ABSTRACT
Background: Bipolar disorder (BD) confers risk for accelerated atherosclerosis and early cardiovascular disease (CVD). In adults, mood symptom burden is associated with CVD. Here we examine endothelial dysfunction, considered an early predictor of CVD, in relation to mood states and symptoms among youth with BD.
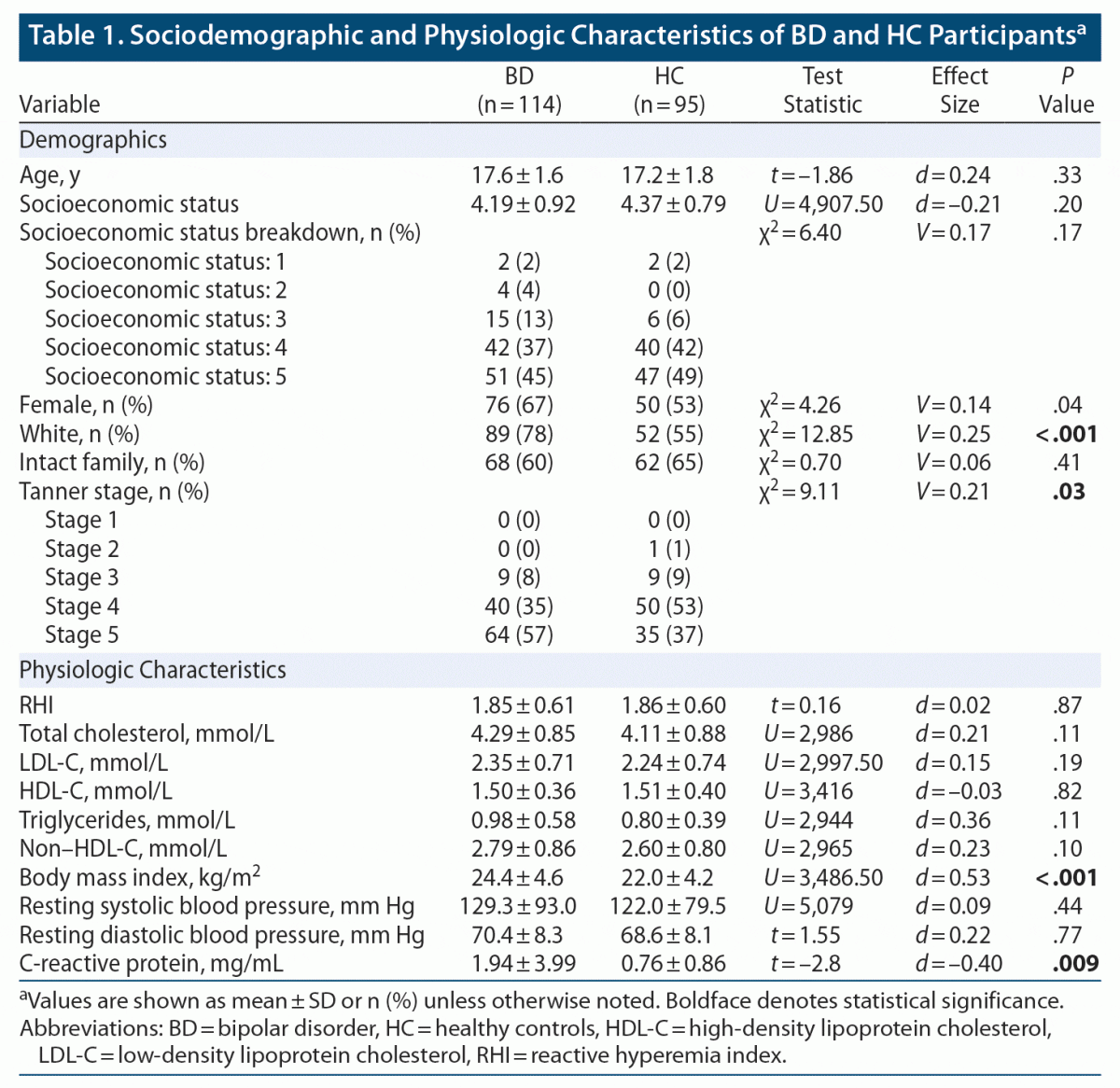
Methods: Participants were 209 youth, aged 13–20 years, including 114 with BD and 95 healthy controls (HC) recruited between 2012 and 2020. Diagnoses and mood symptoms were ascertained using validated, semi-structured interviews based on DSM-IV-TR criteria. Reactive hyperemia index (RHI), a measure of endothelial function, was assessed non-invasively via pulse amplitude tonometry (PAT). RHI was compared across 4 groups: BD-euthymic (n = 34), BD-depressed (n = 36), BD-hypomanic/mixed (n = 44), and HC (n = 95) controlling for age, sex, and obesity. Analyses also examined for RHI-mood associations in the overall BD group.
