Objective: To investigate the relationship between microRNA107 (miRNA107) and BACE1 messenger RNA (mRNA) gene expressions in plasma and their diagnostic capability to distinguish subjects with amnestic mild cognitive impairment from healthy controls.
Method: We recruited 97 patients with Alzheimer’s disease according to diagnostic criteria of DSM-IV and National Institute of Neurologic and Communicative Disorders and Stroke/Alzheimer’s Disease and Related Disorders Association, 116 subjects with amnestic mild cognitive impairment, and 81 healthy controls from January 2012 to December 2012. The real-time PCR was used to quantify miRNA107 and BACE1 mRNA. The power of classification accuracies between patients with amnestic mild cognitive impairment and healthy controls was performed using linear discriminate analysis single or combining both the expression miRNA107 and BACE1 mRNA.
Results: For patients with Alzheimer’s disease, the miRNA107 expressions in plasma and cerebral spinal fluid were correlated (Pearson correlation = 0.665, P = .034). There were statistically significant correlations between plasma miRNA107 and BACE1 mRNA gene expression in Alzheimer’s disease, amnestic mild cognitive impairment, and healthy control groups (r value = −0.316 [P = .002], −0.615 [P < .001], and −0.367 [P = .001], respectively). The overall classification accuracy of miRNA107 to discriminate between patients with amnestic mild cognitive impairment and healthy controls was 91.9%, with sensitivity of 98.3% and specificity of 82.7%.
Conclusions: The miRNA107 expression in plasma has a high capability to discriminate between patients with amnestic mild cognitive impairment and healthy controls.
Trial Registration: ClinicalTrials.gov identifier: NCT01819545
The Feasibility of Utilizing Plasma MiRNA107 and BACE1 Messenger RNA Gene Expression for Clinical Diagnosis of Amnestic Mild Cognitive Impairment

ABSTRACT
Objective: To investigate the relationship between microRNA107 (miRNA107) and BACE1 messenger RNA (mRNA) gene expressions in plasma and their diagnostic capability to distinguish subjects with amnestic mild cognitive impairment from healthy controls.
Method: We recruited 97 patients with Alzheimer’s disease according to diagnostic criteria of DSM-IV and National Institute of Neurologic and Communicative Disorders and Stroke/Alzheimer’s Disease and Related Disorders Association, 116 subjects with amnestic mild cognitive impairment, and 81 healthy controls from January 2012 to December 2012. The real-time PCR was used to quantify miRNA107 and BACE1 mRNA. The power of classification accuracies between patients with amnestic mild cognitive impairment and healthy controls was performed using linear discriminate analysis single or combining both the expression miRNA107 and BACE1 mRNA.
Results: For patients with Alzheimer’s disease, the miRNA107 expressions in plasma and cerebral spinal fluid were correlated (Pearson correlation = 0.665, P = .034). There were statistically significant correlations between plasma miRNA107 and BACE1 mRNA gene expression in Alzheimer’s disease, amnestic mild cognitive impairment, and healthy control groups (r value = −0.316 [P = .002], −0.615 [P < .001], and −0.367 [P = .001], respectively). The overall classification accuracy of miRNA107 to discriminate between patients with amnestic mild cognitive impairment and healthy controls was 91.9%, with sensitivity of 98.3% and specificity of 82.7%.
Conclusions: The miRNA107 expression in plasma has a high capability to discriminate between patients with amnestic mild cognitive impairment and healthy controls.
Trial Registration: ClinicalTrials.gov identifier: NCT01819545
J Clin Psychiatry 2015;76(2):135-141
© Copyright 2015 Physicians Postgraduate Press, Inc.
Submitted: September 26, 2013; accepted May 23, 2014 (doi:10.4088/JCP.13m08812).
Corresponding author: Shifu Xiao, MD, PhD, Department of Geriatric Psychiatry, Shanghai Mental Health Center, Shanghai Jiao Tong University School of Medicine; Alzheimer’s Disease and Related Disorders Center, Shanghai Jiao Tong University, Shanghai, China, 600 South Wan Ping Rd, Xu Hui District, Shanghai, China, 200030 ([email protected]).
Alzheimer’s disease is the most common form of dementia worldwide. Pathologically, the disease is defined by the intracellular accumulation of aggregated and hyperphosphorylated protein tau and the extracellular deposition of amyloid β (Aβ) peptides.1 For the latter, the secretase enzymes have been the focus of intense research for years. Related to the widely accepted hypothesized major pathology of Alzheimer’s disease, the generation and subsequent accumulation of Aβ was reportedly through sequential cleavage of the amyloid precursor protein (APP) by β-site APP-cleaving enzyme 1 (BACE1) and γ-secretase.2 In fact, it has been demonstrated that regulation of expression levels of proteins involved in this Aβ generation process is playing an important role in Alzheimer’s disease.3
The BACE1 messenger RNA (mRNA) is regulated by microRNA (miRNA).4 Accumulating evidence has suggested that alterations of miRNAs could contribute to risks for Alzheimer’s disease. In fact, deregulations in Alzheimer’s disease have been reported5-7 for a number of specific miRNAs. Researchers investigating miRNAs in postmortem brain tissues in patients with Alzheimer’s disease reported their decreases as compared to healthy controls and their variability over different human brain areas. More interestingly, studies5-7 from several groups demonstrated that miRNA expressions are altered not only in the brains of patients with Alzheimer’s disease but also in the cerebral spinal fluid (CSF) and especially in blood plasma of these patients, raising the possibility of their easy use for diagnosis in a clinical setting.
Among these miRNAs, miRNA107 targets genes directly related to disease such as BACE1 mRNA. It interacts with 3‘ ²UTR of BACE1 mRNA.8 In previous microarray studies,9,10 miRNA107 levels were found to be substantially reduced in the temporal cortex of the patients affected by Alzheimer’s disease. However, the difficulty, high costs, and invasiveness of obtaining data for brain tissues and CSF made the microarray data unfeasible for routine uses such as in clinical settings.11 Therefore, it will be of great interest to identify disease-associated changes in miRNA expression measurements from routine laboratory tests. In this regard, measurements from plasma data are more readily available in the clinical setting. We therefore set up this study to (1) explore the possibility of whether plasma measurements are correlated with CSF measurements for miRNA107 and BACE1 mRNA separately and (2) examine whether each of these 2 plasma measures or their combination is capable of especially discriminating between subjects with amnestic mild cognitive impairment and healthy controls, between subjects with Alzheimer’s disease and healthy controls, between subjects with Alzheimer’s disease/amnestic mild cognitive impairment and healthy controls, or even between subjects with Alzheimer’s disease and those with amnestic mild cognitive impairment. We were especially interested in examining such feasibility in distinguishing subjects with amnestic mild cognitive impairment from healthy control, as it is relatively more difficult to diagnose amnestic mild cognitive impairment in contrast to healthy controls than to diagnose Alzheimer’s disease from healthy controls.

