Objective: We performed a double-blind, randomized, placebo-controlled trial to assess the efficacy of topiramate in the treatment of crack cocaine dependence.
Method: Sixty men who were dependent on cocaine (DSM-IV) (exclusive use of crack cocaine) were selected. The subjects were randomly assigned to either a topiramate group (subjects received 50-200 mg of topiramate per day for 12 weeks) or a control group (subjects received placebo). The initial daily treatment dose was 50 mg, and this dose was increased weekly at increments of 25 to 50 mg, based on the subject’s tolerability, to a maximum of 200 mg. All of the subjects also participated in motivational interviews and group therapy. The primary outcome measures were detection of benzoylecgonine in the urine, study retention, frequency of cocaine smoking, amount of cocaine use, and mean amount of money spent on cocaine per week. The study was conducted from February 2013 to February 2014.
Results: Twenty-nine subjects in the topiramate group and 29 subjects in the control group completed the study. Longitudinal assessment revealed that retention was not significant (odds ratio [OR] = 1.072, P = .908) between the 2 groups. Negative results from a urine test for benzoylecgonine (a cocaine metabolite), which is a measure of cocaine abstinence, were more frequently obtained from the topiramate group (OR = 8.687, P < .001). Topiramate reduced the quantity of cocaine used (mean reduction = −3.108 g, P < .001), the frequency of cocaine use (mean = −0.784 times per week, P = .005), and the amount of money spent on cocaine (mean [US dollars] = −$25.38, P = .015; this variable did not achieve statistical significance after Bonferroni correction) compared with the placebo during the 12 weeks (or 84 days) of the assessment. However, the differences in reductions between the 2 groups in the quantity of cocaine used, the frequency of cocaine use, and money spent on cocaine over time (time ×— group interaction) were present only during the first 4 weeks, and none of these variables by 12 weeks. The studied groups did not differ with regard to secondary end points, such as study dropout and the number of subjects who reported side effects.
Conclusions: The present findings indicate that topiramate is effective and safe and thus reinforce previous data suggesting that topiramate is a potentially useful treatment for crack cocaine dependence. However, we found that topiramate is only useful as an adjunctive treatment during the first 4 weeks of the treatment. Future studies with larger samples are needed to confirm these results.
Trial Registration: Registro Brasileiro de Ensaios Cl×nicos (ReBEC) RBR-3vwfjs and UTN: U1111-1131-4443

Efficacy of Topiramate in the Treatment of Crack Cocaine Dependence:
A Double-Blind, Randomized, Placebo-Controlled Trial

ABSTRACT
Objective: We performed a double-blind, randomized, placebo-controlled trial to assess the efficacy of topiramate in the treatment of crack cocaine dependence.
Method: Sixty men who were dependent on cocaine (DSM-IV) (exclusive use of crack cocaine) were selected. The subjects were randomly assigned to either a topiramate group (subjects received 50-200 mg of topiramate per day for 12 weeks) or a control group (subjects received placebo). The initial daily treatment dose was 50 mg, and this dose was increased weekly at increments of 25 to 50 mg, based on the subject’s tolerability, to a maximum of 200 mg. All of the subjects also participated in motivational interviews and group therapy. The primary outcome measures were detection of benzoylecgonine in the urine, study retention, frequency of cocaine smoking, amount of cocaine use, and mean amount of money spent on cocaine per week. The study was conducted from February 2013 to February 2014.
Results: Twenty-nine subjects in the topiramate group and 29 subjects in the control group completed the study. Longitudinal assessment revealed that retention was not significant (odds ratio [OR] = 1.072, P = .908) between the 2 groups. Negative results from a urine test for benzoylecgonine (a cocaine metabolite), which is a measure of cocaine abstinence, were more frequently obtained from the topiramate group (OR = 8.687, P < .001). Topiramate reduced the quantity of cocaine used (mean reduction = −3.108 g, P < .001), the frequency of cocaine use (mean = −0.784 times per week, P = .005), and the amount of money spent on cocaine (mean [US dollars] = −$25.38, P = .015; this variable did not achieve statistical significance after Bonferroni correction) compared with the placebo during the 12 weeks (or 84 days) of the assessment. However, the differences in reductions between the 2 groups in the quantity of cocaine used, the frequency of cocaine use, and money spent on cocaine over time (time ×— group interaction) were present only during the first 4 weeks, and none of these variables by 12 weeks. The studied groups did not differ with regard to secondary end points, such as study dropout and the number of subjects who reported side effects.
Conclusions: The present findings indicate that topiramate is effective and safe and thus reinforce previous data suggesting that topiramate is a potentially useful treatment for crack cocaine dependence. However, we found that topiramate is only useful as an adjunctive treatment during the first 4 weeks of the treatment. Future studies with larger samples are needed to confirm these results.
Trial Registration: Registro Brasileiro de Ensaios Cl×nicos (ReBEC) RBR-3vwfjs and UTN: U1111-1131-4443
J Clin Psychiatry 2016;77(3):398-406
dx.doi.org/10.4088/JCP.14m09377
© Copyright 2016 Physicians Postgraduate Press, Inc.
aDepartment of Psychiatry, Federal University of Tocantins, Palmas, Brazil
bDepartment of Psychiatry, Federal University of São Paulo, Sao Paulo, Brazil
cPsychosocial Care Center for Alcohol and Drugs (CAPS-AD) in Palmas, State of Tocantins, Brazil
dLiNC—Laboratório Interdisciplinar de Neurociências Cl×nicas (Interdisciplinary Laboratory of Clinical Neurosciences), Department of Psychiatry, Federal University of São Paulo, Sao Paulo, Brazil
*Corresponding author: Leonardo Baldaçara, MD, PhD, Federal University of Tocantins, CUP—Medicina, Av. NS 15 ALC NO 14, 109 Norte, Caixa Postal 114, Palmas—TO, Brazil, CEP 77001-090 ([email protected]).