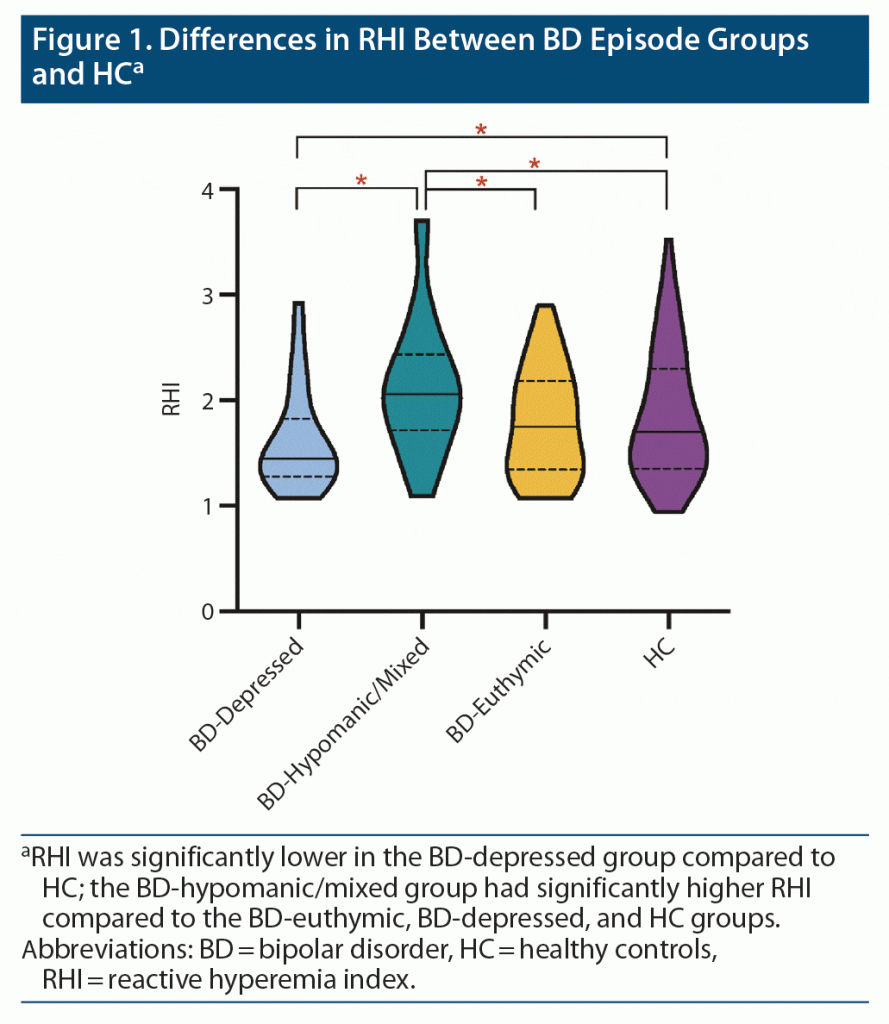
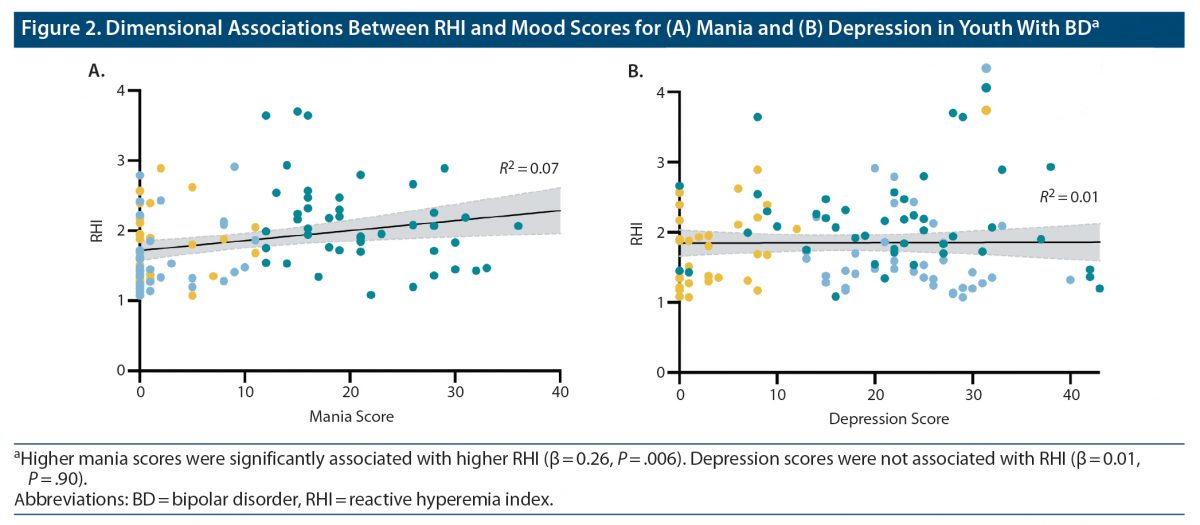
Results: RHI was significantly different between groups (F3,202 = 4.47, P = .005, ηp2 = 0.06). Specifically, RHI was lower in the BD-depressed group compared to HC (P = .04, d = 0.4). Additionally, the BD-hypomanic/mixed group had higher RHI compared to the BD-euthymic (P = .02, d = 0.55), BD-depressed (P < .001, d = 0.79), and HC (P = .04, d = 0.55) groups. Lastly, within the BD group, higher RHI was associated with higher mania scores (P = .006, β = 0.26), but not depression scores. All analyses remained significant in sensitivity analyses further controlling for cardiovascular risk factors and for current lithium, second-generation antipsychotic, and any medication use.
Conclusions: We found that symptomatic youth with BD have anomalous RHI, which varies according to mood polarity. Future studies in larger samples, with prospective repeated measures, should investigate whether endothelial dysfunction partially subserves the psychiatric symptoms and cardiovascular risk observed in BD.
J Clin Psychiatry 2023;84(3):22m14608
To cite: Kennedy KG, Karthikeyan S, Sultan AA, et al. Endothelial function in youth with bipolar disorder: preliminary evidence for mood polarity differences. J Clin Psychiatry. 2023;84(3):22m14608.
To share: https://doi.org/10.4088/JCP.22m14608
© 2023 Physicians Postgraduate Press, Inc.
aCentre for Youth Bipolar Disorder, Centre for Addiction and Mental Health, Toronto, Ontario, Canada
bDepartment of Pharmacology, University of Toronto, Toronto, Ontario, Canada
cLabatt Family Heart Centre, Hospital for Sick Children, Toronto, Ontario, Canada.
dDepartment of Pediatrics, University of Toronto, Toronto, Ontario, Canada
eInstitute for Policy Research & Department of Psychology, Northwestern University, Evanston, Illinois
fDepartment of Psychiatry, University of Toronto, Toronto, Ontario, Canada
*Corresponding author: Benjamin I. Goldstein, MD, PhD, Centre for Addiction and Mental Health, 100 Stokes St, M6J 1H4, Toronto, ON, Canada ([email protected]).
Bipolar disorder (BD) is a chronic mood disorder associated with excessive and premature cardiovascular mortality.1 Cardiovascular disease (CVD) is excessively prevalent in BD, occurs over a decade prematurely in people with BD, and exceeds what can be explained by traditional cardiovascular risk factors (eg, hypertension, obesity, smoking), psychiatric medications, and substance use.2,3 A recent scientific statement from the American Heart Association4 recognized BD in youth to be a moderate-risk condition for accelerated atherosclerosis and early CVD. However, it remains unclear as to what underlying factors may be driving the excess and prematurity of CVD in individuals with BD. Assessment of endothelial function in youth with BD can provide valuable insights into the nascent stages of CVD prior to decades of elevated cardiovascular risk.5
Endothelial dysfunction, an indicator of early atherosclerosis, is a leading noninvasive vascular measure in youth.6 Importantly, endothelial function predicts CVD mortality independent of traditional cardiovascular risk factors (CVRFs).7 Endothelial function is measured using several methods, most commonly brachial arterial ultrasound (yielding measures of flow-mediated dilation) and pulse amplitude tonometry (PAT; yielding measures of reactive hyperemia index [RHI])8; for each measure, lower values indicate poorer endothelial function. In distinction from brachial arterial ultrasound measures of endothelial function, PAT serves as an assay of microvascular function.