- The plasma measurements were correlated with the CSF measurements for microRNA107 (miRNA107) and β-site APP-cleaving enzyme 1 (BACE1) messenger RNA (mRNA) separately.
- Plasma measure of expression of miRNA107 and BACE1 mRNA is capable of discriminating between subjects with amnestic mild cognitive impairment and healthy controls.
METHOD
Participants and Recruitment
This study was registered at ClinicalTrials.gov (identifier: NCT01819545).
We recruited 97 patients with Alzheimer’s disease; 116 patients with amnestic mild cognitive impairment from the tertiary hospital of Shanghai Mental Health Center, Shanghai Jiao Tong University School of Medicine (Alzheimer’s Disease and Related Disorders Center); and 81 cognitively healthy elderly subjects from the Shanghai Changning district, Huangpu district, and Putuo district from January 2012 to December 2012. Patients enrolled by the hospital were via self-referral or referral from family or physician. This study was approved by the institution’s ethics committee, and informed consent was obtained from all subjects and/or their legal guardians.
Probable Alzheimer’s disease was diagnosed according to criteria from the Diagnostic and Statistical Manual for Mental Disorders, Fourth Edition (DSM-IV), and from the National Institute of Neurologic and Communicative Disorders and Stroke/Alzheimer’s Disease and Related Disorders Association.12
Amnestic mild cognitive impairment was diagnosed based on the following criteria13: (1) memory complaint, preferably corroborated by a spouse or relative; (2) objective memory impairment; (3) normal general cognitive function; (4) intact activities of daily living; and (5) absence of dementia. We have amended the amnestic mild cognitive impairment diagnostic criteria of the Petersen Mini-Mental State Examination (MMSE)14 cutoff score in order to be consistent with the low level of education in elderly Chinese people. Research15 has found that with the Chinese version of MMSE, subjects with Alzheimer’s disease who had not been educated exhibited MMSE scores of < 18, those with elementary school education exhibited MMSE scores of < 21, and those with higher than middle school education exhibited MMSE scores of < 25. In the present study, the amnestic mild cognitive impairment analysis was carried out on uneducated subjects with MMSE cutoff scores of ≥ 18, elementary school-educated subjects with MMSE cutoff scores of ≥ 21, and higher than middle school-educated subject with MMSE cutoff scores of ≥ 25.
The cognitively healthy elderly subjects formed the healthy control group and were independently functioning community dwellers with no neurologic or psychiatric conditions.
All participants underwent a screening process that included a review of their medical history, physical and neurologic examinations, laboratory tests, and magnetic resonance imaging (MRI) scans (see below). The clinical assessment of mild cognitive impairment or dementia included neuropsychological tests, as well as behavioral and psychiatric interviews conducted by attending psychiatrists. Patients with Alzheimer’s disease recorded scores of < 4 on the Hachinski Ischemia Scale16 and showed no history of significant systemic or psychiatric conditions or traumatic brain injuries that could compromise brain function. The Clinical Dementia Rating17 and Montreal Cognitive Assessment18 were used to assess all of the participants. The neuropsychological battery which was used only to determine mild cognitive impairment subtype in the present study included Wechsler Memory Scale (WMS) Verbal Associates immediate and 30-minute delayed test,19 Rey Auditory Verbal Learning and 30-minute Delayed Test,20 WMS-Digit Span,19 Category Naming Test-Animals,21 and Clock Drawing Test.22 We rated cognitive impairment of the patients with mild cognitive impairment in 7 domains (memory, attention, language, visual-spatial, orientation, calculation, and executive function) according to the neuropsychological battery and MMSE. On the basis of the assessment, we retained subjects with amnestic mild cognitive impairment and others were excluded, such as those who had impairment in a single nonmemory domain (single nonmemory domain mild cognitive impairment subtype) and those who had impairment in 2 or more domains (multiple domains, slightly impaired mild cognitive impairment subtype). Healthy control subjects had no history of cognitive decline, neurologic disorders, or uncontrolled systemic medical disorders. All subjects included in this study had no more than 2 lacunar ischemia (of diameter < 1 cm) as revealed by MRI fluid-attenuated inversion recovery (FLAIR) sequence. The MRI FLAIR (Siemens MAGNETOM VERIO 3T, SIEMENS, Munich, Freistaat Bayern, Germany) data acquisition setting was with the following parameters: matrix size = 256 ×— 192, number of excitations = 1, field of view = 24 cm, echo time = 140 ms, repetition time = 8,600 ms, inversion time = 2,200 ms.
Blood Plasma Collection and Lumbar Punctures for CSF Collection
An ethylenediaminetetraacetic acid (Zibo, Shandong, China) anticoagulated blood sample (5 mL) was obtained from each subject, and, after standing for 30-60 minutes, each sample was centrifuged for 20 minutes at 4°C low temperature and spun at 3,000 rpm. Each 200 μL sample was taken into a 2-mL eppendorf tube and immediately frozen stored at −80°C refrigerator tested.
To examine the correlation between plasma and CSF measures, we performed lumbar punctures only on a subset of subjects with Alzheimer’s disease (45 of 97), not on healthy controls and subjects with amnestic mild cognitive impairment (due to the difficulty of getting consent from any of them). Eight mL CSF was collected into glass tubes. Cerebral spinal fluid samples were kept in 4°C for a maximum of 1 hour, until centrifuged for 10 minutes at 3,000 g in 4°C, and transferred to and stored in polypropylene tubes as 2 mL aliquots at −80°C.
Quantitative Real-Time Polymerase Chain Reaction
BACE1 mRNA quantitative real-time polymerase chain reaction. Total mRNAs were extracted using TRIZOL Reagent (Invitrogen Life Technologies; Carlsbad, California) and was quantified using the NanoDrop (Waltham, Massachusetts) ND-1000 spectrophotometer. Quality control was performed by assessing the optical density ratio of 260/280 nm. Quantitative real-time polymerase chain reaction (PCR) was conducted using 2 ×— PCR master mix (Super Array, Valencia, California) and the ABI PRISM7900 system (Applied Biosystems; Foster City, California). For each sample, real-time PCR was performed together with reference gene β-actin for target mRNA (BACE1 mRNA) and its relative amount of the mRNA determined by 2–ΔΔ threshold cycle methods.