Cocaine abuse and dependence is a chronic brain disease that has been a global public health problem for several decades.1 According to the United Nations Office on Drugs and Crime World Drug Report 2014, cocaine use remained stable over 2012, with 14 million to 21 million estimated users globally, and it is high in North and South America (1.8% and 1.2% annual prevalence rates, respectively), Oceania (1.5%), and Western and Central Europe (1.0%).2
The purified, alkaloidal, extrapotent form of cocaine, denoted crack cocaine, is produced from cocaine hydrochloride through conversion processes that make it suitable for smoking.2 This conversion is usually accomplished by mixing powdered cocaine with baking soda or ammonia in water. However, in Brazil, the use of other compounds, such as gasoline, kerosene, and quicklime, has been reported, and these methods may be cheaper but are markedly more harmful.3
In Brazil, the use of stimulants is increasing, and crack cocaine is one of the most widely used forms, which has extremely negative health, economic, and social consequences.4 A recent epidemiologic study conducted in Brazil revealed that the prevalence of crack cocaine use was 2.2% in the overall population (excluding the elderly population) and that cocaine addiction was identified in 41.4% of users in 2013.4
Despite significant advances within the last decade in our understanding of the neurobiological changes that are associated with substance abuse and dependence, few pharmacologic interventions have been proven to be effective in the treatment of cocaine dependence, particularly crack cocaine.5,6 Although the mechanism of action of topiramate is not yet completely understood, increasing lines of evidence have demonstrated that it acts as a broad-spectrum anticonvulsant that modulates the function of both voltage-gated and ligand-gated γ-aminobutyric acid-A receptors (GABAA) as well as glutamate neurotransmitters, which, in turn, modulate the brain’s reward system. As a result, topiramate has been considered a potentially useful treatment for cocaine and alcohol dependence.5,7-11
Furthermore, animal studies have suggested that agents that either antagonize the excitatory effects of glutamate or facilitate the inhibitory action of GABA within the mesocorticolimbic dopamine system can reduce the reinforcing effects of cocaine by reducing the release of corticolimbic dopamine.12,13 In humans, preliminary findings from several studies have suggested that topiramate is an effective treatment for alcohol,14,15 methamphetamine,16 and cocaine dependence.1,17
The previous studies that have evaluated the efficacy of topiramate in the treatment of cocaine dependence have been largely preliminary in nature. Therefore, the main goal of the present clinical trial was to evaluate the efficacy of topiramate in the treatment of crack cocaine dependence. Its tolerability and safety were assessed as secondary end points. On the basis of findings of previous studies that involved both animals and humans, we hypothesize that topiramate will be an effective treatment for cocaine dependence.
METHOD
Design
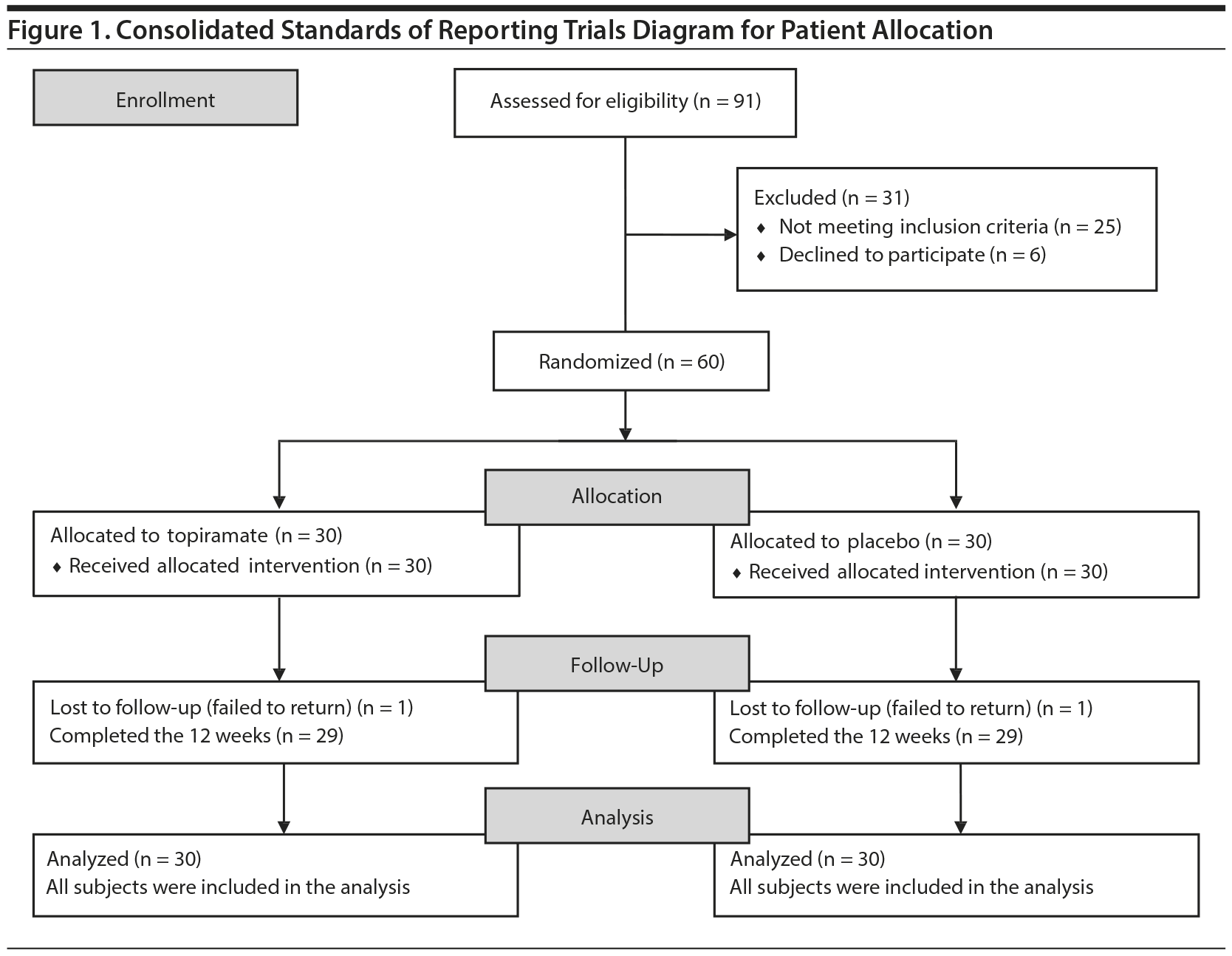
This study was a double-blind, randomized, placebo-controlled trial involving subjects with a DSM-IV diagnosis of cocaine dependence who used crack cocaine exclusively. This clinical study was conducted from February 2013 to February 2014, and randomization was achieved by the allocation of permuted blocks. Each drug regimen was assigned to blocks of 6 patients and distributed in the following order: topiramate, placebo, placebo, topiramate, topiramate, and placebo. This assignment was repeated until all of the individuals (60) were assigned a drug regimen. The trial flow of this study is shown in Figure 1.
Participants
Sixty male outpatients with a DSM-IV diagnosis of cocaine dependence (who exclusively used crack cocaine) were recruited using the Composite International Diagnostic Interview (CIDI).18 Patients spontaneously sought care at the Psychosocial Care Center for Alcohol and Drugs (CAPS-AD) in Palmas, Brazil. First, the outpatients were assessed by a psychiatrist. After signing an informed consent form, which was approved by our institutional review board, the participants were enrolled in the study. Additionally, all of the subjects underwent motivational interviews (8 sessions) and group therapy (12 sessions) treatments that were performed by qualified professionals.
This study utilized the following inclusion criteria: a diagnosis of cocaine dependence according to the DSM-IV, a self-reported exclusive use of crack cocaine, age between 18 and 50 years, and voluntary agreement to participate in the study after signing an informed consent form. The following exclusion criteria were utilized: self-reported use of another form of cocaine (snorted or intravenous), severe physical illness, other Axis I psychiatric disorders (except nicotine dependence), history of intolerance to topiramate, and concomitant use of psychotropic medications during the trial period.