Systematic reviews and meta-analyses have reported that adults with clinical depression have poorer endothelial function versus healthy controls (HC)9 and that higher levels of depression symptoms are associated with poorer endothelial function.10 Furthermore, one prospective study11 found that an increase in depressive symptoms was associated with decreased endothelial functioning a year later. Thus far, few studies have assessed endothelial function in individuals with BD. Two studies in adults with BD12,13 reported no significant differences in endothelial function compared to HC, and a third14 found no associations between endothelial function and mood symptoms. In contrast, one study15 found that lower endothelial function was associated with higher manic/hypomanic symptoms in adults with BD.
While there are fewer studies in youth, the literature on this topic has evolved in recent years. In a large prospective study16 of healthy adolescent females (N = 135) at high risk of developing a mood disorder, greater depressive symptoms were associated with lower concurrent endothelial function over time. Similar findings regarding depression symptoms and endothelial function have been observed in other studies in healthy youth17 and youth with clinical depression18; however, some discordant findings do exist.19 In the two prior studies on this topic in adolescents with BD,20,21 our group reported no significant differences in endothelial function between BD patients and HC. Further research in larger youth samples is warranted to examine whether endothelial function is relevant to particular mood states and/or subtypes of BD.
Youth with BD have elevated levels of proinflammatory markers like C-reactive protein (CRP) and traditional CVRFs including obesity, hyperglycemia, hypertension, and dyslipidemia.22,23 Additionally, CRP and CVRFs are associated with worse mood symptoms,14,20,24,25 neurocognition,26,27 and brain structure28,29 in youth with BD. Given that poorer endothelial function is associated with greater CRP and CVRFs,6,9,30–32 it is important to assess whether endothelial function is independently associated with mood states/symptoms among youth with BD.
The present study aimed to evaluate endothelial function via PAT (ie, RHI) in a large sample of youth with BD compared to HC. We examined endothelial function across BD mood states versus HC, and, within the BD group, we examined the association between endothelial function and mood symptom scores. We hypothesized that youth with BD, specifically those with current depression and/or hypomania, would have lower RHI versus HC. We further hypothesized that lower RHI values would be associated with greater depression and mania/hypomania mood scores in youth with BD.
METHODS
Participants
Consent was obtained from all participants and their parent and/or guardian prior to participating. Ethical approval was granted by Sunnybrook Research Institute Research Ethics Board (REB 347–2011, 408–2011, 032–2012, 409–2013, 405–2014, 435–2015). All data were collected at Sunnybrook Research Institute. However, all data were transferred with the Centre for Youth Bipolar Disorder’s relocation to the Centre for Addiction and Mental Health (CAMH). Thus, ethical approval was also granted by CAMH Research Ethics Board (REB 152/2020, 163/2020, 164/2020, 165/2020, 168/2020, 170/2020),
A total of 209 youth participants (114 BD, 95 HC), aged 13–20 years, English-speaking, and of any race/ethnicity, were included in this dataset. All participants (BD and HC) completed semistructured diagnostic interviews (described subsequently in the Methods) to ensure they met the criteria for BD and/or did not meet exclusion criteria for HC. BD participants who met diagnostic criteria for BD-I, BD-II, or BD not otherwise specified (NOS) were recruited from a tertiary subspecialty clinic (Center for Youth Bipolar Disorder) in Toronto, Ontario. HC participants were recruited through advertisements in the community, primarily using posters on the local public transit system, but also using local flyers and social media advertisements. Participant data for the current study were collated from 6 independent studies from our research group (conducted between 2012 and 2020) that included measures of endothelial function by PAT. With the exception of one prior preliminary publication (n = 30 BD),21 there have been no prior publications of our PAT data.
Exclusion criteria for participants in this dataset varied across the respective studies they participated in. All studies had the following exclusion criteria: (i) inability to provide informed consent; (ii) chronic inflammatory illness; (iii) use of anti-inflammatory, antiplatelet, antihypertensive, or hypoglycemic medication; (iv) infectious illness in the past 14 days; and (v) neurologic or cognitive impairment. Two of the studies had the additional exclusion criterion of (vi) contraindications to magnetic resonance imaging (MRI; eg, cardiac pacemaker or other implanted device). To be included as HC, participants could not have any major psychiatric disorders (eg, BD, major depressive disorder [MDD], psychosis, or recent substance use disorders) or first- or second-degree family history of BD.
Psychiatric Measures
Present and lifetime history of psychiatric diagnoses were determined using the Schedule for Affective Disorders and Schizophrenia for School-Age Children–Present and Life Version (K-SADS-PL),33 a semistructured interview. Extended mood sections, the K-SADS Depression Rating Scale (DRS)34 and K-SADS Mania Rating Scale (MRS),35 were used in place of the mood sections of the K-SADS-PL. DRS and MRS symptom scores during the worst week in the past month were used as measures of current mood state. Diagnoses were determined using Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, Text Revision (DSM-IV-TR), criteria.36 DSM-IV-TR criteria were used, as the DSM-5 version of the K-SADS-PL was available starting only in December 2016; this sample was recruited from 2012 through 2020. BD-NOS was defined using operationalized criteria outlined in the Course and Outcome of Bipolar Youth (COBY) study.37 The Children’s Global Assessment Scale (CGAS) was used as a global assessment of psychosocial functioning and current and lifetime psychiatric symptoms.38 A licensed child and adolescent psychiatrist confirmed all diagnoses during consensus conferences prior to inclusion in the study.
In addition to diagnoses, information regarding treatment, physical and/or sexual abuse, and smoking was obtained during the K-SADS-PL interview.33 The Family History Screen method39 was used to assess family psychiatric history for all first- and second-degree relatives. The Hollingshead Four-Factor Index40 was used to ascertain socioeconomic status (SES). Both the participant and a parent/guardian were independently interviewed to obtain information for the aforementioned instruments.
Physical Measurements
Measurements of height and weight were collected for all participants using a Tanita digital scale (Tanita Corporation of America, Inc; Arlington Heights, Illinois) and a wall-mounted stadiometer, respectively. Body mass index (BMI) was calculated as weight in kilograms divided by the square of height in meters. Participants were asked to locate their waist, and waist circumference was measured using a flexible tape measure. Blood pressure was assessed based on current clinical guidelines in children and adolescents41 using an automated sphygmomanometer between 9:00 am and noon after participants were rested and seated for a minimum of 10 minutes. All anthropometric measurements were taken twice, and the average of the two readings was used for data analyses.