MicroRNA107 quantitative real-time PCR. Total miRNAs were extracted using TRIZOL Reagent and quantified using the NanoDrop ND-1000 spectrophotometer. Quality control was performed by assessing the optical density ratio of 260/280 nm. Quantitative real time PCR was conducted using Universal cDNA Synthesis Kit, 16-32 rxns (Exiqon, Vedbaek, Denmark), and the Gene Amp PCR System 9700 (Applied Biosystems; Foster City, California). For each sample, real-time PCR was performed together with reference gene miRNA423-5p for target miRNA (miRNA107) and its relative amount of the miRNA determined by 2–ΔΔ threshold cycle methods.
APOE Type and BACE1 Protein Concentration
We also used PCR-restriction fragment length polymorphism to determine the apolipoprotein E (APOE) types of subjects.
We made extra efforts to have the blood samples assayed a second time for BACE1 protein. We used the BACE1 protein ELISA kits (LBL 27752 human BACE1 assay kit; Takasaki-Shi, Gunma, Japan) to assay the BACE1 protein concentration from a subset of our study participants: 16 patients with Alzheimer’s disease, 30 patients with amnestic mild cognitive impairment, and 32 healthy control subjects.
Data Analysis
For examining the plasma/CSF associations for miRNA107 and BACE1 mRNA each alone for patients with Alzheimer’s disease, we used simple Pearson correlation analysis.
Demographic characteristics, except gender ratio and age, and clinical characteristics across the Alzheimer’s disease, amnestic mild cognitive impairment, and control groups were assessed by analysis of covariance (ANCOVA) to account for age and gender effects. The ANCOVA was followed by least significant difference post hoc tests. Gender ratio and age were assessed using χ2 test and analysis of variance separately. Similarly, miRNA107 and BACE1 mRNA expression and BACE1 protein differences between the groups in plasma were tested also by ANCOVA (accounting for age and gender) and followed least significant difference. In addition to testing the group difference and for the purpose of adopting these plasma-based measurements as biomarkers for the feasibility of clinical diagnosis, we assessed especially the accuracies of classification between healthy controls and amnestic mild cognitive impairment group, between healthy controls and Alzheimer’s disease group, between amnestic mild cognitive impairment and Alzheimer’s disease groups, and between healthy controls and the pooled amnestic mild cognitive impairment/Alzheimer’s disease group, using each of these 2 measurements alone or combined together. In assessing the classification accuracies, we used the receiver operating characteristic approach for estimating the sensitivity and specificity for each plasma expression alone. For combining the 2 together, we applied the simple linear discriminate analysis approach. The Youden’s Index was used to ensure the critical point of diagnosis accuracy for plasma miRNA107 and/or BACE1 mRNA gene expression. When doing each of all the analyses above, leave-1-out procedure was conducted for the reported sensitivity and specificity. In addition, we also randomly partitioned 60% of the subjects (n = 119) into training and the remaining 40% (n = 78) for testing. The discriminate model generated by the training group was subsequently applied to the subjects in the testing group.
RESULTS
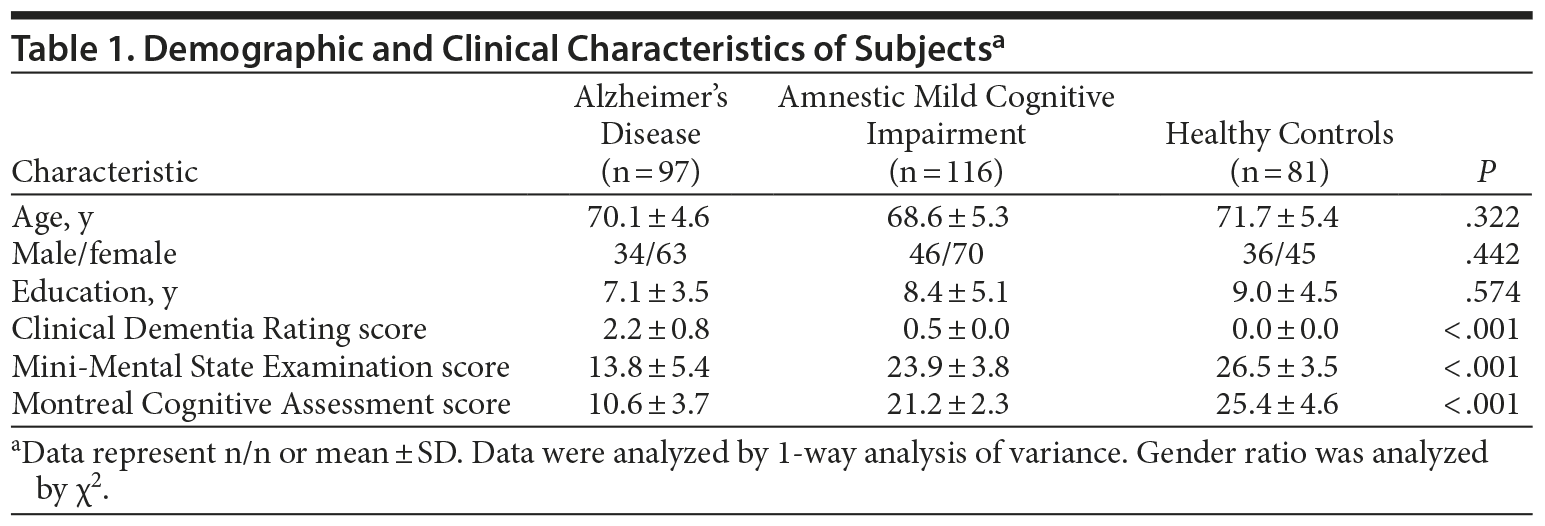
There were no significant differences in age, gender, and education level among the 3 groups (Table 1). In patients with Alzheimer’s disease, the plasma and CSF Pearson correlation coefficient for miRNA107 expression was 0.665 (P = .034). The correlation for BACE1 mRNA, however, was not significant (r = 0.107, P = .585).
