Objective: Herein we provide the 2015 update for the Florida Best Practice Psychotherapeutic Medication Guidelines (FPG) for major depressive disorder (MDD). The FPG represent evidence-based decision support for practitioners providing care to adults with MDD.
Participants: The consensus meeting included representatives from the Florida Agency for Health Care Administration (FAHCA), advocacy members, academic experts in MDD, and multidisciplinary mental health clinicians, as well as health policy experts. The FAHCA provided funding support for the FPG.
Evidence: Evidence was limited to results from adequately powered, randomized, double-blind, placebo-controlled trials; in addition, pooled-, meta-, and network-analyses were included. Recommendations were based on consensus arrived at by the multistakeholder Florida Expert Panel. Articles selected were identified on the electronic search engine PubMed with the dates 2010 to present. The search terms were major depressive disorder, psychopharmacology, antidepressants, psychotherapy, neuromodulation, complementary alternative medicines, pooled-analysis, meta-analysis, and network-analysis. Bibliographies of the identified articles were manually searched for additional citations not identified in the original search.
Consensus Process: A consensus meeting comprising all representatives took place on September 25-26, 2015, in Tampa, Florida. Guiding principles (eg, emphasis on the most rigorous evidence for efficacy, safety, and tolerability) were discussed, defined, and operationalized prior to review of extant data. As MDD often pursues a recurrent and chronic course, principles of practice, measurement-based care, and comprehensive assessment and management of overall physical and mental health were emphasized. Evidence supporting pretreatment major depressive episode specifiers (eg, mixed features, anxious distress) and the role of pharmacogenomics (and other biological-behavioral markers) in informing treatment selection were comprehensively discussed. Algorithmic priority was assigned to agents with relatively greater therapeutic index (ie, efficacy) and minimal propensity for safety and tolerability disadvantages.
Conclusions: The updated 2015 FPG provide concise, pragmatic, evidence-based decision support for treatment selection and sequencing for adults with MDD. Principles of practice include measurement-based care, priority to both psychiatric and medical comorbidity, identification of DSM-5-defined specifiers (eg, mixed features), suicide risk assessment, and evaluation of cognitive symptoms. The FPG have purposefully aimed to minimize emphasis on "expert opinion" and instead differentially emphasized extant evidence for pharmacologic treatments.

Florida Best Practice Psychotherapeutic Medication Guidelines for Adults With Major Depressive Disorder
ABSTRACT
Objective: Herein we provide the 2015 update for the Florida Best Practice Psychotherapeutic Medication Guidelines (FPG) for major depressive disorder (MDD). The FPG represent evidence-based decision support for practitioners providing care to adults with MDD.
Participants: The consensus meeting included representatives from the Florida Agency for Health Care Administration (FAHCA), advocacy members, academic experts in MDD, and multidisciplinary mental health clinicians, as well as health policy experts. The FAHCA provided funding support for the FPG.
Evidence: Evidence was limited to results from adequately powered, randomized, double-blind, placebo-controlled trials; in addition, pooled-, meta-, and network-analyses were included. Recommendations were based on consensus arrived at by the multistakeholder Florida Expert Panel. Articles selected were identified on the electronic search engine PubMed with the dates 2010 to present. The search terms were major depressive disorder, psychopharmacology, antidepressants, psychotherapy, neuromodulation, complementary alternative medicines, pooled-analysis, meta-analysis, and network-analysis. Bibliographies of the identified articles were manually searched for additional citations not identified in the original search.
Consensus Process: A consensus meeting comprising all representatives took place on September 25-26, 2015, in Tampa, Florida. Guiding principles (eg, emphasis on the most rigorous evidence for efficacy, safety, and tolerability) were discussed, defined, and operationalized prior to review of extant data. As MDD often pursues a recurrent and chronic course, principles of practice, measurement-based care, and comprehensive assessment and management of overall physical and mental health were emphasized. Evidence supporting pretreatment major depressive episode specifiers (eg, mixed features, anxious distress) and the role of pharmacogenomics (and other biological-behavioral markers) in informing treatment selection were comprehensively discussed. Algorithmic priority was assigned to agents with relatively greater therapeutic index (ie, efficacy) and minimal propensity for safety and tolerability disadvantages.
Conclusions: The updated 2015 FPG provide concise, pragmatic, evidence-based decision support for treatment selection and sequencing for adults with MDD. Principles of practice include measurement-based care, priority to both psychiatric and medical comorbidity, identification of DSM-5-defined specifiers (eg, mixed features), suicide risk assessment, and evaluation of cognitive symptoms. The FPG have purposefully aimed to minimize emphasis on “expert opinion” and instead differentially emphasized extant evidence for pharmacologic treatments.
J Clin Psychiatry 2017;78(6):703-713
dx.doi.org/10.4088/JCP.16cs10885
© Copyright 2017 Physicians Postgraduate Press, Inc.
aMood Disorder Psychopharmacology Unit, University Health Network, Department of Psychiatry, University of Toronto, Ontario, Canada bDepartment of Psychiatry, University of Toronto, Ontario, Canada cDepartment of Pharmacology, University of Toronto, Ontario, Canada dInstitute of Medical Science, University of Toronto, Ontario, Canada eDepartment of Psychiatry, Veterans Affairs Palo Alto Health Care System, Palo Alto, California fDepartment of Psychiatry and Behavioral Sciences, Stanford University School of Medicine, Palo Alto, California gDepartment of Psychiatry, University of Florida College of Medicine, Gainesville
*Corresponding author: Roger S. McIntyre, MD, FRCPC, University Health Network, 399 Bathurst St, Toronto, ON, Canada, M5T 2S8 ([email protected]).
