ABSTRACT
Objective: As veterans have high rates of posttraumatic stress disorder (PTSD) and historically poor treatment outcomes and high attrition, alternative treatments have gained much popularity despite lack of rigorous research. In this study, a recently developed and manualized 8-session group Equine-Assisted Therapy for PTSD (EAT-PTSD) was tested in an open trial to assess its preliminary feasibility, acceptability, and outcomes for military veterans.
Methods: The study was conducted from July 2016 to July 2019. Sixty-three treatment-seeking veterans with PTSD enrolled. PTSD diagnosis was ascertained using the Structured Clinical Interview for DSM-5, Research Version (SCID-5-RV) and confirmed using the Clinician-Administered PTSD Scale (CAPS-5). Mean age was 50 years, and 23 patients (37%) were women. Clinician and self-report measures of PTSD and depression were assessed at pretreatment, midtreatment, and posttreatment and at a 3-month follow-up. An intent-to-treat analysis and a secondary analysis of those who completed all 4 clinical assessments were utilized.
Results: Only 5 patients (8%) withdrew from treatment, 4 before midtreatment and 1 afterward. Posttreatment assessment revealed marked reductions in both clinician-rated and self-reported PTSD and depression symptoms, which persisted at 3-month follow-up. Specifically, mean (SD) CAPS-5 scores fell from 38.6 (8.1) to 26.9 (12.4) at termination. Thirty-two patients (50.8%) showed clinically significant change (≥ 30% decrease in CAPS-5 score) at posttreatment and 34 (54.0%) at follow-up.
Conclusions: Manualized EAT-PTSD shows promise as a potential new intervention for veterans with PTSD. It appears safe, feasible, and clinically viable. These preliminary results encourage examination of EAT-PTSD in larger, randomized controlled trials.
Trial Registration: ClinicalTrials.gov identifier: NCT03068325
J Clin Psychiatry 2021;82(5):21m14005
To cite: Fisher PW, Lazarov A, Lowell A, et al. Equine-assisted therapy for posttraumatic stress disorder among military veterans: an open trial. J Clin Psychiatry. 2021;82(5):21m14005.
To share: https://doi.org/10.4088/JCP.21m14005
© Copyright 2021 Physicians Postgraduate Press, Inc.
aNew York State Psychiatric Institute, New York, New York
bDepartment of Psychiatry, Columbia University Irving Medical Center, New York, New York
cSchool of Psychological Sciences, Tel Aviv University, Tel Aviv, Israel
dDepartment of Epidemiology, Columbia University Irving Medical Center, New York, New York
‡First authors—equal contribution
*Corresponding author: Amit Lazarov, PhD, Department of Psychiatry, Columbia University Irving Medical Center, 1051 Riverside Drive, New York, NY, 10032 ([email protected]).
Posttraumatic stress disorder (PTSD) is a prevalent and highly debilitating disorder, impairing social, occupational, and other important areas of functioning.1 In the United States, PTSD has a lifetime prevalence of nearly 9% and a 12-month prevalence rate of about 3.5%.1 As military personnel face increased risk for trauma exposure through combat, injury, captivity, and sexual assault,2–4 PTSD rates are even higher for this population (10%–30%).
Despite the development of several psychotherapies and pharmacotherapies for PTSD,5–8 research has consistently shown that more than one-third of treated PTSD patients never fully remit, demonstrating a median illness duration of 3 to 5 years despite treatment.9,10 Many others avoid or reject treatment.5,11 For example, while exposure-based therapy, the gold-standard PTSD treatment,12 is often effective, about half of patients do not benefit, with many dropping out before treatment completion.13–16 Military veterans have shown even weaker treatment effectiveness and higher dropout rates.17–19 Moreover, veterans with PTSD often avoid seeking treatment due to mistrust, stigma, concerns about the treatment experience, low emotional readiness, and logistical barriers or simply because years of nonspecific or ineffective treatments have demoralized them.11,20
As many patients avoid or are proven refractory to standard treatments, a host of complementary and alternative PTSD treatments have arisen and spread widely.21 One such treatment, equine-assisted therapy (EAT), is being increasingly used for a wide range of physical and mental health conditions, including PTSD.22,23 EAT advocates assert that horse-human interaction experiences during therapy can potentially foster insight and behavioral changes in patients, as these interactions offer a platform for eliciting thoughts, feelings, and behaviors related to patients’ lives outside treatment.24,25 Furthermore, horses are considered to be especially conducive to this process as they are naturally hypervigilant and sensitive to verbal and nonverbal cues, providing patients instantaneous feedback during the horse-human interactions, which, in turn, afford patients and therapists opportunities to foster emotional awareness, reflection, and attunement to thoughts, behaviors, and forms of communication.23,26,27
While EAT has gained popularity and exuberant proponents over the years,28 there have been no well-specified treatment manuals of how to deliver EAT, nor has there been adequate safety, feasibility, and efficacy research of EAT. Like many other complementary and alternative treatments, which often either have gone untested or have undergone poorly conceived, likely biased research, lacking adequate standardization or clear therapeutic goals, EAT has also lacked standardization and rigorous testing. Extant EAT research focusing on several mental health conditions, including PTSD, is scarce and generally poorly designed, characterized by small sample sizes, inconsistent assessments, unstandardized treatment procedures, and researcher conflicts of interest. Extant evidence for the efficacy of animal-assisted therapies for PTSD, including EAT, has been mostly anecdotal, mainly focusing on countering dissociative symptoms, emotional numbness, social isolation, and hyperarousal (for a review, see O’Haire et al29). These difficulties, reiterated in several reviews,22,23,26,30–33 have precluded a more mainstream acceptance of EAT. In sum, it is often not clear what EAT comprises or means, let alone whether it works. Thus, more methodologically sound and rigorous research is needed to examine whether EAT may be considered a complementary and/or alternative treatment for PTSD.31,34 A well-designed open trial of a manualized, standardized EAT would be a much needed first step in the right direction.
