Abstract
The age-standardized global prevalence of epilepsy is about 0.3% in women. Seizures are associated with morbidity and mortality risks; so, women with epilepsy (WWE) are usually advised antiepileptic drug (AED) treatment even during pregnancy. Women may also knowingly or unknowingly be exposed during pregnancy to AEDs advised for other on- or off-label indications. In this context, a meta-analysis of 35 adverse gestational outcomes examined in 76 observational studies found that WWE were at increased risk of most of the adverse outcomes, regardless of gestational exposure to AEDs. AEDs, especially in polytherapy, further increased at least a few of the gestational risks, including risks of congenital conditions, neonatal intensive care unit admission, small for gestational age, low birth weight, and neonatal/infant death (it is unclear whether the lack of statistical significance for the remaining risks was because AED exposure was truly limited to these risks or whether the nonsignificant analyses were underpowered). Reassuringly, the increases in risk were mostly in the small to modest range. This meta-analysis pooled unadjusted risks (which would probably be larger than adjusted risks), so readers are informed about expected findings in the population but not about cause-effect relationships that may be cautiously hypothesized from adjusted analyses. A take-home message is that, because of the wide range of outcomes for which risk is increased, WWE should be closely monitored and followed all through pregnancy, regardless of treatment with AEDs. This article also provides readers with suggestions on how to critically interpret literature with regard to 8 matters: confounding by indication and confounding by severity of indication, as specific to the indication for AED prescription; unadjusted and adjusted analyses; the base rate of an outcome in the population; the examination of multiple outcomes; the uniform direction of findings; the sample numbers; the timing of AED exposure; and self-fulfilling prophecies.
J Clin Psychiatry 2024;85(2):24f15411
Author affiliations are listed at the end of this article.
The Global Burden of Disease Study 2016 Epilepsy Collaborators found that, in 2016, the age standardized global prevalence of epilepsy was 0.63%; almost exactly half of affected subjects were women. The prevalence of epilepsy in women was slightly higher during early relative to late reproductive life.1 Given that the occurrence of a seizure can result in physical harm and may even be life-threatening, almost all women with epilepsy (WWE), even those who are pregnant, will need to take antiepileptic drugs (AEDs) to prevent seizures.2
AEDs such as valproate, carbamazepine, lamotrigine, and others are also used on- or off-label to treat mood disorders, migraine and other headaches, other pain syndromes, and other conditions, and so women receiving AEDs, for epilepsy or other indications, may knowingly or unknowingly be exposed to these drugs during early pregnancy and later. This makes it important for women and health care professionals to be aware of the possible consequences of gestational exposure to AEDs. This article therefore examines recent literature on adverse pregnancy outcomes associated with untreated and treated epilepsy in WWE.
Notes About Confounding
At the risk of drawing attention to the obvious, readers must remember that all studies in the field are observational; for ethical and other reasons, it is almost impossible to conduct randomized controlled trials of AEDs during pregnancy. Unfortunately, cause and effect cannot be confidently determined in observational studies, even in adjusted analyses; all that can be stated is that an exposure was (or was not) associated with an outcome. This is because a statistically significant adverse pregnancy outcome may result not from AED exposure but from confounding by indication.3 That is, when WWE are compared with women without epilepsy, the WWE are an already compromised group; an adverse pregnancy outcome observed may have resulted from epilepsy and its correlates rather than from the AED that was used in the treatment of the epilepsy.
When WWE, treated with AEDs during pregnancy, are compared with WWE who were untreated (this study design controls for confounding by indication), an adverse pregnancy outcome observed may have resulted from more severe epilepsy or from less well-controlled epilepsy rather than from the AED that was used in its treatment. The WWE who were untreated may have been a biased group; they may not have received AEDs during pregnancy because their seizures were mild, infrequent, or in remission for a year or longer. That is, the adverse outcome in the WWE who were treated may have arisen because of confounding by severity of indication.3 This concern also applies to comparisons of WWE who receive 1 AED with WWE who receive a different AED; the choice of AED may have been driven by the nature and severity of epilepsy. This concern likewise applies to WWE who receive AED polytherapy vs monotherapy; WWE needing AED polytherapy may have a more severe form of seizure disorder.