Major depressive disorder (MDD) is a common and often severe disorder associated with high rates of nonrecovery, limited treatment response, recurrence, and chronic subsyndromal or syndromal symptomatology.1 Results from patient-reported outcome studies indicate that despite achieving “symptomatic remission,” most individuals affected by MDD report decreased quality of life and impaired psychosocial and workplace function. It is amply documented that MDD is a leading cause of human disability globally and significantly debases human capital.2,3
In addition to the enduring disability associated with MDD, compelling evidence also indicates that MDD is highly associated with psychiatric and medical comorbidity.4 For example, the American Heart Association consensus statement (2015)5 identified MDD as an independent tier II risk factor for cardiovascular and atherosclerotic disease in young populations. Notwithstanding the public health priority of MDD, as well as the increasing public, academic, and policy attention given to MDD, misdiagnosis or delayed diagnosis and failure to incorporate appropriate measurement-based care are significant modifiable deficiencies in current practice. Moreover, guideline-discordant care is frequently reported in MDD, unnecessarily contributing to failure to achieve patient- and society-defined treatment outcomes in MDD.6,7
The American Psychiatric Association (APA) Practice Guideline for the Treatment of Patients with Major Depressive Disorder, Third Edition (https://www.guideline.gov/
summaries/summary/24158/Practice-guideline-for-the-treatment-of-patients-with-major-depressive-disorder-third-edition), was last updated in October 2010. The APA Practice Guideline is similar to most other international MDD guidelines insofar as it is a conflation of both evidence- and expert opinion-based recommendations.8-11 Expert opinion is informed by extensive and comprehensive experience in the assessment, diagnosis, treatment, and management of individuals with MDD. In addition, mood-disorder experts are familiar with scientific, clinical, and therapeutic developments in their subspecialty. Notwithstanding, expert opinion neither replaces nor supersedes extant empirical evidence. Moreover, expert opinion is susceptible to systematic biases, and the patient population often served by experts in the field is not necessarily representative of most individuals with MDD.
Conversely, MDD guidelines derived exclusively from randomized controlled pharmacologic trials also have significant limitations. For example, the majority of large, high-quality studies evaluating pharmacologic agents in MDD are sponsored by industry wherein the principal aim is to seek regulatory approval and/or marketing authorization by establishing efficacy superiority of an agent (with acceptable safety and tolerability risk) compared to placebo. The foregoing studies provide substantial attention to internal validity; consequently, the majority of individuals with mood disorders seen in “real-world” clinical settings are not necessarily eligible for registration trials.12 This limitation in ecological validity is a consequence of the differential emphasis placed on enhancing assay sensitivity in registration trials rather than on generalizability (ie, “external validity”).
Consequently, practicing clinicians often utilize decision support derived from MDD populations with clinical characteristics (eg, substance use disorders, suicidal ideation) divergent from patients that they encounter. Moreover, there is a paucity of adequately powered, head-to-head comparator trials addressing critical questions posed by patients, families, health care providers, and other stakeholders as to the preferred agent for a specific patient. This gap in comparative effectiveness evidence impedes the ability to accurately adjudicate important therapeutic differences between available agents. An additional concern that is raised when interpreting extant trial data is the unavailability of negative study findings.13
A further limitation is that most clinical studies evaluating pharmacologic treatments for mood disorders do not have multiple-year follow-up. Consequently, there is insufficient data informing relapse-and-recurrence prevention14 and possible effects on illness trajectory. A major limitation of clinical research in psychiatry, broadly, has been the disproportionate emphasis on symptomatic improvement with relatively less information regarding patient-reported outcomes such as quality of life and general and workplace functioning. Individuals affected by mood disorders are often seeking treatments that not only reduce their distress but also are capable of improving their overall function, self-regard, and general adaptation; unfortunately, extant research provides insufficient decision support across these critical domains.15 Mindful of the foregoing set of limitations, the guiding principle of the Florida Best Practice Psychotherapeutic Medication Guidelines (FPG) for the treatment of MDD was to adhere to the most rigorous of evidence (with limitations noted) with a pragmatic understanding that such data may not necessarily align entirely with each encountered patient.
Most treatment guidelines for MDD are written almost exclusively by experts and academics, often without any contribution from multiple stakeholders who utilize or reimburse for the treatments. For example, relatively few treatment guidelines in MDD are the result of an iterative process involving payers (ie, public, private), advocacy groups (eg, patients, families), experts in public policy, practitioners in community settings, and academics and experts. Implementation theory would posit that adoption of, and concordance with, guideline-based recommendations is optimized when all stakeholders are participants in the iterative process. A barrier to implementation of treatment guidelines broadly is the perception by stakeholders that recommendations are often overinclusive, lack sufficient specificity, and are not aligned with efforts to personalize treatments for busy clinical practice. In contradistinction to the APA Practice Guideline, as well as other guidelines in MDD (eg, Canadian Network for Mood and Anxiety Treatments [CANMAT]16), the FPG purposefully included multiple stakeholders (eg, advocacy, public health policy experts) throughout the iterative and decision-making process.

