The use of second-generation antipsychotics has not eliminated tardive dyskinesia (TD), and the prevalence of the disorder is higher than commonly realized. The involuntary movements of TD can decrease patients’ quality of life, cause embarrassment, and lead to social withdrawal. Clinicians must evaluate patients taking DRBAs for TD risk factors and regularly screen them for TD using a rating scale. Familiarity with tools and diagnostic criteria will enable clinicians to conduct a differential diagnosis. Once a diagnosis is made, medications approved by the US Food and Drug Administration can be used to treat the condition. These medications are effective, but clinicians should be aware of key differences. A baseline assessment and regular follow-up evaluations will allow the clinician to monitor the patient’s progress and make adjustments to meet treatment goals.
Find more articles on this and other psychiatry and CNS topics:
The Journal of Clinical Psychiatry
The Primary Care Companion for CNS Disorders
CME Background
Articles are selected for credit designation based on an assessment of the educational needs of CME participants, with the purpose of providing readers with a curriculum of CME articles on a variety of topics throughout each volume. Activities are planned using a process that links identified needs with desired results.
To obtain credit, read the article, correctly answer the questions in the Posttest, and complete the Evaluation.
This Academic Highlights section of The Journal of Clinical Psychiatry presents the highlights of the teleconference series “Revisiting Tardive Dyskinesia: Focusing on the Basics of Identification and Treatment,” which was held in July and August 2019. This report was prepared and independently developed by the CME Institute of Physicians Postgraduate Press, Inc., and was supported by an educational grant from Teva Pharmaceuticals.
The program was chaired by Leslie L. Citrome, MD, MPH, Department of Psychiatry and Behavioral Sciences, New York Medical College, Valhalla. The faculty also included Stephen R. Saklad, PharmD, BCPP, Pharmacotherapy Division, The University of Texas at Austin College of Pharmacy, and Pharmacotherapy Education and Research Center, School of Medicine, UT Health San Antonio.
CME Objective
After studying this article, you should be able to:
‘ ¢Recognize risk factors for TD in patients being treated with antipsychotics
‘ ¢Evaluate patients for TD at recommended intervals with standardized tools and criteria
‘ ¢Review TD treatment options to select the most appropriate agent for each patient
Accreditation Statement
The CME Institute of Physicians Postgraduate Press, Inc., is accredited by the Accreditation Council for Continuing Medical Education to provide continuing medical education for physicians.
Release, Expiration, and Review Dates
This educational activity was published in February 2020 and is eligible for AMA PRA Category 1 Creditâ„¢ through April 30, 2022. The latest review of this material was January 2020.
Financial Disclosure
All individuals in a position to influence the content of this activity were asked to complete a statement regarding all relevant personal financial relationships between themselves or their spouse/partner and any commercial interest. The CME Institute has resolved any conflicts of interest that were identified. In the past year, Marlene P. Freeman, MD, Editor in Chief, has received research funding from JayMac and Sage; has been a member of the advisory boards for Otsuka, Alkermes, and Sunovion; has been a member of the Independent Data Safety and Monitoring Committee for Janssen; has been a member of the Steering Committee for Educational Activities for Medscape; and, as a Massachusetts General Hospital (MGH) employee, works with the MGH National Pregnancy Registry, which is sponsored by Teva, Alkermes, Otsuka, Actavis, and Sunovion, and works with the MGH Clinical Trials Network and Institute, which receives research funding from multiple pharmaceutical companies and the National Institute of Mental Health. No member of the CME Institute staff reported any relevant personal financial relationships. Faculty financial disclosure appears at the end of this activity.
J Clin Psychiatry 2020;81(2):TV18059AH3C
To cite: Citrome LL, Saklad SR. Revisiting tardive dyskinesia: focusing on the basics of identification and treatment. J Clin Psychiatry. 2020;81(2):TV18059AH3C
To share: https://doi.org/10.4088/JCP.TV18059AH3C
© Copyright 2020 Physicians Postgraduate Press, Inc.
Tardive dyskinesia (TD) is a potentially permanent, hyperkinetic movement disorder associated with the use of dopamine-receptor blocking agents (DRBAs).1 It is often believed that TD is associated only with antipsychotics; however, other DRBAs are also associated with this neurologic disorder, such as the promotility agent metoclopramide.
The prevalence of TD is higher than commonly thought. A meta-analysis2 of 41 studies included over 11,000 patients with an average age of 43 years. Two-thirds of these patients were men, and more than three-quarters had a schizophrenia spectrum disorder. The overall prevalence of TD in this sample was 25%. The TD rate for those with current use of a second-generation antipsychotic (SGA) was lower than in those with current use of a first-generation antipsychotic (FGA; 21% vs 30%, respectively). The small number of patients with current SGA use and no prior FGA exposure had a TD prevalence rate of 7.2%,2 perhaps because they represent a different group of patients who have not been exposed to antipsychotics for as long as the other cohorts who have been exposed to FGAs.
Clinicians must detect TD early to minimize the risk of the patient’s DRBA-induced movement disorder worsening or becoming permanent. The management of TD is a challenge for clinicians whose patients rely on DRBAs for treatment of their chronic conditions. In this Academic Highlights, Drs Citrome and Saklad discuss best practices for improving the early recognition and diagnosis of TD and tailoring treatment strategies, including the use of valbenazine and deutetrabenazine, which have been approved by the US Food and Drug Administration (FDA) for the treatment of TD in adults.
IDENTIFYING TARDIVE DYSKINESIA
Dr Saklad explained that many people with TD are unaware of their abnormal movements until someone else points them out. A family member described noticing the symptoms and seeking help:
Family Perspectives
“From when it first started and I noticed the jerking movements and everything, I was very concerned, and of course as they got worse, I thought there’s got to be something that they can do, but I mean we even asked different doctors—and one of them even told us that this movement and jerking could last the rest of his life . . . and when they told me that, that really scared me.”3
A study4 of patients with schizophrenia (N = 607) at a state mental hospital in Singapore reported that 40% met criteria for TD, but 67% of these patients were unaware of their abnormal movements. The finding that two-thirds of patients with schizophrenia lacked awareness of their TD suggests that many patients are not likely to voluntarily mention this side effect at clinical visits and, according to Dr Saklad, underscores the importance of proactive screening by clinicians.