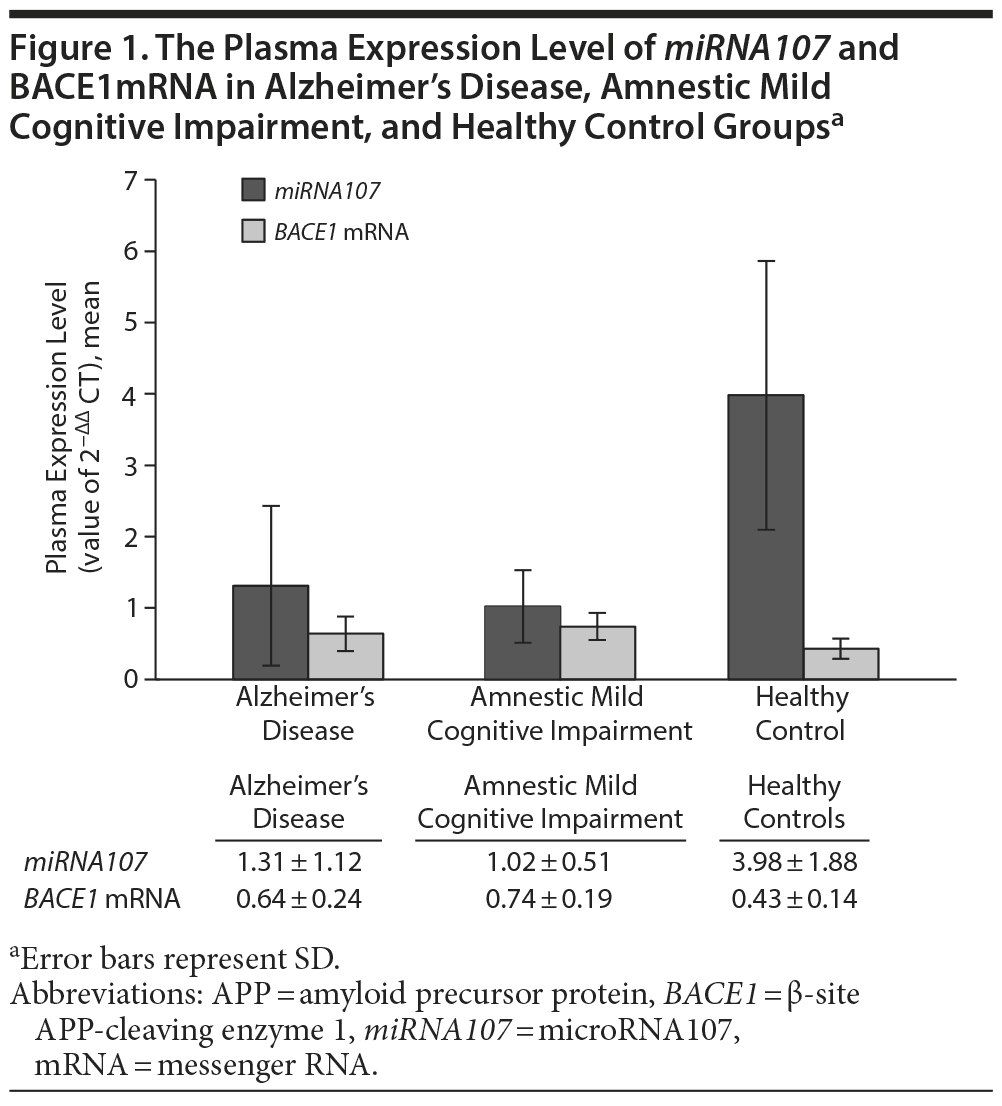
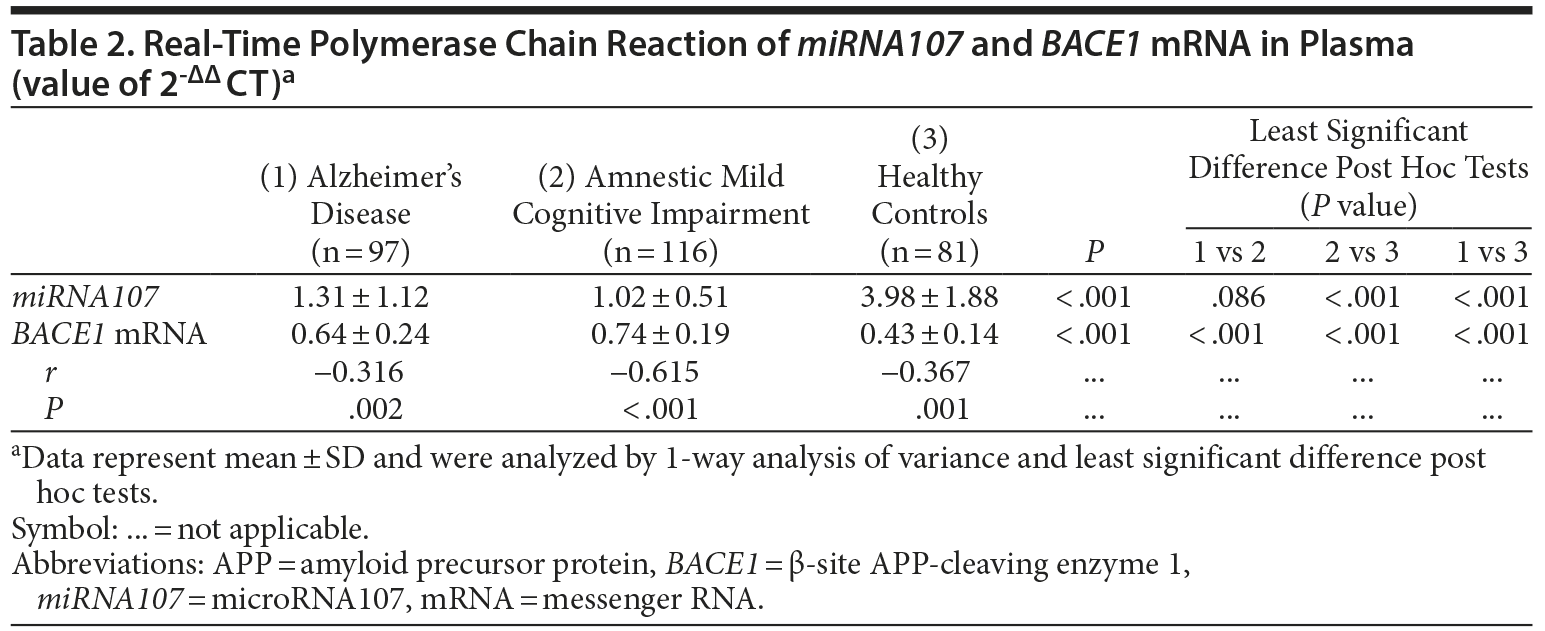
The mean ± SD levels of plasma miRNA107 expression in Alzheimer’s disease group, amnestic mild cognitive impairment group, and healthy controls were 1.31 ± 1.12, 1.02 ± 0.51, and 3.98 ± 1.88, respectively (P < .001; Figure 1, Table 2). We noted that the plasma expression of miRNA107 was significantly different between the amnestic mild cognitive impairment and healthy controls (P < .001) and between Alzheimer’s disease and healthy controls (P < .001), but not between Alzheimer’s disease and amnestic mild cognitive impairment (P = .086).