It is important to recognize that confounding by indication will differ across indications; therefore, AED associated gestational risks might differ between WWE and women with other indications for AED use. This is especially important when unadjusted risks are examined (see below). Regrettably, this concern has been poorly studied.
Interpretation of Unadjusted Analyses
Most studies present unadjusted and adjusted analyses. In unadjusted analyses, as an example, the pregnancy outcome is directly compared between AED exposed and AED-unexposed groups. Because patients were not randomized to their respective groups, the groups commonly differ systematically in many regards. In adjusted analyses, using regression, attempts are made to control or adjust for systematic biases (that is, the confounding variables and other covariates) that might have influenced the outcome under study.
An unadjusted analysis tells us about the magnitude of risk of an adverse outcome in the real world, when WWE experience not just epilepsy but all the problems associated with being WWE. An adjusted analysis tells us how AED exposure influences the magnitude of risk of an adverse outcome after the influence of (recorded) biasing variables has been removed. So, unadjusted analyses tell WWE what to expect, and adjusted analyses help us better understand whether the exposure truly affects the risk and, if so, by how much. Thus, the unadjusted and adjusted analyses are each important but for different reasons. The distinction is especially important when the relationship between risk factor and outcome is large or statistically significant in unadjusted analysis but becomes smaller or statistically nonsignificant in adjusted analysis.
A note is made here that adjusted analyses can never be perfect. This is because nothing can control or adjust for confounding by indication or confounding by severity of indication; at best, propensity score matching can be performed, but this also has limitations.4 Also in adjusted analyses, it is not possible to control for unmeasured and unknown variables that could influence outcomes. Finally, in adjusted analyses, controlling for inadequately or improperly defined and measured variables will inevitably be imperfect.
The Importance of the Base Rate
If an event is common in the population, then even a small increase in risk can be clinically important. For example, if the base rate for spontaneous abortion is about 20% in the general population of women who conceive,5 then an exposure that increases the risk by 50% (RR = 1.50) would move the rate from 20% to 30%; the number needed to harm (NNH) is 10. In contrast, if the base rate for congenital heart defects in the general population of newborns is about 1%,6 then an exposure that increases the risk by 50% would move the rate from 1% to only 1.5%; the NNH is 200. So, it is important to know the base rate in the population in order to understand what the change in absolute risk might be, and hence to understand the clinical significance of the finding.
The base rate is important for another reason, as well. The results of observational studies and of meta analyses thereof are often presented as odds ratios (ORs). ORs numerically magnify the true (relative) risk. The difference between the OR and the true risk is small if the event of interest is rare in the population (e.g. base rate <10%) but can be large if the event is common.
The Examination of Multiple Outcomes
Many studies (and meta-analyses) of pregnancy outcomes in WWE examine multiple outcomes; some, numbering in dozens. It is customary in research to specify a primary outcome and to then test that outcome against a P value of .05; other outcomes are tested in exploratory analyses at a P value of .05, without correction for a Type 1 (false positive) error, or in more conservative analyses at P values that are corrected using Bonferroni or Hochberg methods.7
So, how might readers interpret the many studies in the field that neither specify primary and secondary outcomes nor correct for multiple testing? There is no good answer here. One possibility is for readers to themselves determine whether findings remain statistically significant after P value corrections; this is easy to do.7 Another possibility is to accept uncorrected values for statistical significance when outcomes may not be related, as with risk of antepartum hemorrhage and risk of major congenital malformations (MCMs). A third possibility is for readers with a specific question to examine the specific outcome against a P value of .05, regardless of whether this was a primary or secondary outcome in the study. An example is the reader who is interested in a specific outcome associated with a specific drug during a specific trimester of pregnancy; to the reader, this is the main or primary outcome of interest, regardless of whether the finding was presented in the main analyses or in supplementary data of the study under examination.
The Matter of Uniform Direction of Findings
As a spinoff from the previous section, readers often think that, if a study examines multiple outcomes, then chance findings should lie on either side of the null; that is, if AED exposure truly has no effect on gestational outcomes, the exposure should, by chance, be associated with increased risk in some analyses and with decreased risk in other analyses. However, AED exposure is almost always associated with only increased risk and often with significantly increased risk. This correctly suggests that “there is something going on there.” A wrong interpretation is that the uniform direction of findings implies a cause-effect relationship between AED exposure and adverse outcome. The right interpretation is that the something that is going on is probably confounding (especially by indication and by severity of indication) in unadjusted analyses and residual confounding (confounding from inadequately measured, unmeasured, and unknown confounds) in adjusted analyses, possibly supplemented by a true drug (adverse) effect.
