mixed features, in accordance with DSM-5 criteria, as confirmed by the Structured Clinical Interview for Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition,33 were included in the study. Patients had at least 1 prior documented manic episode (with or without psychotic symptoms) that required treatment prior to screening and had a Young Mania Rating Scale (YMRS) total score34 ≥ 20, with ≥ 4 on at least 2 of 4 YMRS items (irritability, speech, content, disruptive/aggressive behavior); Clinical Global Impressions-Severity (CGI-S) score35 of ≥ 4 at baseline; and a Montgomery-Asberg Depression Rating Scale (MADRS) total score36 < 18. Patients were excluded if they met criteria for rapid cycling or had a DSM-5 diagnosis other than bipolar I disorder that was the primary focus of treatment within the previous 6 months. Exclusion criteria included electrocardiogram (ECG) abnormalities, chemical dependency (preceding 6 months), history of treatment-resistant psychotic symptoms based on poor response to 2 antipsychotic treatments over the last 2 years, risk of self-harm or harm to others, mental disability (moderate to severe), or inability to communicate, give informed consent, and/or participate fully in assignments due to these factors. Positive urine screening for drugs other than tetrahydrocannabinol and as-needed benzodiazepines or likely requirement for continuous treatment with any other psychotropic drug, including antidepressants or mood stabilizers, resulted in exclusion. Patients were not excluded due to current diagnosis or history of tardive dyskinesia or drug-induced EPS or ongoing treatment with anticholinergics.
The study protocol and all amendments were reviewed by the Independent Ethics Committee or Institutional Review Board for each center. The study was conducted according to the ethical principles of the Declaration of Helsinki.37 Informed consent was obtained from each patient in writing before any study-specific procedures were performed.
Interventions and Dose Selection
A fixed dose of 24 mg/d (12 mg twice daily) iloperidone (or 12 mg/d [6 mg twice daily] in CYP2D6 poor metabolizers [n = 15/206 for iloperidone], consistent with the current prescribing guidelines23) was given. A titration schedule consisting of 1 → 3 → 6 → 9 mg doses twice daily was used to reach target dose over 4 days (2 days for CYP2D6 poor metabolizers). All patients were hospitalized to ensure compliance with dosing and discontinued other antipsychotic treatment prior to their first dose of study medication. Rescue medications allowed on an as-needed basis included zolpidem for insomnia; short acting benzodiazepines (lorazepam unless unavailable) for agitation, anxiety, and severe restlessness; and anticholinergics (benztropine mesylate unless unavailable) for EPS. An optional 52-week long-term, open-label phase followed the randomized portion of the study.
Outcome Measures
Efficacy was evaluated using YMRS total score change from baseline to week 4. YMRS was also evaluated at weeks 1, 2, and 3. Other efficacy parameters included CGI-S score, Clinical Global Impression of Change (CGI-C) (equivalent to the Clinical Global Impressions-Improvement [CGI-I]),35 YMRS responder analysis, and the MADRS.
Safety was assessed via frequency of treatment-emergent adverse events (TEAEs) and serious adverse events (SAEs). Additional safety metrics included the frequency and severity of clinically notable or abnormal vital signs, urinalysis, hematology, and chemistry laboratory parameters; 12-lead ECG results; orthostatic response (≥ 20 mm Hg fall systolic blood pressure and/or ≥ 10 mm Hg fall in diastolic blood pressure); physical examination findings during treatment; and EPS as measured by Simpson-Angus Scale (SAS),38 Abnormal Involuntary Movement Scale (AIMS),39 and the Barnes Akathisia Rating Scale (BARS),40 as well as the Columbia-Suicide Severity Rating Scale (C-SSRS)41 and concomitant medication usage.
Analysis Populations
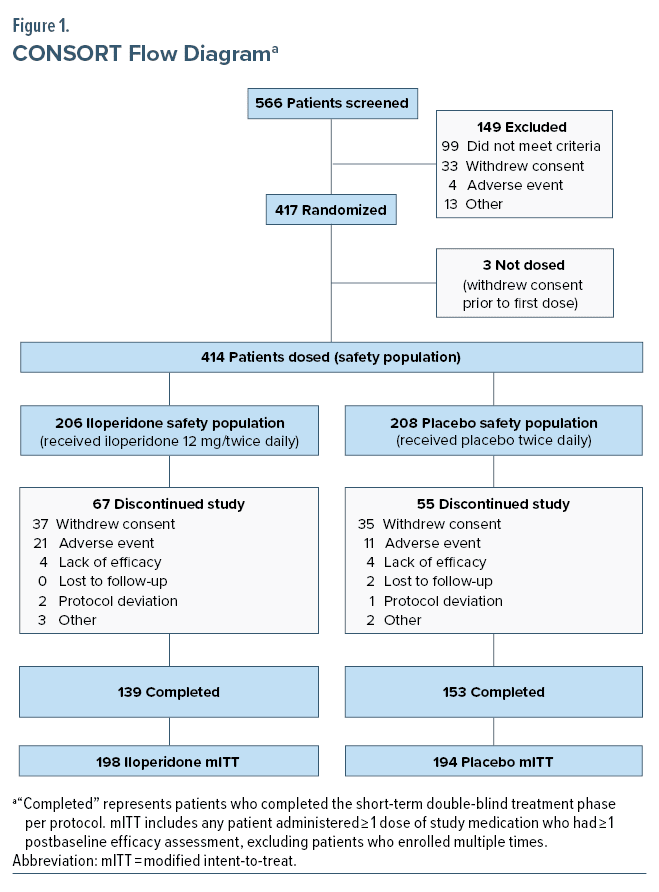
All efficacy analyses were based on the modified-intent-to-treat (mITT) population. The mITT population was defined as any randomized patient who received ≥ 1 dose of study medication and completed ≥ 1 post-baseline efficacy measurement. All safety measures were based on the safety population, defined as any randomized patient who received ≥ 1 dose of study medication.