To address this gap, we recently developed and manualized a group EAT for PTSD (EAT-PTSD) comprising eight 90-minute weekly group sessions and then evaluated it in a very small sample (N = 8 patients from two EAT groups), finding no treatment dropout, high patient satisfaction, and decreases in clinician-assessed PTSD and depressive symptoms from pre- to posttreatment.35 These encouraging initial findings led to further refinement of the treatment manual. We then undertook the first rigorous open trial of standardized, manualized EAT-PTSD tailored for military veterans with PTSD using well-validated clinician-rated and self-report assessment measures. This moderately large (N = 63) open trial of EAT-PTSD had 4 assessment points: pretreatment, midpoint, posttreatment, and 3-month follow-up. We expected symptom reduction to follow treatment and to persist 3 months later.
METHODS
Eligibility Screening
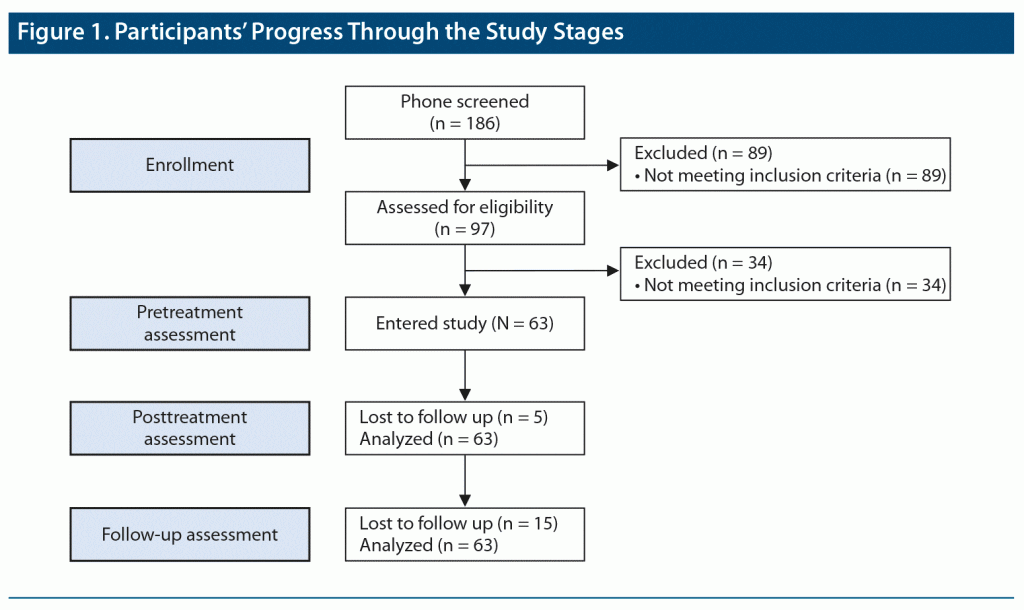
The study was conducted from July 2016 to July 2019. Figure 1 depicts progress through the study stages. Patients were recruited through clinical referrals from Veterans Administration (VA) centers, other programs affiliated with our center (the New York Presbyterian Military Family Wellness Center at Columbia Veterans Research Center), flyers, print and online advertisements, and through word of mouth. Phone screening for study participation used the PTSD Checklist for DSM-5 (PCL-5).36 Individuals scoring ≥ 30, indicating likely PTSD, were invited to an in-person clinical assessment by an independent evaluator, a PhD- or MA-level psychologist trained to ≥ 85% reliability with a senior clinician on all interview-based measures. Primary and comorbid diagnoses were ascertained using the Structured Clinical Interview for DSM-5, Research Version (SCID-5-RV).37 PTSD diagnosis was confirmed using the Clinician-Administered PTSD Scale (CAPS-5).38
Inclusion criteria were (a) primary diagnosis of PTSD, (b) age 18–70 years, (c) reported military experience, and (d) English fluency. Current pharmacotherapy or psychotherapy was permitted providing it had been stable for at least 3 months; patients were asked to hold the regimen stable for the study duration. Importantly, none of those in concurrent psychotherapy were receiving a strict manualized well-defined progressive protocol for PTSD treatment, such as prolonged exposure (PE) or cognitive-behavioral therapy (CBT), but rather were receiving long-term, stable supportive talk therapy in different settings. Exclusion criteria were (a) history of psychotic disorder or current unstable bipolar disorder, determined by SCID-5-RV; (b) 17-item Hamilton Depression Rating Scale (HDRS)39 score > 25; (c) elevated suicide risk, determined by clinical assessment; (d) severe substance or alcohol use disorder within the past 6 months, or moderate use disorder within the past 2 months (SCID-5-RV); and (e) physical limitations impeding participation in equine-assisted activity.
All clinical evaluations took place at New York State Psychiatric Institute (NYSPI). Participants received $100 compensation per assessment. The NYSPI Institutional Review Board approved the study. Participants provided written informed consent (ClinicalTrials.gov identifier: NCT03068325).