Some patients are aware of the symptoms and do seek treatment. An individual living with TD described his experience of reporting the symptoms and not receiving a diagnosis and treatment:
Patient Perspectives
“My mom took me to the doctor and, at the time, he just said that it was probably a side effect of some of the medicine I was on and that he couldn’ t really do anything about it.”3
Impact of TD on Functioning and Quality of Life
The abnormal, involuntary movements of TD contribute to functional impairment, negatively affecting patients’ quality of life. To investigate the burden of TD in a real-world population of patients taking antipsychotics, a study5 evaluated health-related quality of life in participants without visible signs of involuntary movements (Cohort 1) and participants with visible signs and clinical assessment of possible TD (Cohort 2). A higher percentage in Cohort 2 reported having problems in mobility, self-care, usual activities, and pain/discomfort than those in Cohort 1.5 Those with probable TD also had greater impairment in work/school, social life, and family life/home responsibilities compared to those without TD.
Risk Factors for TD
The risk-benefit ratio of DRBAs must be evaluated and individualized with each patient. In an effort to prevent TD, clinicians must consider the type and dose of medication, as well as whether other interventions (eg, psychosocial) could be used to treat the patient’s illness instead of a DRBA.6 Dr Saklad emphasized the need for clinicians to communicate clearly with patients and document their discussions about treatment options. All patients taking DRBAs, he said, should be educated on the risk for and symptoms of TD.
The pathophysiology of TD is unknown but is believed to involve postsynaptic dopamine receptor hypersensitivity.7 Other possible explanatory models for TD include abnormalities in GABA and/or cholinergic neuronal activity, neurotoxicity and oxidative stress, changes in synaptic plasticity, and defective neuroadaptive signaling, with potential overlap with all of these mechanisms.8
According to Dr Saklad, factors increasing the risk of TD may involve the patient directly or may be related to treatment, illness, or other factors. Both patient and other factors can be non-modifiable or modifiable.9 The most important risk factors for developing TD are advancing age and cumulative exposure to DRBAs. A more complete list and additional details follow.
Non-modifiable factors. Non-modifiable patient factors include the following9:
- Genetic dopamine polymorphism in dopamine synthesis, vesicular packaging, and receptors
- CYP polymorphisms reducing antipsychotic clearance
- Older age
- Female sex
- Ancestry (African > Caucasian > Asian)
Other non-modifiable factors include the following9:
- Negative symptoms of schizophrenia
- Intellectual disability and brain damage
- Mood disorder diagnosis
- Cognitive dysfunction along with mood disorder
- Longer duration of severe psychiatric illness
Modifiable factors. Modifiable patient factors include comorbid diabetes, smoking, and alcohol and substance abuse.9 Although these factors are common in patients living with brain disorders, Dr Saklad emphasized that they are areas in which clinicians can work with patients to try to improve.
Modifiable treatment factors that are associated with increased risk of TD include the development of acute extrapyramidal symptoms (EPS) and the use of FGA (over SGA) medications.9 Higher antipsychotic doses are also associated with increased risk. People who metabolize medications less effectively have a higher risk. To reduce TD risk, Dr Saklad advised clinicians to only use DRBAs when clearly indicated and to minimize the dose and duration of exposure to DRBA medications as clinically appropriate.
Concomitant anticholinergic medications can increase the risk of TD.9 Anticholinergic medications that cross the blood-brain barrier can include agents used to treat acute drug-induced parkinsonism, acute dystonia, Parkinson’s disease, gastrointestinal problems, and enuresis. According to Dr Saklad, clinicians should avoid these medications if possible because they can worsen symptoms of TD and also cause cognitive impairment and many other problems.10
Diagnostic Tools for TD
Dr Saklad recommended that clinicians be familiar with diagnostic criteria, differential diagnosis, and use of an assessment tool such as the Abnormal Involuntary Movement Scale11 (AIMS).
Criteria. The Schooler-Kane criteria12 for TD are widely used in clinical practice to determine probable TD. Schooler-Kane criteria require (1) at least 3 months of cumulative exposure to a DRBA; (2) at least moderate dyskinetic movements in 1 body area (≥ 3 score on an AIMS dyskinesia item) or mild dyskinetic movements in 2 body areas (≥ 2 score on ≥ 2 AIMS dyskinesia items); and (3) absence of other conditions that might cause involuntary movements.