The mean ± SD levels of plasma BACE1 mRNA expression in Alzheimer’s disease group, amnestic mild cognitive impairment group, and healthy controls were 0.64 ± 0.24, 0.74 ± 0.19, and 0.43 ± 0.14, respectively (P < .001; Figure 1, Table 2). We noted that the plasma expression of BACE1 mRNA was significantly different between amnestic mild cognitive impairment and healthy controls (P < .001), between Alzheimer’s disease and healthy controls (P < .001), and between Alzheimer’s disease and amnestic mild cognitive impairment (P < .001).
There were statistically significant correlations between plasma miRNA107 and BACE1 mRNA gene expression in Alzheimer’s disease (r = −0.316, P = .002), amnestic mild cognitive impairment (r = −0.615, P < .001), and healthy controls (r = −0.367, P = .001), respectively (Table 2). The correlation over all subjects is r = −0.566 (P < .001).
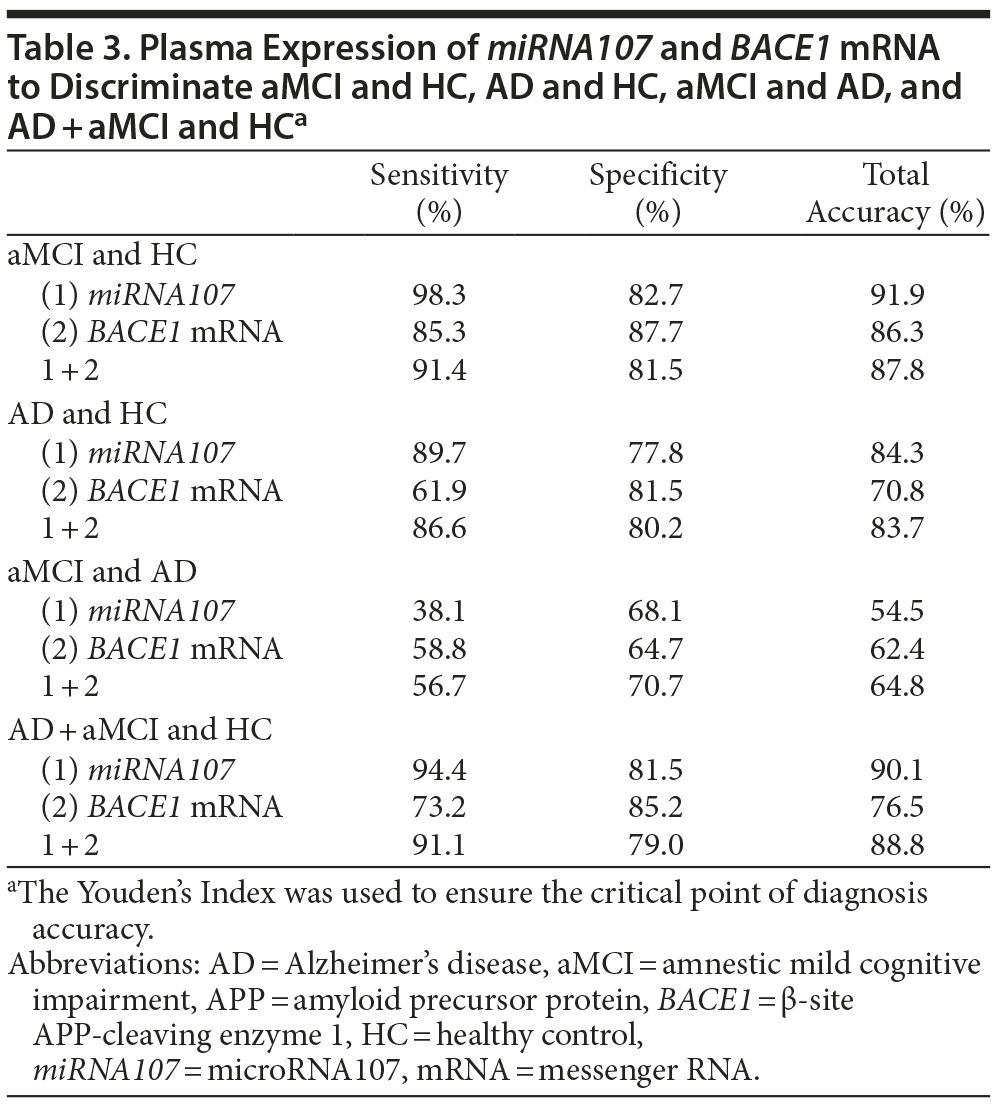
Using the leave-1-out procedure, we found that the sensitivity and specificity of distinguishing amnestic mild cognitive impairment and healthy controls is 98.3% and 82.7%, respectively, for miRNA107 and 85.3% and 87.7%, respectively, for BACE1 mRNA. When these 2 gene expressions were combined, the sensitivity and specificity became 91.4% and 81.5% (Table 3). See Table 3 for the classification results for other group pairs. In addition, we used 60% of subjects (119 in the amnestic mild cognitive impairment and healthy control groups) randomly selected to construct the discriminator and then assessed its performance on the remaining 40% of subjects (78 in the amnestic mild cognitive impairment and healthy control groups). The 44 of 46 subjects with amnestic mild cognitive impairment and 25 of 32 healthy controls were correct classified. Thus, the estimated sensitivity was 95.6%, the specificity 78.1%, and the overall accuracy 87.2%. The Youden’s Index23 was used to ensure the critical point of diagnosis accuracy.
