ABSTRACT
Objective: There is limited evidence for the efficacy of the novel dual orexin receptor antagonists (DORAs) suvorexant and lemborexant in preventing delirium. We examined the efficacy of DORAs in preventing delirium in critically ill patients at an advanced emergency and critical care center.
Methods: In this retrospective observational study, patients 18 years of age or older admitted to the emergency center between July 2018 and November 2021 with hospitalization duration of at least 72 h were included. Kaplan-Meier curves were plotted and log rank tests were performed to compare between patients with and without DORA treatment. Cox regression analyses adjusting for factors associated with delirium risk were also performed.
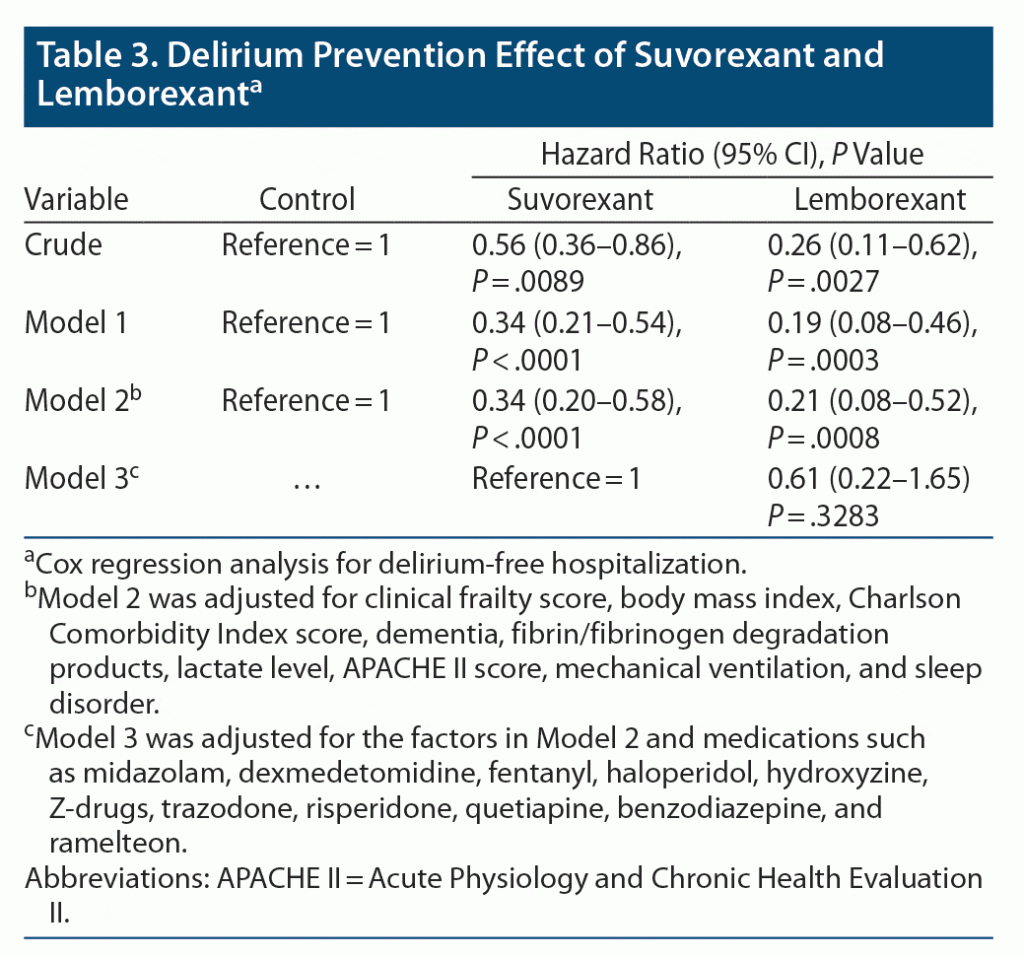
Results: Of the 633 enrolled patients, 82 were treated with suvorexant and 41 with lemborexant. Cox regression analysis showed that, without adjustment, the hazard ratios (95% CIs) for the development of delirium were 0.56 (0.36–0.86) for patients treated with suvorexant and 0.26 (0.11–0.62) for those treated with lemborexant. After adjustment for delirium risk factors, the hazard ratios (95% CIs) remained low at 0.34 (0.20–0.58) for suvorexant and 0.21 (0.08–0.52) for lemborexant.
Conclusions: Both suvorexant and lemborexant may be effective in preventing delirium in critically ill adult patients in an advanced critical care center.
J Clin Psychiatry 2023;84(1):22m14471
To cite: Matsuoka A, Tobita S, Sogawa R, et al. Evaluation of suvorexant and lemborexant for the prevention of delirium in adult critically ill patients at an advanced critical care center: a single-center, retrospective, observational study. J Clin Psychiatry. 2023;84(1):22m14471.
To share: https://doi.org/10.4088/JCP.22m14471
© 2022 Physicians Postgraduate Press, Inc.
aDepartment of Emergency and Critical Care Medicine Faculty of Medicine, Saga University, Saga City, Japan
bDepartment of Pharmacy, Saga University Hospital, Saga City, Japan
cDepartment of Psychiatry, Faculty of Medicine, Saga University, Saga City, Japan
*Corresponding author: Ayaka Matsuoka, MD, Assistant Professor, Department of Emergency and Critical Care Medicine, Faculty of Medicine, Saga University, 5-1-1 Nabeshima, Saga City, Saga Prefecture 849-8501, Japan ([email protected]).