Interventions
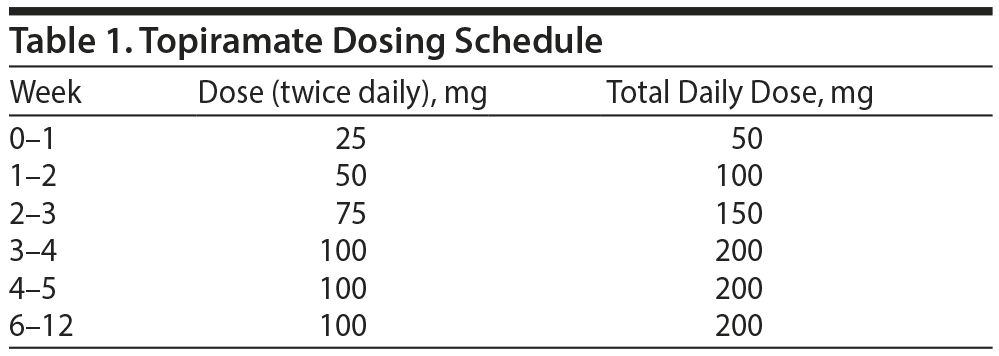
The subjects were randomly assigned to either a group that was treated with topiramate at a dose of 50 to 200 mg per day for 12 weeks or a control group that received placebo. Daily topiramate treatment was started at a dose of 50 mg and increased weekly in increments of 25 to 50 mg according to the subject’s tolerability (to a maximum of 200 mg daily). During weeks 4 through 12, the subjects received their maximum tolerated dose, which was determined during the first 4 weeks of the study (Table 1). During each visit with the research team, the subjects returned any unused tablets to monitor treatment adherence.
The investigational product was packaged in identical boxes that were coded using numbers. At the beginning of the study, the participants’ demographic information, medical history, and family history of psychiatric disorders were obtained. All of the eligible subjects were subjected to a neurologic examination.
All of the subjects were evaluated weekly by a trained psychiatrist. Both the subject and the psychiatrist were blinded to the subject’s treatment assignment. The clinical safety of the treatment was assessed by spontaneous reporting and an open inquiry regarding adverse events as well as by physical examination and an assessment of vital signs on each visit. Adverse events and retention were carefully monitored throughout the study. Reasons for subject discontinuation included the following: withdrawal of consent, safety and/or tolerability issues, lack of efficacy, and significant protocol deviation.
During the 12-week treatment, 8 motivational interview sessions19 and 12 group therapy sessions20 were conducted. The subjects who were discontinued from the study continued attending the motivational interview sessions.
End Points
The primary end points included the following measures: detection of benzoylecgonine (a cocaine metabolite, cutoff value ≥ 300 ng/mL, qualitative measurement) in the urine twice a week (a total of 24 measurements), retention (ie, number of patients who completed the study without data loss resulting in a compromised analysis), frequency of cocaine smoking (ie, mean number of days per week), amount of cocaine (ie, mean daily use, in grams), and mean amount of money spent on cocaine per week. The secondary end points included the occurrence of adverse events and the number of dropouts (ie, patients for whom outcome data were missing after a certain point).
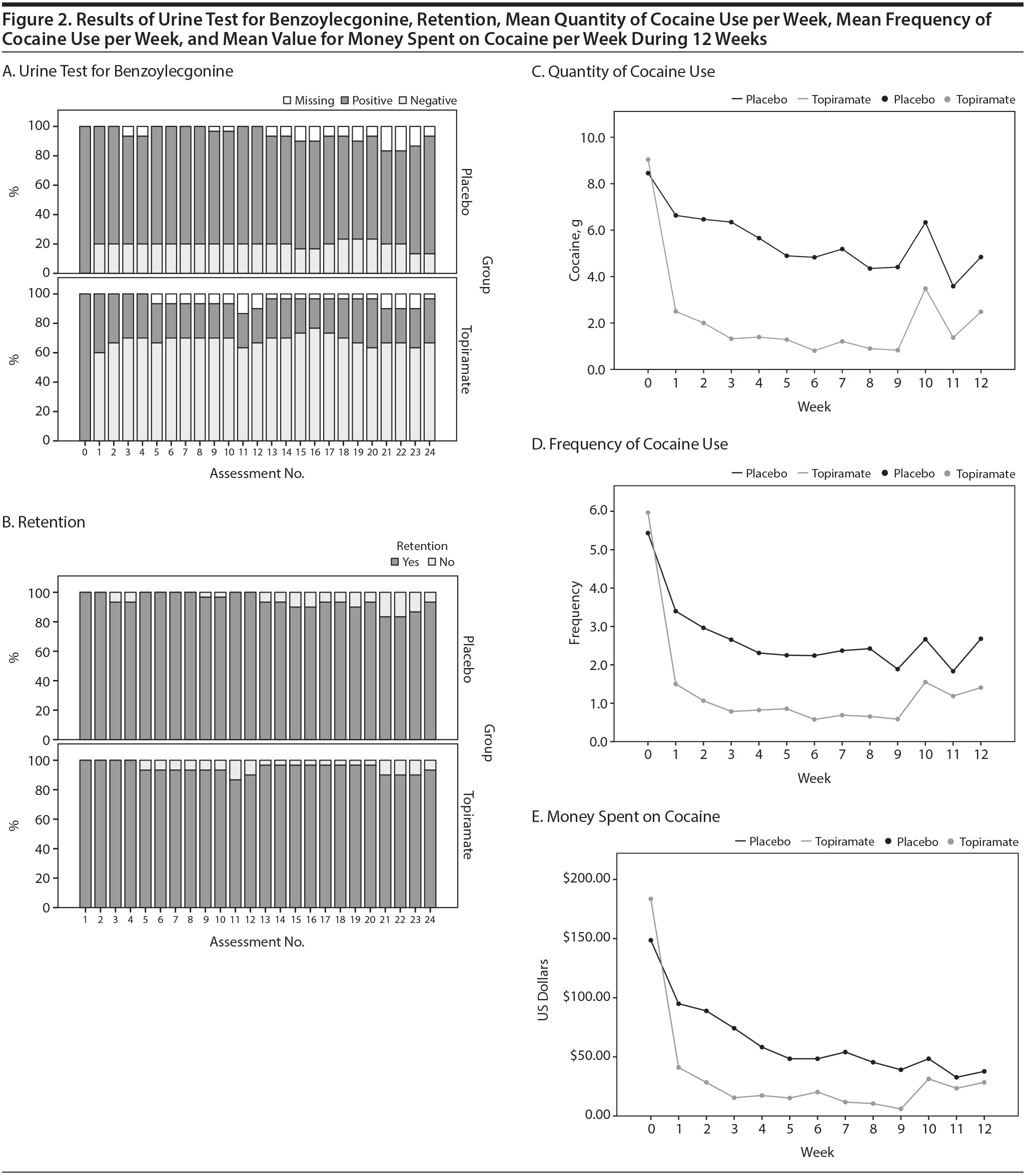
Drug screening was performed twice a week, on Monday morning and Thursday afternoon. A detection test, which reported only the presence or absence of drugs in the urine, was used. If the result was positive and the patient’s daily report was negative (discordant data), the subject was confronted. See Figure 2 for data regarding the urine drug test results (ie, 24 measurements for all of the subjects who completed the study). Approximately 5% of the data was discordant; however, after being confronted, all of the subjects confirmed that they had used the drug.