- An updated guideline informing decisions in the treatment of major depressive disorder in adults has not been available in the United States since 2010, despite the introduction of the DSM-5 (2013) and several antidepressants and adjunctive agents.
- The Florida Best Practice Psychotherapeutic Medication Guidelines provide up-to-date decision support for the safe and effective treatment of adults with major depressive disorder, with particular consideration given to chronic disease management, measurement-based care, attention to psychiatric and medical comorbidity, and newer nosologic entities (eg, mixed features).
Cognizant of the foregoing limitations in the consensus-based process previously mentioned, the overarching principle shaping the FPG was to optimize stakeholder input (eg, expert opinion, payer involvement, clinician preferences). Consequently, the FPG participants aimed to develop a set of algorithms that comport with, and reflect, the most rigorous evidence available in the published biomedical literature. Moreover, the FPG were informed by the aim to offer succinct, unambiguous, and specific recommendations.
The rationale for the Florida Medicaid Drug Therapy Management Program for Behavioral Health (MDTMP) has been described in detail elsewhere.17 Briefly, the Florida legislature authorized the development of the MDTMP, of which the aim was “to improve the quality and efficiency of the prescribing of mental health drugs, and to improve the health outcomes of Medicaid beneficiaries with a mental illness.”17(p921) The members of the MDTMP, as well as the authors of the FPG for MDD herein, were highly familiar with the existing treatment guidelines for MDD across the United States, European Union, Asia, Australia, Canada, and elsewhere.9-11,16,18 A further impetus for creating the FPG was the absence of an updated US-based MDD treatment guideline and the introduction of many mechanistically dissimilar agents for the treatment of MDD. The FPG, in contradistinction to most other guidelines, are updated biannually, providing opportunity for timely modification of treatment recommendations, informed by the most recent available evidence. The overarching goal of the FPG is to support mental health care providers (notably primary care providers) in making treatment decisions that are safe and evidence based and that maximize benefit and minimize harm to patients.
CONSENSUS PROCESS
The current iteration of the FPG for MDD reflects a biennial revision of existing FPG inaugurated in 2012, last updated in 2013 (http://media.mycme.com/documents/168/florida_best_practice_psychoth_41790.pdf). The 2015 group of stakeholders is referred to as the Florida Expert Panel and comprises nationally and internationally recognized experts in mood disorders, Florida psychiatrists in private practice or working at community mental health centers, academics, pharmacists, medical directors at managed care organizations, and obstetrician-gynecologists (the names of the meeting attendees and meeting presentations are available on the program website at www.medicaidmentalhealth.org).
The 2015 Florida Expert Panel met in Tampa, Florida, on September 25-26, 2015, to review and update the adult MDD guidelines, last completed in 2013. (The FPG for adults with bipolar disorder were last published in 2015.) The Florida Expert Panel discussed treatment evidence in the context of prior data published since the previous FPG and reached a consensus about whether to revise and adopt a particular set of guideline recommendations. The aim was to produce a final FPG representing a comprehensive, up-to-date, succinct synthesis of the extant literature, with differential emphasis given to the highest level of clinical evidence (eg, randomized controlled trials; systematic reviews as well as pooled-, meta-, and network-analyses) and expert consensus on the strength of the evidence.19
The FPG for MDD begin with general principles of practice (Table 1). Also, the FPG for MDD are organized hierarchically based on the strength of the scientific evidence for efficacy, safety, and tolerability regarding any treatment option. Level 1 treatments have compelling evidence of efficacy, as well as safety and tolerability, and are given preference over treatments with lower levels of evidence or relatively inferior safety and tolerability. The levels of evidence have been codified as follows:
- Level 1 describes initial treatment recommendations for which there is established efficacy and relative safety (based on replicated, large randomized controlled trials).
- Level 2 is based on 2 considerations: first, if drugs with established efficacy have safety concerns that should limit their use compared to level 1 agents; second, if drugs from level 1 are ineffective or not well tolerated. Compared to level 1, the data on treatment efficacy in level 2 are less robust (based on smaller randomized controlled trials, smaller effect sizes, etc) or the data on comparative safety concerns are more robust and suggest safety concerns for level 2 treatments.
- Level 3 is considered if levels 1 and 2 are ineffective or not well tolerated. Treatments at this level have limited efficacy data or more tolerability limitations than levels 1 and 2.
- Level 4 is considered if levels 1 through 3 are ineffective or not well tolerated; however, the treatments are not as well empirically supported at this time and are listed because of expert opinion or use in clinical practice.
Hierarchical levels of evidence are not a tacit statement of their algorithmic sequence. Instead, as with all guidelines for medical disorders, the FPG for MDD take into account individual patient characteristics such as prior response to treatment, patient preferences, and other aspects of care (eg, comorbidity) when selecting and sequencing treatments throughout the algorithm.
Safety is a particular focus of the FPG. Safety (as distinguished from tolerability) became a concern directly affecting recommendations in the 2014 FPG for the treatment of bipolar disorder (described therein). For example, olanzapine-fluoxetine, a US Food and Drug Administration (FDA)-approved agent for treatment-resistant depression is a level 2 recommendation. In contradistinction, aripiprazole and brexpiprazole, agents also with high-quality studies, are given priority on the basis of clinically significant weight gain and metabolic hazards associated with olanzapine. The previously published FPG for adults with bipolar disorder17 list olanzapine-fluoxetine, an FDA-approved agent for the treatment of bipolar I depression, as a level 2A recommendation.