Outcome Measures
Primary outcome—clinician-rated PTSD. PTSD symptom severity, measured by the CAPS-5,38 was the primary outcome. The CAPS-5, a structured interview diagnosing PTSD based on DSM-5 criteria, is widely used in research. It has demonstrated excellent reliability, convergent and discriminant validity, diagnostic utility, and sensitivity to change.40 Cronbach α in the current sample was 0.74, 0.83, 0.95, and 0.91, for pretreatment, midpoint, posttreatment, and follow-up assessments, respectively.
Clinically significant change (CSC) was defined a priori as ≥ 30% CAPS score reduction at posttreatment as per CSC scoring practices in prior PTSD studies.41–43
Secondary outcome—self-reported PTSD. PTSD symptoms were also assessed using PCL-5,36 a 20-item questionnaire assessing presence and severity of PTSD symptoms. With well-documented psychometric properties, it is considered a valid, reliable PTSD instrument.44 Cronbach α in this sample was ≥ 0.92 at all assessments.
Depression. Clinician-rated depressive symptoms were measured using the HDRS,39 which has demonstrated strong internal consistency and interrater and test-retest reliability.45 Cronbach α in the current sample was 0.69, 0.79, 0.92, and 0.85 at the assessment intervals. Depression was further assessed using the self-report Beck Depression Inventory-II (BDI-II).46 The 21-item BDI-II has high internal consistency and good test-retest reliability.47 The sample Cronbach α was ≥ 0.93 at all assessments.
Equine-Assisted Therapy for PTSD (EAT-PTSD)
Treatment comprised 8 weekly, manual-guided, 90-minute group sessions35 conducted at a large nearby equestrian center, to which participants received roundtrip transportation. The study comprised 16 EAT groups, each including 3 to 5 veterans. All groups but one were of mixed gender. Sessions were co-led by a licensed mental health professional (LMHP—a licensed professional counselor, or licensed clinical social worker) and a qualified equine specialist (ES). The role of the mental health professional is to facilitate patients’ process and reflection on what they experience and feel, typically through short, targeted observations, questions, or gentle directions. The ES guides participants in horse communication and behavior, demonstrates the equine exercises, and offers coaching, encouragement, and advice for engaging in horse-related exercises. An additional equestrian center horse wrangler attended sessions to enhance safety. Each EAT group included the same two horses in all sessions.
Manualized EAT-PTSD is an experiential treatment and hence does not elicit, focus on, or discuss PTSD-associated trauma. Rather, it seeks to increase affective awareness of self and others, improve communication, help regulate emotional response, improve critical thinking/problem solving, and increase self-confidence/self-efficacy while engaging with the horses. Session 1 orients patients to EAT-PTSD (rationale, description, possible benefits). It provides psychoeducation (eg, common reactions to trauma, development and maintenance of PTSD) and a barn tour and ends with meeting the horses in a private “round pen.” Subsequent sessions all take place in the round pen, with each session including (a) review of the content of previous sessions, (b) a grounding exercise focusing attention on current physical sensations, and (c) introduction of increasingly complex encounters and interactions with horses, coupled with team feedback and direction. Each session ends with an opportunity for participants to review and discuss their experiences during the session (“closing circle”).
Early phase treatment sessions (sessions 2–3) focus on acquainting patients with the horses, on grooming exercises, and on learning “leading”—directing the horses with a rope or a wand. The middle phase (sessions 4–7) furthers patient mastery and comfort with the horses in individual and teamwork exercises. For example, patients learn to use a wand to distance the horse, creating personal space, or to collaboratively maneuver a horse onto a tarpaulin, fostering teamwork and cooperation. The final session includes a graduation ceremony celebrating patients’ treatment progress and accomplishments (See Arnon et al35).
All treatment sessions were videotaped and reviewed by research personnel to ensure adherent delivery of the intervention. The treatment teams attended weekly supervision calls led by the research team (P.W.F. and A.L.) to discuss each session and provide feedback.
Data Analysis
The Little MCAR (missing completely at random) test on total scores for the CAPS-5, PCL-5, HDRS, and BDI-II from each time point following the baseline assessment (ie, midpoint, posttreatment, and follow-up analysis) was used to test whether data were missing at random.48
In line with previous open-trial studies exploring novel psychotherapies for different psychopathologies,48–52 we used an intent-to-treat analysis, thereby analyzing data from all 63 patients. As missing data were minimal and missing at random (see Results section), we conducted the intent-to-treat analysis with full information maximum likelihood (FIML), a gold standard approach to handling missing data.53 In addition, we repeated analyses for patients who completed assessments at all 4 time points (n = 48). Treatment effects were explored using a repeated-measures analysis of variance (ANOVA) with time (pretreatment, midpoint, posttreatment, and follow-up) as a within-subjects factor. Post hoc tests using the Bonferroni correction were used to compare the different time points. All statistical tests were 2-sided, using α ≤ .05. Effect sizes are reported using η2p and Cohen d when appropriate.
RESULTS
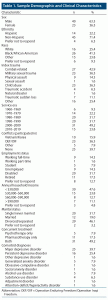
Of 186 phone-screened veterans, 97 were invited for clinical intake, yielding a final sample of 63 veterans with PTSD (mean [SD] age =50.01 [12.38] years; range, 27–70 years; n = 23 [37%] female) (Figure 1). Of enrolled veterans, 47 were receiving concurrent treatment: psychotherapy alone (n = 5), pharmacotherapy alone (n = 11), or both (n = 31). Forty-eight had at least 1 comorbid disorder. See Table 1 for a more complete description of the sample.