Statistical Methods
The primary efficacy parameter for the double-blind phase was change from baseline to week 4 (day 28) in YMRS total score. A restricted maximum likelihood (REML)-based mixed-effects model for repeated measures (MMRM) was applied to analyze the primary efficacy endpoint in the mITT population and included the following time points: days 7, 10, 14, 21, and 28. The MMRM model included the fixed, categorical effects of treatment group, visit, treatment group–by-visit interaction, and pooled sites, as well as the fixed, continuous covariates of baseline score and the baseline score–by-visit interaction.
Secondary efficacy endpoints included change from baseline in YMRS total, CGI-S, and MADRS total score and were analyzed in a similar manner to the primary endpoint. CGI-C was collected post-baseline only and therefore analyzed using a similar model without including baseline terms. For the categorical endpoints, such as YMRS response, the Cochran-Mantel-Haenszel test was applied adjusting for pooled site at each visit.
Safety analysis assessed significant changes from baseline in vital signs, clinical laboratory results, ECG results, and SAS, AIMS, BARS, and C-SSRS evaluations. Fridericia’s QT correction was performed using the equation QTcF = QT interval/(RR interval)1/3.
Tests for statistical significance were performed at 2-sided 5% significance level; confidence intervals (CIs) were 2-sided 95% CIs.
RESULTS
Participants