The Importance of Sample Numbers
Beyond examining the magnitude and statistical significance of a risk, readers should examine the number of studies, the number of pregnancies, and the number of adverse outcomes detected that contributed data to the analysis. Findings based on smaller numbers of exposed pregnancies, and especially smaller numbers of adverse outcomes detected, are less likely to be valid estimates of the population than findings based on larger numbers. Analyses based on small numbers are also likely to be underpowered and hence associated with false negative findings.
The Timing of Exposure
For many if not for most outcomes, the timing of exposure is important. As examples, the first trimester, and especially weeks 4–10 of gestation, is critical for MCMs, and the second trimester, for neurodevelopmental disorders. So, a study may well find no association between gestational exposure to an AED and MCMs if the AED exposure occurred only from the last trimester onward. However, most studies, in primary analyses, tend to present results for exposure “anytime during pregnancy”; a common reason for this is that trimester-wise data analyses might be underpowered. Readers should keep in mind that the results of “anytime” exposure analyses may sometimes reflect confounding by indication more than a specific drug effect.
Self-Fulfilling Prophecies
Medical professionals who take care of WWE and their offspring through pregnancy, childbirth, and afterward are fully aware of both epilepsy diagnosis and medication status of their patients. They are therefore more likely to look for, detect, and record adverse outcomes and to advise precautionary measures such as induced labor, cesarean section, or neonatal intensive care unit (NICU) admission to avert adverse outcomes. WWE who are aware of the risks may readily agree for precautionary measures. As a result, at least some adverse outcomes, or proxies thereof, may result from what might be described as self-fulfilling prophecies.
Epilepsy, Antiepileptic Drugs, and Pregnancy: 2015 Meta-Analysis
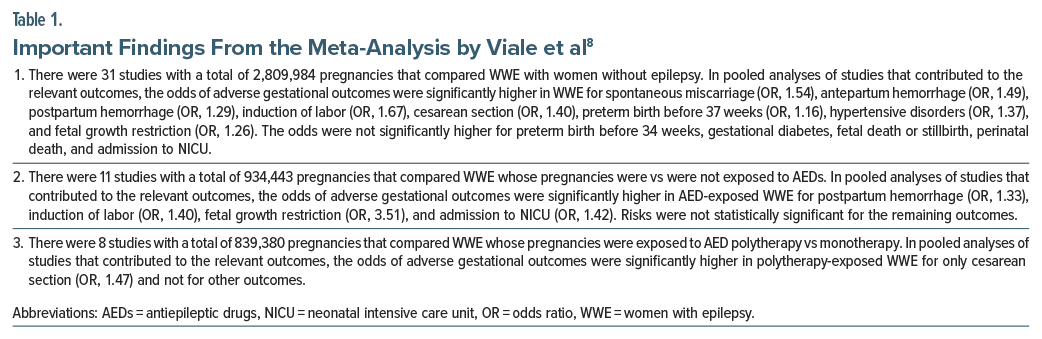
Almost a decade ago, a systematic review and meta analysis8 of 13 prospective and 25 retrospective studies (pooled N = 2,837,325 pregnancies), published in 39 articles, examined pregnancy outcomes in WWE. Important findings from the meta-analysis are presented in Table 1. In summary, relative to women without epilepsy, WWE had significantly elevated risks of 8 of 13 adverse maternal and fetal outcomes. The increases in risk were mostly small to modest, with most ORs in the 1.20–1.50 range. When confounding by indication was addressed by comparing WWE receiving vs not receiving AEDs, there were only 4 adverse outcomes the risks of which reached statistical significance; and when AED polytherapy vs monotherapy exposure was compared, the risk was significantly raised only for cesarean section.
Readers are reminded that nonsignificant risks in different sets of analyses are not necessarily due to a true absence of risk or a result of controlling for confounding through choice of a more appropriate comparison group; nonsignificance could also be a result of inadequate statistical power because of fewer studies, fewer exposed pregnancies, and fewer detected outcomes available for the analyses. Readers may also note that the significant risks were examined at P < .05; that is, no correction was applied for multiple outcome testing.