Attrition Rates
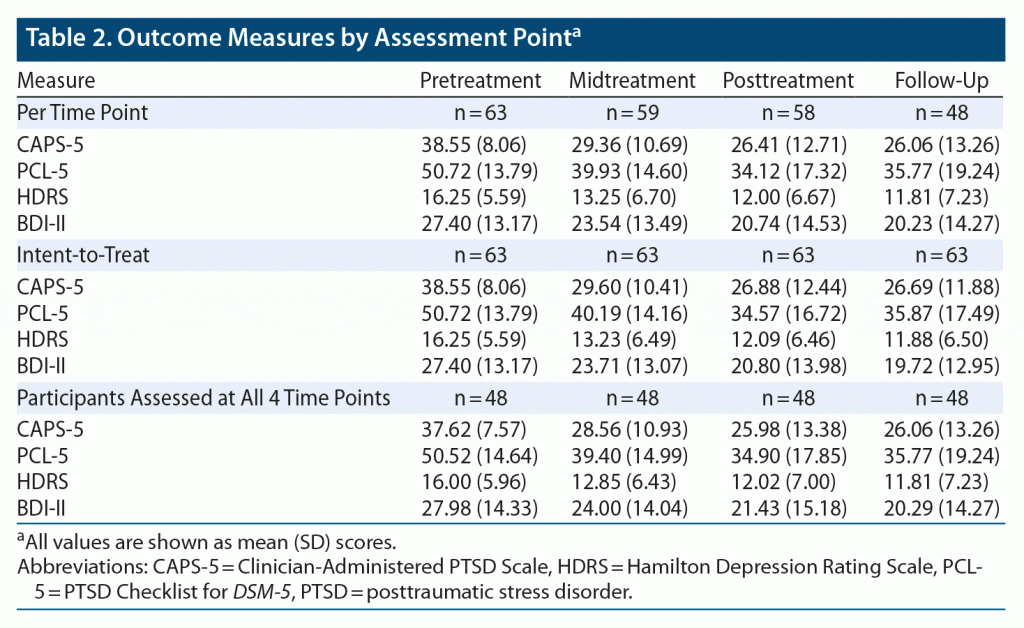
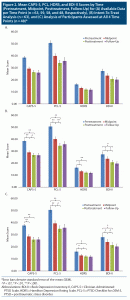
Table 2 and Figure 2A depict outcome measures over time. All 63 subjects completed baseline assessments. Attrition was low: only 5 patients (7.9%) discontinued EAT, 4 patients (6.3%) before midpoint (with 1 dropping out following baseline assessment, attending no EAT-PTSD sessions) and 1 patient (1.6%) after. Forty-eight patients (76.2%) provided 3-month follow-up data. The Little MCAR test indicated that data were missing at random (χ214 = 17.53, P = .23). In addition, there were no adverse events or safety concerns reported by any of the participants or staff members.
Treatment Effects
Table 2 and Figure 2B present results for CAPS-5, PCL-5, HDRS, and BDI-II scores at each of the 4 time points for the intent-to-treat analysis (n = 63). Table 2 and Figure 2C present results for patients who were assessed on all 4 time points. Results were highly similar (see Supplementary Appendix 1).
Mean (SD) CAPS-5 scores decreased from 38.6 (8.1) at baseline to 26.9 (12.4) at termination, with 29 participants (46.0%) and 23 participants (36.5%) scoring below the cutoff score of 25 at posttreatment and follow-up, respectively. Repeated-measures ANOVA with time as the within-subjects factor was significant (F3,186 = 30.41, P < .0001, η2p = 0.33). Post hoc tests with Bonferroni correction revealed a significant pre- to posttreatment reduction (P < .0001, d = 1.11), which persisted at 3-month follow-up (P = .88). This pre- to posttreatment reduction was evident at midpoint (P < .0001, d = 0.96), with further trend-level significant reduction at posttreatment (P = .050, d = 0.24). Based on CAPS-5 scores, 32 veterans (50.8%) demonstrated CSC at posttreatment and 34 (54.0%) at 3-month follow-up.
Mean (SD) PCL-5 scores decreased from 50.7 (13.8) at baseline to 34.6 (16.7) at termination. Repeated-measures ANOVA was significant (F3,186 = 30.85, P < .0001, η2p = 0.33). Post hoc analyses revealed significant reduction from pre- to posttreatment (P < .0001, d = 1.05), maintained at 3-month follow-up (P = .39). This pre- to posttreatment significant reduction in symptoms was evident at midpoint assessment (P < .0001, d = 0.70), with additional reduction from mid- to posttreatment (P < .0001, d = 0.34).
Depression Measures
Mean (SD) HDRS scores decreased from 16.3 (5.6) at baseline to 12.1 (6.5) at termination (F3,186 = 13.82, P < .0001, η2p = 0.18). Bonferroni-corrected post hoc tests revealed significant pre- to posttreatment reduction (P < .0001, d = 0.69), evident at midtreatment (P = .003, d = 0.50) without additional reduction from mid- to posttreatment (P = .13), which was maintained at 3-month follow-up (P = .72).
Mean (SD) BDI-II scores similarly decreased from 27.4 (13.2) at baseline to 20.8 (14.0) at termination (F3,186 = 15.92, P < .0001, η2p = 0.20). Post hoc analyses revealed a significant pre- to posttreatment reduction (P = .001, d = 0.49), maintained at 3-month follow-up (P = .22). This pre- to posttreatment significant reduction was evident at the midpoint assessment (P = .005, d = 0.28), with additional reduction from midpoint to posttreatment assessment (P = .001, d = 0.22).