Patient disposition, demographics, and disease characteristics at baseline are characterized in Table 1 and Figure 1. Of 392 patients in the mITT population, 333 (85%) were enrolled at 20 sites in the United States, 49 (12.5%) were enrolled at 6 sites in Bulgaria, and 10 (2.5%) were enrolled at 1 site in Poland. Disorder characteristics were similar between groups at baseline. Mean YMRS total score, CGI-S, and MADRS scores were similar between iloperidone and placebo groups at baseline evaluation.
Primary Efficacy Endpoint: Week 4 YMRS Change From Baseline
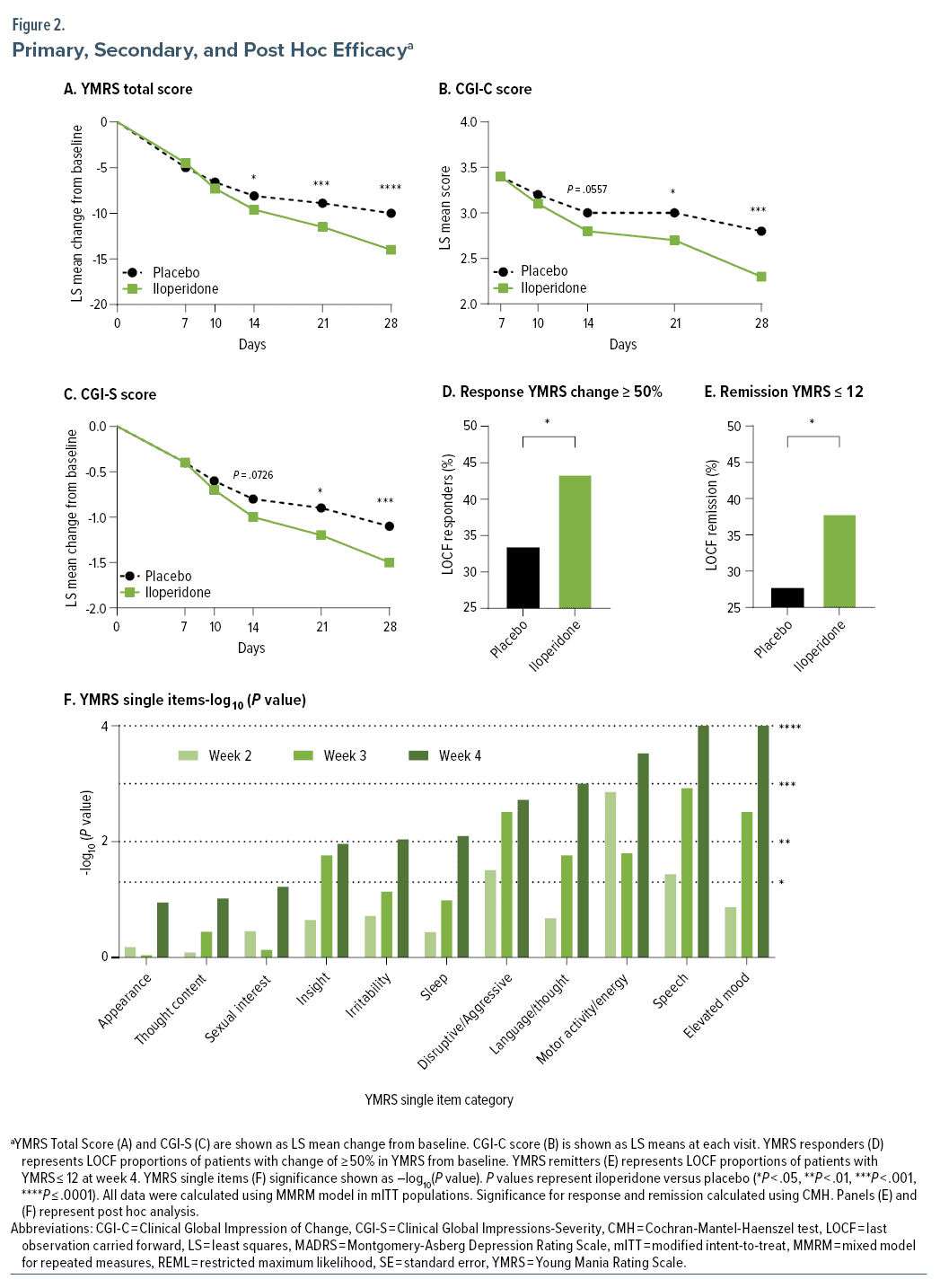
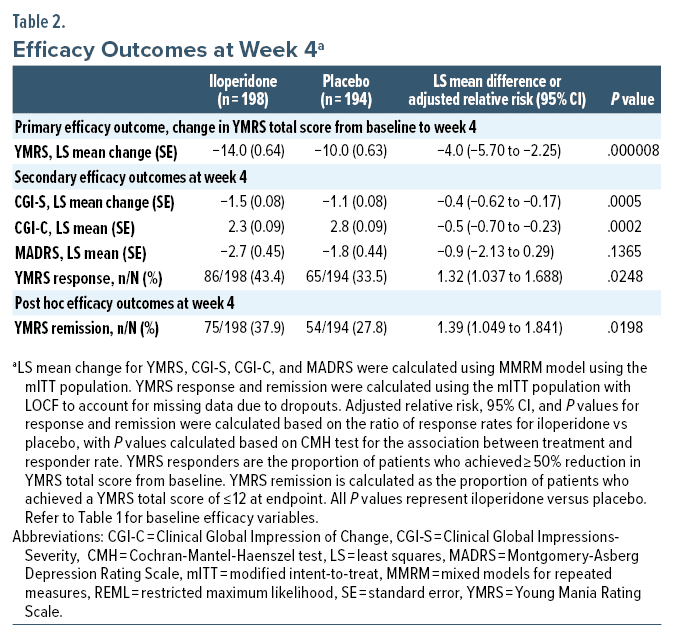
Change in YMRS total score from baseline to week 4 was statistically significant for iloperidone compared with placebo using the MMRM approach (Figure 2A, Table 2).
Secondary Efficacy Endpoint: YMRS Change From Baseline Prior to Week 4
Statistically significant difference between iloperidone and placebo was observed on days 14, 21, and 28 (Figure 2A).
Other Secondary Efficacy Endpoints
Statistically significant differences between iloperidone and placebo were also seen on all other secondary measures of efficacy at day 28 (CGI-S, CGI-C, YMRS responders), except for change from baseline in MADRS (Figure 2B–D, Table 2).
Safety Outcomes
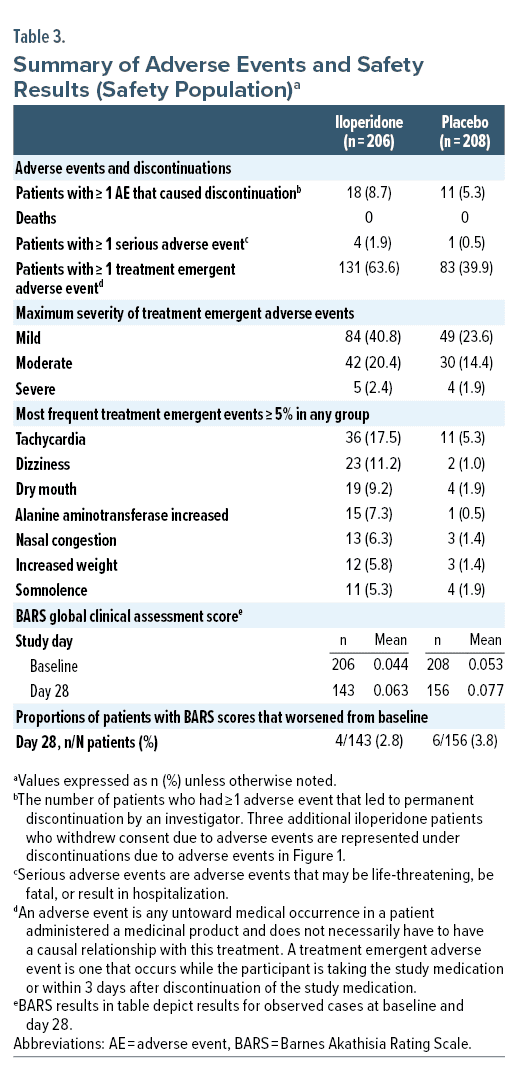
In the iloperidone group, 67.5% of patients experienced at least 1 adverse event, compared to 48.6% of patients in the placebo group. Patients withdrew from the study at a rate of 32.9% for iloperidone treated patients and 27.1% for placebo treated patients. In the iloperidone group, 18 (8.7%) patients had TEAEs leading to study drug discontinuation, compared to 11 (5.3%) patients in the placebo group. No TEAE associated with discontinuation occurred in more than 2 patients in either treatment group. No patient in the study experienced any AE resulting in death.