Blood samples were collected in a subset of participants (of the 6 independent studies, 4 included bloodwork and 1 had optional bloodwork) for assessment of CVRFs and/or CRP. Blood collection via antecubital venipuncture was performed between 9:00 am and 11:00 am. Participants were instructed to fast for at least 10 hours prior to the blood draw and to refrain from tobacco, alcohol, or any illicit drug use for 24 hours prior to their visit. Samples were sent to the hospital blood laboratory for analysis of fasting blood glucose, triglycerides, high-density lipoprotein (HDL-C), low-density lipoprotein (LDL-C), total cholesterol, and CRP. CRP was assayed using an immunoturbidimetric assay (CRPL3 Tina-quant C-Reactive Protein Gen. 3; Roche Diagnostics; Indianapolis, Indiana) on a Roche Cobas 702 analyzer; the detection limit for CRP was 0.2 pg/mL.
Pulse Amplitude Tonometry
RHI, a measure of endothelial function, was assessed by pulse amplitude tonometry (PAT) using the EndoPAT 2000 device (Itamar Medical; Caesarea, Israel). PAT was performed after a minimum of 30 minutes following blood pressure measurement and prior to blood collection. The procedure was conducted by trained research personnel in a quiet, dimly lit, temperature-controlled room. Participants rested in a supine position, and a deflated blood pressure cuff was positioned on their left arm (arm to be occluded). The upper arm (ie, brachial artery) was the location of occlusion for the majority of participants; however, the occlusion site was switched to the forearm in 2018 for better tolerability (ie, less discomfort). A prior study in healthy adults42 reported no significant difference in the RHI between forearm and upper-arm occlusion. Nevertheless, to account for any potential effects, we included occlusion location as a covariate in sensitivity analyses.
Noninvasive pneumatic finger probes connected to the EndoPAT device were placed on the index fingers of both hands and inflated. PAT signals were recorded continuously for a duration of 16 minutes, consisting of a baseline calibration period of 6 minutes, followed by 5 minutes of occlusion during which the blood pressure cuff was inflated to 220 mm Hg and a 5-minute post-occlusion period after deflation of the cuff. RHI values for each participant were calculated by the provided software using an automated computer algorithm. Briefly, a PAT ratio was calculated for the occluded arm using the mean pulse wave amplitudes pre- and post-occlusion. This ratio was then divided by the same ratio for the contralateral arm to compensate for potential systemic changes, yielding the RHI value. Lower RHI values reflect a greater risk for coronary atherosclerosis and poorer endothelial function. Data for all participants were manually screened for the absence of any operational errors by two independent research personnel masked to participant status prior to inclusion in any data analysis.
Statistical Analysis
Clinical study data were collected and managed using REDCap, (Research Electronic Data Capture), a secure web-based software platform designed to support data capture for research studies.43 Data analyses were performed using IBM SPSS Statistics, version 27 (IBM Corp.; Armonk, New York).
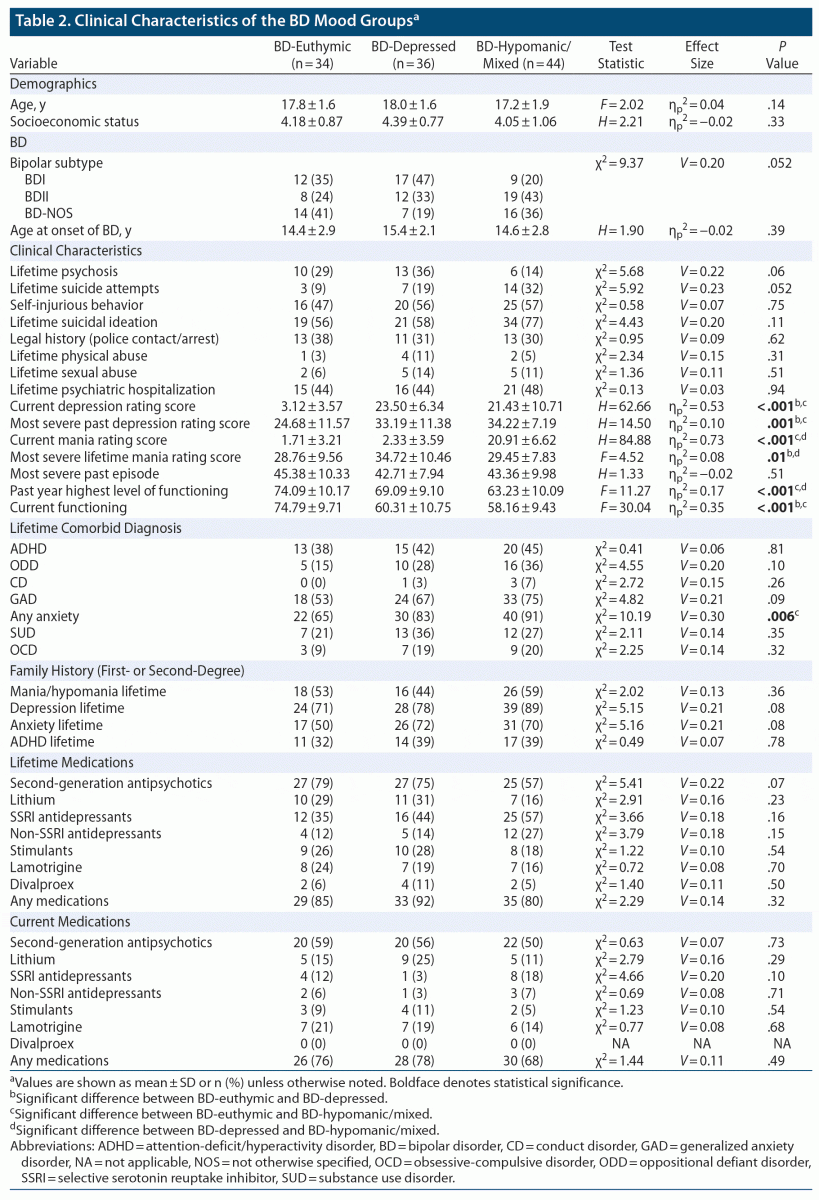
Classification of participants with BD into BD-euthymic, BD-depressed, or BD-hypomanic/mixed episode groups was based on the K-SADS-PL depression and mania rating scales. BD-euthymic was defined as a score of < 13 on both the K-SADS-PL depression and K-SADS-PL mania scales. BD-depressed was defined as a score of ≥ 13 on the K-SADS-PL depression rating scale but < 13 on the K-SADS-PL mania rating scale. BD-hypomanic/mixed was defined as a score of ≥ 13 on the K-SADS-PL mania rating scale regardless of depression score.