Although a large number of first-line treatment options are available for adults with MDD, the evidentiary base informing treatment steps after failure of the first-line treatment, and beyond, is considerably less robust. Moreover, despite minimal evidence supporting the notion that pretreatment phenomenology sufficiently informs treatment selection, specific principles and treatments would be recommended if supported by the evidence. Similar to the previous FPG for bipolar disorder, the guidelines for MDD herein provide decision support and education.
The Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5) defines and provides operational criteria for up to 9 specifiers as part of a major depressive episode (MDE). The consensus among the Florida Expert Panel was that there were insufficient data supporting the notion that specifiers significantly influence treatment selection.16,18,20,21 Exceptions, however, were noted for the psychotic (mood congruent, mood incongruent) and mixed features specifiers. The consensus was that there was unequivocal evidence of illness validators, as well as response characteristics, for adults with MDD and psychosis that warrant a separate treatment algorithm when compared to MDD without psychosis.
It was also a consensus that MDD with mixed features exhibited differential illness validators, a more complex illness presentation, higher risk for suicidality, and insufficient outcome with conventional antidepressants when compared to MDD without mixed features. The foregoing consensus provided the impetus and rationale for the DSM-5 taskforce to introduce the mixed features specifier during an MDE as part of MDD. The Florida Expert Panel recognized that the evidentiary base supporting treatment options for MDD with mixed features specifier was minimal and largely comprised predominantly descriptive and uncontrolled studies, with only 1 well-controlled trial22 that we are aware of to date. Nonetheless, the differential illness trajectory (eg, increased probability of subsequent hypomanic or manic episodes) and the relatively higher probability of symptom intensification, destabilization, and unmasking of bipolar disorder warranted particular attention to the mixed features specifier. Consequently, the FPG for MDD were divided into 3 subsections: MDD without mixed features specifier and nonpsychotic, MDD with mixed features and nonpsychotic, and MDD with psychosis.
Guiding principles common to each of the 3 foregoing MDD algorithms are to assess and monitor for the presence or history of hypomania or mania, to assess and treat psychiatric and medical comorbidity, and to evaluate for the presence of psychosis, mixed features, and suicidality. Measurement-based care for assessing symptoms, side effects, and adherence is strongly recommended and has been demonstrated to significantly improve health outcome.23 Several measurement tools are available for evaluating the presence and severity of depressive symptoms (eg, Patient Health Questionnaire-9, Quick Inventory of Depressive Symptomatology-Self-Report). Safety monitoring should include ongoing surveillance of anthropometrics, metabolic parameters, and, where indicated, electrocardiographic monitoring. Clinicians are encouraged to specifically probe for and monitor tolerability concerns commonly associated with antidepressants, eg, sexual dysfunction, gastrointestinal, and insomnia. Adherence should be evaluated at each visit by probing the percentage of days that individuals have been discordant with recommended treatment.
An additional principle of practice is integration of primary and specialist health care as part of a collaborative working relationship with patients and families.24 During the initial assessment, and during the provision of ongoing care, psychosocial assessment, determination of social support systems, potential threats to continuity of (or access to) care, and the provision of tools and supplementary materials to foster self-management are encouraged. It is recognized by the Florida Expert Panel that the level of evidence supporting adjunctive exercise (ie, aerobic or resistance) as well as other lifestyle and health behavior modifications (eg, dietary modification, sleep hygiene/behavior, smoking cessation) as singular modalities of therapy does not comport with level 1 evidence.25,26 Notwithstanding, the health benefits afforded by the foregoing, as well as their beneficial effects on general well-being, health, and quality of life, warrant discussion and, in many cases, specific recommendations at patients’ first treatment visit as part of a comprehensive management plan.
The Florida Expert Panel recognized that cognitive symptoms are common during an MDE. During the past several years, a compelling body of literature27,28 has indicated that cognitive symptoms in MDD are prevalent and often persistent despite resolution of mood and neurovegetative symptoms. It is further noted that cognitive symptoms in MDD are a principal mediator of psychosocial impairment and workplace disability.29-32 The Florida Expert Panel recognized that a gold standard (eg, comprehensive, multidimensional, brief) neurocognitive measure capable of screening for cognitive symptoms as well as detecting change across time with intervention is not readily available.27 Moreover, evidence supporting the premise that screening for cognitive dysfunction in MDD moderates health outcomes is not available. Nonetheless, the Florida Expert Panel concluded that particular emphasis should be given to cognitive symptoms in MDD as part of the overall assessment.