The APOE status was not significant difference among 3 groups (χ2 test, P = .750). We used the APOE status in the analysis of variance statistical models. The APOE status did not produce the effect in the variance statistical models. The APOE status in the Alzheimer’s disease group was APOE*2/*2, n = 0; APOE*3/*3, n = 89; APOE*4/*4, n = 2; APOE*2/*3, n = 3; APOE*2/*4, n = 2; and APOE*3/*4, n = 1. The APOE status in the amnestic mild cognitive impairment group was APOE*2/*2, n = 0; APOE*3/*3, n = 107; APOE*4/*4, n = 2; APOE*2/*3, n = 4; APOE*2/*4, n = 3; and APOE*3/*4, n = 0. The APOE status in the healthy control group was APOE*2/*2, n = 1; APOE*3/*3, n = 74; APOE*4/*4, n = 0; APOE*2/*3, n = 4; APOE*2/*4, n = 2; and APOE*3/*4, n = 0.
For the post hoc analysis on the BACE1 plasma protein concentration for a subset of the subjects, there were no significant differences in age, gender, and education level among the 3 groups, a finding consistent with our results for the entire samples. The plasma BACE1 protein concentration was significantly different (P = .026) among Alzheimer’s disease (n = 16), amnestic mild cognitive impairment (n = 30), and healthy control (n = 32) groups, with the group pairwise differences between Alzheimer’s disease and healthy control groups (P = .025) and between amnestic mild cognitive impairment and healthy control groups (P = .022), but not between Alzheimer’s disease and amnestic mild cognitive impairment groups (P = .724). The mean ± SD levels of plasma BACE1 protein concentration in Alzheimer’s disease, amnestic mild cognitive impairment, and healthy control groups were 5.07 ± 1.78 ng/mL, 4.89 ± 2.01 ng/mL, and 3.89 ± 1.25 ng/mL, respectively.
DISCUSSION
The main aim of the study was to search for plasma miRNA biomarkers that can be used to primarily distinguish between subjects with amnestic mild cognitive impairment and healthy controls as well as between subjects with Alzheimer’s disease and healthy controls or between subjects with Alzheimer’s disease/amnestic mild cognitive impairment and healthy controls in clinical settings. The results obtained in our experiments have demonstrated for the first time that minimally invasive tests based on analysis of cell-free miRNA107 and BACE1 mRNA expression in plasma are feasible for distinguishing especially amnestic mild cognitive impairment from healthy control.
In spite of the positive findings, we noted that the plasma and CSF measure association was significant only for miRNA107 but not for BACE1 mRNA using data from patients with Alzheimer’s disease. The lack of such association for BACE1 mRNA is probably due to the inability of the molecular BACE1 mRNA to freely go through blood-brain barrier because of the molecular size.24 Regardless, our findings should be treated as preliminary and exploratory. Their use and understanding require additional studies to establish its validity. And our results and discussions should be interpreted with this caution.
Amyloid plaques and neurofibrillary tangles are considered to be the most important hallmarks of Alzheimer’s disease pathophysiology. The amyloid hypothesis states that the 39-43 amino acid Aβ peptides, ie, the main constituent of amyloid plaques, are responsible for the initiation of a neurotoxic cascade that ultimately leads to neuronal death and dementia.25,2 Considerable evidence indicates that Aβ production is critical to the brain deterioration related to Alzheimer’s disease.26 Secretase plays a central role in this process. Amyloid β is generated by the sequential cleavage of APP, a type I integral transmembrane protein that becomes the exclusive target in Alzheimer’s disease pathology. The cleavage occurs within the ectodomain of APP mediated by BACE1 (β-secretase, memapsin-2, aspartyl protease 2).27–31 Due to heterogeneous γ-secretase cleavage, Aβ species of different lengths are produced, including the pathogenic 42-amino acid peptide Aβ42.2 Because BACE1 is rate limiting for this disease process, considerable effort has been expended on elucidating its role in Alzheimer’s disease and identification of therapeutic agents that specifically target this enzyme. In our research, we did not find significant association between plasma and CSF measures for BACE1 mRNA using data from patients with Alzheimer’s disease. It has been reported that it is difficult for the BACE1 mRNA to go across the blood-brain barrier because of its molecular size.24 Another possible cause of this lack of association between CSF and plasma was that BACE1 mRNA is also prevalent in platelets and other peripheral cell types.
The down-regulation of miRNA107 is frequently observed in the brain of Alzheimer’s disease, as well in patients suffering from mild cognitive impairment.8,32,33 Among several candidates, miRNA107 targets genes directly related to disease, such as BACE1.34 In this study, we found miRNA107 plasma measures were significantly different between patients with Alzheimer’s disease and healthy controls and between subjects with mild cognitive impairment and healthy controls. Furthermore, miRNA107 can distinguish amnestic mild cognitive impairment from healthy controls with high accuracy. Together with these findings, the observation that miRNA107 expression in plasma is decreased in amnestic mild cognitive impairment makes this miRNA an interesting candidate for future mechanistic and, eventually, diagnostic studies.
With the use of a total of 294 plasma samples from Alzheimer’s disease, amnestic mild cognitive impairment, and healthy controls in our study, we found significant expression correlation between miRNA107 and BACE1 mRNA within each group and over all subjects. To our knowledge, no previous publication has reported such correlation finding. It is also interesting to note that miRNA107 expression was lower in subjects with amnestic mild cognitive impairment than in healthy controls or in subjects with Alzheimer’s disease. Similarly, changes of BACE1 mRNA among the 3 groups were also not monotonic with regard to the disease severity. The nonmonotonicity, however, coincided with the regulatory relationships in the 2 genes.
It is important to note that most of elderly patients and age-matched controls had various nonneurologic conditions unrelated to amnestic mild cognitive impairment. In our study, the other comorbidities in the 3 groups included diabetes, hyperlipidemia, the lichen planus of tongue, gallstone, breast fibroma, hernia, hearing loss, gastric ulcer, myoma of uterus, anemia, hyperplasia of prostate, sciatica, cholecystitis, cataract, protrusion of intervertebral disc, and gout. There were no significant differences among the 3 groups. Since prevalences of these comorbidities were the same among these patient groups, our study demonstrated the plasma test capability of detecting amnestic mild cognitive impairment in subjects with other accompanying diseases. Our results support the ability of the expression in plasma of miRNA107 and BACE1 mRNA to differentiate amnestic mild cognitive impairment (and Alzheimer’s disease) from an age-matched control. However, additional larger studies are necessary for further validation of our results, including a prospective longitudinal study. The differentiation of Alzheimer’s disease from other type dementias is an important but separate issue that was not addressed in this study. It is expected that a combination of multiple miRNAs in plasma could be useful for such differential diagnosis. For example, additional promising miRNAs along with those described in the present study could be potentially used for detecting other neurodegenerative diseases and for differential diagnosis.