The criteria for TD in the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition,13 are less specific than the Schooler-Kane criteria in the time requirement for DRBA exposure, stating that involuntary movements develop with the use of neuroleptic medication for “at least a few months” and that the time may be shorter for older adults.14
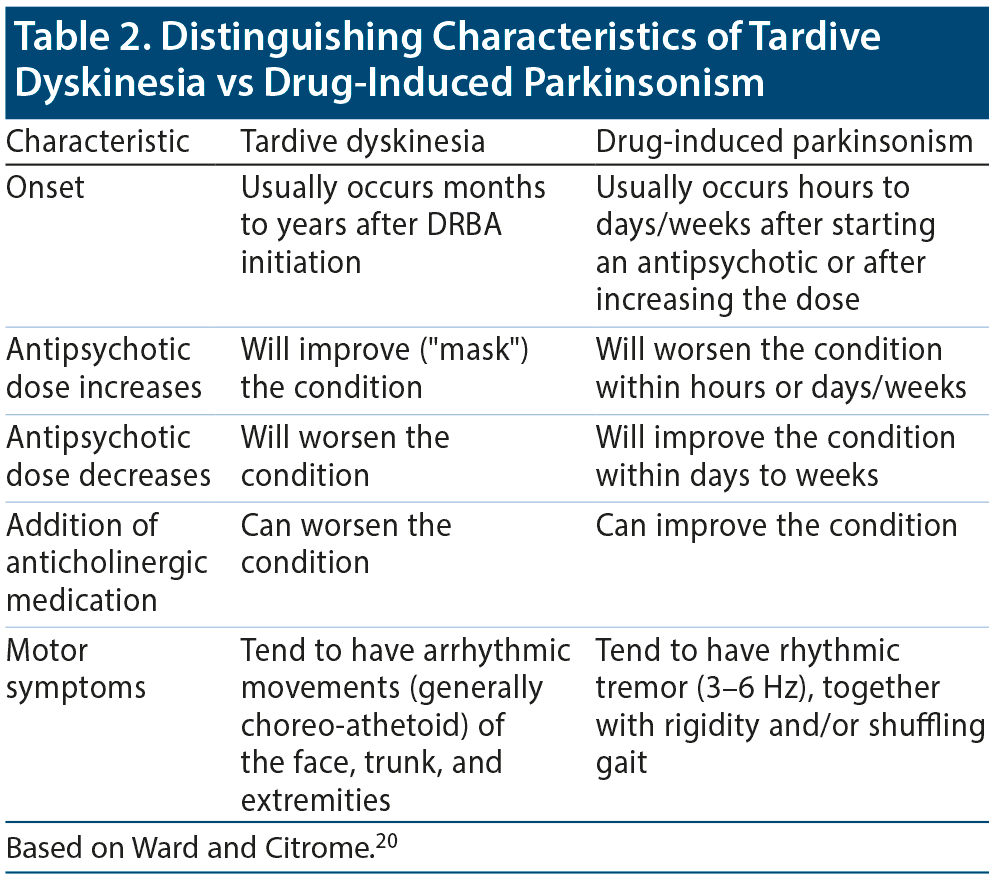
The involuntary movements are frequently observed in the tongue, jaw, and perioral area, as well as in the trunk and the extremities. Common orofacial movements include chewing; lip smacking, puckering, or pursing; tongue protrusion; grimacing; or cheek bulging.15 Other signs include contracting, twisting, or writhing movements of the fingers, hands, arms, or legs. These involuntary movements are usually choreiform or athetoid and are usually clearly distinguishable from the regular rhythmic 3-6 Hz movements seen with drug-induced parkinsonism.13
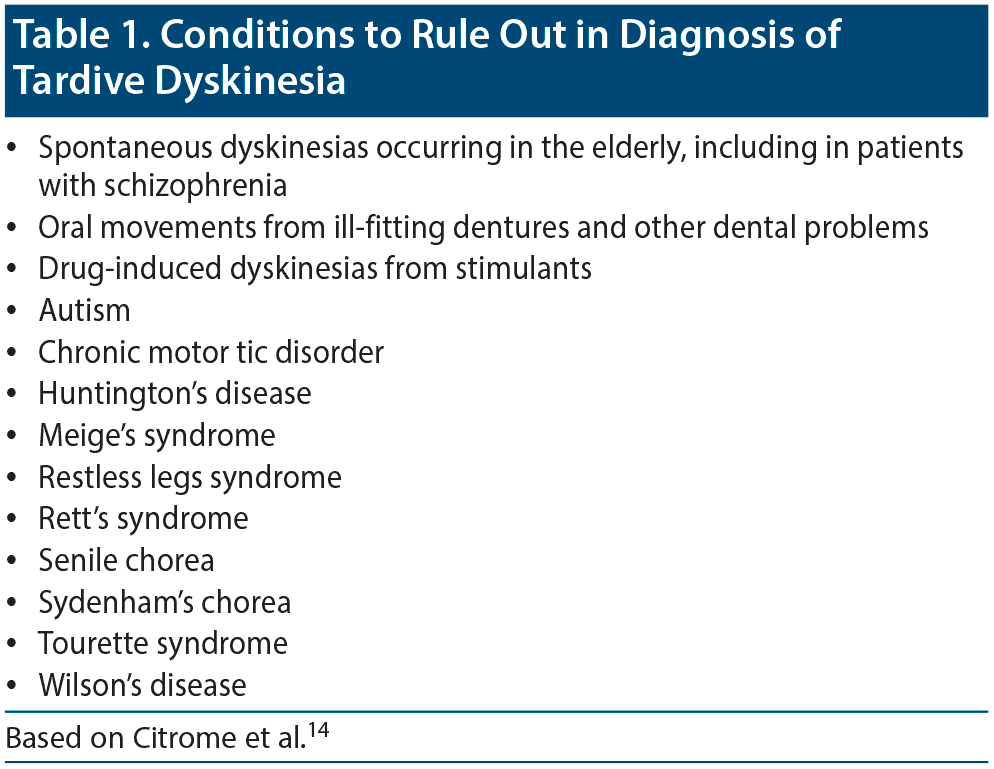
Differential diagnosis. Many conditions and syndromes can cause abnormal movements that resemble TD and must be ruled out; thus, TD is a diagnosis of exclusion (Table 1).14
Dr Saklad stated that spontaneous dyskinesias are often difficult to differentiate from TD. Spontaneous dyskinesias are fairly common in elderly patients and patients with schizophrenia who have never been exposed to DRBAs and are often noted in the same body areas typically associated with TD.
While TD is the most common abnormal movement syndrome,16 clinicians should be familiar with the other tardive syndromes.17,18 Drug-induced dyskinesias from stimulants can present a diagnostic challenge because amphetamines and methamphetamines interact with some of the same neurotransmitter transporter systems that are involved in the treatment of TD.19
Distinguishing characteristics can help clinicians determine if patients have TD versus drug-induced parkinsonism (previously called pseudoparkinsonism; Table 2).20
Abnormal Involuntary Movement Scale. A rating scale cannot be exclusively used to diagnose TD, but it can establish and quantify the presence of abnormal movements and be used in follow-up. Dr Saklad pointed out that other rating scales besides the AIMS may also be used (such as the Dyskinesia Identification System: Condensed User Scale21 [DISCUS]) as long as training is provided. Whatever scale is used, a baseline assessment is essential to document the presence of any movements before DRBA treatment begins.