This meta-analysis8 is overshadowed by the more recent and far larger meta-analysis by Mazzone et al,9 presented in the next section. This meta-analysis8 is included only because, to judge from a sentence in the discussion section of the paper, the authors pooled adjusted ORs (the issue was not otherwise considered in the statistical methods subsection, nor did the corresponding author respond to an enquiry).
Epilepsy, Antiepileptic Drugs, and Pregnancy: 2023 Meta-Analysis
Mazzone et al9 described a systematic review and meta-analysis of 76 observational studies of which 21 were prospective cohort studies, 45 were retrospective cohort studies, 9 were case-control studies, and 1 was a cross-sectional study. Most studies (n = 62) were considered to be at low risk of bias. Results were examined for 21 maternal and fetal outcomes and 14 neonatal outcomes. Most analyses, presented in Tables 2 and 3, were based on data pooled from tens of thousands of pregnancies; the rest, presented in Tables 4 and 5, were based on data that were mostly pooled from a few thousand pregnancies.
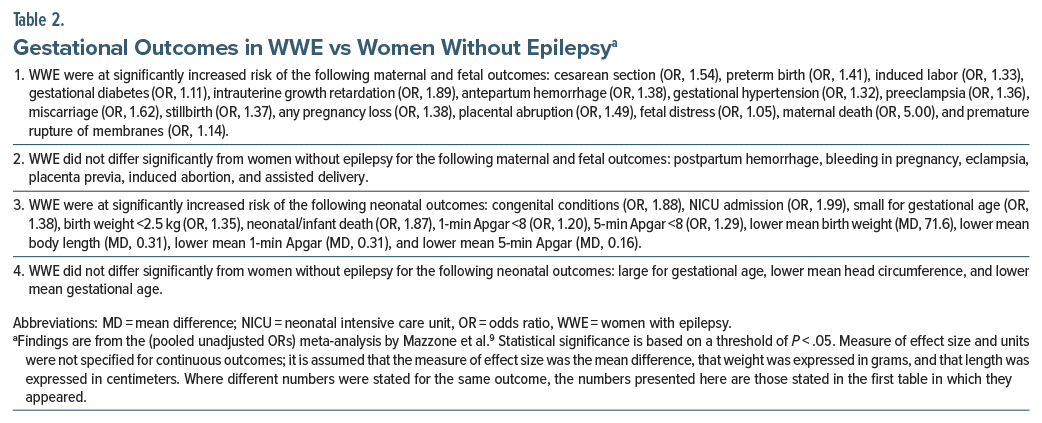
Important findings for WWE vs women without epilepsy are presented in Table 2. In summary, WWE were found to be at significantly increased risk of 15 of 21 maternal and fetal outcomes and 11 of 14 neonatal outcomes. Most of the statistically significant ORs were in the 1.30–1.60 range, indicating small to modest increase in risk, and most mean differences appeared too small to be of clinical significance. The 95% CIs were narrow for almost all outcomes, indicating that the estimates were precise; however, the OR was high, and the 95% CI was very wide for an uncommon outcome, risk of maternal death (OR, 5.00; 95% CI, 1.38–18.04), indicating substantial imprecision.