Adverse events were classified as common if they occurred in > 5% of any given group. In iloperidone treated patients, these events were tachycardia 17.5% (36/206 patients), dizziness 11.2% (23/206 patients), dry mouth 9.2% (19/206), alanine aminotransferase increased 7.3% (15/206 patients), nasal congestion 6.3% (13/206), increased weight 5.8% (12/206 patients), and somnolence 5.3% (11/206 patients) (Table 3).
There were 5 total serious adverse events (SAEs) in the safety population, with 4 occurring in iloperidone group (4/206 patients) and 1 occurring in the placebo group (1/208 patients) (Table 3). Two SAEs reported in the iloperidone group were identified as related to study medication (sedation and spontaneous penile erection), and 2 SAEs were identified as unrelated (gastrointestinal hemorrhage and respiratory distress). One patient in the placebo group had an SAE of bipolar disorder (depression).
Change from baseline in clinical laboratory parameters was generally similar across treatment groups. Compared to placebo, mild to moderate increases in alanine aminotransferase (ALT) and prolactin were observed in some iloperidone treated patients. A mean weight increase (SD) was observed for iloperidone treated patients (4.60 ± 4.271 kg) compared to placebo (1.63 ± 3.578 kg). No major differences were seen in mean fasting glucose for iloperidone and placebo from baseline to day 28 (iloperidone: 0.39 ± 0.933 mmol/L; placebo: 0.30 ± 1.240 mmol/L).
The average increase in ventricular rates from baseline was slightly higher for iloperidone treated patients compared to placebo during titration, but progressively decreased for iloperidone patients, with an average increase in ventricular rate of 4.7 and 1.5 beats per minute for iloperidone and placebo at week 4, respectively. The mean change in QT interval and QT interval corrected for heart rate using Fridericia’s correction (QTcF) from baseline to day 28 was −0.1 msec and +8.3 msec for iloperidone treated patients, respectively, and −3.5 msec and −1.0 msec for placebo treated patients, respectively. Post-randomization changes in QTcF interval of ≥ 60 msec from baseline were observed for 3 iloperidone patients and 0 placebo patients (1 instance for each patient).
Incidence of orthostatic response was higher for iloperidone patients than placebo patients during titration (range of 1.5%–6.4% vs 2.4%–3.1% for iloperidone and placebo treated patients, respectively), but was low and placebo-like on week 2, 3, and 4 visits, observed in 5.6% and 4.5% of iloperidone and placebo treated patients at week 4, respectively.
An AE of akathisia was reported in 9 (4.4%) and 0 iloperidone and placebo patients, respectively, and no patient discontinued due to akathisia. No statistically significant difference was identified between iloperidone and placebo groups for proportions of patients with worsening from baseline to any visit for BARS scores (Table 3).
Rates of EPS were low and similar to placebo for iloperidone patients. Mild TEAE of extrapyramidal disorder occurred in 2 patients in the iloperidone group and 0 patients in placebo group but did not result in dose modification or permanent discontinuations. No statistically significant difference was identified between iloperidone and placebo groups for change from baseline to any endpoint on the SAS or AIMS.
A total of 6 (3.4%) and 0 (0%) of patients in iloperidone and placebo groups received benztropine (3/6 patients for 1 to 4 total days and 3/6 patients for 18 to 23 total days); all 6 patients completed the study. One additional iloperidone patient had initiated benztropine ≥ 6 days before baseline and continued anticholinergic treatment throughout the study and completed.
No suicidal behavior was reported, and C-SSRS results were similar for patients receiving iloperidone and placebo throughout the study. Suicidal ideation was reported in 1 iloperidone patient and in 1 placebo patient.
DISCUSSION
In this phase 3 study, 24 mg/d iloperidone demonstrated efficacy for the acute treatment of bipolar mania in adults compared to placebo. Iloperidone was observed to be safe and well tolerated in adult patients with bipolar mania, consistent with previous evidence demonstrating iloperidone is safe and well tolerated in individuals with schizophrenia,42 and no new safety risks were identified.
YMRS total score change from baseline to 4 weeks was significantly greater for the iloperidone group compared to placebo. Statistical significance was detected as early as 14 days from the initial dose of study medication and was maintained throughout the remainder of the double-blind phase. A post hoc analysis of change from baseline in YMRS total score excluding patients given benzodiazepines revealed no difference in treatment effect for iloperidone mITT patient subgroups (Supplementary Table 1). Post hoc analysis also showed statistically significant improvement regardless of the presence or absence of psychotic features at baseline for iloperidone subgroups (Supplementary Table 2).
Other outcome measures provide further support for the efficacy of iloperidone in treating bipolar mania. Improvement on CGI-S, CGI-C, and YMRS response was greater for iloperidone vs placebo. Numerically higher change on MADRS score was observed at endpoint for iloperidone compared to placebo, although the treatment difference did not achieve statistical significance. This likely reflects that the study was not designed to evaluate patients experiencing moderate to severe depressive symptoms, limiting the ability to draw conclusions about iloperidone’s effect in this domain.
Post hoc analysis examined change from baseline to weeks 2, 3, and 4 in YMRS single items, which showed statistically significant superiority of iloperidone vs placebo groups as early as day 14 in the disruptive/aggressive behavior, increased motor activity energy, and speech items. Single item scores continued to improve on day 21 and achieved statistical significance in 8 of the 11 single items on day 28 (P = .0110 for insight, P < .01 for irritability, sleep, disruptive/aggressive behavior, language/thought disorder, increased motor activity energy, speech, and elevated mood) (Figure 2F).