GUIDELINES
Major Depressive Disorder
Timely and accurate diagnoses, as well as consensually agreed upon, objective, and measurable therapeutic objectives that aim for symptomatic, syndromal, and functional recovery, are emphasized.33
Level 1 (initial treatment). Available evidence would support either manual-based psychotherapy (eg, cognitive-behavioral therapy, interpersonal therapy) or monotherapy with a conventional antidepressant for mild to moderate depressive episodes (Table 2). Evidence for superior efficacy of either modality as first-line treatment is not available.34,35 The Florida Expert Panel recognizes that drug-placebo differences in antidepressant efficacy are greater in individuals with higher baseline levels of depressive symptoms. There remains an absence of compelling evidence that any single antidepressant or class is superior in efficacy to another.35
It is also recognized that higher baseline depression severity would proscribe manual-based psychotherapy as first-line treatment. Pragmatically, individuals with more severe pretreatment depression are often unable to sufficiently engage psychosocial interventions (eg, cognitive-behavioral therapy), warranting initial treatment with antidepressant therapy (a relatively new psychosocial treatment, cognitive remediation therapy, which specifically targets cognitive dysfunction, has demonstrated preliminary evidence36 of improvement in cognitive functions in adults with MDD). Moreover, individuals with psychotic features should not be offered manual-based psychotherapy as a primary treatment option.37,38 In addition, a pragmatic recommendation is that individuals with severe, nonpsychotic MDD who are not able to actively participate in psychosocial treatments should not be offered manual-based psychotherapy as a first-line and exclusive modality of treatment. The Florida Expert Panel also recognized that most conventional antidepressants have not been sufficiently studied with respect to their ability to provide independent, clinically relevant benefit on measures of cognition. Similar to comments earlier regarding the absence of head-to-head studies evaluating efficacy on conventional depression outcomes, there is minimal evidence directly comparing the relative benefits of antidepressants on measures of cognition.
Available evidence indicates that partial therapeutic improvement after 2-4 weeks warrants dose optimization or inclusion of level 2 treatment recommendations. The foregoing recommendation is supported by replicated evidence39,40 that insufficient symptomatic outcome (ie, defined specifically as less than 20% reduction in scores on depression rating scales) after approximately 2-4 weeks of antidepressant therapy has robust negative prediction of nonresponse after 8 weeks. It is also highly recommended to consider propensity for drug-drug interactions and teratogenicity throughout the algorithm.
Level 2 (if level 1 is ineffective or not well tolerated; has high-quality evidence and established safety). Nonadherence is recognized as a principal detractor to achieving therapeutic success and should be systematically assessed. At level 2, full-dose optimization should be realized with the index agent. Level 2 decisions include either switching to a different antidepressant monotherapy or combining manual-based psychotherapy or an FDA-approved second-generation antipsychotic (SGA) for MDD (ie, aripiprazole or brexpiprazole).41,42 Available evidence43,44 indicates that if beneficial effects with SGAs are not observed after 1-2 weeks, dose optimization of the SGA should be considered. Meta-analytic data45,46 indicate that SGA efficacy may be greater as a function of the number of previously failed antidepressants. The Florida Expert Panel recognizes that although combination antidepressant prescription is commonplace, the evidence base supporting the benefit of combined antidepressants that are either prescribed sequentially or coadministered at the initiation of therapy is insufficient. The evidence,47,48 however, strongly suggests that the use of multiple antidepressants concurrently is associated with higher risks of adverse events. In addition, available evidence25,26 and results of meta-analyses would also support the adjunctive inclusion of exercise-based therapy at this level.
Level 3 (if levels 1 and 2 are ineffective or not well tolerated). The Florida Expert Panel was of the view that the guiding principles of adherence and dose optimization should be revisited and considered for psychiatric consultation at this level. US Food and Drug Administration-approved SGAs for MDD with higher propensity for weight gain or metabolic dysregulation (eg, olanzapine) are recommended at this level. Tricyclic antidepressants (TCAs), monoamine oxidase inhibitor (MAOI), electroconvulsive therapy (ECT), and repetitive transcranial magnetic stimulation (rTMS) are recommended as level 3 treatments.49-51 In addition, coadministration of lithium, triiodothyronine (T3), l-methylfolate, or S-adenosylmethionine (SAMe) are recommended at this level, although the data supporting their use are much less robust than for the treatments listed above in this level. A point of emphasis is that the evidence for ECT and TMS is far superior to the evidence supporting lithium, T3, l-methylfolate, or SAMe in MDD. The rationale for including the foregoing agents at this level with neuromodulatory (eg, rTMS) options is largely in response to relative lack of access to neuromodulatory treatments and limited patient acceptability of some modalities of neuromodulation (eg, ECT).
Level 4 (if levels 1-3 are ineffective or not well tolerated). The combination of level 1-3 treatments should be considered while avoiding contraindicated combinations (eg, combination of MAOI and SSRI). Triple pharmacologic therapy, as well as other neuromodulatory approaches (eg, vagus nerve stimulation), are recommended at this level.
It is well established that at least half of individuals with MDD are susceptible to relapse and recurrence of clinically significant depressive symptoms after recovery from the index episode. Individuals with recurrence vulnerability factors would be candidates for continuation or maintenance therapy. For any individual with ongoing subsyndromal symptoms, a history of illness multiple recurrence, or a need for multiple-level treatments to achieve symptomatic, syndromal, and functional recovery, indefinite treatment may be warranted. It is also recognized that recurrence prevention in an individual responding acutely to pharmacotherapy may be achieved by the sequential administration of manual-based psychotherapies.52,53 For now, however, the most robust body of evidence would support continuation or maintenance of pharmacotherapeutic regimens (and their respective doses) that resulted in remission during the acute phase.