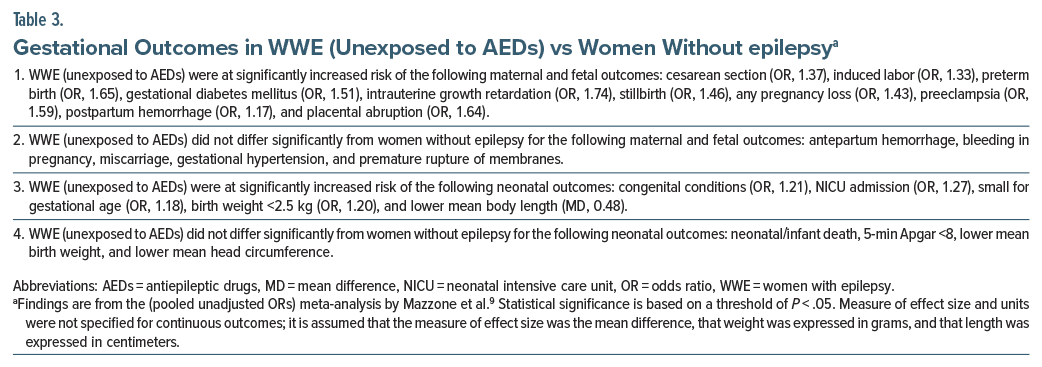
Important findings for WWE (unexposed to AEDs) vs women without epilepsy are presented in Table 3. In summary, WWE who were unexposed to AEDs were found to be at significantly increased risk of 10 of 15 maternal and fetal outcomes and 5 of 9 neonatal outcomes. Almost all the statistically significant ORs were in the 1.20–1.75 range, indicating small to modest increase in risk, and the only statistically significant mean difference appeared too small to be of clinical significance. All CIs were narrow, indicating precision of estimates.
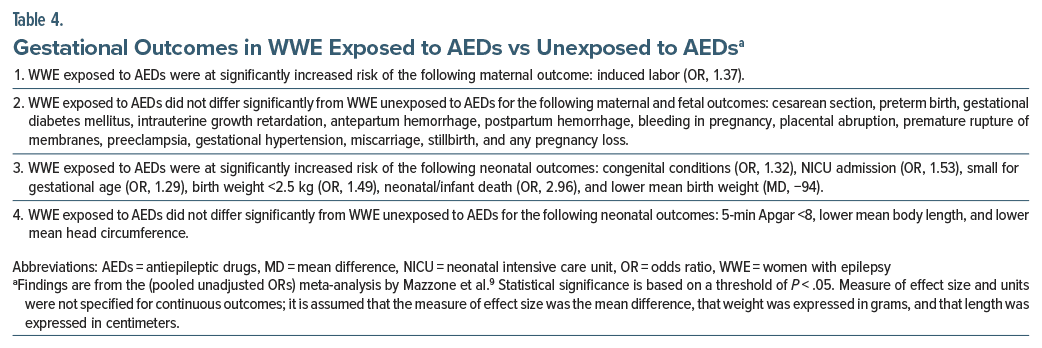
Important findings for WWE exposed to AEDs vs unexposed to AEDs are presented in Table 4. In summary, WWE who were exposed to AEDs were found to be at significantly increased risk of only 1 of 15 maternal and fetal outcomes and 6 of 9 neonatal outcomes. The statistically significant ORs were approximately in the 1.30–1.50 range, that is, they were small to modest in magnitude. The OR (2.96) for neonatal/infant death was, however, high. The lower mean birth weight, by 94 g, also appeared clinically significant.
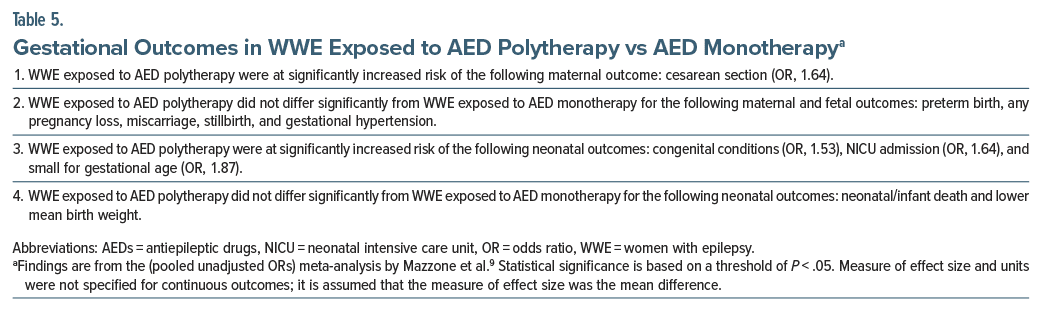
Important findings for WWE exposed to AED polytherapy vs AED monotherapy are presented in Table 5. These analyses were based on samples of only a few thousand patients or fewer. In summary, WWE exposed to AED polytherapy were at increased risk of only 1 of 6 maternal and fetal outcomes and 3 of 5 neonatal outcomes; the increase in risk was modest, with statistically significant ORs lying in the 1.50–1.90 range.