Understanding the safety and tolerability of second-generation antipsychotics, particularly for long term or maintenance treatment, remains one of the major concerns for clinicians and patients.3,43–45 Outcomes of interest include those related to weight gain and metabolic disturbances,46 QT/QTc and cardiovascular safety,18,47 akathisia/EPS and tardive dyskinesia,48,49 and postural and non-postural changes in blood pressure.50,51
In this study, patients had mild to moderate weight gain compared to baseline, in alignment with previous studies of iloperidone in schizophrenia patient populations.
QTcF findings were similar to previously reported results at doses of 12 mg twice daily, including some adaptation to QTcF increases at day 28 evaluations24 and mean change in QTcF decreasing to 7 to 9 ms within 4 weeks after initiating treatment.24,47 The results of this study also support that QTc increases can be managed in practice by following recommended prescribing directions to avoid contraindicated metabolic inhibitors and to reduce the dosage by half in patients with impaired CYP2D6 metabolism.23
Though much improved compared to early antipsychotics, second-generation antipsychotics can still cause considerable adverse motor side effects.48,49 However, among all second-generation antipsychotics, iloperidone’s akathisia profile is favorable.28 In this study, the incidence of patients whose BARS scores worsened from baseline was low in the iloperidone group, with an incidence similar to placebo. Antipsychotic-induced akathisia has been reported more frequently in patients with bipolar disorder relative to schizophrenia populations treated with the same medication.29,52 The underlying reasons for observed differences in the two patient populations remains incompletely characterized, but clinical observations support that increased agitation and akathisia associated with depressive episodes complicates clinical evaluations of patients with bipolar disorder.53–55
Iloperidone has a unique receptor binding profile that includes strong affinity for the α1-adrenergic receptor.23 α1 Receptor antagonism is associated with a variety of physiological effects including decreased peripheral vascular resistance, which results in acute and sustained decreases in blood pressure. Selective α-adrenergic receptor antagonists have been developed to treat benign prostatic hyperplasia and hypertension.56 Central α1-adrenergic inhibition has also been postulated to have CNS effects. For example, some agents have been tested in the reduction of agitation in Alzheimer’s disease patients and in the reduction of nightmares in posttraumatic stress disorder patients.57–60 For iloperidone and other atypical antipsychotics, α-adrenergic receptor inhibition in the CNS has been hypothesized to contribute to their relatively low rates of akathisia and EPS.28–31,61 While the benefits of α-adrenergic inhibition vary across different conditions, antagonism of these receptors is also observed to result in decreased orthostatic response of blood pressure.25 Similar to other α-adrenergic receptor antagonists, orthostatic responses were seen more frequently during titration in iloperidone treated patients and then declined substantially to placebo-like rates on evaluations conducted after day 10. This supports previous findings that patients adapt to symptoms of postural blood pressure changes associated with α1-adrenergic inhibition despite the sustained decreases in blood pressure observed in hypertensive patients treated with such agents.62–65
This study provides evidence that iloperidone is effective as an intervention in acute treatment of bipolar mania in adults. Limitations include the exclusion of patients with significant comorbidities such as substance use disorders. Long-term efficacy in the prevention of manic or depressive episodes was not assessed. Of note, iloperidone has been approved for maintenance treatment of schizophrenia since 2016.23
This study provides evidence that iloperidone improves the symptoms of bipolar mania in adults and can be a useful treatment option for people with bipolar disorder. The safety profile for iloperidone was consistent with previous studies.
Article Information
Published Online: January 15, 2024. https://doi.org/10.4088/JCP.23m14966
© 2024 Physicians Postgraduate Press, Inc.
Submitted: June 20, 2023; accepted November 8, 2023.
To Cite: Torres R, Czeisler EL, Chadwick SR, et al. Efficacy and safety of iloperidone in bipolar mania: a double-blind, placebo-controlled study. J Clin Psychiatry. 2024;85(1):23m14966
Author Affiliations: Vanda Pharmaceuticals, Inc, Washington, DC (Torres, Czeisler, Chadwick, Smieszek, Xiao, C. M. Polymeropoulos, Birznieks, M. H. Polymeropoulos); Department of Psychiatry, University of California, San Diego (Stahl).
Corresponding Author: Rosarelis Torres, PhD, 2200 Pennsylvania NW, Suite 300-E, Washington, DC 20037 ([email protected]).
Relevant Financial Relationships: Drs Torres, Chadwick, Smieszek, Xiao, C. M. Polymeropoulos, and M. H. Polymeropoulos; Mr Birznieks; and Ms Czeisler are full-time employees of Vanda Pharmaceuticals Inc. and may hold company stock and/or stock options. Dr Stahl has served as a consultant to Acadia, Alkermes, Allergan, AbbVie, Arbor Pharmaceuticals, Axovant, Axsome, Celgene, Concert, Clearview, EMD Serono, Eisai Pharmaceuticals, Ferring, Impel NeuroPharma, Intra-Cellular Therapies Inc., Ironshore Pharmaceuticals, Janssen, Karuna, Lilly, Lundbeck, Merck, Otsuka, Pfizer, Relmada, Sage Therapeutics, Servier, Shire, Sunovion, Takeda, Taliaz, Teva, Tonix, Tris Pharma, Vanda Pharmaceuticals, and Viforpharma; is a board member of Genomind; has served on speakers bureaus for Acadia, Lundbeck, Otsuka, Perrigo, Servier, Sunovion, Takeda, Teva, and Vertex; and has received research and/or grant support from Acadia, Avanir, Braeburn Pharmaceuticals, Eli Lilly, Intra-Cellular Therapies Inc., Ironshore, ISSWSH, Neurocrine, Otsuka, Shire, Sunovion, and TMS NeuroHealth Centers.