The Florida Expert Panel also recognizes that disparate complementary alternative medicines (CAMs) and behavioral or psychosocial interventions are often utilized by individuals with MDD. The Florida Expert Panel concluded that insufficient evidence exists at this time for most CAMs as well as psychosocial interventions (eg, yoga therapy, music therapy) other than manualized, evidence-based psychotherapies. The Florida Expert Panel also recognized the growing interest in agents that target glutamatergic systems (eg, ketamine, rapastinel). The Florida Expert Panel concluded that there was insufficient evidence to recommend ketamine for the treatment of MDD. It should be recognized that ketamine remains an investigational approach and has significant safety and tolerability concerns.54-57
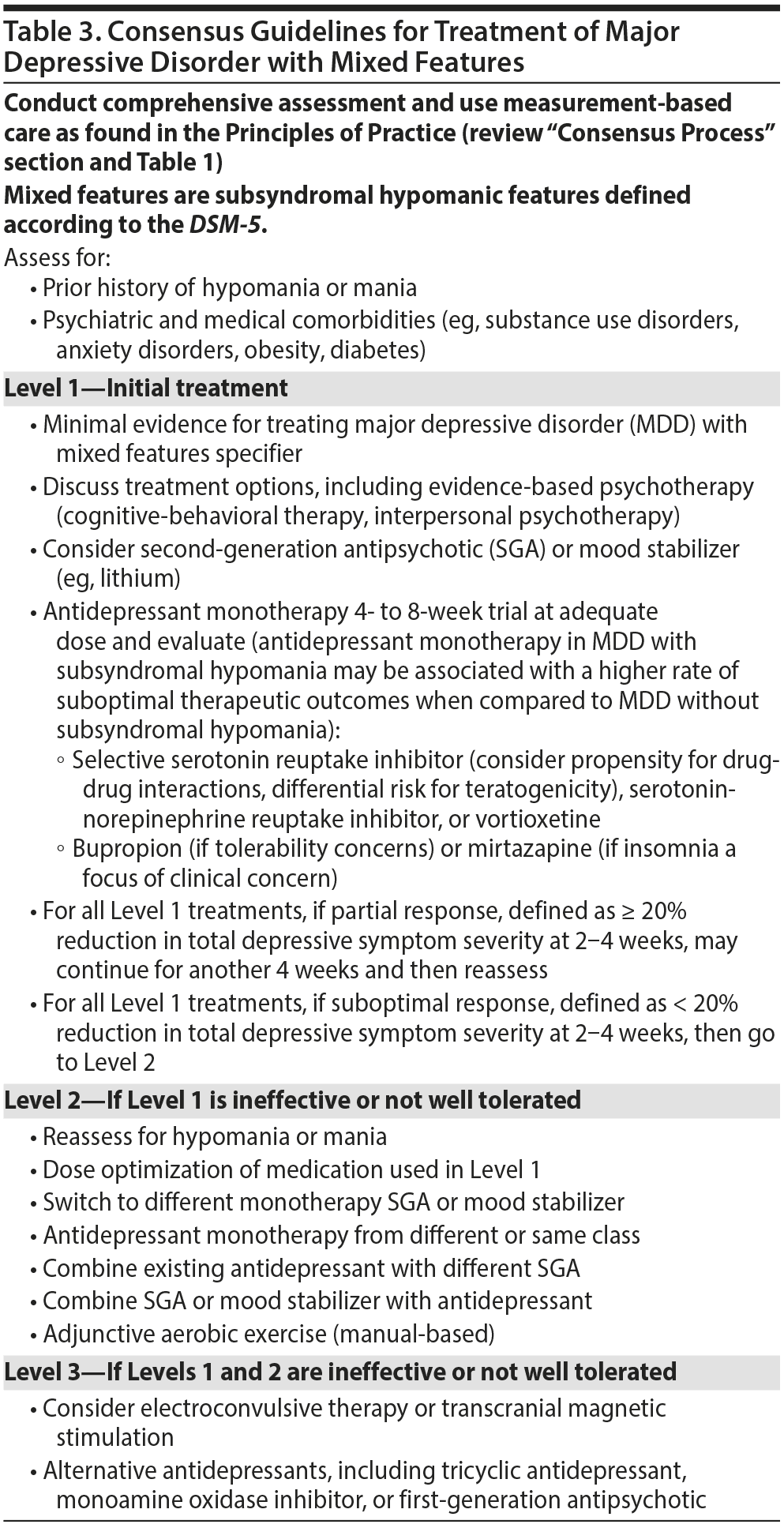
Major Depressive Disorder With Mixed Features
Mixed features (ie, subsyndromal hypomania) are common, affecting approximately 20%-40% of individuals during an MDE with no prior history of hypomanic or manic episodes.58 Careful assessment for prior history of hypomania or mania, family history of bipolar disorder, and other probabilistic features of bipolar disorder is warranted in any individual with MDD with mixed features. The Florida Expert Panel recognizes the paucity of evidence supporting treatment strategies for MDD with mixed features. Consequently, the hierarchical levels of recommendation for treating MDD with mixed features are similar to those for MDD without mixed features (Table 3).
The Florida Expert Panel also recognizes that conventional antidepressants have not been systematically studied in patients with MDD and currently depressed with DSM-5-defined mixed features specifier. The available evidence59,60 regarding antidepressants in adults with MDD and mixed features, albeit largely descriptive and uncontrolled, suggests that outcomes in MDD with mixed features may be less reliable when compared to outcomes in MDD without mixed features. Moreover, individuals with intradepressive episode subsyndromal hypomanic or manic symptoms may be at higher risk for antidepressant-induced mood destabilization; however, insufficient characterization of this risk with DSM-5-defined mixed features is a significant limitation.8,61 Notwithstanding, the Florida Expert Panel concluded that available evidence does not warrant recommendations proscribing conventional antidepressants in MDD with mixed features. In addition, the hazards posed by antidepressants in susceptible individuals with bipolar spectrum disorders invited the need to specifically alert the possibility that, for some individuals, conventional antidepressants with MDD and mixed features specifier may not be a sufficient therapeutic selection. Given the limited available data on MDE with mixed features, the Florida Expert Panel took a pragmatic position that clinicians require decision support in individuals presenting with MDD and mixed features.