In addition to conducting an initial assessment before treatment, the APA guideline22 recommends that patients be evaluated for TD every 6 months while receiving an FGA and every 12 months while receiving an SGA. Patients with risk factors for TD should be examined every 3 months while receiving an FGA and every 6 months while taking an SGA. Dr Saklad noted that clinicians may assess more frequently as indicated.
The AIMS takes about 10 minutes to administer. Items 1-7 are the specific dyskinesia items for 7 different muscle groups, and the other questions relate to overall severity, incapacitation, awareness of movements, and dental issues.11,23 Below are general steps for clinicians to follow:
- Observe patients unobtrusively at rest either before or after administering the exam, such as while they are sitting in the waiting room.
- Have patients sit in a firm unpadded chair or stool without arms and remove their shoes and socks.
- Ask patients if they have experienced involuntary movements and, if so, whether they are bothered by them.
- Ask patients about any problems with their teeth or gums, if they have dentures, and if they are chewing anything.
- Look for evidence of involuntary movements in the patient’s entire body while asking them to perform tasks that include sticking out their tongues, tapping their thumbs to each finger, and extending their limbs.11,24 These tasks occupy the patient’s attention and decrease their ability to suppress abnormal movements. Moreover, voluntary movements in one muscle group may facilitate the production of involuntary movements in other muscle groups, and these “activating maneuvers” can simulate what happens to persons with TD when they go about with their routine activities, such as getting dressed, walking, talking, and eating.
For scoring movements on the AIMS 5-point scale (from 0 = none/normal to 4 = severe), Dr Saklad recommended clinicians keep in mind that minimal is the extreme edge of normal. So, a questionable movement would be rated as 1 = minimal, and 2 = mild is the first point where movements are definitively abnormal. For item 8 of the AIMS (global judgment of the severity of abnormal movements), the overall severity is the highest rating assigned in items 1-7.25 Item 9, global judgment of incapacitation due to abnormal movements, is rated from 0 = none/normal to 4 = severe. Item 10 rates the patient’s awareness of abnormal movements and level of distress due to the abnormal movements on an adjusted scale of 0 = no awareness to 4 = awareness with severe distress. Items 11 and 12 document dental status. Additional items have been included in other versions of the AIMS.23
Case Practice Question
Discussion of the best response is at the end of the activity.
Case 1. Frank is a 32-year-old man with bipolar disorder and TD. You want to assess how the TD symptoms may be impacting his functioning. What rating scale will best help you evaluate this aspect of Frank’s care over the long term?
a.Functional Impairments Scale for Tardive Dyskinesia
b.The AIMS
c.Simpson-Angus Scale
d.Sheehan Disability Scale
TREATMENT FOR TARDIVE DYSKINESIA
The American Academy of Neurology (AAN) published evidence-based recommendations26 in 2013 regarding management of tardive syndromes, including TD. These guidelines also described treatment options that are not recommended and those that lack sufficient data to support (or refute) their use. Since the publication of the guidelines, valbenazine and deutetrabenazine were approved by the US FDA for the treatment of TD in adults. Recently, new evidence was combined with existing guidelines to provide updated recommendations.27 Notably, the reviewers found insufficient evidence to support or refute the treatment strategies of withdrawing causative agents or switching from an FGA to an SGA. An individual living with TD described her experience with switching her DRBA:
Patient Perspectives
“I stopped the medicine that I was taking for the depression that they [doctors] said resulted in the tremors . . . and then started taking another one. And then that seemed to help me a whole lot more. . . . After I started taking this last medicine, it was able to stop the tremors and stuff, but I still have some of the facial twitches on my mouth that don’t quite go away, but it’s really helped it a lot more.”3
The Evolution of TD Treatment Options
Options prior to FDA-approved medications. Some evidence-based options that were outlined in the 2013 AAN recommendations included clonazepam, Ginkgo biloba, amantadine, and tetrabenazine.26 Not recommended were diltiazem, galantamine, and eicosapentaenoic acid. The following options had insufficient data to support or refute their use for the management of TD: bromocriptine, thiamine, baclofen, and vitamin E. The medication benztropine is sometimes incorrectly used for TD, but it is not approved nor effective for the treatment of TD; Dr Citrome explained that it may increase the risk of TD and can even make TD symptoms worse.20
FDA-approved TD medications. Approved by the FDA in 2017, valbenazine and deutetrabenazine are reversible vesicular monoamine transporter type 2 (VMAT2) inhibitors and are related to tetrabenazine, an FDA-approved drug for the treatment of chorea in Huntington’s disease but not for TD (at least in the US).28 The normal function of VMAT2 is to transport monoamines, such as dopamine, into synaptic vesicles for release into the synaptic cleft when the neuron fires. VMAT2 inhibitors block the transport of dopamine into synaptic vesicles, thereby reducing the amount of dopamine released into the synapse, particularly in the dorsal striatum, a key area of the brain that controls motor movements. This mechanism of action is believed to ultimately decrease the occurrence of dyskinetic movement.29
Two individuals described how treatment for TD has improved their lives:
Patient Perspectives
“I thought while I had those tremors and everything that I wasn’ t ever going to get past this, that I was going to have to live that way the rest of my life. And it’s like I just didn’ t think I could handle that forever, until I got put on this last medicine and it was able to calm everything down. But I did not think I would live through that if I didn’ t have help.”3
“When I first started the medicine, I wouldn’ t go anywhere in public. I pretty much stayed in my house and tried to hide the movements, but . . . about a week after I got on this medicine, I started being able to go out in public. I went to the mall with my mom and walked around and, nope, didn’ t feel awkward about it. I’ ve been able to do a lot of things like play baseball or softball or football or volleyball with my nieces and nephews, and that’s something I couldn’ t do before.”3
Tetrabenazine. While not an approved medication in the United States for TD,30 tetrabenazine is approved in some countries for this purpose. Dr Citrome explained that its use is limited by substantial side effects and short half-life.31 Its short half-life means that it requires frequent dosing and can result in high peaks in plasma concentrations, which, he said, can be problematic in terms of tolerability.