Sample Size
The sample size was calculated based on the effect sizes reported in prior clinical trials, which had reported that the efficacy of topiramate was approximately 45% in the treatment group and 15% in the placebo group. To achieve a statistical power of 90%, a standard α = .10 and 1 − β = .90 were used.

- Currently, no medications for the treatment of crack cocaine addiction have been approved by the US Food and Drug Administration (FDA).
- Topiramate has been approved by the FDA for the treatment of epilepsy and migraine headaches, but it may also be useful for the treatment of crack cocaine dependence.
- Topiramate appears to be effective as an adjunctive for crack cocaine addiction during the first 4 weeks of the treatment.
Statistical Analyses
The aim of our statistical approach was to evaluate repeated measures. For such an evaluation, we employed generalized estimating equations using a first-order autoregressive relationship. For the 24 binary variables that were collected (eg, urine examination result [an outcome related to the abstinence] and retention), logistic models were utilized. Abstinence over time, which was measured based on the urine examination results, was coded using the following system: “1” for a negative urine examination result, “0” for a positive result, and a missing value for “faults.” To represent retention, the same 24 binary variables from the urine examination were recoded to the following values: “1” for patients who attended the session (regardless of a positive/negative urine examination result) and “0” for patients who were absent.
To analyze the frequency of cocaine use (a count variable), a Poisson log-linear model was used, and linear regression was used to analyze the continuous variables (ie, amount of cocaine used [in grams] and amount of money spent on cocaine [in US dollars] over 12 weeks [13 total assessments, including the baseline assessment]).
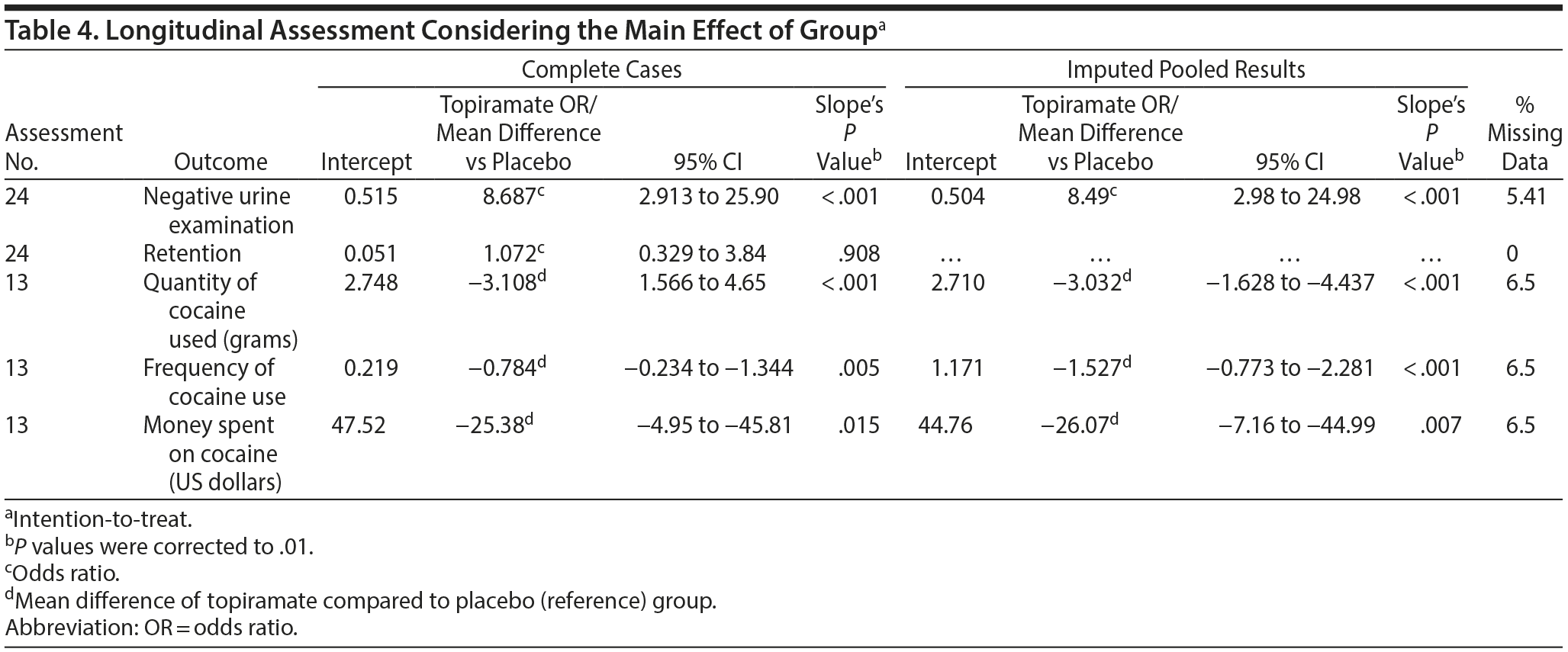
Two analytic paradigms were adopted to assess the effectiveness of topiramate: a complete case analysis and an intention-to-treat (ITT) analysis. For the ITT, a multiple imputation procedure that involved sequential regression imputation, which is also called fully conditional specification, was used. This approach fills in the data on a variable-by-variable basis; each time, the imputation model is matched to a variable’s distribution form. The imputed continuous outcomes were constrained by a minimum of 0 because it is impossible to obtain negative values for days, grams, or US dollars. Five imputed datasets were created, as recommended by Rubin21,22; thus, the data were pooled to produce estimates (see Table 4, right side). The multiple imputations assumed a missing at random data mechanism.
Lastly, we calculated the number needed to treat (NNT) for the subjects who were free of cocaine at the end of 4th, 10th, and 12th weeks as well as the number of dropouts. In addition, we calculated the number needed to harm (NNH) to evaluate the emergence of adverse events by comparing both groups using the χ2 test. We also used the χ2 test to compare differences between the 2 groups. All of the analyses were performed using SPSS 22.0 software (IBM Corporation). Due to the multiple correlated outcomes evaluated, the statistical significance level was corrected to .01 (Bonferroni correction for 5 outcomes and significance level of .05) for evaluation of the effectiveness of topiramate in the longitudinal analysis.
Ethical Issues
This study was conducted according to the principles described in the Declaration of Helsinki and Good Clinical Practice standards. It was approved by the institutional review board of the Federal University of Tocantins (N° 015/2012, Brazilian National Research Ethics Commission [CONEP] N° 05157812.7.0000.5519) and registered at the Brazilian Registry of Clinical Trials (Registro Brasileiro de Ensaios Cl×nicos—ReBEC, RBR-3vwfjs and UTN: U1111-1131-4443).