A single published study62 with the SGA ziprasidone demonstrated clinically significant efficacy in mitigating depressive (and hypomanic) symptoms in a mixed population with MDD or bipolar II disorder experiencing an MDE with hypomanic symptoms. A recently published randomized controlled trial22 compared lurasidone to placebo in the treatment of adults with DSM-IV-TR-defined MDD experiencing a depressive episode and presenting with 2-3 hypomanic symptoms. Individuals meeting eligibility criteria were randomly assigned to double-blind treatment with monotherapy lurasidone 20-60 mg or placebo for up to 6 weeks. This first placebo-controlled trial in adults with MDD and subthreshold hypomanic symptoms noted a significant advantage at week 6 in total depression scores (effect size = 0.8) as well as across secondary measures (eg, Clinical Global Impressions-Severity of Illness scale, Hamilton Anxiety Rating Scale, and Sheehan Disability Scale scores). Individuals receiving lurasidone did not exhibit mood destabilization (ie, hypomanic symptom amplification), and instead overall hypomanic symptom severity (as measured by the Young Mania Rating Scale) was significantly reduced in lurasidone-treated individuals. The results of this study, however, cannot be generalized to MDD without the mixed features specifier.
Recognizing the limitations of the existing literature, as well as the need for decision support for this commonly encountered subgroup of patients, the Florida Expert Panel pragmatically concluded that SGAs and/or mood stabilizers (eg, lithium) could be considered as a level 1 or level 2 treatment in MDD with mixed features. The Florida Expert Panel would recommend a first-line agent recommended by the FPG for bipolar disorder in an individual with suspected bipolar spectrum illness.17 The Florida Expert Panel would recommend a conventional antidepressant as a first-line agent in MDD with or without mixed features. For individuals with well-circumscribed mixed features as part of MDD, SGAs, and/or mood stabilizing options should be considered alongside conventional antidepressants. The expectation is that rigorous controlled trials will provide evidence supplanting the pragmatic and clinical recommendations that currently exist in the FPG algorithm for MDD with mixed features.
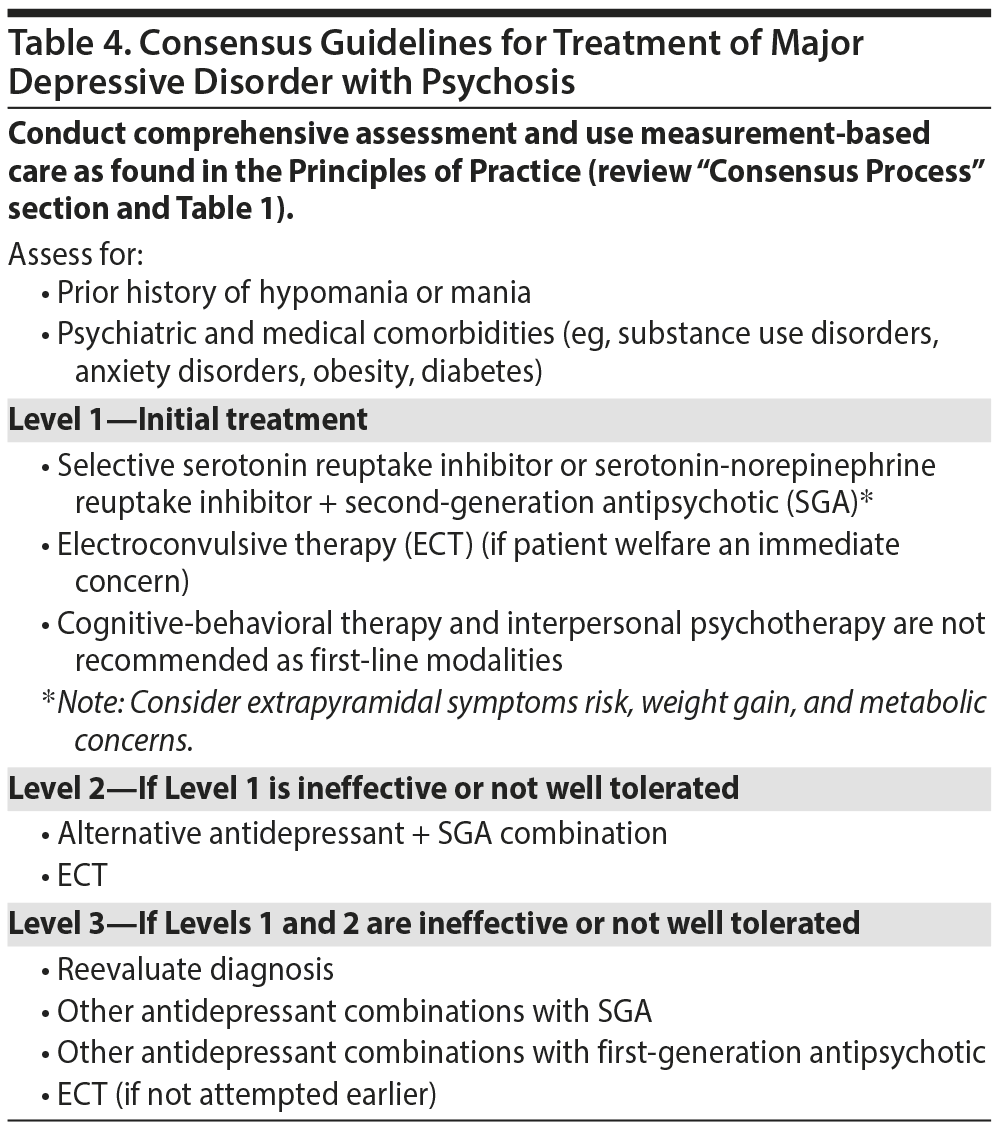
Major Depressive Disorder With Psychotic Features
It is recognized by the Florida Expert Panel that MDD with psychotic features (ie, mood congruent and mood incongruent) identifies a subgroup of individuals with a highly complex and severe illness presentation and suboptimal response to antidepressant monotherapy.63 The risk for suicidality exists in every individual with MDD; it is noted, however, that persons with MDD with psychosis are at relatively higher risk of suicidality, inviting need for careful risk assessment.