Valbenazine and Deutetrabenazine Evidence
The treatment of TD with valbenazine and deutetrabenazine is supported by clinical trial evidence with much larger sample sizes than in studies of tetrabenazine, noted Dr Citrome.32,33 These newer medications were examined in randomized, double-blind, placebo-controlled trials, using both flexible-dose and fixed-dose designs.32-37 The results showed significantly greater reductions in TD symptoms in patients receiving valbenazine (fixed-dose, with target of 80 mg/d)35 and deutetrabenazine (flexibly dosed within the range of 6 mg BID to 24 mg BID, with food)36 than in patients who received placebo.
Adverse effects. In 3 placebo-controlled studies of valbenazine, somnolence was the most common adverse effect (10.9%, more than twice the rate with placebo).38 Few patients discontinued the trials because of adverse events (3% for valbenazine vs 2% for placebo).38
In trials of deutetrabenazine,36,37 2 adverse events occurred in 4% of patients treated with the drug and at an incidence greater than that observed with placebo: nasopharyngitis and insomnia.39 Few patients required a dose reduction because of adverse events (4% for deutetrabenazine vs 2% for placebo).39 Few patients discontinued the trials because of adverse events (3.6% for deutetrabenazine vs 3.1% for placebo).33
Efficacy. The efficacy of deutetrabenazine and valbenazine can be indirectly compared by examining the number of patients who experienced at least a 50% reduction in the AIMS dyskinesia score from baseline to endpoint and calculating the number needed to treat (NNT) versus placebo and the respective 95% confidence intervals (CIs). The results of the phase 3 fixed-dose studies (ie, a 6-week valbenazine study and a 12-week deutetrabenazine study) resulted in an NNT of 5 for both VMAT2 inhibitors at doses considered efficacious.32,33,35,37 Specifically, these NNTs are based on pooled data from the 12 mg BID and 18 mg BID deutetrabenazine doses and the 40 mg/d and 80 mg/d valbenazine doses. An NNT of 5 for a medication vs placebo can be considered to be a moderate “effect size” and denotes a potentially useful intervention.40
Clinical Strategies
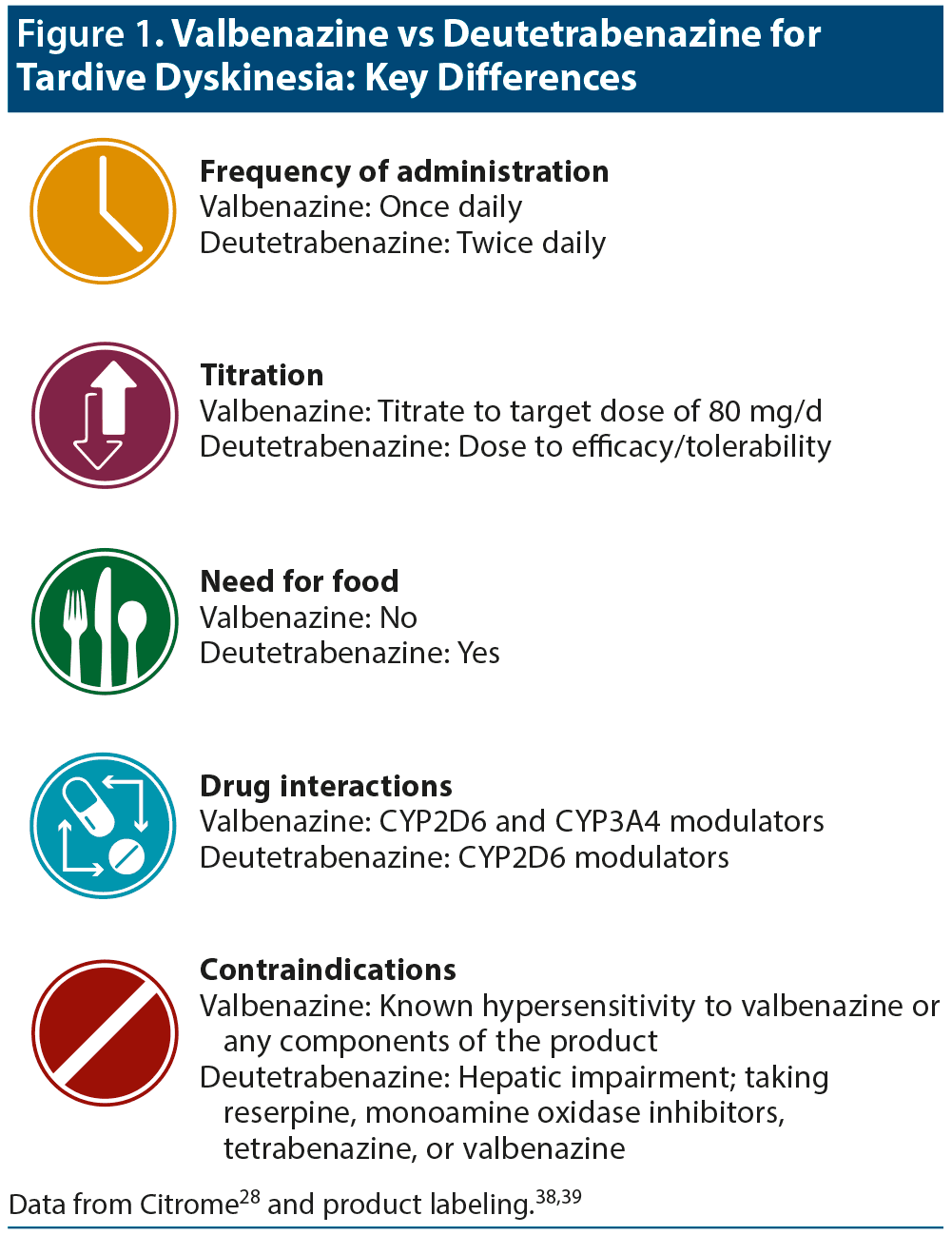
According to Dr Citrome, deutetrabenazine and valbenazine are both effective medications, but clinicians should be aware of key differences (Figure 1).28,38,39 Differences include dose titration and daily frequency, need for dosing with meals, potential drug interactions, and contraindications.