Delirium is a syndrome characterized by acute onset of deficits in attention, awareness, and cognition that fluctuate in severity over a short period of time. Delirium is a common symptom in critically ill patients in the intensive care unit (ICU),1,2 and its incidence has been reported to range from 50% to 80%, depending on the method of measurement and the target population.3,4 In intensive care, the development of delirium is an important issue because it leads to prolonged ICU and hospital stays, higher medical costs, longer duration of ventilatory management, increased mortality after discharge, and long-term cognitive impairment after discharge.5–9 Although effective treatment for delirium has not yet been established,10 preventive measures have been shown to decrease the incidence of delirium and potentially shorten its duration.11,12
Preventive measures for delirium can be broadly divided into pharmacologic and non-pharmacologic interventions, which have been the subject of various studies.13,14 Multiple risk factors are believed to be involved in the development of delirium.15,16 The importance of the ABCDEF bundle (Assess, Prevent, and Manage Pain; Both Spontaneous Awakening Trials and Spontaneous Breathing Trials; Choice of Analgesia and Sedation; Delirium: Assess, Prevent, and Manage; Early Mobility and Exercise; and Family Engagement and Empowerment), a framework of risk factors and management approaches for delirium in the ICU, is increasingly being recognized.4,17,18 Element D indicates delirium, and it has been shown that the incidence of delirium may be reduced by improving sleep quality. In other words, element D includes the importance of sleep.13 Several sleep-related drugs have been reported in previous studies to have preventive effects against delirium.19–25 The Clinical Practice Guidelines for the Prevention and Management of Pain, Agitation/Sedation, Delirium, Immobility, and Sleep Disruption in Adult Patients in the ICU (PADIS) do not recommend the use of melatonin to improve sleep.18 However, increasing evidence for the prevention of delirium with the melatonin agonist ramelteon has recently been published.20,21,23
Orexin is a neuropeptide involved in the regulation of the sleep-wake cycle; its binding to orexin receptors promotes wakefulness.26 Previous studies have shown that the dual orexin receptor antagonists (DORAs) suvorexant and lemborexant are useful for sleep disturbances.26,27 Suvorexant has been reported to have a preventive effect against delirium.23–25 Terada et al28 reported that lemborexant is also effective in sleep disturbances in cancer patients with delirium. However, few articles have reported the efficacy of both suvorexant and lemborexant in preventing delirium in critically ill patients in an advanced critical care center. Therefore, in this study, we examined the efficacy of suvorexant and lemborexant in preventing delirium in critically ill patients.
METHODS
Study Design, Setting, and Participants
This observational study was performed in the advanced emergency and critical care center at Saga University Hospital. Patients admitted to our emergency center between July 2018 and November 2021 were included. In this study population, patients who did not receive DORAs or were treated with a DORA after delirium onset were defined as the control group. Patients who were treated with a DORA after admission were defined as the DORA group, which was divided into the suvorexant and lemborexant groups according to the drug used. DORAs were prescribed at the discretion of the clinician, and DORA compliance was estimated by confirming from the nurse’s records that the drug was actually administered and not simply whether or not it was prescribed. In this study, patients were defined as being at high risk for delirium caused by alcohol withdrawal if they drank heavily on a daily basis and the clinician considered that prevention of alcohol withdrawal delirium was necessary. This study was approved by the Ethics Committee of Saga University Hospital (approval number: 2021–06-R-13). All eligible patients were given the opportunity to opt out; informed consent was not required for this retrospective observational study.
Delirium Diagnosis and Data Collection
Delirium was assessed according to the Confusion Assessment Method for the ICU (CAM-ICU).29 First, (1) acute mental status changes and (2) lack of attention were assessed. When these were detected, (3) fluctuations in the level of consciousness and (4) disorganized thinking were evaluated by assessing for jarring conversations, disorganized behavior, and risky behavior. The medical records included detailed descriptions of the patient’s actual conversations and movements regarding (1) through (4) as well as outcomes of evaluation with the Richmond Agitation Sedation Scale (RASS).30 A physician analyzed these medical records and determined that a patient was CAM-ICU positive if all 4 (ie, 1–4) were detected. This step was performed by two independent physicians to check for concordance in the determination of CAM-ICU positivity. Medical records were updated 2 or 3 times per day, in the morning and in the afternoon, depending on a patient’s nursing needs. Additional records were kept when there was a change in patient status.
Staff members who made the original entries in the RASS and CAM-ICU evaluations were dedicated emergency center nurses who had received training on delirium from psychiatric nurses experienced in conducting these evaluations. In addition, training on medical record keeping was provided, and only nurses who had reached the standards set by the hospital performed medical record entries. At the time this study was conducted, such medical record entry and education had already become routine practice in the wards.
Patient background information including age, sex, body mass index (BMI), clinical frailty score, visual impairment, hearing impairment, medical history, Charlson Comorbidity Index score,31 smoking history, alcohol consumption history, and benzodiazepine medication status prior to admission was extracted from medical records. The clinical frailty score is a tool that assesses patient frailty on a 9-point scale from 1 (very fit) to 9 (terminally ill) based on comorbidities, physical function, and cognitive function.32,33 Vital signs and blood test findings at the time of admission were also extracted. The Acute Physiology and Chronic Health Evaluation (APACHE) II score34 and Sequential Organ Failure Assessment (SOFA) score35 were calculated to determine illness severity at admission. Diseases and conditions that triggered admission to the emergency center were retrieved from the records, and we extracted the treatment administered before the onset of delirium or, if the patient had not yet developed delirium, before leaving the emergency center. Information regarding sleep disorder, defined as difficulty in initiating or maintaining sleep, waking up too early, and circadian rhythm disruption, was available in the medical records.