DISCUSSION
This open trial examined the first rigorously defined, manualized group EAT intervention for PTSD (EAT-PTSD) in a relatively large sample of military veterans, with clinician-rated and self-report outcome measures of PTSD and depression. Results suggest EAT-PTSD is potentially safe and well-tolerated, with large–effect size improvement on standard ratings. Posttreatment findings generally corroborate those of our previous small pilot study (N = 8),35 showing significant symptom reduction from pre- to posttreatment. Unlike in our earlier pilot study, treatment benefits across all outcome measures largely persisted for 3 months following treatment. Although the group mean CAPS-5 score (26.9) remained above the diagnostic threshold of 25, 50.8% and 54.0% of veterans demonstrated clinically significant change at posttreatment and follow-up, respectively, with 46.0% and 36.5% scoring below the cutoff score at posttreatment and follow-up, respectively. Yet, as this trial was an open trial, present findings need to be regarded with caution. Clinical efficacy of EAT needs to be more exhaustively examined in a randomized controlled trial to allow a more formal testing of its efficacy.
Presented findings also suggest high EAT patient tolerability, low attrition, and safety. Dropout rates were low, which is an encouraging preliminary finding in contrast to the roughly 20% treatment dropout rates among PTSD patients broadly13 and 30%–40% among veterans specifically.17,54,55 Yet, these findings warrant caution, as the open trial design allowed participants to actively select treatment without facing randomization to a placebo or unwanted alternative condition.56 We also found patients to be eager to enroll in the EAT-PTSD program, excited by the opportunity for equine engagement. While some patients expressed preference for group treatment over individual psychotherapy, some were more excited about group EAT as an adjunct to ongoing individual treatment. In addition, many patients also expressed looking forward to the EAT sessions, wishing for a longer treatment duration (ie, more than 8 weeks). Finally, while some risk of injury may exist due to propinquity to and handling horses, this risk level is hard to ascertain. Still, wranglers were included in the EAT protocol due to safety concerns, and horses were specifically chosen for their temperament, having no history of aggression. Indeed, no incidents were reported in any of the 16 EAT study groups included in this study.
While this open trial generated encouraging results, why EAT-PTSD yielded benefits is less clear. However, the primary aim of the present study was to explore, using a well-designed and methodologically sound experimental design, whether EAT-PTSD indeed leads to potential therapeutic benefits for veterans, as previous less rigorous research and polemics on EAT have claimed. Hence, we did not address possible underlying constructs or potential mechanisms of action, raising some intriguing questions. For example, is there, as some equine advocates insist, something unique about horses as powerful but fearful prey animals that particularly resonates with veterans with PTSD?24,25 Or would dogs,57 dolphins,58 or dromedaries yield similar outcomes? Does the effect derive from something inherent in the equine interactions, from the human group process, or from the idyllic, bucolic environment, an escape from urban pressures? Might EAT facilitate renewed attachment relationships for traumatized humans who feel distrustful and detached?59 At this juncture, we can only speculate. Considerable follow-up research is needed to address these and related questions pertaining to underlying constructs and mechanisms of change. Still, we believe that the present study is an important addition to the very limited reliable evidence of the benefits and safety of EAT for veterans with PTSD, providing a much-needed foundation for further explorations of EAT.
Notwithstanding the aforementioned limitations, our recently published substudy60 yields some preliminary conjectures. We examined a subsample of the present study (N = 20) using longitudinal neuroimaging (ie, structural magnetic resonance imaging [sMRI], resting state functional MRI [rs-fMRI], and diffusion tensor imaging [DTI]) to explore possible mechanisms and predictors of EAT outcome.60 Results showed a significant increase in caudate functional connectivity (FC) and reduction in gray matter density of the thalamus and the caudate at posttreatment. This increase of caudate FC was further positively associated with clinical improvement at posttreatment and at 3-month follow-up. Higher baseline caudate FC was associated with greater PTSD symptom reduction posttreatment. These exploratory findings suggest that EAT-PTSD may target reward circuitry responsiveness and produce a caudate pruning effect from pre- to posttreatment. Yet, these exploratory findings should be further examined in future research using additional behavioral tasks and clinical measures of related concepts such as anhedonia, as well as observer and self-report measures of emotional regulation, critical thinking and problem-solving, and self-efficacy.
The study has some limitations. The lack of a control arm in which participants receive no active therapy is a major one. While an open trial is appropriate for this preliminary stage of treatment development, the absence of a control condition precludes testing the clinical efficacy of EAT, especially as many participants had concurrent (stable) psychological and/or pharmacologic treatment. Open trials sometimes deliver overly rosy results, and symptom change could conceivably reflect passage of time. Still, symptom reduction was clinically significant and persisted at 3-month follow-up. Second, independent evaluators could not be blinded to the open treatment. Third, so as not to prevent patients from seeking treatment post-EAT, the study could not control for any additional treatment patients may have received between posttreatment and follow-up assessments. Any additional treatment following the termination of EAT, while not affecting the posttreatment assessment, may have affected clinical status at follow-up unrelated to the specific effects of EAT. Relatedly, we did not exclude participants with stable psychotherapy or psychopharmacology from entering the study. As most (n = 47) received either or both, this may have also affected emergent results. On the other hand, these could also be considered ineffective or insufficient interventions, as patients had typically been receiving them chronically and still met study entry criteria. We did verify that concurrent treatment had been unchanged for at least 3 months and that no study participants were receiving a clearly defined progressive time-limited PTSD treatment such as PE or CBT. Fourth, the potential benefits of EAT may be geographically restricted to venues accessible to horse stables. Relatedly, the study did not assess costs in relation to benefits. Even if future research finds EAT efficacious, it may prove to be an expensive endeavor. Fifth, EAT may have appealed to veterans with an a priori affinity for horses, and findings may be specific to such patients. Finally, several administrative factors of the EAT protocol, less than central to the therapy itself, may have contributed to the observed low attrition rates and symptom reduction. These may include the “outdoor” recreational aspects of EAT, which incorporated a trip outside participants’ urban setting, atypical of most PTSD interventions; the relatively high assessment payments (summing potentially to $400); and the simple pleasure of being in a bucolic setting and being around animals, including horses. These factors may have encouraged study participation and completion. Yet, the stable symptom reduction on the CAPS-5, PCL-5, HDRS, and BDI-II and in rates of CSC at 3-month follow-up assessment lend support for the therapeutic value of EAT-PTSD.