Dr Citrome highlighted the following important facts about deutetrabenazine and valbenazine28,41,42:
- Both medications are effective and well-tolerated in reducing abnormal TD movements, which often cause substantial physical, social, and psychological impairment (irrespective of the patient’s underlying psychiatric diagnosis)
- Patients taking VMAT2 inhibitors can remain on antipsychotic therapy, which will reduce the risk of psychiatric decompensation; there is no need to discontinue, reduce, or change the treatment regimen. This is especially important in persons with stable schizophrenia for whom changes in antipsychotic regimens can be difficult to manage
- The treatment goal with VMAT2 inhibitors is to reduce the severity and impact of TD
- Treatment may not eliminate all signs of TD
- Attempting to completely suppress all TD symptoms will likely result in overtreatment and a greater potential for adverse effects
- Customize medication selection by considering
- Ease of adherence
- The ability to fine-tune the daily dose
- Side-effect profile
- If the patient is already taking mood stabilizers and/or antidepressants, he or she can continue therapy with them
- Neither of the FDA-approved TD medications, relative to placebo, destabilizes depression, mania, or psychosis or induces suicidality36,43
- Long-term data are reassuring; no new safety signals or concerns emerged with either medication44,45
- Tolerability and efficacy could vary among individual patients.
A family member of an individual living with TD described how treatment has lifted the burden that TD had placed on his uncle’s life:
Family Perspectives
“Since he began treatment, he’s back to the person that we love. He’s interacting with everybody more. Everybody notices it… he’s a great person to be around . . . But, when the movements started, it was none of that. He wasn’ t talking, he wasn’ t moving, he didn’ t want to go nowhere. He barely ate. You had to pour his drink for him, he couldn’ t hold a cup. Everything was just crazy. So, we’ re just . . . everybody’s so happy that he’s back. He’s ready to go places, he wants to travel, wants to work. So, I’ m just happy for him.”3
Assessing functional impairment. After a patient’s treatment for TD is initiated, Dr Citrome emphasized the need for follow-up assessments to be conducted on a regular basis. It is not adequate to measure the AIMS dyskinesia items alone, he said. The follow-up questions regarding functional impairments attributable to TD must be asked (interference with daily activities such as eating, drinking, speaking, breathing, dressing, writing, working, and socializing). Dr Citrome reiterated that clinicians must be proactive in discussing these issues with patients.
Case Practice Question
Discussion of the best response is at the end of the activity.
Case 2. Susan is a 50-year-old woman with schizophrenia who is now experiencing dyskinetic movements of her mouth and jaw. She is also having difficulties tying her shoelaces because of intermittent dyskinetic movements of her fingers. You make the diagnosis of TD, and you want to select treatment. Which of the following would be among the best options?
a.Valbenazine, deutetrabenazine, and possibly benztropine
b.Valbenazine, deutetrabenazine, and possibly vitamin E
c.Valbenazine, deutetrabenazine, and possibly amantadine
d.Valbenazine, deutetrabenazine, and possibly galantamine
Clinical Points
- Assume that TD exists in your practice and will continue to occur because of greater use of antipsychotic medications.
- Consider and communicate the risks and benefits of DRBA use with patients.
- Be aware of risk factors for TD that can and cannot be modified.
- Regularly assess for TD using a rating scale like the AIMS in patients taking DRBAs.
- Prevent TD if possible by minimizing acute drug-induced parkinsonian symptoms by selecting agents with lower risk for this problem and minimizing the use of anticholinergic medication.
- Treat TD as quickly as possible after it appears with effective, well-tolerated, approved medications.
Discussion of Case Practice Questions
Case 1: Preferred response is b.
The AIMS already includes items that address not only subjective distress and awareness of TD but also any incapacitation due to abnormal movements. No extra scale is necessary.
Case 2: Preferred response is c.
Both valbenazine and deutetrabenazine are FDA-approved specifically for the management of TD. Amantadine has some evidence of efficacy in ameliorating TD. Benztropine can worsen TD; vitamin E has insufficient evidence for use in treating TD; and galantamine is not recommend for use in treating TD.
Published online: Februay 18, 2020
Disclosure of off-label usage: The chair has determined that, to the best of his knowledge, the only interventions that are approved by the US Food and Drug Administration for the treatment of tardive dyskinesia are valbenazine and deutetrabenazine.
Credit Designation
The CME Institute of Physicians Postgraduate Press, Inc., designates this journal-based CME activity for a maximum of 1 AMA PRA Category 1 Creditâ„¢. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
Note: The American Academy of Physician Assistants (AAPA) accepts certificates of participation for educational activities certified for AMA PRA Category 1 Creditâ„¢ from organizations accredited by ACCME or a recognized state medical society. Physician assistants may receive a maximum of 1 hour of Category I credit for completing this program.