Medical information was recorded 3 times a day, and additional entries were added if there was any change in a patient’s physical or mental condition.
Statistical Analysis
On the basis of previous studies, we assumed an 18% incidence of delirium in the DORA group and a 32% incidence of delirium in the control group.25 The sample size required to ensure a power of 0.8 in the current study was calculated to be 37 for both the DORA and control groups. Medians and interquartile ranges (IQRs) were calculated for continuous variables, and frequencies and percentages were calculated for categorical variables. Univariate analysis was used to compare patient background, severity of illness, treatment, medications used, incidence of delirium, and mortality between the DORA and control groups. The Wilcoxon test was performed for continuous variables and the χ2 test for categorical variables.
The cohort was then divided into two groups, one with delirium and the other without delirium, and univariate analysis was performed to identify factors contributing to the development of delirium. Spearman tests were performed on these factors to assess multicollinearity. Kaplan-Meier curves were plotted for the suvorexant, lemborexant, and control groups, and log rank tests were used to compare them. Then, Cox regression analysis was performed to calculate the hazard ratio of suvorexant and lemborexant for the development of delirium using a proportional hazards model. In the Cox regression analysis, the hazard ratio of suvorexant or lemborexant use for the development of delirium was calculated with no adjustment at all in the crude model.
In the subsequent Models 1 and 2, factors identified in previous studies as risk factors for developing delirium in the ICU6,36 and factors predicted in the present study to contribute to delirium were used as adjustment factors. In Model 1, we used factors such as patient’s background, severity of illness, treatment, clinical frailty score, BMI, Charlson Comorbidity Index score, dementia, fibrin/fibrinogen degradation products (FDP), lactate level, APACHE II score, mechanical ventilation, and sleep disorder as adjustment factors. In Model 2, we included the adjustment factors of Model 1 plus concomitant medications such as midazolam, dexmedetomidine, fentanyl, haloperidol, hydroxyzine, Z-drugs, trazodone, risperidone, quetiapine, benzodiazepine, and ramelteon. The medications used as adjustment factors in Model 2 are all medications that have been employed in our emergency center for delirium and altered mental status and are routinely used by emergency physicians and psychiatrists.
All statistical analyses were performed using the JMP Pro version 14 software package (SAS Inc).
RESULTS
Participants
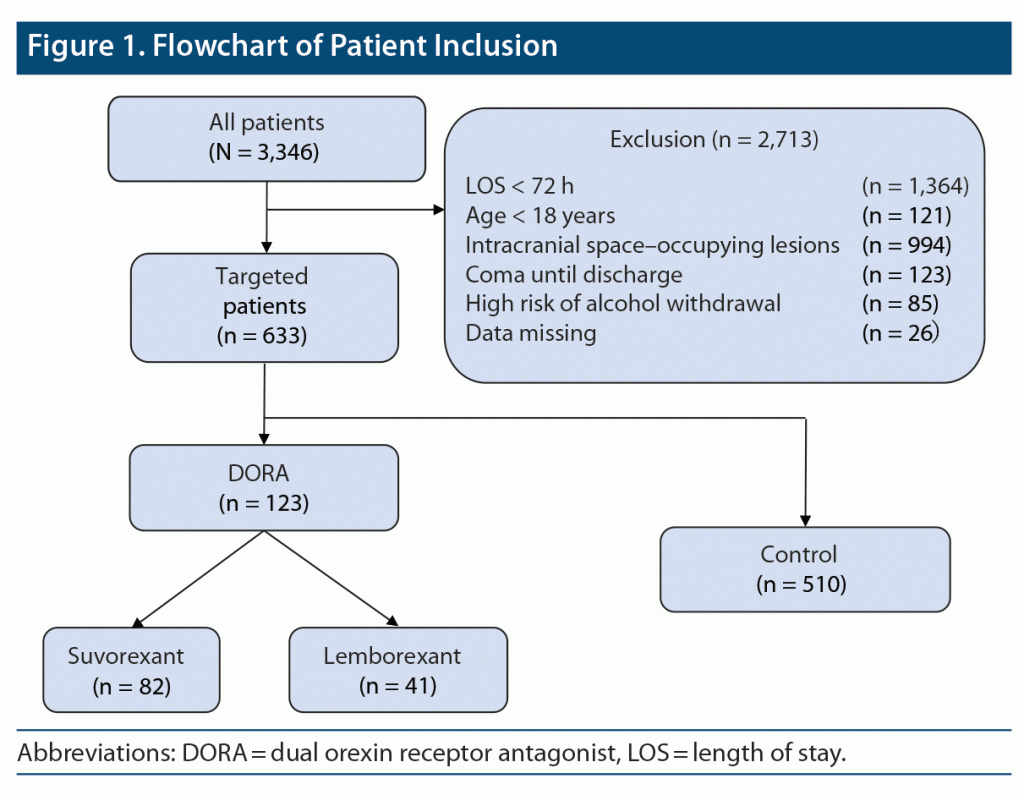
Between July 2018 and November 2021, 3,346 patients were admitted to our emergency and critical care center. Patients discharged from the emergency center in less than 72 h (n = 1,364), under 18 years of age (n = 121), with intracranial space–occupying lesion (n = 994), with impaired consciousness continuing until discharge (n = 123), at high risk of alcohol withdrawal delirium (n = 85), admitted during drug or stimulant abuse (n = 0), or who were pregnant (n = 0) or prisoners (n = 0) were excluded from the study. Patients with incomplete medical records and missing data on admission (n = 26) were also excluded. Ultimately, 633 patients were included in the analysis. Of these, 123 patients were in the DORA group, including 82 in the suvorexant group and 41 in the lemborexant group, and 510 patients were in the control group (Figure 1).