Between-group differences were examined using χ2 tests for categorical variables and independent-samples t tests or F tests for continuous variables; Mann-Whitney U tests and Kruskal-Wallis H tests were used when assumptions of normality were violated. Effect sizes were reported as a Cohen d or ηp2 for continuous variables and the Cramer V for categorical variables. Two approaches were used to investigate RHI and mood in youth with BD versus HC. First, to test for differences in RHI across youth with BD in different mood states and HC, general linear models (GLMs) were used, with RHI as the dependent variable and mood state (ie, euthymia, depression, hypomanic/mixed or HC) as a predictor with age, sex, and BMI as covariates. Second, the RHI-mood association was analyzed within the BD group by substituting mood state with mood scores (ie, depression or mania) as the predictor. Lastly, sensitivity analyses were conducted to evaluate the effect of various medications (including current second-generation antipsychotic [SGA], lithium, antidepressant, antimanic, and any medication use), CVRFs (lipids, blood pressure, smoking), CRP, and occlusion location.
RESULTS
Demographic and Clinical Characteristics
This study included 209 youth, 114 with BD (38 BD-I, 39 BD-II, 37 BD-NOS) and 95 HC; a total of 167 youth (84 HC, 82 BD) also had measures of serum lipids, and a total of 177 youth (84 HC, 93 BD) had CRP measures. Sociodemographic and physiologic characteristics of participants with BD and HC are presented in Table 1. Compared to HC, the BD group had fewer White participants (78% vs 55%; P < .001, V = 0.25), higher Tanner stage development (Stage 5: 65% vs 35%; P = .03, V = 0.21), higher BMI (24.4 ± 4.6 vs 22.0 ± 4.2; P < .001, d = 0.53), and higher CRP (1.94 ± 3.99 vs 0.76 ± 0.86; P = .009, d = –0.40). RHI and other demographic and physiologic characteristics did not differ significantly. Within the BD group, there were 34 BD-euthymic, 36 BD-depressed, and 44 BD-hypomanic/mixed participants. Additionally, within the BD-depressed and BD-hypomanic/mixed groups, 72 youth with BD were experiencing clinically significant depression. Clinical characteristics of the BD mood groups are presented in Table 2. There were significant differences in current depression scores (P < .001, ηp2 = 0.53), most severe lifetime depression scores (P = .001, ηp2 = 0.10), current mania scores (P < .001, ηp2 = 0.73), most severe lifetime mania scores (P = .01, ηp2 = 0.08), past-year highest level of functioning (P < .001, ηp2 = 0.17), current functioning (P < .001, ηp2 = 0.35), and any anxiety (P = .006, V = 0.30). All other clinical characteristics were not significantly different.
Between-Group Differences in RHI
Significant between-group differences in RHI were found when controlling for age, sex, and BMI (F3,202 = 4.47, P = .005, ηp2 = 0.06; Figure 1). Post hoc pairwise comparisons revealed that RHI was significantly lower in the BD-depressed group (P = .04, d = 0.4) compared to the HC group. In addition, the BD-hypomanic/mixed group had significantly higher RHI compared to the BD-euthymic (P = .02, d = 0.55), BD-depressed (P < .001, d = 0.79), and HC group (P = .04, d = 0.55).
Dimensional Associations Between RHI and Mood
Given the significant between-group differences in RHI, the association of RHI with mania and depression mood scores was further examined within the overall BD group (n = 114). Higher RHI was associated with higher mania scores (β = 0.26, P = .006), whereas there was no significant association with depression mood scores (β = 0.01, P = .90) (Figure 2).
Sensitivity Analyses
We undertook sensitivity analyses to examine the effect of current medication use (lithium, SGA, antidepressants, stimulant, antimanic, and any medication use) and CVRFs (LDL-C, HDL-C, triglycerides, CRP, and systolic and diastolic blood pressure) on RHI. Each medication or CVRF was separately included as an additional covariate in our analyses. Lastly, we also conducted sensitivity analyses incorporating the site of occlusion (ie, bicep vs forearm) and duration of occlusion as an additional covariate. All sensitivity analyses are summarized in Table 3. Higher diastolic blood pressure was associated with lower RHI (F3,201 = 9.50, P = .002, η2p = 0.05) in the mood states analysis, and current selective serotonin reuptake inhibitor (SSRI) use was associated with greater RHI (β = 0.19, P = .047) in the within BD group mania analysis. Both the mood state differences in RHI and the RHI-mania association remained significant in all sensitivity analyses.
DISCUSSION
This study investigated the association between endothelial function and mood states/symptoms in youth with BD. In analyses investigating differences in RHI in BD mood states and HC, we found evidence of mood polarity–specific differences: the BD-hypomanic/mixed group had significantly higher RHI compared to the BD-euthymic, BD-depressed, and HC groups, whereas the BD-depressed group had significantly lower RHI compared to the HC group. Furthermore, within the overall BD cohort, higher RHI was associated with higher mania scores. Importantly, these findings remained significant after controlling for various confounders such as obesity, blood pressure, lipids, and psychiatric medications (ie, current use of SGAs, lithium, antidepressants, antimanics, or any medications). These findings advance knowledge regarding the association between mood states and endothelial function among youth early in their course of BD and invite future studies probing underlying mechanisms.