MOC Approval Statement
Through the American Board of Medical Specialties (“ABMS”) ongoing commitment to increase access to practice relevant Continuing Certification Activities through the ABMS Continuing Certification Directory, Revisiting Tardive Dyskinesia: Focusing on the Basics of Identification and Treatment has met the requirements as a MOC Part II CME Activity (apply toward general CME requirement) for the following ABMS Member Boards:
MOC Part II CME Activity
Psychiatry and Neurology
Financial Disclosure
Dr Citrome is a consultant for Acadia, Alkermes, Allergan, Avanir, BioXcel, Eisai, Impel, Indivior, Intra-Cellular Therapies, Janssen, Lundbeck, Luye, Merck, Neurocrine, Noven, Osmotica, Otsuka, Pfizer, Sage, Shire, Sunovion, Takeda, Teva, and Vanda; is a member of the speaker bureaus for Acadia, Alkermes, Allergan, Janssen, Lundbeck, Merck, Neurocrine, Otsuka, Pfizer, Sage, Shire, Sunovion, Takeda, and Teva; and is a stock shareholder of Bristol-Myers Squibb, Eli Lilly, Johnson & Johnson, Merck, and Pfizer. Dr Saklad is a consultant for Texas Health and Human Services, San Antonio State Hospital, Alkermes, Genomind, Intra-Cellular Therapies, and Otsuka; has received grant/research support from Alkermes; and is a member of the speakers/advisory boards for Alkermes and Neurocrine.
Review Process
The faculty member(s) agreed to provide a balanced and evidence-based presentation and discussed the topic(s) and CME objective(s) during the planning sessions. The faculty’s submitted content was validated by CME Institute staff, and the activity was evaluated for accuracy, use of evidence, and fair balance by the Chair and a peer reviewer who is without conflict of interest.
The opinions expressed herein are those of the faculty and do not necessarily reflect the opinions of the CME provider and publisher or the supporter.
REFERENCES
1. Lerner PP, Miodownik C, Lerner V. Tardive dyskinesia (syndrome): current concept and modern approaches to its management. Psychiatry Clin Neurosci. 2015;69(6):321-334. PubMed CrossRef
2. Carbon M, Hsieh C-H, Kane JM, et al. Tardive dyskinesia prevalence in the period of second-generation antipsychotic use: a meta-analysis. J Clin Psychiatry. 2017;78(3):e264-e278. PubMed CrossRef
3. McEvoy JP. Living With Tardive Dyskinesia. The CME Institute: Patients website. https://www.cmeinstitute.com/patients/Pages/home.aspx. Published 2018. Accessed October 18, 2019.
4. Chong SA, Remington G, Mahendran R, et al. Awareness of tardive dyskinesia in Asian patients with schizophrenia. J Clin Psychopharmacol. 2001;21(2):235-237. PubMed CrossRef
5. Caroff SM, Cutler A, Lenderking WR, et al. Quality of life and functional impairment results: a prospective real-world dyskinesia screening study and registry in patients taking antipsychotic agents. Value Health. 2018;21(suppl 1):S188. CrossRef
6. Correll CU, Rubio JM, Kane JM. What is the risk-benefit ratio of long-term antipsychotic treatment in people with schizophrenia? World Psychiatry. 2018;17(2):149-160. PubMed CrossRef
7. Margolese HC, Chouinard G, Kolivakis TT, et al. Tardive dyskinesia in the era of typical and atypical antipsychotics, part 1: pathophysiology and mechanisms of induction. Can J Psychiatry. 2005;50(9):541-547. PubMed CrossRef
8. Rana AQ, Chaudry ZM, Blanchet PJ. New and emerging treatments for symptomatic tardive dyskinesia. Drug Des Devel Ther. 2013;7:1329-1340. PubMed CrossRef
9. Solmi M, Pigato G, Kane JM, et al. Clinical risk factors for the development of tardive dyskinesia. J Neurol Sci. 2018;389:21-27. PubMed CrossRef
10.Cornett EM, Novitch M, Kaye AD, et al. Medication-induced tardive dyskinesia: a review and update. Ochsner J. 2017;17(2):162-174. PubMed
11.Guy W. ECDEU Assessment Manual for Psychopharmacology. Revised Edition. Washington, DC: US Department of Health, Education, and Welfare; 1976.
12.Schooler NR, Kane JM. Research diagnoses for tardive dyskinesia. Arch Gen Psychiatry. 1982;39(4):486-487. PubMed CrossRef
13.American Psychiatric Association. Diagnostic and Statistical Manual for Mental Disorders. Fifth Edition. Washington, DC: American Psychiatric Association; 2013.
14.Citrome L, Dufresne R, Dyrud JM. Tardive dyskinesia: minimizing risk and improving outcomes in schizophrenia and other disorders. Am J Manag Care. 2007;12(suppl). http://www.ajmc.com/journals/supplement/2007/2007-12-vol12-n1-decisionmakernews/dec07-2760p1-12. Accessed October 21, 2019.
15.Kane JM. Assessing patients for tardive dyskinesia. J Clin Psychiatry. 2017;78(9):e1428. PubMed CrossRef
16.Waln O, Jankovic J. An update on tardive dyskinesia: from phenomenology to treatment. Tremor Other Hyperkinet Mov (N Y). 2013;3:tre-03-161-4138-1. PubMed
17.Truong DD, Frei K. Setting the record straight: the nosology of tardive syndromes. Parkinsonism Relat Disord. 2019;59:146-150. PubMed CrossRef
18.American Academy of Neurology. Treating and managing tardive syndromes. 2013. https://www.aan.com/Guidelines/Home/GetGuidelineContent/613. Accessed October 21, 2019.