Funding/Support: This study was funded by Vanda Pharmaceuticals, Inc. (Washington, DC).
Role of the Funders/Sponsors: The sponsor was responsible for the design, analysis, interpretation, and publication of this study. Final approval for the decisions to submit the manuscript was the sole decision of the authors.
Previous Presentations: Posters presented at the American Association of Psychiatric Pharmacists meeting, Atlanta, Georgia, April 16–19, 2023; American Society of Clinical Psychopharmacology meeting, Miami, Florida, May 30– June 2, 2023; SLEEP meeting, Indianapolis, Indiana, June 3–7, 2023; and International Society for Bipolar Disorders meeting, Chicago, Illinois, June 22– 25, 2023.
Acknowledgments: Vanda Pharmaceuticals and the authors thank the patients, study sites, and investigators who participated in this clinical trial. Vanda Pharmaceuticals and the authors also thank Leslie Citrome, MD, MPH, who provided medical writing and editorial assistance for this manuscript under the direction of the authors, funded by Vanda Pharmaceuticals.
ORCID: Sean R. Chadwick: https://orcid.org/0000-0003-2424-7983; Sandra M. Smieszek: https://orcid.org/0000-0002-8006-0454; Stephen M. Stahl: https://orcid.org/0000-0002-6536-6973
Supplementary Material: Available at Psychiatrist.com.
CLINICAL POINTS
- Numerous agents are available for bipolar mania, although many patients cannot find a suitable treatment option.
- Iloperidone can provide a new option to treat bipolar mania with a favorable side effect profile for the categories of akathisia/extrapyramidal side effects, weight gain, and sedation.
References (65)

- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. Fifth Edition, Text Revision. American Psychiatric Association; 2022.
- Leboyer M, Kupfer DJ. Bipolar disorder: new perspectives in health care and prevention. J Clin Psychiatry. 2010;71(12):1689–1695. PubMed CrossRef
- McIntyre RS, Berk M, Brietzke E, et al. Bipolar disorders. Lancet. 2020;396(10265):1841–1856. PubMed CrossRef
- Lichtenstein P, Yip BH, Björk C, et al. Common genetic determinants for schizophrenia and bipolar disorder in Swedish families: a population-based study. Lancet. 2009;373(9659):234–239. PubMed CrossRef
- Stahl EA, Breen G, Forstner AJ, et al; eQTLGen Consortium; BIOS Consortium; Bipolar Disorder Working Group of the Psychiatric Genomics Consortium. Genome-wide association study identifies 30 loci associated with bipolar disorder. Nat Genet. 2019;51(5):793–803. PubMed CrossRef
- Mullins N, Forstner AJ, O’Connell KS, et al; HUNT All-In Psychiatry. Genome-wide association study of more than 40,000 bipolar disorder cases provides new insights into the underlying biology. Nat Genet. 2021;53(6):817–829. PubMed CrossRef
- Rowland TA, Marwaha S. Epidemiology and risk factors for bipolar disorder. Ther Adv Psychopharmacol. 2018;8(9):251–269. PubMed CrossRef
- Pini S, de Queiroz V, Pagnin D, et al. Prevalence and burden of bipolar disorders in European countries. Eur Neuropsychopharmacol. 2005;15(4):425–434. PubMed CrossRef
- Bebbington P, Ramana R. The epidemiology of bipolar affective disorder. Soc Psychiatry Psychiatr Epidemiol. 1995;30(6):279–292. PubMed CrossRef
- Kessler RC, Chiu WT, Demler O, et al. Prevalence, severity, and comorbidity of 12-month DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62(6):617–627. PubMed CrossRef
- Merikangas KR, Akiskal HS, Angst J, et al. Lifetime and 12-month prevalence of bipolar spectrum disorder in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2007;64(5):543–552. PubMed CrossRef
- Dong M, Lu L, Zhang L, et al. Prevalence of suicide attempts in bipolar disorder: a systematic review and meta-analysis of observational studies. Epidemiol Psychiatr Sci. 2019;29:e63. PubMed CrossRef
- Messer T, Lammers G, Müller-Siecheneder F, et al. Substance abuse in patients with bipolar disorder: a systematic review and meta-analysis. Psychiatry Res. 2017;253:338–350. PubMed CrossRef
- Coello K, Kjærstad HL, Stanislaus S, et al. Thirty-year cardiovascular risk score in patients with newly diagnosed bipolar disorder and their unaffected first-degree relatives. Aust N Z J Psychiatry. 2019;53(7):651–662. PubMed CrossRef
- Hayes JF, Miles J, Walters K, et al. A systematic review and meta-analysis of premature mortality in bipolar affective disorder. Acta Psychiatr Scand. 2015;131(6).