The authors9 presented a pooled analysis of adjusted ORs for 10 gestational outcomes in WWE vs women without epilepsy. The ORs attenuated slightly or remained much the same but became nonsignificant for gestational hypertension and increased markedly from 5.00 (95% CI, 1.38–18.04) to 9.41 (95% CI, 5.36–16.53) for maternal death.
Caveats: 1
The impressively large meta-analysis by Mazzone et al9 pooled unadjusted estimates; the theoretical arguments for and against this approach are discussed elsewhere.10 For reasons explained in the earlier section on unadjusted vs adjusted analyses, the findings of this meta-analysis should be interpreted as representing real-world outcomes rather than cause and effect relationships. Again, for reasons explained in the earlier section on confounding by indication, the findings of this meta-analysis should be generalized only to WWE and not to women with other indications for AED use.
The meta-analysis9 presented outcomes for related variables that were not explicitly defined and differentiated. Examples of such variables are miscarriage, stillbirth, and any pregnancy loss, and preeclampsia, eclampsia, and gestational hypertension. How the reader interprets these different terms may not necessarily be identical with how the authors interpreted them. Perhaps the authors merely extracted and presented data for variables that were ‘as defined’ by the authors of the original studies on which the meta-analysis was based.
Many of the analyses presented in Tables 4 and 5 may have been underpowered. Finally, all findings are reported significant against a threshold of P < .05, and so some findings may be false positives.
Caveats: 2
The meta-analyses8,9 reviewed in this article did not present findings for type of seizure disorder, individual drug exposures, doses of drugs, and trimester of exposure. This means that the meta-analysis results will be more useful to epilepsy clinics and other health care providers who plan for WWE in general than to health care providers who manage individual WWE. Different gestational risks could be expected to show different sensitivity to different kinds of seizures, and to exposure to different AEDs in different doses and in different trimesters of pregnancy. In this context, the next article in this column will present recent research findings on MCMs associated with gestational exposure to individual AEDs.
The meta-analyses8,9 reviewed in this article did not present information on other outcomes that are relevant to gestational exposure to AEDs. These outcomes include differences in intellectual development in exposed and unexposed children, risks of autism spectrum disorder and attention-deficit/ hyperactivity disorder, and risks of other childhood and adolescence-onset psychiatric disorders. All of these adverse outcomes have been associated with AED use during pregnancy, especially with specific AEDs such as valproate and topiramate.11–13
A Bonus Note
Clinicians who use AEDs to treat WWE during pregnancy should be aware that dose-normalized concentrations of AEDs drop significantly as pregnancy progresses. Drugs affected include lamotrigine, lacosamide, levetiracetam, oxcarbazepine, zonisamide, and carbamazepine. The results are less clear for topiramate.14
Take-Home Message
A take-home message is that WWE are at increased risk of a large number of adverse gestational outcomes, whether or not they are exposed to AEDs during pregnancy. The unadjusted ORs for significantly increased risk are mostly small to modest in magnitude (eg, 1.30–1.60). The wide range of increased risks means that WWE require to be closely monitored and followed all through pregnancy, regardless of treatment with AEDs.
AEDs, and especially AED polytherapy, further increase at least a few of the gestational risks, important among which are congenital conditions, NICU admission, small for gestational age, low birth weight, and neonatal/infant death. It is presently unclear whether the lack of statistical significance for the rest of the gestational risks is because AED exposure is truly limited to these risks or whether the analyses for the rest of the risks were underpowered.
Given that untreated seizures are associated with maternal morbidity and mortality risks in addition to the gestational risks associated with untreated epilepsy, and given that the absolute AED-associated increase in risk is small for most outcomes, it may be prudent to treat epilepsy during pregnancy, with careful choice of drug and dosing schedule. All treatment decisions, nevertheless, need to be made in a process shared between health care providers and the women whom they advise.
Concluding Notes
It is hoped that the information provided in this article will help WWE and women who use AEDs during pregnancy understand the many different risks to which they and their fetus/neonate are exposed at different stages of pregnancy and afterward. Proper understanding of risks will allow for institution of preventive and protective measures, and for planning for early detection of and intervention for adverse outcomes, should any occur.
Article Information
Published Online: June 5, 2024. https://doi.org/10.4088/JCP.24f15411
© 2024 Physicians Postgraduate Press, Inc.
