Abstract
Women with epilepsy (WWE) are usually advised antiepileptic drug (AED) treatment even during pregnancy. It is therefore important to know what the major congenital malformation (MCM) risks might be with untreated epilepsy, and with first-trimester exposure to different AEDs in monotherapy. This article reviews recent findings from a large multinational registry, a large multinational population based study, and a large meta-analysis. In summary, data from the meta-analysis suggest that the MCM rate is 2%–3% in women without epilepsy and about 3% in WWE who were unexposed to AEDs during pregnancy. Data from the meta analysis also suggest that the MCM rate is approximately population level at 2.6%–3.5% with levetiracetam and lamotrigine and that it is about 4%–5% with carbamazepine, 2.8%–4.8% with oxcarbazepine, about 4% with topiramate, about 5%–7% with phenytoin, about 6%–9% with phenobarbital, and nearly 10% with valproate. The MCM risk with valproate is significantly higher than that with other AEDs (including topiramate and phenobarbital) that significantly increase the risk. Data from the registry suggest that risks are dose-dependent with valproate, phenobarbital, and carbamazepine and that the risk with valproate may be as high as 25% at doses >1,450 mg/d. Valproate is also associated with a wide range of MCMs. Data from the population-based study were generally confirmatory. Strengths and limitations of the studies are considered. The findings of these studies encourage the consideration of levetiracetam or lamotrigine monotherapy for WWE who are pregnant and strongly discourage the consideration of the older AEDs, especially phenytoin and phenobarbitone, and most especially valproate. These considerations also apply to all WWE of childbearing age because it may not be easy to change AEDs when pregnancy is planned and because pregnancy is often unplanned.
J Clin Psychiatry 2024;85(3):24f15432
Author affiliations are listed at the end of this article.
The previous article in this column1 discussed meta analyses2,3 of the risk of nearly 3 dozen maternal, fetal, and neonatal outcomes in pregnant women with epilepsy (WWE). In summary, relative to women without epilepsy, WWE were at increased risk of almost all the adverse gestational outcomes examined. In WWE, relative to no exposure, exposure to antiepileptic drugs (AEDs) during pregnancy was associated with fewer adverse gestational outcomes. Finally, relative to exposure in monotherapy, exposure to AEDs in polytherapy was associated with still fewer adverse gestational outcomes. The decreasing breadth of risks across these successive waves of analysis could reflect either increasing control for confounding by indication and the severity thereof or inadequately powered analyses with diminishing sample sizes.
In these meta-analyses,2,3 important adverse outcomes were increased risks of major congenital malformations (MCMs), neonatal intensive care unit admission, small for gestational age, low birth weight, and neonatal/infant death. There were several limitations to the findings; notably, neither meta-analysis presented risks for individual AEDs, nor were there dose dependence analyses. This is important because WWE and their health care providers need to know the risks associated with specific drugs in specific doses in order to make informed choices.
This article therefore presents recent data on an important gestational outcome, MCMs, in association with individual AEDs prescribed in monotherapy to WWE. The data are drawn from 3 important studies: a large, comprehensive, Cochrane meta-analysis4 and 2 studies published after the Cochrane review.5,6 The latter are considered first.
THE EURAP STUDY
This cohort study5 was an update of a previous report from the International Registry of Antiepileptic Drugs and Pregnancy, which collects data from >1,500 collaborators in 47 countries. A strength of this study, relative to other cohort studies, is that data for important variables were collected prospectively. This allowed not only a granular examination of outcomes but also a granular adjustment for covariates and confounds.
The sample comprised 8,483 WWE, exposed to AEDs (but not to known teratogens) during the first trimester of pregnancy, and enrolled within week 16 of gestation. The mean age of the sample was 30 years. The sample was 87% white. Data from these women were available for 9,840 pregnancies. Strikingly, folate dosing was inappropriate for 62% of the pregnancies. In this context, appropriate dosing was defined as at least 0.4 mg/d, started at least 3 months before conception, and continued all through the first trimester.
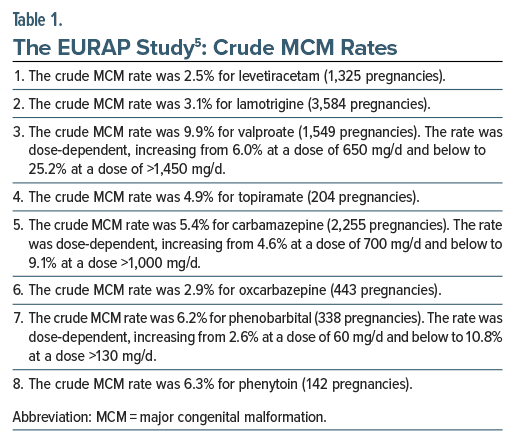
The crude MCM rates, as recorded in the study, are presented in Table 1. Reassuringly, the MCM rate for 2 newer AEDs was approximately the same as that for women in the general population: that for levetiracetam was 2.5% (95% CI [confidence interval], 1.8%–3.5%) and that for lamotrigine was 3.1% (95% CI, 2.5%–3.7%). The narrow CIs indicate the precision of the estimate, and the upper bound of each CI suggests minimal cause for concern even in the worst-case scenario. Of much concern, the MCM rate with valproate was 6.0% with doses of 650 mg/d and lower and 25.2% with doses of >1,450 mg/d.