Present findings build on prior evidence of endothelial dysfunction in individuals with mood disorders. Prior meta-analyses have found that adults with clinical depression have poorer endothelial function versus HC9 and that higher symptoms of depression are associated with poorer endothelial function.10 Similarly, studies have found that youth with a current major depressive episode have poorer endothelial function versus HC18 and that symptoms of depression are associated with poorer endothelial function.17 In contrast, prior studies have generally not found endothelial dysfunction in adults with BD, either in comparison to HC or across mood states.13,14 However, one study15 found that lower endothelial function was associated with higher levels of manic/hypomanic symptoms in adults with BD. Given the sparse literature in BD, it remains unclear whether the difference between current findings in youth and prior findings in adults is methodological or a true difference. However, mood polarity differences in vascular-related metrics have been observed before. For example, cerebral blood flow, which is influenced by endothelial function,44 has also been found to be higher during mania and lower during depression in adults.45
There may be several mechanisms underlying the observation that greater manic symptom severity is associated with higher endothelial function. From a psychological perspective, as summarized in a recent American Heart Association scientific statement,46 positive psychological health has been associated with indices of positive cardiovascular health, whereby positive psychological health can improve cardiovascular health through behavioral factors (ie, greater exercise) or biologically by positively influencing the autonomic nervous system.46 Indeed, prior research has demonstrated that positive affect, such as joy47 and laughter,48,49 is associated with increased endothelial function in normative samples. Hypomanic symptoms in this outpatient sample, including elation, may have similar beneficial associations with endothelial function. Importantly, the current study does not address mania, but rather hypomanic symptoms. Given prior evidence that the prospective burden of manic episodes is associated with increased cardiovascular mortality50 and with cardiac autonomic dysregulation,51,52 we speculate that current findings may not extend to full-fledged mania. Nonetheless, future studies are warranted to evaluate endothelial function among youth with more severe manic symptoms and evaluate specific symptoms of hypomania/mania individually, as there may be heterogeneity in their associations with RHI.
Further mechanisms underlying the observed polarity differences in mood and endothelial function may relate to physiological differences between mood states. As Kraepelin contemplated in his seminal text over a century ago, “If one prefers the assumption of chemical causes, one might think that the same poison, which engenders the alternation of psychic states, affects also the arterial walls.”53(p163) While CVRFs and inflammation are elevated in youth with BD22,23 and are associated with mood symptoms14,20,24,25 and poorer endothelial function6,9,30–32 in BD, few blood-based biomarkers differ according to mood polarity. While we anticipated that CVRFs and CRP would be associated with differences in endothelial function, all findings persisted even after controlling for CVRFs and CRP. However, a recent study from our group25 found that triglycerides and the triglyceride-to–HDL-C ratio were higher in the BD-euthymic and BD-hypomanic/mixed groups versus the HC group. Although, given that elevated atherogenic lipid profiles are associated with increased CVD and poorer endothelial functioning,6,7 it is unlikely that it explains why better endothelial functioning was observed within the BD-hypomanic/mixed group. Future research investigating biological mechanisms underlying anomalous endothelial function in BD is warranted.
Several factors may explain why endothelial function was lower within the BD-depressed group but not associated with depression within the overall BD group. First, there may be a ceiling effect. In the BD group, 70% currently had clinically significant depression symptoms. As such, while there is a range of depression severity within the BD group, there may be limited variance as it relates to depression-RHI association given the sample characteristics, which could explain present findings. A second possible explanation relates to the high rate of co-occurring hypomanic symptoms within the current sample. Given that mania was associated with higher endothelial functioning, it may be confounding the association between depression and endothelial function. Future studies including purely hypomanic and purely depressed subgroups are warranted. Lastly, the heterogeneity of depression (ie, individual differences in specific depression symptoms) may relate to the lack of association. We did not evaluate each depression symptom individually, and there may be variability in specific symptoms that is relevant to the vascular-depression association, including low self-esteem,54 anger,55,56 and insomnia.57
Limitations
Several limitations of this study require consideration. First, the cross-sectional design of the study precludes making inferences regarding the temporal relationship between RHI and mood. Future studies with repeated measures of RHI are necessary to inform our understanding of life-course relationships of endothelial function in BD across mood states. Second, the severity of manic symptoms in this study was constrained to hypomania, as mania was an exclusion criterion in this outpatient study. Moreover, there were few participants with pure hypomania. As such, we cannot draw conclusions about mania or pure hypomania. Third, the HC group had a significantly higher proportion of White participants and a lower overall Tanner stage than the BD group. As a consequence, the HC group may not be a representative control sample. Lastly, while we endeavored to control for important established correlates of RHI such as demographics (eg, age, sex), CVRFs (eg, lipids, blood pressure, obesity, CRP), and current use of psychiatric medications (eg, SGA, antidepressants, lithium, any medication use), we cannot rule out the possibility that residual confounding remains.
CONCLUSION
The results of this study advance knowledge regarding the vascular-bipolar link by investigating endothelial function in relation to mood among youth early in their course of illness. Present findings suggest the need for future prospective studies to inform our understanding of the direction, and biological underpinnings, of the observed associations. Given that the increased risk and prematurity of CVD in BD exceed what can be explained by traditional CVRFs, novel measures of endothelial function have the potential to inform our understanding of the vascular-bipolar link and its interface with mood symptoms and to facilitate optimized CVD risk stratification in BD.7 Ultimately, this line of research adds to the existing evidence for the potential value of integrating cardiovascular-related therapeutic approaches in BD.
Submitted: July 25, 2022; accepted December 13, 2022.
Published online: May 1, 2023.
Author contributions: Dr Kennedy wrote the manuscript and performed all relevant analyses. Mr Karthikeyan assisted with the literature search, writing the manuscript, and creating figures. Dr Sultan created the tables and assisted with manuscript preparation. Drs McCrindle and Miller provided critical feedback and intellectual content to the manuscript. Dr Goldstein is the principal investigator, participated in the conceptualization of the study, and provided critical feedback and intellectual content to the manuscript.
Relevant financial relationships: Drs Kennedy, Sultan, McCrindle, Miller, and Goldstein and Mr Karthikeyan declare no conflicts of interest.
Funding/support: This study was funded by the Canadian Institutes of Health Research (MOP 136947, PJT 162110, MOP 119525) and a Miner’s Lamp Innovation Fund from the University of Toronto.
Role of the sponsor: The funding sources were not involved in study design and the conduct of the research.
Acknowledgments: The authors would like to thank all our participants, their families, and the staff at the Center for Youth Bipolar Disorder.
Additional information: Dr Goldstein acknowledges his position as RBC Investments Chair in Children’s Mental Health and Developmental Psychopathology at The Centre for Addiction and Mental Health (CAMH), a joint Hospital-University Chair between the University of Toronto, CAMH, and the CAMH Foundation. The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
Clinical Points
- Youth with bipolar disorder are at increased risk for cardiovascular disease, which in turn confers greater mood symptom burden. While cardiovascular disease is rare in youth, endothelial dysfunction, an early predictor of cardiovascular disease, begins in youth. Evaluating whether endothelial dysfunction is present in youth with bipolar disorder may yield new therapeutic opportunities.
- Compared to controls, youth with bipolar disorder in a depressive episode had poorer endothelial function, whereas those in a hypomanic/mixed episode had better endothelial function.