To Cite: Andrade C. Epilepsy, antiepileptic drugs, and adverse pregnancy outcomes, 1: examination and interpretation of recent research. J Clin Psychiatry. 2024;85(2):24f15411.
Author Affiliation: Department of Clinical Psychopharmacology and Neurotoxicology, National Institute of Mental Health and Neurosciences, Bangalore, India ([email protected]).
 Each month in his online column, Dr Andrade considers theoretical and practical ideas in clinical psychopharmacology with a view to update the knowledge and skills of medical practitioners who treat patients with psychiatric conditions.
Each month in his online column, Dr Andrade considers theoretical and practical ideas in clinical psychopharmacology with a view to update the knowledge and skills of medical practitioners who treat patients with psychiatric conditions.Department of Clinical Psychopharmacology and Neurotoxicology, National Institute of Mental Health and Neurosciences, Bangalore, India ([email protected]).
Financial disclosure and more about Dr Andrade.
References (14)

- GBD 2016 Epilepsy Collaborators. Global, regional, and national burden of epilepsy, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019;18(4):357–375. PubMed CrossRef
- Tomson T, Battino D, Bromley R, et al. Management of epilepsy in pregnancy: a report from the international league against epilepsy task force on women and pregnancy. Epileptic Disord. 2019;21(6):497–517. PubMed CrossRef
- Andrade C. Confounding by indication, confounding variables, covariates, and independent variables: knowing what these terms mean and when to use which term. Indian J Psychol Med. 2024;46(1):78–80. PubMed
- Andrade C. Propensity score matching in nonrandomized studies: a concept simply explained using antidepressant treatment during pregnancy as an example. J Clin Psychiatry. 2017;78(2):e162–e165. PubMed CrossRef
- Buss L, Tolstrup J, Munk C, et al. Spontaneous abortion: a prospective cohort study of younger women from the general population in Denmark. Validation, occurrence and risk determinants. Acta Obstet Gynecol Scand. 2006;85(4):467–475. PubMed CrossRef
- Liu Y, Chen S, Zühlke L, et al. Global birth prevalence of congenital heart defects 1970–2017: updated systematic review and meta-analysis of 260 studies. Int J Epidemiol. 2019;48(2):455–463. PubMed CrossRef
- Andrade C. Multiple testing and protection against a type 1 (false positive) error using the Bonferroni and Hochberg corrections. Indian J Psychol Med. 2019;41(1):99–100. PubMed CrossRef
- Viale L, Allotey J, Cheong-See F, et al; EBM CONNECT Collaboration. Epilepsy in pregnancy and reproductive outcomes: a systematic review and meta-analysis. Lancet. 2015;386(10006):1845–1852. PubMed CrossRef
- Mazzone PP, Hogg KM, Weir CJ, et al. Comparison of perinatal outcomes for women with and without epilepsy: a systematic review and meta-analysis. JAMA Neurol. 2023;80(5):484–494. PubMed
- Menon V, Andrade C, Thennarasu K. Polycystic ovarian syndrome and autism spectrum disorder in the offspring: should the primary outcome have been different? Mol Psychiatry. 2021;26(5):1438–1439. PubMed CrossRef
- Bjørk MH, Zoega H, Leinonen MK, et al. Association of prenatal exposure to antiseizure medication with risk of autism and intellectual disability. JAMA Neurol. 2022;79(7):672–681. PubMed
- Dreier JW, Bjørk MH, Alvestad S, et al. Prenatal exposure to antiseizure medication and incidence of childhood- and adolescence-onset psychiatric disorders. JAMA Neurol. 2023;80(6):568–577. PubMed CrossRef
- Honybun E, Cockle E, Malpas CB, et al. Neurodevelopmental and functional outcomes following in utero exposure to antiseizure medication: a systematic review. Neurology. 2024;102(8):e209175. PubMed CrossRef
- Pennell PB, Karanam A, Meador KJ, et al. Antiseizure medication concentrations during pregnancy: results from the Maternal Outcomes and Neurodevelopmental Effects of Antiepileptic Drugs (MONEAD) Study. JAMA Neurol. 2022;79(4):370–379. PubMed
This PDF is free for all visitors!
Save
Cite