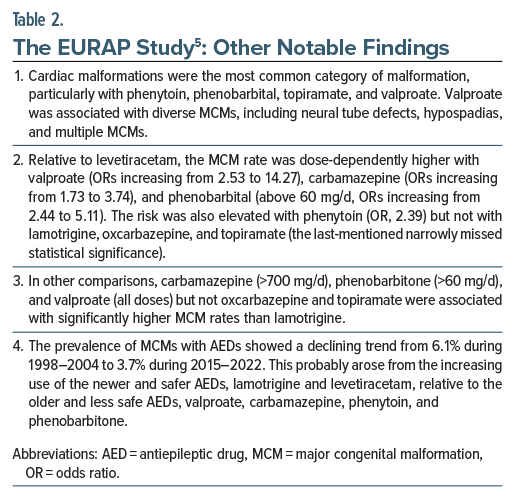
Other notable findings from the study are presented in Table 2. The authors5 presented many comparisons of different AEDs in different doses. These are too many to list; interested readers may refer to the original article. These comparisons could be important when, for WWE, choices are considered between one drug at one dose and another drug at another dose.
OBSERVATIONS ON THE EURAP STUDY
Among the studies reviewed in this article, the EURAP cohort study5 is of special interest because, although it was smaller than the Nordic study6 and, of course, the Cochrane meta-analysis,4 the prospective design allowed the collection of data on important independent variables, covariates, and confounds that are unavailable in health care databases and national registers that provide material for retrospective cohort studies. As examples, data were available for parental history, type of epilepsy, seizure control during the first trimester, and appropriate use of periconceptional folate supplementation. Not only were all of these included as covariates in the main MCM analyses but also their respective contributions to the outcome could be examined.
The EURAP study5 found that parental history of MCMs was associated with a significantly increased risk of MCMs in the offspring (odds ratio [OR], 3.43); unfortunately, family history of epilepsy was not studied, and so we do not know whether the genetic predisposition to MCMs might be related to epilepsy or is independent of it.
Type of epilepsy (generalized vs focal and unknown vs focal) was not associated with increased risk of MCMs, diminishing a potential role for confounding by indication, and suggesting that other descriptors of epilepsy, including treatment with AEDs, drive the risk.
Occurrence of tonic-clonic seizures during the first trimester was not associated with increased risk of MCMs, diminishing a potential role for confounding by severity of indication, and again suggesting that other descriptors of epilepsy, including treatment with AEDs, drive the risk.
The appropriate use of periconceptional folate supplementation was not associated with decreased risk of MCMs. This finding was unexpected. It may have arisen because the definition of appropriateness (from at least 3 months before conception to at least the end of the first trimester, in the dose of at least 0.4 mg/d) was too strict, allowing the comparison group to include women with reasonably adequate folate use. It may have arisen because folate use was examined only in the context of overall MCMs and not specifically for, as an example, neural tube defects. It may have arisen because the optimal dose of folate may be higher than 0.4 mg/d, periconceptionally, in women exposed to AEDs.7 And, finally, it may be that folate supplementation does not assure protection against AED-associated MCMs. Health care professionals, therefore, should not assume that AEDs such as valproate are safe in women who take 0.4 mg/d (or 4 mg/d, as many guidelines recommend for at-risk pregnancies) periconceptionally.
Importantly, all of these findings were obtained from analyses in which AED use was the variable of primary interest and parental history, type of epilepsy, seizure control during the first trimester, and appropriate use of periconceptional folate supplementation were covariates. An interpretation of covariate ORs is, at best, suggestive and, at worst, fallacious. This is because of the “Table 2 Fallacy.”8 In order to identify how a variable influences outcomes, that variable should be the variable of primary interest and covariates appropriate to that variable should be added. As studied by Battino et al,5 the covariates were appropriate not for each other but for the AED exposures. Therefore, the findings for parental history, type of epilepsy, seizure control during the first trimester, and appropriate use of periconceptional folate supplementation should be viewed with caution.
To their credit, the authors5 used an appropriate method (the Benjamini-Hochberg procedure) to reduce the risk of a type 1 (false-positive) statistical error associated with the multiple hypothesis testing reported above. This displays a sense of academic responsibility that is seldom seen in papers reporting multiple outcomes in observational studies.