RESULTS
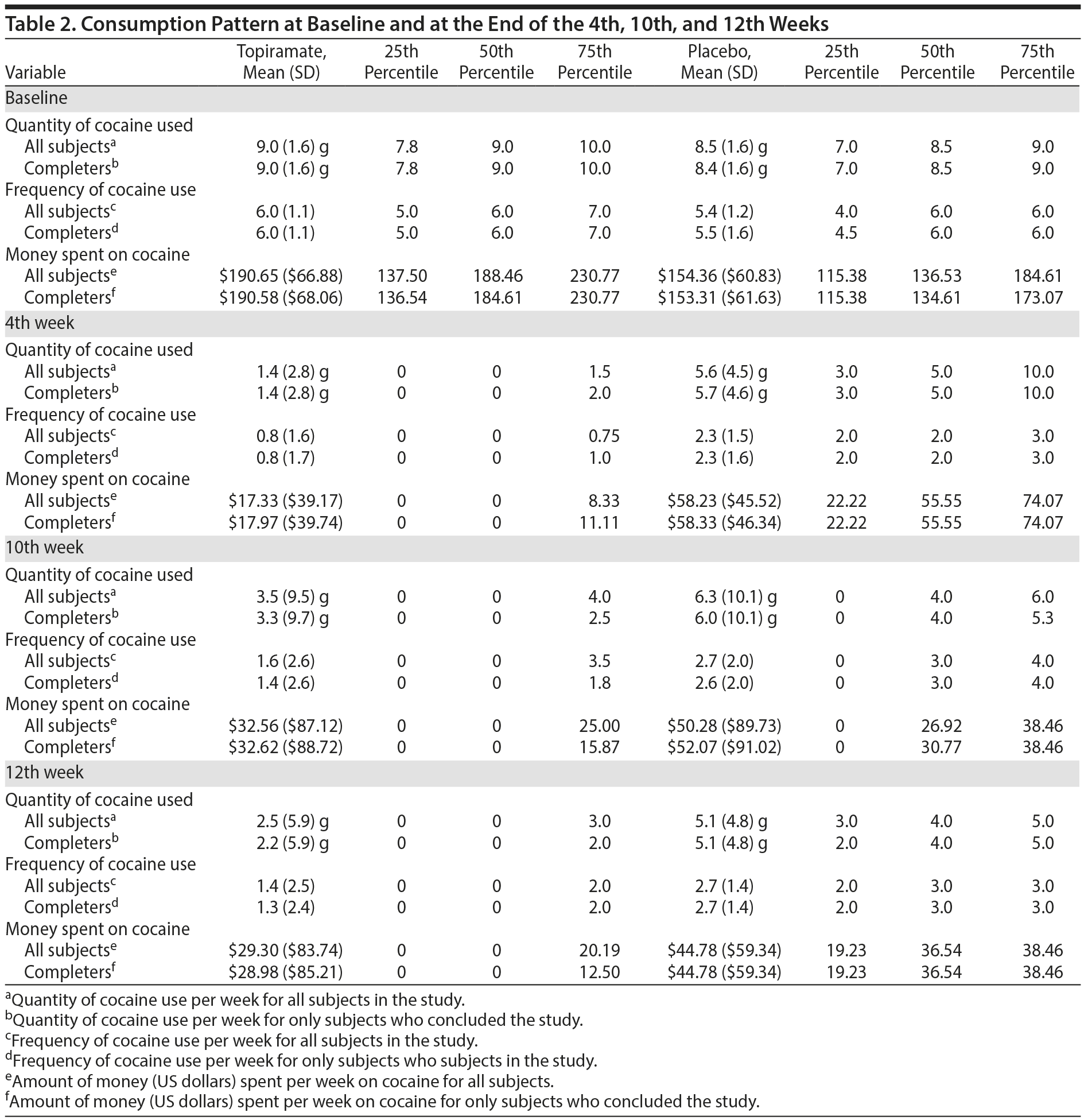
Consumption Pattern at Baseline and at the End of the 4th, 10th, and 12th Weeks
The mean ages of the study participants were 28.6 years (SD = 4.7) in the topiramate group and 30.30 years (SD = 3.9) in the placebo group (Z = 1.57, U = 555.50, P = .12). Comparisons were made using the Mann-Whitney test. Table 2 presents the consumption patterns of both groups at baseline and at the end of the 4th, 10th, and 12th weeks. Twenty-nine subjects in each group completed the study, and there were a total of 2 dropouts (χ2 = 0, P = 1.000). For further details, see Table 2.
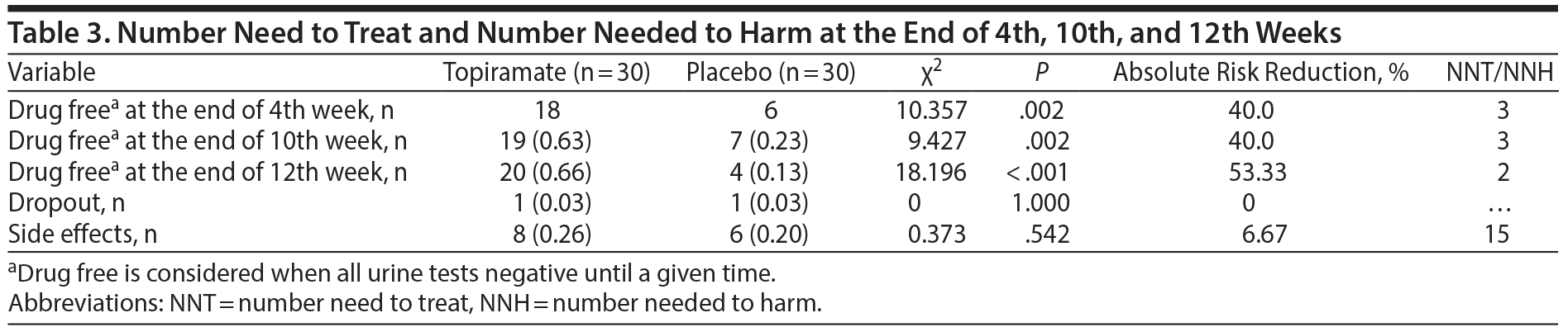
Number Needed to Treat and Number Needed to Harm
During the end of the fourth week, 18 subjects in the topiramate group were considered to be drug free (ie, all urine tests were negative until a given time), whereas only 6 subjects in the placebo group were drug free (χ2 = 10.357, P = .002, absolute risk reduction [ARR] = 40.0%, NNT = 3). During the end of the 10th week, 19 subjects in the topiramate group were considered to be drug free, whereas only 7 subjects were drug free in the placebo group (χ2 = 9.427, P = .002, ARR = 40.0%, NNT = 3). Finally, during the end of the 12th week, 20 subjects in the topiramate group were considered to be drug free, whereas only 4 subjects in the placebo group were drug free (χ2 = 18.196, P < .001, ARR = 53.33%, NNT = 2) (Table 3).