19.Müller T. Valbenazine granted breakthrough drug status for treating tardive dyskinesia. Expert Opin Investig Drugs. 2015;24(6):737-742. PubMed CrossRef
20.Ward KM, Citrome L. Antipsychotic-related movement disorders: drug-induced parkinsonism vs tardive dyskinesia: key differences in pathophysiology and clinical management. Neurol Ther. 2018;7(2):233-248. PubMed CrossRef
21.Kalachnik JE, Sprague RL. The Dyskinesia Identification System Condensed User Scale (DISCUS): reliability, validity, and a total score cut-off for mentally ill and mentally retarded populations. J Clin Psychol. 1993;49(2):177-189. PubMed
22.Lehman AF, Lieberman JA, Dixon LB, et al; American Psychiatric Association; Steering Committee on Practice Guidelines. Practice guideline for the treatment of patients with schizophrenia, second edition. Am J Psychiatry. 2004;161(suppl):1-56. PubMed
23.Kane JM, Correll CU, Nierenberg AA, et al; Tardive Dyskinesia Assessment Working Group. Revisiting the Abnormal Involuntary Movement Scale: proceedings from the Tardive Dyskinesia Assessment Workshop. J Clin Psychiatry. 2018;79(3):17cs11959. PubMed CrossRef
24.Menzies V, Farrell SP. Schizophrenia, tardive dyskinesia, and the Abnormal Involuntary Movement Scale (AIMS). J Am Psychiatr Nurses Assoc. 2002;8(2):51-56. CrossRef
25.Munetz MR, Benjamin S. How to examine patients using the Abnormal Involuntary Movement Scale. Hosp Community Psychiatry. 1988;39(11):1172-1177. PubMed
26.Bhidayasiri R, Fahn S, Weiner WJ, et al; American Academy of Neurology. Evidence-based guideline: treatment of tardive syndromes: report of the Guideline Development Subcommittee of the American Academy of Neurology. Neurology. 2013;81(5):463-469. PubMed CrossRef
27.Bhidayasiri R, Jitkritsadakul O, Friedman JH, et al. Updating the recommendations for treatment of tardive syndromes: a systematic review of new evidence and practical treatment algorithm. J Neurol Sci. 2018;389:67-75. PubMed CrossRef
28.Citrome L. Clinical management of tardive dyskinesia: Five steps to success. J Neurol Sci. 2017;383:199-204. PubMed CrossRef
29.Jankovic J. Movement disorders in 2016: progress in Parkinson disease and other movement disorders. Nat Rev Neurol. 2017;13(2):76-78. PubMed CrossRef
30.Xenazine (tetrabenazine): highlights of prescribing information. September 2018. https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=ac768bab-8afa-4446-bc7f-caeeffec0cda. Accessed January 6, 2020.
31.Kaur N, Kumar P, Jamwal S, et al. Tetrabenazine: spotlight on drug review. Ann Neurosci. 2016;23(3):176-185. PubMed CrossRef
32.Citrome L. Valbenazine for tardive dyskinesia: a systematic review of the efficacy and safety profile for this newly approved novel medication: what is the number needed to treat, number needed to harm and likelihood to be helped or harmed? Int J Clin Pract. 2017;71(7):ijcp.12964.
33.Citrome L. Deutetrabenazine for tardive dyskinesia: a systematic review of the efficacy and safety profile for this newly approved novel medication: What is the number needed to treat, number needed to harm and likelihood to be helped or harmed? Int J Clin Pract. 2017;71(11):ijcp.13030.
34.O’ Brien CF, Jimenez R, Hauser RA, et al. NBI-98854, a selective monoamine transport inhibitor for the treatment of tardive dyskinesia: A randomized, double-blind, placebo-controlled study. Mov Disord. 2015;30(12):1681-1687. PubMed CrossRef
35.Hauser RA, Factor SA, Marder SR, et al. KINECT 3: a phase 3 randomized, double-blind, placebo-controlled trial of valbenazine for tardive dyskinesia. Am J Psychiatry. 2017;174(5):476-484. PubMed CrossRef
36.Fernandez HH, Factor SA, Hauser RA, et al. Randomized controlled trial of deutetrabenazine for tardive dyskinesia: the ARM-TD study. Neurology. 2017;88(21):2003-2010. PubMed CrossRef
37.Anderson KE, Stamler D, Davis MD, et al. Deutetrabenazine for treatment of involuntary movements in patients with tardive dyskinesia (AIM-TD): a double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Psychiatry. 2017;4(8):595-604. PubMed CrossRef
38.Ingrezza (valbenazine): highlights of prescribing information. December 2018. https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=4c970164-cafb-421f-9eb5-c226ef0a3417. Accessed October 18, 2019.
39.Austedo (deutetrabenazine): highlights of prescribing information. July 2019. https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=7ea3c60a-45c7-44cc-afc2-d87fa53993c0. Accessed October 18, 2019.
40.Citrome L, Ketter TA. When does a difference make a difference? Interpretation of number needed to treat, number needed to harm, and likelihood to be helped or harmed. Int J Clin Pract. 2013;67(5):407-411. PubMed CrossRef
41.Margolius A, Fernandez HH. Current treatment of tardive dyskinesia. Parkinsonism Relat Disord. 2019;59:155-160. PubMed CrossRef
42.Niemann N, Jankovic J. Treatment of tardive dyskinesia: a general overview with focus on the vesicular monoamine transporter 2 inhibitors. Drugs. 2018;78(5):525-541. PubMed CrossRef
43.McIntyre RS, Remington G, Correll CU, et al. 67 effects of long-term valbenazine on psychiatric status in patients with tardive dyskinesia and a primary mood disorder. CNS Spectr. 2019;24(1):210-211. CrossRef
44.Hauser RA, Fernandez HH, Stamler D, et al. 45 long-term treatment with deutetrabenazine is associated with continued improvement in tardive dyskinesia: results from an open-label extension study. CNS Spectr. 2019;24(1):200-201. CrossRef
45.Fernandez HH, Stamler D, Davis MD, et al. Long-term safety and efficacy of deutetrabenazine for the treatment of tardive dyskinesia. J Neurol Neurosurg Psychiatry. 2019;90(12):1317-1323. PubMed CrossRef
This PDF is free for all visitors!
Save
Cite