THE NORDIC STUDY
The Nordic study6 is briefly discussed here; the findings support those reported in the EURAP study.5 This study was a very large population-based observational study of a 1996–2020 cohort drawn from health registers in Denmark, Finland, Iceland, Norway, and Sweden. The sample comprised women who were unexposed to AEDs (n = 4,866,362) and women with first-trimester exposure in monotherapy to lamotrigine (n = 8,339), valproate (n = 2,031), topiramate (n = 509), carbamazepine (n = 2,674), oxcarbazepine (n = 1,313), and levetiracetam (n = 1,040). Indications for exposure included epilepsy, mood disorder, and migraine. Analyses were adjusted for measured confounds.
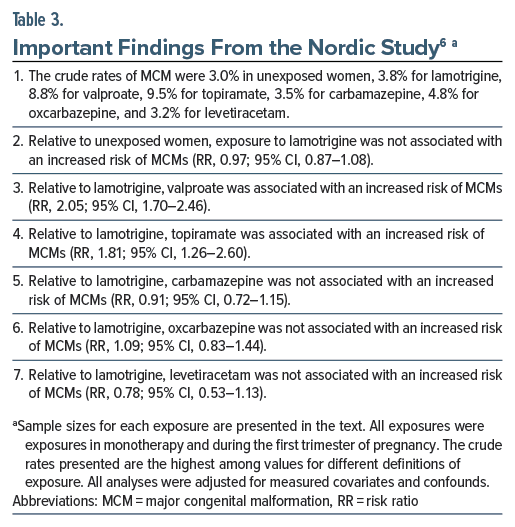
Important findings from the study are presented in Table 3. In summary, relative to unexposed women, exposure to lamotrigine was not associated with increased risk of MCMs. Relative to lamotrigine exposure, exposure to carbamazepine, oxcarbazepine, and levetiracetam was not associated with increased risk of MCMs. Relative to exposure to lamotrigine, exposure to valproate and topiramate were each associated with an approximately doubled risk of MCMs.
Notably, the increased risks with valproate and topiramate were dose-dependent. Valproate was associated with several specific MCMs, including cardiac MCMs, central nervous system MCMs, cleft lip and palate, club foot, and hypospadias.
THE COCHRANE META-ANALYSIS
Bromley et al4 identified 49 studies, published in 128 papers, that examined MCM outcomes in WWE treated with AEDs in monotherapy; comparison groups were WWE untreated with AEDs and women without epilepsy. The review was updated to February 2022. The authors presented results separately for prospective cohort studies (n = 17,963) and retrospective epidemiological health record studies (n = 7,913).
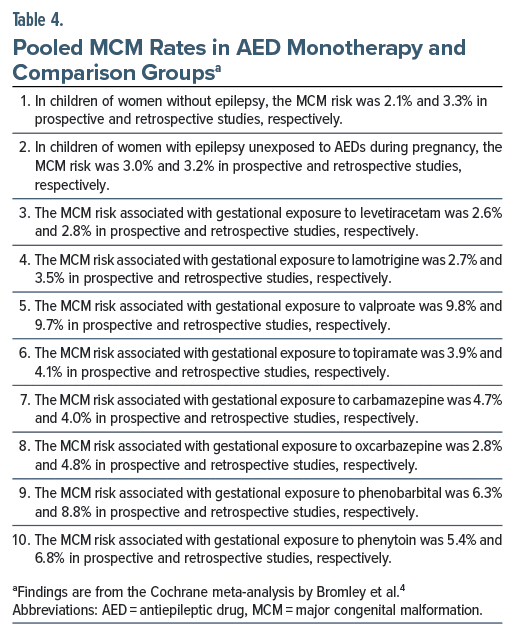
The pooled MCM rates for different AEDs are presented in Table 4. In summary, the MCM rate was 2%–3% in women without epilepsy and about 3% in WWE who were unexposed to AEDs during pregnancy. The MCM rate was 2.6%–3.5% with levetiracetam and lamotrigine, 2.8%–4.8% with oxcarbazepine and carbamazepine, 3.9%–4.1% with topiramate, 5.4%–8.8% with phenytoin and phenobarbital, and 9.7%–9.8% with valproate. Risks appeared dose dependent with valproate and carbamazepine.
Some AEDs were associated with specific MCMs. As examples, valproate was associated with spinal, skeletal, cardiac, and facial MCMs. Phenobarbital was associated with cardiac MCMs and topiramate with facial MCMs.
Comparisons among AEDs suggested no significant difference in the MCM rates between levetiracetam and lamotrigine. These 2 drugs were not associated with a higher risk of MCMs relative to untreated WWE. Levetiracetam was not associated with a higher risk of MCMs relative to women without epilepsy. Lamotrigine was associated with a higher risk of MCMs relative to women without epilepsy in prospective but not in retrospective studies.