There was insufficient evidence concerning the number of dropouts (ie, there was only 1 dropout from each group), suggesting that topiramate was well tolerated. Adverse events were usually mild and evenly distributed between the topiramate and placebo groups. Side effects were observed in 8 subjects (26%) in the topiramate group and 6 subjects (20%) in the placebo group (χ2 = 0.373, P = .542, NNH = 15). For further details, see Table 3.
The adverse events that were most commonly reported by the subjects in the topiramate group included headache (n = 2), fatigue (n = 2), diarrhea (n = 1), dizziness (n = 2), and nausea (n = 2). None of the subjects developed symptoms of renal lithiasis. In the placebo group, the adverse events reported were headache (n = 3), fatigue (n = 2), and dizziness (n = 2).
Longitudinal Assessment
In our longitudinal assessment, under a complete case analysis paradigm (ie, some values throughout the weeks were considered missing), only the retention outcome was not significantly different between the groups (odds ratio [OR] = 1.072, P = .908) (Table 4). Logistic regression analysis of the urine test results (utilizing a positive result as a reference status) throughout the 12 weeks of the study revealed an OR of 8.687 (P < .001) in favor of topiramate; therefore, patients assigned to the topiramate group were 8.68-fold more likely to obtain a negative result compared with those in the placebo group. Furthermore, the quantity of cocaine used (mean reduction = −3.108 g, P < .001) and the frequency of use (mean reduction = −0.784 times per week, P = .005) were significantly different between the 2 groups. However, after Bonferroni correction (P values corrected to .01), the outcome “money spent on cocaine” (mean reduction [US dollars] = −$25.38, P = .015) did not achieve statistical significance.
After the inclusion of the small number of missing values (preserving the intention-to-treat paradigm), only retention was not statistically significant, and these results agreed with those of the complete case analysis. For more details, see Table 4.
We performed a more detailed analysis of the interaction between the covariate time and group assignment. However, a lack of evidence was found regarding the prediction of an interaction between group and time in the study for a negative urine test (β = 0.029, P = .098). A significant reduction in the quantity of cocaine used (mean reduction = −2.403 g, P < .001 [main effect of the group]) was still observed when controlling for the covariate time in the study (β = −0.333, P < .001), indicating that there was a decrease of 0.333 g in the quantity of cocaine used per week, regardless of the group assignment. The interaction between time and group assignment was not statistically significant for this model (β = 0.98, P = .357). The following outcomes showed an interaction with time: frequency (β = −0.258, P = 0 < .001) and money spent (β = −22.70, P < .001). For frequency and money spent, time was found to be a statistically significant outcome predictor, indicating that there was a reduction of 0.258 in the frequency of cocaine use per week and of $9.08 per week, regardless of the group assignment. However, the time ×— group interaction was not significant for frequency (β = 0.066, P = .114) and money spent (β = 0.704, P = .656).
Lastly, we performed a subanalysis of the time ח group interaction considering only the first 4 weeks (period of topiramate adjustment up to 200 mg). The results revealed that the interaction was not statistically significant for predicting a negative urine test (β = 0.073, P = .056). However, the time ח group interaction was statistically significant for the prediction of the quantity of cocaine used (β = 1.149, P < .001), the reduction in the frequency of cocaine use (β = 0.467, P < .001), and the reduction in the money spent on cocaine (β = 17.291, P < .001). Further details are shown in Figure 2.
DISCUSSION
The present findings suggest that topiramate is effective and safe and, thus, reinforce previous data suggesting that topiramate is potentially useful as an adjunctive treatment for cocaine dependence. The differences between the groups were present throughout the entire duration of treatment. However, with the exception of the quantity of cocaine used, frequency, and money spent in the first 4 weeks, there was no interaction between time and group assignment; a statistically significant interaction term indicates that the groups are changing over time and that they are changing in different ways.
We observed that some subjects discontinued the use of cocaine early in the treatment (Table 3 and Figure 2), whereas others used the drug for a longer time period. In the latter group (those who did not stop using the drug), topiramate demonstrated the potential to reduce cocaine use in the first 4 weeks of treatment when the dose was increased to 200 mg. In subsequent weeks, this interaction over time was not present, suggesting that prolonged use of topiramate may not be beneficial or that the maximum dosage of 200 mg had a limited effect. We hypothesized that higher doses of topiramate could decrease the amount of cocaine used over time, and this possibility should be explored in future research.
Several studies have suggested that topiramate is a potentially effective pharmacologic intervention for the treatment of addiction.12,13 In an observational, prospective, 6-month, multicenter study, Bobes et al23 evaluated users of heroin, cocaine, and alcohol participating in rehabilitation programs. Their findings suggested that topiramate is well tolerated and effective because the urine detection of the substance used decreased from 94.1% (n = 64) to 39.6% (n = 19) after 6 months, nonserious adverse effects were reported by 28% of the subjects, and 33.3% of the subjects reported relapse.5,23
In a double-blind, placebo-controlled pilot study, 40 cocaine addicts were evaluated during 13 weeks of treatment with topiramate at a dose of 200 mg/d; from the third week of treatment, the abstinence rates were observed to be greater in the intervention group compared with the placebo group.1 In another trial, the same researchers found that topiramate plus cognitive-behavioral therapy appeared to reduce cocaine use in some patients with comorbid cocaine and alcohol dependence.14 Recently, Johnson et al17 found that topiramate (at a dose of up to 300 mg daily for 6 to 12 weeks) was more effective than placebo in a large sample because the results from urine tests indicated that 16.6% of the patients in the topiramate group and 5.8% of the patients in the placebo group were cocaine free, with an OR of 3.12.
In contrast with the present findings, Nuijten et al7 found that topiramate treatment was associated with a low retention rate and that topiramate neither improved treatment retention nor reduced cocaine use in ITT analyses. Post hoc, exploratory analyses in their study suggested a moderating effect with comorbid opioid dependence, whereby a significant effect of topiramate on cocaine use was observed only in patients with comorbid opioid dependence. These researchers concluded that the potential therapeutic role of topiramate in the treatment of cocaine dependence was limited. However, the study conducted by Nuijten et al7 differed from our study in several ways. First, they examined male and female subjects with an average age greater than 40 years. Second, subjects who used other substances (eg, alcohol, cannabis, and heroin) were enrolled. Third, the retention rate in their study was significantly lower than that obtained in other trials, including the present study.
The side effects that are most frequently reported with topiramate use are drowsiness, paresthesia, and difficulty concentrating.17,23,24 In the present study, topiramate showed a favorable tolerability profile. The adverse events were mild and evenly distributed between the topiramate and placebo groups, which is in agreement with reports from previous studies.1,17 All of the commonly reported adverse events affected less than 10% of all of the subjects. No serious adverse events were reported.