- Symptomatic youth with bipolar disorder have anomalous endothelial function independent of obesity, psychiatric medications, and traditional cardiovascular risk factors.
References (57)

- Ösby U, Brandt L, Correia N, et al. Excess mortality in bipolar and unipolar disorder in Sweden. Arch Gen Psychiatry. 2001;58(9):844–850. PubMed CrossRef
- Goldstein BI, Schaffer A, Wang S, et al. Excessive and premature new-onset cardiovascular disease among adults with bipolar disorder in the US NESARC cohort. J Clin Psychiatry. 2015;76(2):163–169. PubMed CrossRef
- Goldstein BI, Fagiolini A, Houck P, et al. Cardiovascular disease and hypertension among adults with bipolar I disorder in the United States. Bipolar Disord. 2009;11(6):657–662. PubMed CrossRef
- Goldstein BI, Carnethon MR, Matthews KA, et al; American Heart Association Atherosclerosis; Hypertension and Obesity in Youth Committee of the Council on Cardiovascular Disease in the Young. Major depressive disorder and bipolar disorder predispose youth to accelerated atherosclerosis and early cardiovascular disease: a scientific statement from the American Heart Association. Circulation. 2015;132(10):965–986. PubMed CrossRef
- Goldstein BI, Baune BT, Bond DJ, et al. Call to action regarding the vascular-bipolar link: a report from the Vascular Task Force of the International Society for Bipolar Disorders. Bipolar Disord. 2020;22(5):440–460. PubMed CrossRef
- Urbina EM, Williams RV, Alpert BS, et al; American Heart Association Atherosclerosis, Hypertension, and Obesity in Youth Committee of the Council on Cardiovascular Disease in the Young. Noninvasive assessment of subclinical atherosclerosis in children and adolescents: recommendations for standard assessment for clinical research: a scientific statement from the American Heart Association. Hypertension. 2009;54(5):919–950. PubMed CrossRef
- Widlansky ME, Gokce N, Keaney JF Jr, et al. The clinical implications of endothelial dysfunction. J Am Coll Cardiol. 2003;42(7):1149–1160. PubMed CrossRef
- Flammer AJ, Anderson T, Celermajer DS, et al. The assessment of endothelial function: from research into clinical practice. Circulation. 2012;126(6):753–767. PubMed CrossRef
- Waclawovsky AJ, de Brito E, Smith L, et al. Endothelial dysfunction in people with depressive disorders: a systematic review and meta-analysis. J Psychiatr Res. 2021;141:152–159. PubMed CrossRef
- Cooper DC, Tomfohr LM, Milic MS, et al. Depressed mood and flow-mediated dilation: a systematic review and meta-analysis. Psychosom Med. 2011;73(5):360–369. PubMed CrossRef
- Fiedorowicz JG, Ellingrod VL, Kaplan MJ, et al. The development of depressive symptoms during medical internship stress predicts worsening vascular function. J Psychosom Res. 2015;79(3):243–245. PubMed CrossRef
- Murray DP, Metz NS, Haynes WG, et al. Vascular function is not impaired early in the course of bipolar disorder. J Psychosom Res. 2012;72(3):195–198. PubMed CrossRef
- Tong B, Abosi O, Schmitz S, et al. Bipolar disorder and related mood states are not associated with endothelial function of small arteries in adults without heart disease. Gen Hosp Psychiatry. 2018;51:36–40. PubMed CrossRef
- Schmitz SL, Abosi OJ, Persons JE, et al. Impact of mood on endothelial function and arterial stiffness in bipolar disorder. Heart Mind (Mumbai). 2018;2(3):78–84. PubMed CrossRef
- Fiedorowicz JG, Coryell WH, Rice JP, et al. Vasculopathy related to manic/hypomanic symptom burden and first-generation antipsychotics in a sub-sample from the collaborative depression study. Psychother Psychosom. 2012;81(4):235–243. PubMed CrossRef
- Tomfohr LM, Murphy MLM, Miller GE, et al. Multiwave associations between depressive symptoms and endothelial function in adolescent and young adult females. Psychosom Med. 2011;73(6):456–461. PubMed CrossRef
- Tomfohr LM, Martin TM, Miller GE. Symptoms of depression and impaired endothelial function in healthy adolescent women. J Behav Med. 2008;31(2):137–143. PubMed CrossRef
- Waloszek JM, Byrne ML, Woods MJ, et al. Early physiological markers of cardiovascular risk in community based adolescents with a depressive disorder. J Affect Disord. 2015;175:403–410. PubMed CrossRef
- Olive LS, Abhayaratna WP, Byrne D, et al. Do self-reported stress and depressive symptoms effect endothelial function in healthy youth? The LOOK longitudinal study. PLoS One. 2018;13(4):e0196137. PubMed CrossRef
- Hatch JK, Scola G, Olowoyeye O, et al. Inflammatory markers and brain-derived neurotrophic factor as potential bridges linking bipolar disorder and cardiovascular risk among adolescents. J Clin Psychiatry. 2017;78(3):e286–e293. PubMed CrossRef
- Naiberg MR, Hatch JK, Selkirk B, et al. Retinal photography: a window into the cardiovascular-brain link in adolescent bipolar disorder. J Affect Disord. 2017;218:227–237. PubMed CrossRef
- Goldstein BI, Korczak DJ. Links between child and adolescent psychiatric disorders and cardiovascular risk. Can J Cardiol. 2020;36(9):1394–1405. PubMed CrossRef
- Goldstein BI, Lotrich F, Axelson DA, et al. Inflammatory markers among adolescents and young adults with bipolar spectrum disorders. J Clin Psychiatry. 2015;76(11):1556–1563. PubMed CrossRef
- Karthikeyan S, Dimick MK, Fiksenbaum L, et al. Inflammatory markers, brain-derived neurotrophic factor, and the symptomatic course of adolescent bipolar disorder: A prospective repeated-measures study. Brain Behav Immun. 2022;100:278–286. PubMed CrossRef
- Shapiro LR, Kennedy KG, Dimick MK, et al. Elevated atherogenic lipid profile in youth with bipolar disorder during euthymia and hypomanic/mixed but not depressive states. J Psychosom Res. 2022;156:110763. PubMed CrossRef
- Naiberg MR, Newton DF, Collins JE, et al. Elevated triglycerides are associated with decreased executive function among adolescents with bipolar disorder. Acta Psychiatr Scand. 2016;134(3):241–248. PubMed CrossRef