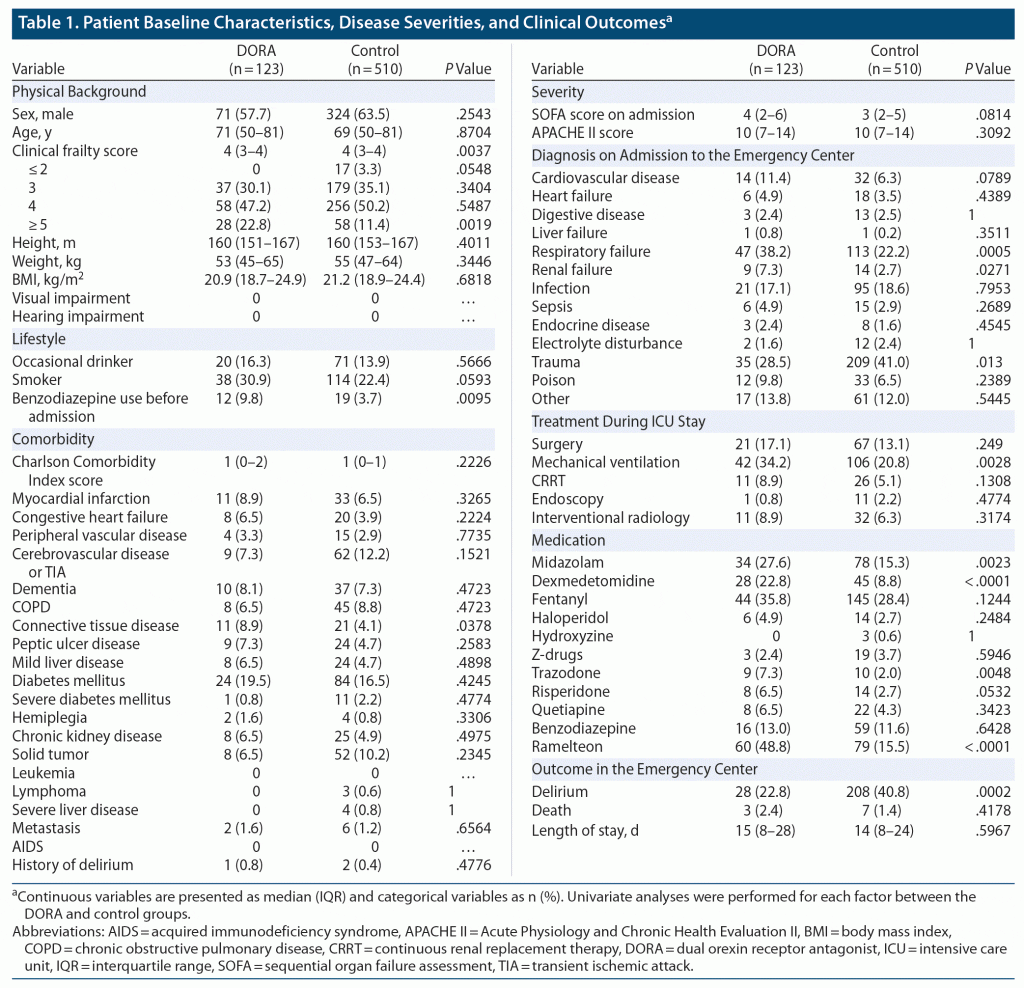
The median (IQR) age of the DORA group was 71 (50–81) years and not significantly different from that of the control group, which was 69 (50–81) years (P = .8704). The clinical frailty score was significantly higher in the DORA group (P = .0037), and patients with a score of 5 or higher were significantly more likely to be in the DORA group (P = .0019).
A significantly higher proportion of patients in the DORA group were prescribed benzodiazepines prior to admission (9.8% vs 3.7%, P = .0095). Acute respiratory failure was significantly more common in the DORA group (38.2% vs 22.2%, P = .0005), and significantly more patients in this group required respiratory management (34.2% vs 20.8%, P = .0028). Significantly more patients in the DORA group also used midazolam (27.6% vs 15.3%, P = .0023), dexmedetomidine (22.8% vs 8.8%, P < .0001), trazodone (7.3% vs 2.0%, P = .0048), and ramelteon (48.8% vs 15.5%, P < .0001) during the observation period. The percentage of patients who developed delirium was significantly higher in the control group (22.3% vs 40.8%, P = .0002; Table 1).