- Yatham LN, Kennedy SH, Parikh SV, et al. Canadian Network for Mood and Anxiety Treatments (CANMAT) and International Society for Bipolar Disorders (ISBD) 2018 guidelines for the management of patients with bipolar disorder. Bipolar Disord. 2018;20(2):97–170. PubMed CrossRef
- Kishi T, Ikuta T, Matsuda Y, et al. Pharmacological treatment for bipolar mania: a systematic review and network meta-analysis of double-blind randomized controlled trials. Mol Psychiatry. 2022;27(2):1136–1144. PubMed CrossRef
- Cipriani A, Barbui C, Salanti G, et al. Comparative efficacy and acceptability of antimanic drugs in acute mania: a multiple-treatments meta-analysis. Lancet. 2011;378(9799):1306–1315. PubMed CrossRef
- American Psychiatric Association. The American Psychiatric Association Practice Guideline for the Treatment of Patients With Schizophrenia, Third Edition. American Psychiatric Association; 2021.
- Citrome L. Iloperidone: a clinical overview. J Clin Psychiatry. 2011;72(suppl 1):19–23. PubMed CrossRef
- Citrome L. Iloperidone for schizophrenia: a review of the efficacy and safety profile for this newly commercialised second-generation antipsychotic. Int J Clin Pract. 2009;63(8):1237–1248. PubMed CrossRef
- Citrome L. Iloperidone: chemistry, pharmacodynamics, pharmacokinetics and metabolism, clinical efficacy, safety and tolerability, regulatory affairs, and an opinion. Expert Opin Drug Metab Toxicol. 2010;6(12):1551–1564. PubMed CrossRef
- Fanapt (iloperidone). Package insert. Vanda Pharmaceuticals Inc; 2017. https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/022192s018s021lbl.pdf
- Cutler AJ, Kalali AH, Weiden PJ, et al. Four-week, double-blind, placebo- and ziprasidone-controlled trial of iloperidone in patients with acute exacerbations of schizophrenia. J Clin Psychopharmacol. 2008;28(suppl 1):S20–S28. PubMed CrossRef
- Stahl SM. Stahl’s Essential Psychopharmacology: Neuroscientific Basis and Practical Applications. Cambridge University Press; 2021.
- Citrome L, Goldberg JF, Stahl SM. Toward convergence in the medication treatment of bipolar disorder and schizophrenia. Harv Rev Psychiatry. 2005;13(1):28–42. PubMed CrossRef
- Svensson TH. Alpha-adrenoceptor modulation hypothesis of antipsychotic atypicality. Prog Neuropsychopharmacol Biol Psychiatry. 2003;27(7):1145–1158. PubMed CrossRef
- Citrome L. Activating and sedating adverse effects of second-generation antipsychotics in the treatment of schizophrenia and major depressive disorder: absolute risk increase and number needed to harm. J Clin Psychopharmacol. 2017;37(2):138–147. PubMed CrossRef
- Chow CL, Kadouh NK, Bostwick JR, et al. Akathisia and newer second-generation antipsychotic drugs: a review of current evidence. Pharmacotherapy. 2020;40(6):565–574. PubMed CrossRef
- Stahl SM. Role of α1 adrenergic antagonism in the mechanism of action of iloperidone: reducing extrapyramidal symptoms. CNS Spectr. 2013;18(6):285–288. PubMed CrossRef
- Musco S, McAllister V, Caudle I. Dopamine-receptor blocking agent-associated akathisia: a summary of current understanding and proposal for a rational approach to treatment. Ther Adv Psychopharmacol. 2020;10:2045125320937575. PubMed CrossRef
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition: DSM-5. American Psychiatric Association; 2013.
- First MB, Williams JBW, Karg RS, et al. Structured Clinical Interview for DSM-5 Disorders, Clinical Trials Version (SCID-5-CT). American Psychiatric Association; 2015.
- Young RC, Biggs JT, Ziegler VE, et al. A rating scale for mania: reliability, validity and sensitivity. Br J Psychiatry. 1978;133(5):429–435. PubMed CrossRef
- Guy W. Clinical Global Impressions. ECDEU Assessment Manual for Psychopharmacology. Revised (DHEW Publication No ADM 76–338). National Institute of Mental Health; 1976:218–222.
- Montgomery SA, Asberg M. A new depression scale designed to be sensitive to change. Br J Psychiatry. 1979;134(4):382–389. PubMed CrossRef
- Holm S. Declaration of Helsinki. In: International Encyclopedia of Ethics. Wiley; 2019.