Early detection of patients with mild cognitive impairment by a noninvasive or minimally invasive screening test is more practical than invasive and expensive tests. Such a test can be not only used in a clinical diagnosis setting but also applied as a preselection tool to enroll patients in clinical trials. In the present study, we found that the plasma expression of miRNA107 had a relationship to its expression in CSF. But there was no relationship of BACE1 mRNA expression between plasma and CSF. We also found that the expression of miRNA107 and BACE1 mRNA had a reverse change after the amnestic mild cognitive impairment stage. When and why the change becomes present should be need for further research. Although our study had a small number of subjects and its results need confirmation from independent study, we found that the plasma BACE1 protein has a potential ability to discriminate between subjects with Alzheimer’s disease and healthy controls.
Recently, the National Institute on Aging and the Alzheimer’s Association developed new diagnostic guidelines for Alzheimer’s disease.35-37 The guidelines contain updated classification of the phases of Alzheimer’s disease, namely, the dementia phase, the symptomatic predementia phase (mild cognitive impairment), and the asymptomatic preclinical phase of Alzheimer’s disease (preclinical mild cognitive impairment). The new guidelines also provide recommendations for the diagnosis of preclinical mild cognitive impairment, mild cognitive impairment, and Alzheimer’s disease dementia and stress the lack of and a great need for reliable biomarkers that can be used for detection of mild cognitive impairment and preclinical phases of Alzheimer’s disease. We believe the current study provides preliminary evidence for the inclusion of plasma-based biomarkers toward this objective.
Additional studies are needed to further test and verify the ability of discriminant amnestic mild cognitive impairment and healthy controls in an independent cohort by plasma miRNA107. Moreover and related to what we discussed earlier about the differential dementia diagnosis, miRNA107 is not specific to Alzheimer’s disease, as it is also hypothesized to regulate progranulin/granulin, a molecule known to be important in non-tau frontotemporal dementia. Thus, this marker may have somewhat limited value in differentiating Alzheimer’s disease from other dementia.10 The possibility of differentiating Alzheimer’s disease from other dementia by the expression of plasma miRNA107 and BACE1 mRNA together with possible additional plasma measures should be examined in separate future studies.
CONCLUSION
This study suggests an association between plasma and CSF expression of miRNA107 and an association between the expressions of miRNA107 and BACE1 mRNA in plasma. Moreover, miRNA107 and BACE1 mRNA are capable of discriminating between healthy controls and patients with amnestic mild cognitive impairment.
Author affiliations: Department of Geriatric Psychiatry (Drs Wang, Dong, Su, Liu, Cheng, Dai, Yang, and Xiao), Shanghai Mental Health Center (Dr Li), Shanghai Jiao Tong University School of Medicine; Alzheimer’s Disease and Related Disorders Center (Drs Wang, Dong, Su, Liu, Cheng, Dai, Yang, and Xiao) and Med-X Research Institution (Dr Chen), Shanghai Jiao Tong University, Shanghai, China; Banner Alzheimer’s Institute (Dr Chen); Neurology Department, Medical Campus, University of Arizona (Dr Chen); Arizona Alzheimer’s Consortium (Dr Chen), Phoenix; and School of Mathematics and Statistics, Arizona State University, Tempe (Dr Chen), Arizona.
Previous presentation: Part of the abstract was presented at the Alzheimer’s Association International Conference on Alzheimer’s Disease; July 13-18, 2013; Boston, Massachusetts.
Potential conflicts of interest: None reported.
Funding/support: Supported by the National Natural Science Foundation of China project 81201030, the Funds for International Cooperation and Exchange of the National Natural Science Foundation of China (project 61210001), the China Ministry of Science and Technology grants 2009BAI77B03 and 2012ZX09303, and the Shanghai Science and Technology Committee grants 08411951100 and 10ZR1425800. The authors gratefully acknowledge the support of Shanghai Jiao Tong University K. C. Wong Medical Fellowship Fund.
Role of the sponsors: The sponsor played no role in design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript.
REFERENCES
1. De Strooper B. Proteases and proteolysis in Alzheimer disease: a multifactorial view on the disease process. Physiol Rev. 2010;90(2):465-494. PubMed doi:10.1152/physrev.00023.2009
2. Dislich B, Lichtenthaler SF. Membrane-bound aspartyl protease BACE1: molecular and functional properties in Alzheimer’s disease and beyond. Front Physiol. 2012;3:8. PubMed doi:10.3389/fphys.2012.00008
3. Bettens K, Brouwers N, Engelborghs S, et al. APP and BACE1 miRNA genetic variability has no major role in risk for Alzheimer disease. Hum Mutat. 2009;30(8):1207-1213. PubMed doi:10.1002/humu.21027
4. Sathya M, Premkumar P, Karthick C, et al. BACE1 in Alzheimer’s disease. Clin Chim Acta. 2012;414:171-178. PubMed doi:10.1016/j.cca.2012.08.013
5. Hébert SS, Sergeant N, Buée L. MicroRNAs and the regulation of tau metabolism. Int J Alzheimers Dis. 2012;2012:406561. PubMed
6. Hébert SS, Papadopoulou AS, Smith P, et al. Genetic ablation of Dicer in adult forebrain neurons results in abnormal tau hyperphosphorylation and neurodegeneration. Hum Mol Genet. 2010;19(20):3959-3969. PubMed doi:10.1093/hmg/ddq311
7. Cogswell JP, Ward J, Taylor IA, et al. Identification of miRNA changes in Alzheimer’s disease brain and CSF yields putative biomarkers and insights into disease pathways. J Alzheimers Dis. 2008;14(1):27-41. PubMed