Valproate was associated with significantly higher risks than all other AEDs, including AEDs such as phenobarbitone and topiramate that were themselves associated with significantly higher risks. There were hundreds of other comparisons between AEDs and the comparison groups, and between one AED and another AED for all MCMs combined as well as for different MCMs separately. Listing the results of all of these is out of the scope of this article; the interested reader is referred to the original article which is freely available online.
Data were also presented for newer and infrequently prescribed AEDs, including AEDs such as gabapentin, vigabatrin, and eslicarbazepine. These findings are also not listed in the present article.
GENERAL NOTES
Another meta-analysis,3 reviewed in an earlier article,1 found that WWE who were unexposed to AEDs were at higher risk of congenital conditions than women without epilepsy. Studies in the field may therefore suffer from confounding by indication.
The studies reviewed in this article need to be viewed through the prism of the caveats expressed in the previous article in this series.1 As an example, the Cochrane meta analysis4 did not explicitly restrict article selection to studies with AED exposure during the first trimester of pregnancy. The Cochrane meta-analysis4 also presented hundreds of comparisons; there would surely have been many false-positive findings among these.
TAKE-HOME MESSAGE
The findings of the studies reviewed in this article encourage the consideration of monotherapy with levetiracetam or lamotrigine for WWE who are pregnant and strongly discourage the consideration of the older AEDs, especially phenytoin and phenobarbitone, and most especially valproate.
Importantly, these considerations apply not just to WWE during pregnancy but also to all women of childbearing age. This is because it may not be easy to change AEDs when pregnancy is planned, more so because pregnancy is often unplanned, and especially so because in some parts of the world, termination of pregnancy may be prohibited by law.
Article Information
Published Online: July 3, 2024. https://doi.org/10.4088/JCP.24f15432
© 2024 Physicians Postgraduate Press, Inc.
To Cite: Andrade C. Epilepsy, antiepileptic drugs, and adverse pregnancy outcomes, 2: major congenital malformations with antiepileptic drug monotherapy.
J Clin Psychiatry. 2024;85(3):24f15432.
Author Affiliations: Department of Clinical Psychopharmacology and Neurotoxicology, National Institute of Mental Health and Neurosciences, Bangalore, India ([email protected]).
 Each month in his online column, Dr Andrade considers theoretical and practical ideas in clinical psychopharmacology with a view to update the knowledge and skills of medical practitioners who treat patients with psychiatric conditions.
Each month in his online column, Dr Andrade considers theoretical and practical ideas in clinical psychopharmacology with a view to update the knowledge and skills of medical practitioners who treat patients with psychiatric conditions.Department of Clinical Psychopharmacology and Neurotoxicology, National Institute of Mental Health and Neurosciences, Bangalore, India ([email protected]).
Financial disclosure and more about Dr Andrade.
References (8)

- Andrade C. Epilepsy, antiepileptic drugs, and adverse pregnancy outcomes: 1. Examination and interpretation of recent research. J Clin Psychiatry. 2024;85(2):24f15411. PubMed CrossRef
- Viale L, Allotey J, Cheong-See F, et al; EBM CONNECT Collaboration. Epilepsy in pregnancy and reproductive outcomes: a systematic review and meta-analysis. Lancet. 2015;386(10006):1845–1852. PubMed CrossRef
- Mazzone PP, Hogg KM, Weir CJ, et al. Comparison of perinatal outcomes for women with and without epilepsy: a systematic review and meta-analysis. JAMA Neurol. 2023;80(5):484–494. PubMed
- Bromley R, Adab N, Bluett-Duncan M, et al. Monotherapy treatment of epilepsy in pregnancy: congenital malformation outcomes in the child. Cochrane Database Syst Rev. 2023;8(8):CD010224. PubMed CrossRef
- Battino D, Tomson T, Bonizzoni E, et al; EURAP Collaborators. Risk of major congenital malformations and exposure to antiseizure medication monotherapy. JAMA Neurol. 2024:e240258.
- Cohen JM, Alvestad S, Cesta CE, et al. Comparative safety of antiseizure medication monotherapy for major malformations. Ann Neurol. 2023;93(3):551–562. PubMed
- Dwyer ER, Filion KB, MacFarlane AJ, et al. Who should consume high-dose folic acid supplements before and during early pregnancy for the prevention of neural tube defects? BMJ. 2022;377:e067728. PubMed CrossRef
- Westreich D, Greenland S. The table 2 fallacy: presenting and interpreting confounder and modifier coefficients. Am J Epidemiol. 2013;177(4):292–298. PubMed CrossRef
This PDF is free for all visitors!
Save
Cite