Nonetheless, group EAT for PTSD, the first manualized EAT, might offer an effective primary intervention or adjunctive therapy for PTSD. Current results suggest EAT-PTSD appears safe, tolerable, well-regarded, and beneficial for veterans meeting DSM-5 PTSD criteria. EAT-PTSD might engage avoidant patients, resistant to more formal treatment modalities, encouraging subsequent openness to additional therapy. These promising open trial findings warrant further testing of this EAT protocol in randomized controlled trials.
Submitted: March 25, 2021; accepted June 3, 2021.
Published online: August 31, 2021.
Author contributions: Dr Fisher: conceptualization, data curation, funding acquisition, investigation, project administration, supervision, writing—original draft. Dr Lazarov: formal analysis, writing—original draft. Dr Lowell: project administration, supervision. Ms Arnon: data curation, project administration. Dr Turner: writing—review and editing. Ms Bergman: data curation, project administration. Mr Ryba: data curation, project administration. Sara Ms Such: data curation, project administration. Ms Marohasy: data curation, project administration. Dr Zhu: writing—review and editing. Dr Suarez-Jimenez: writing—review and editing. Dr Markowitz: conceptualization, writing—review and editing. Dr Neria: conceptualization, funding acquisition, investigation,resources, supervision, writing—review and editing.
Potential conflicts of interest: None.
Funding/support: The study was supported by the Earle I. Mack Foundation, the Jockey Club, the David and Julia Koch Foundation, the Nicholson Family Charity Fund, the Mary & Daniel Loughran Foundation, the Viola Foundation, Gulfstream Park Racing Association, Gerald Parsky, Peter M. Brant, the Ganek Family Foundation, the Live Oak Foundation, the Reid Family Charitable Fund, Meta Aerospace Capital, LTD, and Tactical Air Support.
Role of the sponsor: The funding agencies had no role in the study design; in the collection, analysis and interpretation of data; in the writing of the report; or in the decision to submit the article for publication.
Acknowledgments: We greatly appreciate and thank the veterans who participated in this study. We are also grateful to the following people for their contributions to the project: Debra G. Farber, MS, MCIS, LPC; Jody Jacob-McVey, BS; Bonnie E. Malajian, MSW, LCSW; April Neumann AS; and Susan Stegmeyer, who were members of the EAT treatment teams; Anna Gassib, BS, CEO of the Bergen Equestrian Center in Leonia, New Jersey, where all EAT sessions took place; Allan J. Hamilton (University of Arizona Health Sciences) and Jane F. Hamilton (Rancho Bosque Equestrian Center of Excellence in Tucson, Arizona) for their advice on the EAT treatment protocol; and Anne Poulson, JD, who raised funds to cover project expenses. None of these individuals has any potential conflicts of interest relevant to the subject of this article.
Supplementary material: Available at Psychiatrist.com.
Clinical Points
- Equine-assisted therapy (EAT) has gained popularity over the years as a complementary and alternative treatment for posttraumatic stress disorder (PTSD). However, there have been no well-specified treatment manuals of how to deliver EAT, nor has there been adequate safety, feasibility, and efficacy research on EAT for PTSD.
- The present study addresses this gap in knowledge by using a rigorous open trial of standardized, manualized EAT-PTSD tailored for military veterans, examining changes in PTSD and depressive symptoms across treatment.
- For veterans with PTSD, EAT may serve as a viable alternative or augmentative treatment, lowering both PTSD and depressive symptoms after treatment and 3 months following treatment termination.
References (60)

- American Psychiatric Association. Diagnostic and Statistical Manual for Mental Disorders. Fifth Edition. Washington, DC: American Psychiatric Association; 2013.