Although the mechanism of action of topiramate is not yet completely understood, increasing lines of evidence suggest that it acts on glutamatergic and GABAergic systems to modulate the brain’s reward system. It has been demonstrated12,25 that, at pharmacologically relevant concentrations, topiramate blocks voltage-dependent sodium channels and antagonizes the AMPA (α-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid)/KA (kainate) subtype of the excitatory glutamate receptor, without any apparent effects on N-methyl-d-aspartate (NMDA) activity. Topiramate also inhibits the carbonic anhydrase enzyme (particularly isozymes II and IV), which is not considered an important component of antiepileptic activity. Moreover, topiramate increases the activity, frequency, and capacity of GABA neurotransmission by acting on the subtypes of the GABAA receptor that are insensitive to benzodiazepines.12,25 GABA is the main inhibitory neurotransmitter of the brain and has been investigated as a potential target in the treatment of cocaine addiction. Preclinical studies have shown that GABAergic neurons modulate the dopaminergic system and the rewarding effects of cocaine. Furthermore, chronic exposure to cocaine can affect the function of the GABA system. Cocaine-dependent patients may present with an increase in GABAA receptors. These changes in GABA responses may be associated with the decreased levels of GABA observed in the brains of cocaine addicts.12
Experimental research examining the effects of repeated cocaine exposure has revealed changes in the glutamatergic system, including up-regulation of NMDA receptor binding in regions such as the cerebral cortex, striatum, amygdala, and hippocampus26,27; a decrease in NR1 and/or NR2B/2C expression in regions such as the globus pallidus, subiculum, striatum, and cerebellum27,28; up-regulation of NR1 and down-regulation of glutamate receptors 2-7 (GluR2-7) and KA2 expression in the cerebral cortex27,29; increased phosphorylation of GluR1 in the prefrontal cortex27,30; and an increase in metabotropic GluR5 expression in the hippocampus.27,31 Additionally, stimulation of AMPA receptors in the nucleus accumbens has been shown to reinstate previously extinguished cocaine-seeking behavior; furthermore, an infusion of AMPA antagonists into the nucleus accumbens appears to block the reinstatement induced by priming injections of cocaine, cocaine-associated cues, or infusions of cocaine into the frontal cortex.27,32 In contrast, some studies27,33 have reported that NMDA receptor antagonists, whether systemically administered or infused into the shell of the nucleus accumbens, actually induce the reinstatement of cocaine-seeking behavior. The frontal cortex has been identified as the primary source of glutamatergic afferents to the nucleus accumbens that mediate cocaine-primed reinstatement.27,34-36 Together, these data suggest a critical role of ionotropic GluRs in the nucleus accumbens in mediating the reinforcing effects of cocaine and in the reinstatement of cocaine-seeking behavior due to exposure to cocaine- or drug-associated cues.27,34-36 Glutamatergic transmission in the ventral tegmental area (VTA) also plays a role in cocaine reward, reinforcement, and reinstatement.27,34-36 For example, electrical stimulation of glutamatergic fibers in the ventral subiculum reinstates cocaine-seeking behavior in a manner that is dependent on glutamate transmission in the VTA.28 A blockade of NMDA or AMPA receptors in the VTA blocks the development of cocaine-conditioned place preference and cue-induced reinstatement.27,34-36 The expression and phosphorylation of GluR1 in the VTA also mediates the reinforcing effects of cocaine.27,34-36
The present findings should be interpreted with caution because this study has some limitations. First, the relatively small sample size may represent a constraint in our statistical analyses. Second, the study enrolled only males without Axis I comorbidities and was conducted within a single site, thus limiting the generalizability of our findings. However, the present study examined a well-characterized, homogeneous sample, limiting the effects of confounding variables.
CONCLUSIONS
The present findings suggest that topiramate is effective and safe as an adjunctive treatment for crack cocaine addiction during the first 4 weeks of treatment. This study reinforces previous data1,5,17 suggesting that topiramate is potentially useful in the treatment of cocaine dependence. Additionally, given its relatively low cost, the use of topiramate is suitable in public mental health services within developing countries. Future studies that utilize larger samples and explore higher dosages are warranted to confirm these results.
Submitted: July 3, 2014; accepted May 7, 2015.
Drug names: topiramate (Topamax and others).
Potential conflicts of interest: None reported.
Funding/support: This study was supported by the Ministry of Science and Technology (MCTI) and the National Council for Scientific and Technological Development (CNPq) of Brazil (universal grant number 14/2013 and protocol number 47411/2013-4).
Role of the sponsor: The CNPq sponsored this study and provided only financial support and approval of the study design. The CNPq was not involved in the data collection, statistical analysis, or creation of the final report.
Participating study sites: Federal University of Tocantins, Brazil; Psychosocial Care Center for Alcohol and Drugs (CAPS-AD), Palmas, Tocantins, Brazil; Department of Psychiatry, Federal University of São Paulo, Sao Paulo, Brazil; and LiNC—Laboratório Interdisciplinar de Neurociências Cl×nicas (Interdisciplinary Laboratory of Clinical Neurosciences), Department of Psychiatry, Federal University of São Paulo, Sao Paulo, Brazil.
REFERENCES
1. Kampman KM, Pettinati H, Lynch KG, et al. A pilot trial of topiramate for the treatment of cocaine dependence. Drug Alcohol Depend. 2004;75(3):233-240. PubMed doi:10.1016/j.drugalcdep.2004.03.008
2. World Drug Report 2014. Vienna, Austria: United Nations Office on Drugs and Crime; 2014. United Nations Office on Drugs and Crime Web site. https://www.unodc.org/documents/data-and-analysis/WDR2014/World_Drug_Report_2014_web.pdf. Accessed January 13, 2016.
3. Crack assusta e revela um Brasil desesperado. em discuss×£o! Revista de audiências públicas do Senado Federal. 2011:46. Sanado Federal Brasil Web site. http://www.senado.gov.br/noticias/jornal/emdiscussao/Upload/201104%20-%20agosto/pdf/em%20discuss×£o!_agosto_2011_internet.pdf. Accessed January 13, 2016.