- Simpson GM, Angus JW. A rating scale for extrapyramidal side effects. Acta Psychiatr Scand suppl. 1970;45(s212):11–19. PubMed CrossRef
- Gharabawi GM, Bossie CA, Lasser RA, et al. Abnormal Involuntary Movement Scale (AIMS) and Extrapyramidal Symptom Rating Scale (ESRS): cross-scale comparison in assessing tardive dyskinesia. Schizophr Res. 2005;77(2–3):119–128. PubMed CrossRef
- Barnes TRE. A rating scale for drug-induced akathisia. Br J Psychiatry. 1989;154(5):672–676. PubMed CrossRef
- Posner K, Brent D, Lucas C, et al. Columbia-Suicide Severity Rating Scale (C-SSRS). Published online 2008. https://cssrs.columbia.edu/wp-content/uploads/C-SSRS_Pediatric-SLC_11.14.16.pdf
- Nair A, Salem A, Asamoah AL, et al. An update on the efficacy and safety of iloperidone as a schizophrenia therapy. Expert Opin Pharmacother. 2020;21(15):1793–1798. PubMed CrossRef
- Jauhar S, Young AH. Controversies in bipolar disorder; role of second-generation antipsychotic for maintenance therapy. Int J Bipolar Disord. 2019;7(1):10. PubMed CrossRef
- Maina G, Salvi V, Vitalucci A, et al. Prevalence and correlates of overweight in drug-naïve patients with bipolar disorder. J Affect Disord. 2008;110(1-2):149–155. PubMed CrossRef
- Goldstein BI, Liu SM, Zivkovic N, et al. The burden of obesity among adults with bipolar disorder in the United States. Bipolar Disord. 2011;13(4):387–395. PubMed CrossRef
- Dayabandara M, Hanwella R, Ratnatunga S, et al. Antipsychotic-associated weight gain: management strategies and impact on treatment adherence. Neuropsychiatr Dis Treat. 2017;13:2231–2241. PubMed CrossRef
- Potkin SG, Preskorn S, Hochfeld M, et al. A thorough QTc study of 3 doses of iloperidone including metabolic inhibition via CYP2D6 and/or CYP3A4 and a comparison to quetiapine and ziprasidone. J Clin Psychopharmacol. 2013;33(1):3–10. PubMed CrossRef
- Demyttenaere K, Detraux J, Racagni G, et al. Medication-induced akathisia with newly approved antipsychotics in patients with a severe mental illness: a systematic review and meta-analysis. CNS Drugs. 2019;33(6):549–566. PubMed CrossRef
- Carbon M, Kane JM, Leucht S, et al. Tardive dyskinesia risk with first- and second-generation antipsychotics in comparative randomized controlled trials: a meta-analysis. World Psychiatry. 2018;17(3):330–340. PubMed CrossRef
- Bhanu C, Nimmons D, Petersen I, et al. Drug-induced orthostatic hypotension: a systematic review and meta-analysis of randomised controlled trials. PLoS Med. 2021;18(11):e1003821. PubMed CrossRef
- Gugger JJ. Antipsychotic pharmacotherapy and orthostatic hypotension: identification and management. CNS Drugs. 2011;25(8):659–671. PubMed CrossRef
- Citrome L, Yatham LN, Patel MD, et al. Cariprazine and akathisia, restlessness, and extrapyramidal symptoms in patients with bipolar depression. J Affect Disord. 2021;288:191–198. PubMed CrossRef
- Penders TM, Agarwal S, Rohaidy R. Persistent akathisia masquerading as agitated depression after use of ziprasidone in the treatment of bipolar depression. Neuropsychiatr Dis Treat. 2013;9:463–465. PubMed CrossRef
- Citrome L. Addressing the need for rapid treatment of agitation in schizophrenia and bipolar disorder: focus on inhaled loxapine as an alternative to injectable agents. Ther Clin Risk Manag. 2013;9(1):235–245. PubMed CrossRef
- Bjarke J, Gjerde HN, Jørgensen HA, et al. Akathisia and atypical antipsychotics: relation to suicidality, agitation and depression in a clinical trial. Acta Neuropsychiatr. 2022;34(5):282–288. PubMed CrossRef
- Frishman WH, Kotob F. Alpha-adrenergic blocking drugs in clinical medicine. J Clin Pharmacol. 1999;39(1):7–16. PubMed CrossRef
- Wang LY, Shofer JB, Rohde K, et al. Prazosin for the treatment of behavioral symptoms in Alzheimer’s disease patients with agitation and aggression. Am J Geriatr Psychiatry. 2009;17(9):744–751. PubMed CrossRef
- Richards A, Inslicht S, Ruoff LM, et al. An open-label study of doxazosin extended-release for PTSD: findings and recommendations for future research on doxazosin. Focus Am Psychiatr Publ. 2018;16(1):67–73. PubMed CrossRef
- Smith C, Koola MM. Evidence for using doxazosin in the treatment of posttraumatic stress disorder. Psychiatr Ann. 2016;46(9):553–555. PubMed CrossRef
- Grossberg GT, Kohegyi E, Mergel V, et al. Efficacy and safety of brexpiprazole for the treatment of agitation in Alzheimer’s dementia: two 12-week, randomized, double-blind, placebo-controlled trials. Am J Geriatr Psychiatry. 2020;28(4):383–400. PubMed CrossRef
- Tarazi FI, Stahl SM. Iloperidone, asenapine and lurasidone: a primer on their current status. Expert Opin Pharmacother. 2012;13(13):1911–1922. PubMed CrossRef
- Stanaszek WF, Kellerman D, Brogden RN, et al. Prazosin update: a review of its pharmacological properties and therapeutic use in hypertension and congestive heart failure. Drugs. 1983;25(4):339–384. PubMed CrossRef
- Raskind MA, Millard SP, Petrie EC, et al. Higher pretreatment blood pressure is associated with greater posttraumatic stress disorder symptom reduction in soldiers treated with prazosin. Biol Psychiatry. 2016;80(10):736–742. PubMed CrossRef
- Elliott HL, Donnelly R, Meredith PA, et al. Predictability of antihypertensive responsiveness and alpha-adrenoceptor antagonism during prazosin treatment. Clin Pharmacol Ther. 1989;46(5):576–583. PubMed CrossRef
- Donnelly R, Meredith PA, Elliott HL. Pharmacokinetic-pharmacodynamic relationships of α-adrenoceptor antagonists. Clin Pharmacokinet. 1989;17(4):264–274. PubMed CrossRef
Save
Cite
Advertisement
GAM ID: sidebar-top