Risk Factors for the Development of Delirium
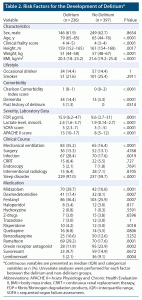
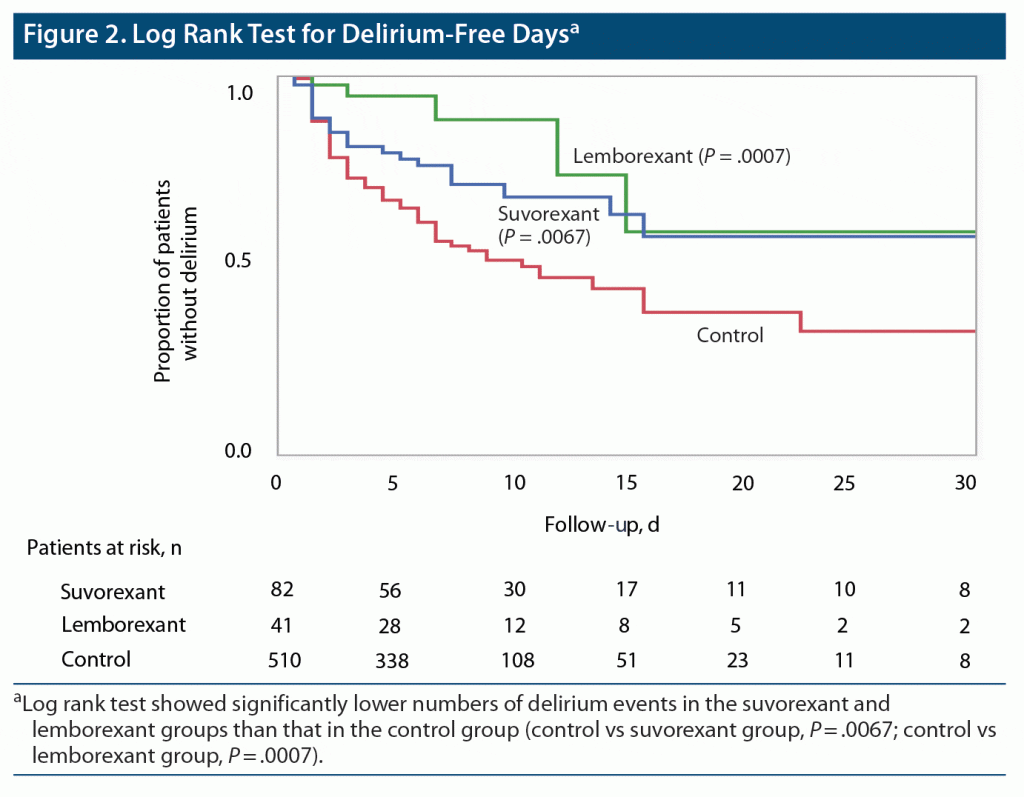
Table 2 shows the association of each factor with the development of delirium. Patients who developed delirium were characterized by older age, higher clinical frailty score, lower BMI, higher Charlson Comorbidity Index score, higher SOFA and APACHE II scores, higher FDP and lactate levels, and a greater likelihood of ventilatory management, infection, and sleep disorder. Regarding drugs used, midazolam, dexmedetomidine, fentanyl, and ramelteon were all used significantly more frequently in the delirium group. In this analysis, delirium-free patients were also significantly more likely to be treated with oral DORAs (23.9% vs 11.9%, P = .0002). When the DORA group was divided into patients treated with suvorexant (suvorexant group) and those treated with lemborexant (lemborexant group), the percentage of patients treated with suvorexant tended to be higher in the non-delirium group (9.8% vs 14.9%, P = .0672). Likewise, the percentage of patients treated with lemborexant was significantly higher in the non-delirium group (2.1% vs 9.1%, P = .0004). The results of the log rank test showed significantly lower numbers of delirium events in the suvorexant and lemborexant groups than that in the control group (control vs suvorexant group, P = .0067; control vs lemborexant group, P = .0007; Figure 2).
Effects of Suvorexant and Lemborexant on the Development of Delirium
To examine the efficacy of suvorexant and lemborexant in suppressing delirium, Cox regression analysis was performed for the control, suvorexant, and lemborexant groups. The hazard ratios (95% CIs) for the development of delirium were 0.56 (0.36–0.86) for the suvorexant group and 0.26 (0.11–0.62) for the lemborexant group compared to the control group. In Model 1, adjusted for patient background, severity of illness, and treatment, the hazard ratios (95% CIs) of suvorexant and lemborexant for the development of delirium were 0.34 (0.21–0.54) and 0.19 (0.08–0.46), respectively. Furthermore, in Model 2, adjusted for concomitant medications in addition to the factors used in Model 1, the hazard ratios (95% CIs) for developing delirium in the suvorexant and lemborexant groups remained significantly lower at 0.34 (0.20–0.58) and 0.21 (0.08–0.52), respectively. The hazard ratio for the development of delirium for lemborexant-treated patients compared to suvorexant-treated patients was 0.61 (0.22–1.65; Table 3).
The hazard ratios (95% CIs) for the development of delirium were as follows: for midazolam-treated patients, 1.15 (0.74–1.78); for dexmedetomidine-treated patients, 1.42 (0.91–2.22); and for ramelteon-treated patients, 0.96 (0.69–1.35; Supplementary Table 1).