- Hoge CW, Castro CA, Messer SC, et al. Combat duty in Iraq and Afghanistan, mental health problems, and barriers to care. N Engl J Med. 2004;351(1):13–22. PubMed CrossRef
- Murdoch M, Polusny MA, Hodges J, et al. Prevalence of in-service and post-service sexual assault among combat and noncombat veterans applying for Department of Veterans Affairs posttraumatic stress disorder disability benefits. Mil Med. 2004;169(5):392–395. PubMed CrossRef
- Suris A, Lind L. Military sexual trauma: a review of prevalence and associated health consequences in veterans. Trauma Violence Abuse. 2008;9(4):250–269. PubMed CrossRef
- Schneier FR, Neria Y, Pavlicova M, et al. Combined prolonged exposure therapy and paroxetine for PTSD related to the World Trade Center attack: a randomized controlled trial. Am J Psychiatry. 2012;169(1):80–88. PubMed CrossRef
- Bradley R, Greene J, Russ E, et al. A multidimensional meta-analysis of psychotherapy for PTSD. Am J Psychiatry. 2005;162(2):214–227. PubMed CrossRef
- Steckler T, Risbrough V. Pharmacological treatment of PTSD—established and new approaches. Neuropharmacology. 2012;62(2):617–627. PubMed CrossRef
- Sullivan GM, Neria Y. Pharmacotherapy of PTSD: current status and controversies. Psychiatr Ann. 2009;39(6):342–347. PubMed CrossRef
- Difede J, Olden M, Cukor J. Evidence-based treatment of post-traumatic stress disorder. Annu Rev Med. 2014;65(1):319–332. PubMed CrossRef
- Kessler RC, Sonnega A, Bromet E, et al. Posttraumatic stress disorder in the National Comorbidity Survey. Arch Gen Psychiatry. 1995;52(12):1048–1060. PubMed CrossRef
- Stecker T, Shiner B, Watts BV, et al. Treatment-seeking barriers for veterans of the Iraq and Afghanistan conflicts who screen positive for PTSD. Psychiatr Serv. 2013;64(3):280–283. PubMed CrossRef
- Rauch SA, Eftekhari A, Ruzek JI. Review of exposure therapy: a gold standard for PTSD treatment. J Rehabil Res Dev. 2012;49(5):679–687. PubMed CrossRef
- Imel ZE, Laska K, Jakupcak M, et al. Meta-analysis of dropout in treatments for posttraumatic stress disorder. J Consult Clin Psychol. 2013;81(3):394–404. PubMed CrossRef
- Najavits LM. The problem of dropout from “gold standard” PTSD therapies. F1000Prime Rep. 2015;7:43. PubMed CrossRef
- Szafranski DD, Smith BN, Gros DF, et al. High rates of PTSD treatment dropout: a possible red herring? J Anxiety Disord. 2017;47:91–98. PubMed CrossRef
- Gros DF, Price M, Yuen EK, et al. Predictors of completion of exposure therapy in OEF/OIF veterans with posttraumatic stress disorder. Depress Anxiety. 2013;30(11):1107–1113. PubMed CrossRef
- Kehle-Forbes SM, Meis LA, Spoont MR, et al. Treatment initiation and dropout from prolonged exposure and cognitive processing therapy in a VA outpatient clinic. Psychol Trauma. 2016;8(1):107–114. PubMed CrossRef
- Steenkamp MM, Litz BT, Hoge CW, et al. Psychotherapy for military-related PTSD: a review of randomized clinical trials. JAMA. 2015;314(5):489–500. PubMed CrossRef
- Steenkamp MM, Litz BT, Marmar CR. First-line psychotherapies for military-related PTSD. JAMA. 2020;323(7):656–657. PubMed CrossRef
- Ouimette P, Vogt D, Wade M, et al. Perceived barriers to care among veterans health administration patients with posttraumatic stress disorder. Psychol Serv. 2011;8(3):212–223. CrossRef
- Wynn GH. Complementary and alternative medicine approaches in the treatment of PTSD. Curr Psychiatry Rep. 2015;17(8):600. PubMed CrossRef
- Bachi K, Terkel J, Teichman M. Equine-facilitated psychotherapy for at-risk adolescents: the influence on self-image, self-control and trust. Clin Child Psychol Psychiatry. 2012;17(2):298–312. PubMed CrossRef
- Selby A, Smith-Osborne A. A systematic review of effectiveness of complementary and adjunct therapies and interventions involving equines. Health Psychol. 2013;32(4):418–432. PubMed CrossRef
- Hallberg L. Walking the Way of the Horse. Bloomington, IN: iUniverse, Inc; 2008.
- Trotter KS. Harnessing the Power of Equine Assisted Counseling: Adding Animal Assisted Therapy to Your Practice. New York, NY: Routledge Taylor & Francis Group; 2012.
- Anestis MD, Anestis JC, Zawilinski LL, et al. Equine-related treatments for mental disorders lack empirical support: a systematic review of empirical investigations. J Clin Psychol. 2014;70(12):1115–1132. PubMed CrossRef
- Schultz PN, Remick-Barlow GA, Robbins L. Equine-assisted psychotherapy: a mental health promotion/intervention modality for children who have experienced intra-family violence. Health Soc Care Community. 2007;15(3):265–271. PubMed CrossRef
- Masini A. Equine-assisted psychotherapy in clinical practice. J Psychosoc Nurs Ment Health Serv. 2010;48(10):30–34. PubMed CrossRef
- O’Haire ME, Guérin NA, Kirkham AC. Animal-assisted intervention for trauma: a systematic literature review. Front Psychol. 2015;6:1121. PubMed CrossRef
- Kendall E, Maujean A, Lakhani A, et al. A systematic review of the efficacy of equine-assisted interventions on psychological outcomes. European Journal of Psychotherapy & Counselling. 2015;17(1):57–79. CrossRef
- Kinney AR, Eakman AM, Lassell R, et al. Equine-assisted interventions for veterans with service-related health conditions: a systematic mapping review. Mil Med Res. 2019;6(1):28. PubMed CrossRef
- Smith-Osborne A, Selby A. Implications of the literature on equine-assisted activities for use as a complementary intervention in social work practice with children and adolescents. Child Adolesc Social Work J. 2010;27(4):291–307. CrossRef
- Staudt M, Cherry D. Equine-facilitated therapy and trauma: current knowledge, future needs. Adv Soc Work. 2017;18(1):403–414. CrossRef
- Marchand WR, Andersen SJ, Smith JE, et al. Equine-assisted activities and therapies for veterans with posttraumatic stress disorder: current state, challenges and future directions. Chronic Stress (Thousand Oaks). 2021;5:2470547021991556. PubMed
- Arnon S, Fisher PW, Pickover A, et al. Equine-assisted therapy for veterans with PTSD: manual development and preliminary findings. Mil Med. 2020;185(5–6):e557–e564. PubMed CrossRef
- Weathers FW, Litz BT, Keane TM, et al. The PTSD Checklist for DSM-5 (PCL-5). 2013. Scale available from the National Center for PTSD. https://www.ptsd.va.gov/professional/assessment/adult-sr/ptsd-checklist.asp
- First M, Williams J, Karg R, et al. Structured Clinical Interview for DSM-5 Research Version (SCID-5 for DSM-5, Research Version; SCID-5-RV). Arlington, VA: American Psychiatric Association; 2015.