- Naiberg MR, Newton DF, Collins JE, et al. Impulsivity is associated with blood pressure and waist circumference among adolescents with bipolar disorder. J Psychiatr Res. 2016;83:230–239. PubMed CrossRef
- Kennedy KG, Islam AH, Grigorian A, et al. Elevated lipids are associated with reduced regional brain structure in youth with bipolar disorder. Acta Psychiatr Scand. 2021;143(6):513–525. PubMed CrossRef
- Kennedy KG, Grigorian A, Mitchell RHB, et al. Association of blood pressure with brain structure in youth with and without bipolar disorder. J Affect Disord. 2022;299:666–674. PubMed CrossRef
- Metzig AM, Schwarzenberg SJ, Fox CK, et al. Postprandial endothelial function, inflammation, and oxidative stress in obese children and adolescents. Obesity (Silver Spring). 2011;19(6):1279–1283. PubMed CrossRef
- Hatch J, Collinger K, Moody A, et al. Non-invasive vascular imaging is associated with cardiovascular risk factors among adolescents with bipolar disorder. Pediatr Cardiol. 2015;36(1):158–164. PubMed CrossRef
- Parrinello CM, Hua S, Carnethon MR, et al. Associations of hyperglycemia and insulin resistance with biomarkers of endothelial dysfunction in Hispanic/Latino youths: results from the Hispanic Community Children’s Health Study/Study of Latino Youth (SOL Youth). J Diabetes Complications. 2017;31(5):836–842. PubMed CrossRef
- Kaufman J, Birmaher B, Brent D, et al. Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Version (K-SADS-PL): initial reliability and validity data. J Am Acad Child Adolesc Psychiatry. 1997;36(7):980–988. PubMed CrossRef
- Chambers WJ, Puig-Antich J, Hirsch M, et al. The assessment of affective disorders in children and adolescents by semistructured interview. Test-retest reliability of the schedule for affective disorders and schizophrenia for school-age children, present episode version. Arch Gen Psychiatry. 1985;42(7):696–702. PubMed CrossRef
- Axelson D, Birmaher BJ, Brent D, et al. A preliminary study of the Kiddie Schedule for Affective Disorders and Schizophrenia for School-Age Children mania rating scale for children and adolescents. J Child Adolesc Psychopharmacol. 2003;13(4):463–470. PubMed CrossRef
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, Text Revision. American Psychiatric Association; 2000.
- Birmaher B, Axelson D, Strober M, et al. Clinical course of children and adolescents with bipolar spectrum disorders. Arch Gen Psychiatry. 2006;63(2):175–183. PubMed CrossRef
- Shaffer D, Gould MS, Brasic J, et al. A children’s global assessment scale (CGAS). Arch Gen Psychiatry. 1983;40(11):1228–1231. PubMed CrossRef
- Weissman MM, Wickramaratne P, Adams P, et al. Brief screening for family psychiatric history: the family history screen. Arch Gen Psychiatry. 2000;57(7):675–682. PubMed CrossRef
- Hollingshead AB. Four Factor Index of Social Status. Yale University; 1975:21–52.
- Flynn JT, Kaelber DC, Baker-Smith CM, et al; Subcommittee on Screening and Management of High Blood Pressure in Children. Clinical practice guideline for screening and management of high blood pressure in children and adolescents. Pediatrics. 2017;140(3):e20171904. PubMed CrossRef
- Faizi AK, Kornmo DW, Agewall S. Evaluation of endothelial function using finger plethysmography. Clin Physiol Funct Imaging. 2009;29(5):372–375. PubMed CrossRef
- Harris PA, Taylor R, Thielke R, et al. Research electronic data capture (REDCap)–a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377–381. PubMed CrossRef
- Andresen J, Shafi NI, Bryan RM Jr. Endothelial influences on cerebrovascular tone. J Appl Physiol (1985). 2006;100(1):318–327. PubMed CrossRef
- Wang Y, Liu X, Li P, et al. Regional Cerebral Blood Flow in Mania: Assessment Using 320-Slice Computed Tomography. Front Psychiatry. 2018;9:296. PubMed CrossRef
- Levine GN, Cohen BE, Commodore-Mensah Y, et al. Psychological health, well-being, and the mind-heart-body connection: a scientific statement from the American Heart Association. Circulation. 2021;143(10):e763–e783. PubMed CrossRef
- Miller M, Mangano CC, Beach V, et al. Divergent effects of joyful and anxiety-provoking music on endothelial vasoreactivity. Psychosom Med. 2010;72(4):354–356. PubMed CrossRef
- Sugawara J, Tarumi T, Tanaka H. Effect of mirthful laughter on vascular function. Am J Cardiol. 2010;106(6):856–859. PubMed CrossRef
- Miller M, Mangano C, Park Y, et al. Impact of cinematic viewing on endothelial function. Heart. 2006;92(2):261–262. PubMed CrossRef
- Fiedorowicz JG, et al. Manic/hypomanic symptom burden predicts cardiovascular mortality with bipolar disorder in the collaborative depression study. Psychosom Med. 2009;71:598–606. PubMed CrossRef
- Chang H-A, Chang CC, Tzeng NS, et al. Heart rate variability in unmedicated patients with bipolar disorder in the manic phase. Psychiatry Clin Neurosci. 2014;68(9):674–682. PubMed CrossRef
- Wazen GLL, Gregório ML, Kemp AH, et al. Heart rate variability in patients with bipolar disorder: From mania to euthymia. J Psychiatr Res. 2018;99:33–38. PubMed CrossRef
- Kraepelin E. Manic depressive insanity and paranoia. J Nerv Ment Dis. 1921;53(4):350. CrossRef
- Ross KM, Liu S, Tomfohr LM, et al. Self-esteem variability predicts arterial stiffness trajectories in healthy adolescent females. Health Psychol. 2013;32(8):869–876. PubMed CrossRef
- Osika W, Montgomery SM, Dangardt F, et al. Anger, depression and anxiety associated with endothelial function in childhood and adolescence. Arch Dis Child. 2011;96(1):38–43. PubMed CrossRef
- Cooper DC, Milic MS, Tafur JR, et al. Adverse impact of mood on flow-mediated dilation. Psychosom Med. 2010;72(2):122–127. PubMed CrossRef
- Routledge FS, Dunbar SB, Higgins M, et al. Insomnia symptoms are associated with abnormal endothelial function. J Cardiovasc Nurs. 2017;32(1):78–85. PubMed CrossRef
Save
Cite
Advertisement
GAM ID: sidebar-top