4. Abdalla RR, Madruga CS, Ribeiro M, et al. Prevalence of cocaine use in Brazil: data from the II Brazilian National Alcohol and Drugs Survey (BNADS). Addict Behav. 2014;39(1):297-301. PubMed doi:10.1016/j.addbeh.2013.10.019
5. Reis AD, Castro LA, Faria R, et al. Craving decrease with topiramate in outpatient treatment for cocaine dependence: an open label trial. Rev Bras Psiquiatr. 2008;30(2):132-135. PubMed doi:10.1590/S1516-44462008005000012
6. Baldaçara L, Diniz TA, Parreira BL, et al. Could disulfiram be a new treatment for crack cocaine dependence?: a pilot study. Rev Bras Psiquiatr. 2013;35(1):97-98. PubMed doi:10.1016/j.rbp.2012.10.006
7. Nuijten M, Blanken P, van den Brink W, et al. Treatment of crack-cocaine dependence with topiramate: a randomized controlled feasibility trial in The Netherlands. Drug Alcohol Depend. 2014;138:177-184. PubMed doi:10.1016/j.drugalcdep.2014.02.024
8. Preti A. New developments in the pharmacotherapy of cocaine abuse. Addict Biol. 2007;12(2):133-151. PubMed doi:10.1111/j.1369-1600.2007.00061.x
9. Raby WN, Rubin EA, Garawi F, et al. A randomized, double-blind, placebo-controlled trial of venlafaxine for the treatment of depressed cocaine-dependent patients. Am J Addict. 2014;23(1):68-75. PubMed doi:10.1111/j.1521-0391.2013.12065.x
10. Karila L, Reynaud M, Aubin HJ, et al. Pharmacological treatments for cocaine dependence: is there something new? Curr Pharm Des. 2011;17(14):1359-1368. PubMed doi:10.2174/138161211796150873
11. Winstanley EL, Bigelow GE, Silverman K, et al. A randomized controlled trial of fluoxetine in the treatment of cocaine dependence among methadone-maintained patients. J Subst Abuse Treat. 2011;40(3):255-264. PubMed doi:10.1016/j.jsat.2010.11.010
12. Herrero AI, Del Olmo N, González-Escalada JR, et al. Two new actions of topiramate: inhibition of depolarizing GABA(A)-mediated responses and activation of a potassium conductance. Neuropharmacology. 2002;42(2):210-220. PubMed doi:10.1016/S0028-3908(01)00171-X
13. Shank RP, Gardocki JF, Streeter AJ, et al. An overview of the preclinical aspects of topiramate: pharmacology, pharmacokinetics, and mechanism of action. Epilepsia. 2000;41(suppl 1):S3-S9. PubMed doi:10.1111/j.1528-1157.2000.tb02163.x
14. Kampman KM, Pettinati HM, Lynch KG, et al. A double-blind, placebo-controlled trial of topiramate for the treatment of comorbid cocaine and alcohol dependence. Drug Alcohol Depend. 2013;133(1):94-99. PubMed doi:10.1016/j.drugalcdep.2013.05.026
15. Breslin FJ, Johnson BA, Lynch WJ. Effect of topiramate treatment on ethanol consumption in rats. Psychopharmacology (Berl). 2010;207(4):529-534. PubMed doi:10.1007/s00213-009-1683-4
16. Elkashef A, Kahn R, Yu E, et al. Topiramate for the treatment of methamphetamine addiction: a multi-center placebo-controlled trial. Addiction. 2012;107(7):1297-1306. PubMed doi:10.1111/j.1360-0443.2011.03771.x
17. Johnson BA, Ait-Daoud N, Wang XQ, et al. Topiramate for the treatment of cocaine addiction: a randomized clinical trial. JAMA Psychiatry. 2013;70(12):1338-1346. PubMed doi:10.1001/jamapsychiatry.2013.2295
18. Quintana MI, Gastal FL, Jorge MR, et al. Validity and limitations of the Brazilian version of the Composite International Diagnostic Interview (CIDI 2.1). Rev Bras Psiquiatr. 2007;29(1):18-22. PubMed doi:10.1590/S1516-44462006005000024
19. Bernstein E, Bernstein J, Levenson S. Project ASSERT: an ED-based intervention to increase access to primary care, preventive services, and the substance abuse treatment system. Ann Emerg Med. 1997;30(2):181-189. PubMed doi:10.1016/S0196-0644(97)70140-9
20. Carroll KM. A Cognitive Behavioral Approach: Treating Cocaine Addiction. Rockville, MD: National Institute on Drug Abuse; 1998.
21. Rubin DB. Multiple imputation after 18+ years. J Am Stat Assoc. 1996;91(434):473-489. doi:10.1080/01621459.1996.10476908
22. Rubin DB. Multiple Imputation for Nonresponse in Surveys. Hoboken, NJ: John Wiley & Sons; 2009.
23. Bobes J, Carre×±o JE, Gutiérrez CE, et al. Study of effectiveness of craving control with topiramate in patients with substance dependence disorders. Actas Esp Psiquiatr. 2004;32(5):299-306. PubMed
24. Shinn AK, Greenfield SF. Topiramate in the treatment of substance-related disorders: a critical review of the literature. J Clin Psychiatry. 2010;71(5):634-648. PubMed doi:10.4088/JCP.08r04062gry
25. Cubells JF. Topiramate for cocaine dependence. Curr Psychiatry Rep. 2006;8(2):130-131. PubMed doi:10.1007/s11920-006-0011-5
26. Itzhak Y, Stein I. Sensitization to the toxic effects of cocaine in mice is associated with the regulation of N-methyl-D-aspartate receptors in the cortex. J Pharmacol Exp Ther. 1992;262(2):464-470. PubMed
27. Gass JT, Olive MF. Glutamatergic substrates of drug addiction and alcoholism. Biochem Pharmacol. 2008;75(1):218-265. PubMed doi:10.1016/j.bcp.2007.06.039
28. Yamaguchi M, Suzuki T, Abe S, et al. Repeated cocaine administration differentially affects NMDA receptor subunit (NR1, NR2A-C) mRNAs in rat brain. Synapse. 2002;46(3):157-169. PubMed doi:10.1002/syn.10132
29. Hemby SE, Horman B, Tang W. Differential regulation of ionotropic glutamate receptor subunits following cocaine self-administration. Brain Res. 2005;1064(1-2):75-82. PubMed doi:10.1016/j.brainres.2005.09.051
30. Zhang X, Lee TH, Davidson C, et al. Reversal of cocaine-induced behavioral sensitization and associated phosphorylation of the NR2B and GluR1 subunits of the NMDA and AMPA receptors. Neuropsychopharmacology. 2007;32(2):377-387. PubMed doi:10.1038/sj.npp.1301101
31. Freeman WM, Brebner K, Lynch WJ, et al. Cocaine-responsive gene expression changes in rat hippocampus. Neuroscience. 2001;108(3):371-380. PubMed doi:10.1016/S0306-4522(01)00432-8
32. Park WK, Bari AA, Jey AR, et al. Cocaine administered into the medial prefrontal cortex reinstates cocaine-seeking behavior by increasing AMPA receptor-mediated glutamate transmission in the nucleus accumbens. J Neurosci. 2002;22(7):2916-2925. PubMed
33. Steketee JD, Kalivas PW. Drug wanting: behavioral sensitization and relapse to drug-seeking behavior. Pharmacol Rev. 2011;63(2):348-365. PubMed doi:10.1124/pr.109.001933
34. Kalivas PW. The glutamate homeostasis hypothesis of addiction. Nat Rev Neurosci. 2009;10(8):561-572. PubMed doi:10.1038/nrn2515
35. Kalivas PW. Glutamate systems in cocaine addiction. Curr Opin Pharmacol. 2004;4(1):23-29. PubMed doi:10.1016/j.coph.2003.11.002
36. Kalivas PW, Lalumiere RT, Knackstedt L, et al. Glutamate transmission in addiction. Neuropharmacology. 2009;56(suppl 1):169-173. PubMed doi:10.1016/j.neuropharm.2008.07.011
Enjoy free PDF downloads as part of your membership!
Save
Cite
Advertisement
GAM ID: sidebar-top