8. Wang WX, Rajeev BW, Stromberg AJ, et al. The expression of microRNA miR-107 decreases early in Alzheimer’s disease and may accelerate disease progression through regulation of beta-site amyloid precursor protein-cleaving enzyme 1. J Neurosci. 2008;28(5):1213-1223. PubMed doi:10.1523/JNEUROSCI.5065-07.2008
9. Wang WX, Huang Q, Hu Y, et al. Patterns of microRNA expression in normal and early Alzheimer’s disease human temporal cortex: white matter versus gray matter. Acta Neuropathol. 2011;121(2):193-205. PubMed doi:10.1007/s00401-010-0756-0
10. Wang WX, Wilfred BR, Madathil SK, et al. miR-107 regulates granulin/progranulin with implications for traumatic brain injury and neurodegenerative disease. Am J Pathol. 2010;177(1):334-345. PubMed doi:10.2353/ajpath.2010.091202
11. Müller M, Kuiperij HB, Claassen JA, et al. MicroRNAs in Alzheimer’s disease: differential expression in hippocampus and cell-free cerebrospinal fluid. Neurobiol Aging. 2014;35(1):152-158. PubMed doi:10.1016/j.neurobiolaging.2013.07.005
12. McKhann G, Drachman D, Folstein M, et al. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology. 1984;34(7):939-944. PubMed doi:10.1212/WNL.34.7.939
13. Petersen RC, Doody R, Kurz A, et al. Current concepts in mild cognitive impairment. Arch Neurol. 2001;58(12):1985-1992. PubMed doi:10.1001/archneur.58.12.1985
14. Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189-198. PubMed doi:10.1016/0022-3956(75)90026-6
15. Katzman R, Zhang MY, Ouang-Ya-Qu, et al. A Chinese version of the Mini-Mental State Examination; impact of illiteracy in a Shanghai dementia survey. J Clin Epidemiol. 1988;41(10):971-978. PubMed doi:10.1016/0895-4356(88)90034-0
16. Hachinski VC, Iliff LD, Zilhka E, et al. Cerebral blood flow in dementia. Arch Neurol. 1975;32(9):632-637. PubMed doi:10.1001/archneur.1975.00490510088009
17. Schafer KA, Tractenberg RE, Sano M, et al; Alzheimer’s Disease Cooperative Study. Reliability of monitoring the clinical dementia rating in multicenter clinical trials. Alzheimer Dis Assoc Disord. 2004;18(4):219-222. PubMed
18. Nasreddine ZS, Phillips NA, Bédirian V, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53(4):695-699. PubMed doi:10.1111/j.1532-5415.2005.53221.x
19. Stone CP, Girdner J, Albrecht R. An alternate form of the Wechsler Memory Scale. J Psychol. 1946;22(2):199-206. PubMed doi:10.1080/00223980.1946.9917307
20. Mungas D. Differential clinical sensitivity of specific parameters of the Rey Auditory-Verbal Learning Test. J Consult Clin Psychol. 1983;51(6):848-855. PubMed doi:10.1037/0022-006X.51.6.848
21. Bayles KA, Tomoeda CK, Trosset MW. Naming and categorical knowledge in Alzheimer’s disease: the process of semantic memory deterioration. Brain Lang. 1990;39(4):498-510. PubMed doi:10.1016/0093-934X(90)90158-D
22. Sunderland T, Hill JL, Mellow AM, et al. Clock drawing in Alzheimer’s disease: a novel measure of dementia severity. J Am Geriatr Soc. 1989;37(8):725-729. PubMed
23. Youden WJ. Index for rating diagnostic tests. Cancer. 1950;3(1):32-35. PubMed doi:10.1002/1097-0142(1950)3:1<32::AID-CNCR2820030106>3.0.CO;2-3
24. Coulson DT, Beyer N, Quinn JG, et al. BACE1 mRNA expression in Alzheimer’s disease postmortem brain tissue. J Alzheimers Dis. 2010;22(4):1111-1122. PubMed
25. Haass C, Selkoe DJ. Soluble protein oligomers in neurodegeneration: lessons from the Alzheimer’s amyloid beta-peptide. Nat Rev Mol Cell Biol. 2007;8(2):101-112. PubMed doi:10.1038/nrm2101
26. Stockley JH, O’ Neill C. Understanding BACE1: essential protease for amyloid-β production in Alzheimer’s disease. Cell Mol Life Sci. 2008;65(20):3265-3289. PubMed doi:10.1007/s00018-008-8271-3
27. Hussain I, Powell D, Howlett DR, et al. Identification of a novel aspartic protease (Asp 2) as beta-secretase. Mol Cell Neurosci. 1999;14(6):419-427. PubMed doi:10.1006/mcne.1999.0811
28. Sinha S, Anderson JP, Barbour R, et al. Purification and cloning of amyloid precursor protein beta-secretase from human brain. Nature. 1999;402(6761):537-540. PubMed doi:10.1038/990114
29. Vassar R, Bennett BD, Babu-Khan S, et al. Beta-secretase cleavage of Alzheimer’s amyloid precursor protein by the transmembrane aspartic protease BACE. Science. 1999;286(5440):735-741. PubMed doi:10.1126/science.286.5440.735
30. Yan R, Bienkowski MJ, Shuck ME, et al. Membrane-anchored aspartyl protease with Alzheimer’s disease beta-secretase activity. Nature. 1999;402(6761):533-537. PubMed doi:10.1038/990107
31. Lin X, Koelsch G, Wu S, et al. Human aspartic protease memapsin 2 cleaves the beta-secretase site of beta-amyloid precursor protein. Proc Natl Acad Sci U S A. 2000;97(4):1456-1460. PubMed doi:10.1073/pnas.97.4.1456
32. Nelson PT, Wang WX. MiR-107 is reduced in Alzheimer’s disease brain neocortex: validation study. J Alzheimers Dis. 2010;21(1):75-79. PubMed
33. Smith P, Al Hashimi A, Girard J, et al. In vivo regulation of amyloid precursor protein neuronal splicing by microRNAs. J Neurochem. 2011;116(2):240-247. PubMed doi:10.1111/j.1471-4159.2010.07097.x
34. Yao J, Hennessey T, Flynt A, et al. MicroRNA-related cofilin abnormality in Alzheimer’s disease. PLoS ONE. 2010;5(12):e15546. PubMed doi:10.1371/journal.pone.0015546
35. McKhann GM, Knopman DS, Chertkow H, et al. The diagnosis of dementia due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7(3):263-269. PubMed doi:10.1016/j.jalz.2011.03.005
36. Albert MS, DeKosky ST, Dickson D, et al. The diagnosis of mild cognitive impairment due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7(3):270-279. PubMed doi:10.1016/j.jalz.2011.03.008
37. Sperling RA, Aisen PS, Beckett LA, et al. Toward defining the preclinical stages of Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7(3):280-292. PubMed doi:10.1016/j.jalz.2011.03.003
Please sign in or purchase this PDF for $40.00.
Save
Cite