DISCUSSION
The study suggests that both suvorexant and lemborexant may have delirium-preventive effects in critically ill patients in the emergency center. In the univariate analysis of the delirium and non-delirium groups, the prescription rate of lemborexant was significantly higher in the non-delirium group, whereas that of suvorexant was not different between the two groups. However, Cox regression analysis showed that both suvorexant and lemborexant were effective in preventing delirium. Moreover, the results of Model 2 suggested that the efficacy of lemborexant in preventing delirium was not significantly different from that of suvorexant. Lemborexant has a higher affinity for orexin receptor 2 than for orexin receptor 1 and is expected to increase non–rapid eye movement sleep and improve sleep compared to suvorexant.37,38 It is also expected to have an immediate effect on sleep onset and reduce somnolence on the following day because of its rapid binding to and dissociation from orexin receptors.26,37 Although the current study did not show a significant difference in efficacy between suvorexant and lemborexant in preventing delirium, further increased use of lemborexant could produce different results.
The factors identified in this study that were involved in the development of delirium were consistent with existing risk factors for developing delirium in the ICU and included higher age, higher clinical frailty score, lower BMI, greater dementia, high APACHE II score, increases ventilator use, and greater sleep disturbances.39–44
Findings of previous studies suggest that dexmedetomidine prevents delirium.45–47 However, in the present study, adjusted Cox regression analyses revealed a lack of delirium prevention by dexmedetomidine. In our practice, dexmedetomidine is rarely used as a single agent but often in combination with other sedatives, such as midazolam, or other sedatives are substituted by dexmedetomidine. Dexmedetomidine is also often used to sedate patients on ventilatory management. Thus, patients who were treated with dexmedetomidine may have been more severely ill and more prone to delirium than those who were not.
In the present study, we found no significant association between ramelteon use and delirium. However, Hatta et al21 reported that ramelteon was effective in preventing delirium in a randomized placebo-controlled trial. In our study, ramelteon was often used in combination with suvorexant or lemborexant. In some cases, it was also prescribed as a single dose for abortive use in patients with sleep disorders. Therefore, the efficacy of ramelteon as a single agent in preventing delirium was not confirmed by our results. In Cox regression analyses, delirium incidence rates were lower in the suvorexant and lemborexant groups after adjusting for ramelteon use. Some studies reported that the combination of suvorexant and ramelteon prevents delirium,23,48 and lemborexant may have a stronger effect when used in combination with ramelteon.
This study had some limitations. First, the diagnosis of delirium onset was assessed by retrospectively checking medical records and following the CAM-ICU. The medical records used for the CAM-ICU evaluation were not recorded at the same time for all patients, and the number of times they were recorded varied from patient to patient. It has been reported that hypoactive delirium is more likely to be missed,49 and it is also possible that patients with hypoactive delirium were overlooked in our study.
Second, because of the single-center retrospective study design and the small number of patients in the lemborexant group, we were unable to adequately compare the efficacies of lemborexant and suvorexant in preventing delirium.
Third, DORAs are oral drugs. Patients who were sedated were administered a DORA through a nasogastric tube. However, patients who were very critically ill and unable to receive the drug via a nasogastric tube were included in the control group in this study. Therefore, it is possible that the control group included a large number of severely ill patients, which may have affected the incidence of delirium.
Furthermore, the improvement in sleep status after oral administration of suvorexant or lemborexant could not be analyzed due to insufficient data. It is important that future research examines the effects of these drugs on sleep improvement and whether they lead to a reduction in delirium duration.
Despite these limitations, this study is the first to suggest that both suvorexant and lemborexant may have a preventive effect against delirium in critically ill patients requiring intensive care in an emergency department. Further research on this topic is warranted.
CONCLUSIONS
Both suvorexant and lemborexant may be effective in preventing delirium in critically ill patients in advanced critical care centers. Further randomized controlled trials are needed to verify the efficacy of lemborexant in preventing delirium.
Submitted: March 29, 2022; accepted July 1, 2022.
Published online: November 7, 2022.
Author contributions: Study conception and design: A.M. (Ayaka Matsuoka), T.M., and A.M. (Akira Monji); data acquisition: A.M. (Ayaka Matsuoka), S.T., R.S., T.M.-H., and K.S.; data analysis: A.M. (Ayaka Matsuoka), S.T., R.S., and C.S.; data interpretation: A.M. (Ayaka Matsuoka), S.T., R.S., C.S., A.M. (Akira Monji), and Y.M.; drafting of the manuscript: A.M. (Ayaka Matsuoka), K.S., and Y.S.; study supervision: Y.S. and A.M. (Akira Monji). All authors approved the final version of the manuscript.
Relevant financial relationships: The authors declare that they have no competing interests.
Funding/support: This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Ethics approval and consent to participate: This study was approved by the Ethics Committee of Saga University Hospital (approval number: 2021-06-R-13) and conducted in accordance with the Declaration of Helsinki. All eligible patients were given the opportunity to opt out.
Acknowledgments: We would like to thank Editage for English-language editing.
Additional information: The datasets are available from the corresponding author on reasonable request.
Supplementary material: Available at Psychiatrist.com.
Clinical Points
- Lemborexant, a dual orexin receptor antagonist (DORA), is used as a sleep medication, but its effect on delirium is unclear.
- The effect of lemborexant on the development of delirium in critically ill patients was investigated at an emergency center.
- The results suggest that, like suvorexant, lemborexant may be useful in the prevention of delirium.