- Weathers FW, Blake DD, Schnurr PP, et al. The Clinician Administered PTSD Scale for DSM-5 (CAPS-5). 2013. Assessment available from https://www.ptsd.va.gov/. https://www.ptsd.va.gov/professional/assessment/adult-int/caps.asp
- Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23(1):56–62. PubMed CrossRef
- Weathers FW, Bovin MJ, Lee DJ, et al. The Clinician-Administered PTSD Scale for DSM-5 (CAPS-5): development and initial psychometric evaluation in military veterans. Psychol Assess. 2018;30(3):383–395. PubMed CrossRef
- Hien DA, Jiang H, Campbell ANC, et al. Do treatment improvements in PTSD severity affect substance use outcomes? a secondary analysis from a randomized clinical trial in NIDA’s Clinical Trials Network. Am J Psychiatry. 2010;167(1):95–101. PubMed CrossRef
- Lazarov A, Suarez-Jimenez B, Abend R, et al. Bias-contingent attention bias modification and attention control training in treatment of PTSD: a randomized control trial. Psychol Med. 2019;49(14):2432–2440. PubMed CrossRef
- Markowitz JC, Petkova E, Neria Y, et al. Is exposure necessary? a randomized clinical trial of interpersonal psychotherapy for PTSD. Am J Psychiatry. 2015;172(5):430–440. PubMed CrossRef
- Bovin MJ, Marx BP, Weathers FW, et al. Psychometric properties of the PTSD Checklist for Diagnostic and Statistical Manual of Mental Disorders-Fifth Edition (PCL-5) in veterans. Psychol Assess. 2016;28(11):1379–1391. PubMed CrossRef
- Trajković G, Starčević V, Latas M, et al. Reliability of the Hamilton Rating Scale for Depression: a meta-analysis over a period of 49 years. Psychiatry Res. 2011;189(1):1–9. PubMed CrossRef
- Beck AT, Steer RA, Brown G. Beck Depression Inventory-II. The Psychological Corporation; 1996.
- Dozois DJA, Dobson KS, Ahnberg JL. A psychometric evaluation of the Beck Depression Inventory-II. Psychol Assess. 1998;10(2):83–89. CrossRef
- Bennett SM, Capriotti M, Bauer C, et al. Development and open trial of a psychosocial intervention for young children with chronic tics: the CBIT-JR Study. Behav Ther. 2020;51(4):659–669. PubMed CrossRef
- Beard C, Fuchs C, Asnaani A, et al. A pilot open trial of cognitive bias modification for panic disorder. Cognit Ther Res. 2016;40(6):792–798. CrossRef
- Storch EA, Lehmkuhl HD, Ricketts E, et al. An open trial of intensive family based cognitive-behavioral therapy in youth with obsessive-compulsive disorder who are medication partial responders or nonresponders. J Clin Child Adolesc Psychol. 2010;39(2):260–268. PubMed CrossRef
- Mennin DS, Fresco DM, Ritter M, et al. An open trial of emotion regulation therapy for generalized anxiety disorder and cooccurring depression. Depress Anxiety. 2015;32(8):614–623. PubMed CrossRef
- Hjeltnes A, Molde H, Schanche E, et al. An open trial of mindfulness-based stress reduction for young adults with social anxiety disorder. Scand J Psychol. 2017;58(1):80–90. PubMed CrossRef
- Enders CK. Applied Missing Data Analysis. New York, NY: The Guilford Press; 2010.
- Goetter EM, Bui E, Ojserkis RA, et al. A systematic review of dropout from psychotherapy for posttraumatic stress disorder among Iraq and Afghanistan combat veterans. J Trauma Stress. 2015;28(5):401–409. PubMed CrossRef
- Olatunji B, Deacon BJ, Ambramowitz JS. The cruelest cure? ethical issues in the implementation of exposure-based treatments. Cognit Behav Pract. 2009;16(2):172–180. CrossRef
- Rutherford BR, Wall MM, Brown PJ, et al. Patient expectancy as a mediator of placebo effects in antidepressant clinical trials. Am J Psychiatry. 2017;174(2):135–142. PubMed CrossRef
- Young C, Horton J. Canine and Equine Therapy for Mental Health: A Review of Clinical Effectiveness. Ottawa, ON: Canadian Agency for Drugs and Technologies in Health; 2019.
- Salgueiro E, Nunes L, Barros A, et al. Effects of a dolphin interaction program on children with autism spectrum disorders: an exploratory research. BMC Res Notes. 2012;5(1):199. PubMed CrossRef
- Milrod B, Keefe JR, Choo TH, et al. Separation anxiety in PTSD: a pilot study of mechanisms in patients undergoing IPT. Depress Anxiety. 2020;37(4):386–395. PubMed CrossRef
- Zhu X, Suarez-Jimenez B, Zilcha-Mano S, et al. Neural changes following equine-assisted therapy for posttraumatic stress disorder: a longitudinal multimodal imaging study. Hum Brain Mapp. 2021;42(6):1930–1939. PubMed CrossRef
Save
Cite
Advertisement
GAM ID: sidebar-top