References (49)

- Rood PJT, Zegers M, Ramnarain D, et al; UNDERPIN-ICU Study Investigators. The impact of nursing delirium preventive interventions in the ICU: a multicenter cluster-randomized controlled clinical trial. Am J Respir Crit Care Med. 2021;204(6):682–691. PubMed CrossRef
- Stollings JL, Kotfis K, Chanques G, et al. Delirium in critical illness: clinical manifestations, outcomes, and management. Intensive Care Med. 2021;47(10):1089–1103. PubMed CrossRef
- Luetz A, Grunow JJ, Mörgeli R, et al. Innovative ICU solutions to prevent and reduce delirium and post-intensive care unit syndrome. Semin Respir Crit Care Med. 2019;40(5):673–686. PubMed CrossRef
- Zhang S, Han Y, Xiao Q, et al. Effectiveness of bundle interventions on ICU delirium: a meta-analysis. Crit Care Med. 2021;49(2):335–346. PubMed CrossRef
- Jackson P, Khan A. Delirium in critically ill patients. Crit Care Clin. 2015;31(3):589–603. PubMed CrossRef
- Kotfis K, Marra A, Ely EW. ICU delirium: a diagnostic and therapeutic challenge in the intensive care unit. Anaesthesiol Intensive Ther. 2018;50(2):160–167. PubMed CrossRef
- Noriega FJ, Vidán MT, Sánchez E, et al. Incidence and impact of delirium on clinical and functional outcomes in older patients hospitalized for acute cardiac diseases. Am Heart J. 2015;170(5):938–944. PubMed CrossRef
- Palakshappa JA, Hough CL. How we prevent and treat delirium in the ICU. Chest. 2021;160(4):1326–1334. PubMed CrossRef
- Shehabi Y, Riker RR, Bokesch PM, et al; SEDCOM (Safety and Efficacy of Dexmedetomidine Compared With Midazolam) Study Group. Delirium duration and mortality in lightly sedated, mechanically ventilated intensive care patients. Crit Care Med. 2010;38(12):2311–2318. PubMed CrossRef
- Al-Qadheeb NS, Balk EM, Fraser GL, et al. Randomized ICU trials do not demonstrate an association between interventions that reduce delirium duration and short-term mortality: a systematic review and meta-analysis. Crit Care Med. 2014;42(6):1442–1454. PubMed CrossRef
- Burry LD, Hutton B, Guenette M, et al. Comparison of pharmacological and non-pharmacological interventions to prevent delirium in critically ill patients: a protocol for a systematic review incorporating network meta-analyses. Syst Rev. 2016;5(1):153. PubMed CrossRef
- Kalisvaart KJ, de Jonghe JF, Bogaards MJ, et al. Haloperidol prophylaxis for elderly hip-surgery patients at risk for delirium: a randomized placebo-controlled study. J Am Geriatr Soc. 2005;53(10):1658–1666. PubMed CrossRef
- Hayhurst CJ, Pandharipande PP, Hughes CG. Intensive care unit delirium: a review of diagnosis, prevention, and treatment. Anesthesiology. 2016;125(6):1229–1241. PubMed CrossRef
- Salvi F, Young J, Lucarelli M, et al. Non-pharmacological approaches in the prevention of delirium. Eur Geriatr Med. 2020;11(1):71–81. PubMed CrossRef
- Guo Y, Fan Y. A preoperative, nurse-led intervention program reduces acute postoperative delirium. J Neurosci Nurs. 2016;48(4):229–235. PubMed CrossRef
- Hsieh SJ, Ely EW, Gong MN. Can intensive care unit delirium be prevented and reduced? lessons learned and future directions. Ann Am Thorac Soc. 2013;10(6):648–656. PubMed CrossRef
- Barr J, Fraser GL, Puntillo K, et al; American College of Critical Care Medicine. Clinical practice guidelines for the management of pain, agitation, and delirium in adult patients in the intensive care unit. Crit Care Med. 2013;41(1):263–306. PubMed CrossRef
- Devlin JW, Skrobik Y, Gélinas C, et al. Clinical Practice Guidelines for the Prevention and Management of Pain, Agitation/Sedation, Delirium, Immobility, and Sleep Disruption in Adult Patients in the ICU. Crit Care Med. 2018;46(9):e825–e873. PubMed CrossRef
- Daymont C, Hwang WT, Feudtner C, et al. Head-circumference distribution in a large primary care network differs from CDC and WHO curves. Pediatrics. 2010;126(4):e836–e842. PubMed CrossRef
- Furuya M, Miyaoka T, Yasuda H, et al. Ramelteon as adjunctive therapy for delirium referred to a consultation-liaison psychiatry service: a retrospective analysis. Int J Geriatr Psychiatry. 2015;30(9):994–995. PubMed CrossRef
- Hatta K, Kishi Y, Wada K, et al; DELIRIA-J Group. Preventive effects of ramelteon on delirium: a randomized placebo-controlled trial. JAMA Psychiatry. 2014;71(4):397–403. PubMed CrossRef
- Khaing K, Nair BR. Melatonin for delirium prevention in hospitalized patients: a systematic review and meta-analysis. J Psychiatr Res. 2021;133:181–190. PubMed CrossRef
- Hatta K, Kishi Y, Wada K, et al. Real-world effectiveness of ramelteon and suvorexant for delirium prevention in 948 patients with delirium risk factors. J Clin Psychiatry. 2019;81(1):19m12865. PubMed CrossRef
- Hatta K, Kishi Y, Wada K, et al; DELIRIA-J Group. Preventive effects of suvorexant on delirium: a randomized placebo-controlled trial. J Clin Psychiatry. 2017;78(8):e970–e979. PubMed CrossRef
- Izuhara M, Izuhara HK, Tsuchie K, et al. Real-world preventive effects of suvorexant in intensive care delirium: a retrospective cohort study. J Clin Psychiatry. 2020;81(6):20m13362. PubMed CrossRef
- Murphy P, Moline M, Mayleben D, et al. Lemborexant, a dual orexin receptor antagonist (DORA) for the treatment of insomnia disorder: results from a Bayesian, adaptive, randomized, double-blind, placebo-controlled study. J Clin Sleep Med. 2017;13(11):1289–1299. PubMed CrossRef
- Scott LJ. Lemborexant: first approval. Drugs. 2020;80(4):425–432. PubMed CrossRef
- Terada T, Hirayama T, Sadahiro R, et al. Pilot study of Lemborexant for insomnia in cancer patients with delirium. J Palliat Med. 2022;25(5):797–801. PubMed CrossRef
- Chen TJ, Chung YW, Chang HR, et al. Diagnostic accuracy of the CAM-ICU and ICDSC in detecting intensive care unit delirium: a bivariate meta-analysis. Int J Nurs Stud. 2021;113:103782. PubMed CrossRef
- Ely EW, Truman B, Shintani A, et al. Monitoring sedation status over time in ICU patients: reliability and validity of the Richmond Agitation-Sedation Scale (RASS). JAMA. 2003;289(22):2983–2991. PubMed CrossRef
- Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–383. PubMed CrossRef
- Church S, Rogers E, Rockwood K, et al. A scoping review of the Clinical Frailty Scale. BMC Geriatr. 2020;20(1):393. PubMed CrossRef
- Shimura T, Yamamoto M, Kano S, et al. Impact of frailty markers on outcomes after transcatheter aortic valve replacement: insights from a Japanese multicenter registry. Ann Cardiothorac Surg. 2017;6(5):532–537. PubMed CrossRef
- Knaus WA, Draper EA, Wagner DP, et al. APACHE II: a severity of disease classification system. Crit Care Med. 1985;13(10):818–829. PubMed CrossRef
- Vincent JL, Moreno R, Takala J, et al. On behalf of the Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine. The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. Intensive Care Med. 1996;22(7):707–710. PubMed CrossRef
- Zaal IJ, Devlin JW, Peelen LM, et al. A systematic review of risk factors for delirium in the ICU. Crit Care Med. 2015;43(1):40–47. PubMed CrossRef
- Beuckmann CT, Suzuki M, Ueno T, et al. In vitro and in silico characterization of lemborexant (E2006), a novel dual orexin receptor antagonist. J Pharmacol Exp Ther. 2017;362(2):287–295. PubMed CrossRef
- Kishi T, Nomura I, Matsuda Y, et al. Lemborexant vs suvorexant for insomnia: a systematic review and network meta-analysis. J Psychiatr Res. 2020;128:68–74. PubMed CrossRef
- Inouye SK, Westendorp RG, Saczynski JS. Delirium in elderly people. Lancet. 2014;383(9920):911–922. PubMed CrossRef
- Kamdar BB, Niessen T, Colantuoni E, et al. Delirium transitions in the medical ICU: exploring the role of sleep quality and other factors. Crit Care Med. 2015;43(1):135–141. PubMed CrossRef
- Łukasik-Głębocka M, Sommerfeld K, Teżyk A, et al. Post-injection delirium/sedation syndrome after olanzapine long-acting intramuscular injection: who is at risk? Basic Clin Pharmacol Toxicol. 2015;117(3):213–214. PubMed CrossRef
- Ní Chróinín D, Francis N, Wong P, et al. Older trauma patients are at high risk of delirium, especially those with underlying dementia or baseline frailty. Trauma Surg Acute Care Open. 2021;6(1):e000639. PubMed CrossRef
- van den Boogaard M, Pickkers P, Slooter AJ, et al. Development and validation of PRE-DELIRIC (PREdiction of DELIRium in ICu patients) delirium prediction model for intensive care patients: observational multicentre study. BMJ. 2012;344(feb09 3):e420. PubMed CrossRef
- Zhang H, Yuan J, Chen Q, et al. Development and validation of a predictive score for ICU delirium in critically ill patients. BMC Anesthesiol. 2021;21(1):37. PubMed CrossRef
- León-Salas B, Trujillo-Martín MM, Del Castillo LPM, et al. Pharmacologic interventions for prevention of delirium in hospitalized older people: a meta-analysis. Arch Gerontol Geriatr. 2020;90:104171. PubMed CrossRef
- Su X, Meng ZT, Wu XH, et al. Dexmedetomidine for prevention of delirium in elderly patients after non-cardiac surgery: a randomised, double-blind, placebo-controlled trial. Lancet. 2016;388(10054):1893–1902. PubMed CrossRef
- Wu YC, Tseng PT, Tu YK, et al. Association of delirium response and safety of pharmacological interventions for the management and prevention of delirium: a network meta-analysis. JAMA Psychiatry. 2019;76(5):526–535. PubMed CrossRef
- Tian Y, Qin Z, Han Y. Suvorexant with or without ramelteon to prevent delirium: a systematic review and meta-analysis. Psychogeriatrics. 2022;22(2):259–268. PubMed CrossRef
- Kosari SA, Amiruddin A, Shorakae S, et al. A rare cause of hypoactive delirium. BMJ Case Rep. 2014;2014(oct19 1):bcr2014205382. PubMed CrossRef
This PDF is free for all visitors!
Save
Cite