Manual-based psychotherapy is not recommended as a first-line therapy for MDD with psychotic features. The combination of a first-line antidepressant with an SGA is a strongly supported recommendation (Table 4).64 The Florida Expert Panel recognizes that there is insufficient comparative evidence of a conventional antidepressant as monotherapy to an SGA in psychotic depression. Risk for extrapyramidal side effects weight gain and metabolic abnormalities are critical considerations when selecting an SGA. Electroconvulsive therapy (ECT) is recommended as an initial treatment for MDD with psychotic features if patient welfare is an immediate concern. Second-level treatments to be considered for MDD with psychosis are an alternative antidepressant + SGA combination or ECT. Level 3 treatment recommendations are similar with consideration for alternative antidepressants.
Evidence to inform recommendations regarding the optimal duration of combination antidepressant-antipsychotic (or ECT) is insufficient. It is also not known as to whether any 1 agent within a co-therapy regimen should be discontinued during maintenance treatment and, if both treatments are discontinued, the temporality of discontinuation. The Florida Expert Panel, however, concluded that co-therapy should continue uninterrupted with periodic assessment in MDD with psychosis.
CONCLUSION
The FPG 2015 for adults with MDD provide updated decision support for practitioners assessing and providing care for adults with MDD. The FPG 2015 integrate multiple stakeholder perspectives and are not created exclusively by academics and specialist experts. The FPG aim to be simple, succinct, evidence based, and pragmatic. A barrier to implementation for many practice guidelines across multiple medical areas has been the perception that they are impractical, over-inclusive, and nonrepresentative. In addition, it is widely known by patients’ families, providers, payers, and other stakeholders that there are meaningful differences between features in tolerability and safety that would significantly impact the selection and sequencing of treatments in MDD. The FPG 2015 have endeavored to give significant consideration to both efficacy as well as tolerability and safety throughout the multiple level recommendations.
Treatment recommendations contained in the FPG 2015 herein are to be understood within the context of the principles of practice, which can be further reviewed at www.medicaidmentalhealth.org. Toward the aim of precision medicine in MDD, there has been tremendous interest in the role of pharmacogenomics informing treatment selection and improving health outcomes.65,66 The Florida Expert Panel concluded that pharmacogenetic testing may be considered on an individual basis, particularly when treatment resistance or intolerability to medications frequently occur. The Florida Expert Panel also recognizes that a need exists for replicated, sufficiently powered randomized controlled trials in order to unequivocally establish that routine pharmacogenomics improves health outcomes and is cost-effective in the treatment of MDD.67,68 The selection of antidepressant therapy for any particular individual will need to be informed by many factors, including, but not limited to, patient preference, safety and tolerability profiles, prior antidepressant utilization, potential for drug-drug interactions, cost and access, and comorbidities. It remains to be established whether any single antidepressant or class is unequivocally superior in subgroups of patients with MDD with any of the DSM-5-defined specifiers or symptomatic presentations (eg, insomnia, anxiety). Consequently, the decision to select antidepressants for such commonly encountered scenarios should be carried out on an individualized basis.
The overarching aim of the FPG 2015 is to improve health outcomes among individuals with MDD. By providing up-to-date decision support as well as concordance with principles of practice, it is believed that the probability of a satisfactory therapeutic outcome (defined by multiple stakeholders) in adults with MDD will increase. The FPG 2015 represent an update from the 2013 iteration of the FPG for adults with MDD. The FPG will continue to be updated biannually with additional planning to incorporate updates and edits on a regular ongoing basis.
Submitted: April 21, 2016; accepted September 8, 2016.
Disclosure of off-label usage: The authors have determined that, to the best of their knowledge, no investigational information about pharmaceutical agents that is outside US Food and Drug Administration-approved labeling has been presented in this article.
Financial disclosure: Within the last 2 years (2014-2016), Dr McIntyre has received research grant support from Lundbeck, JanssenOrtho, Shire, Purdue, AstraZeneca, Pfizer, Otsuka, Allergan, and Stanley Medical Research Institute and has received speaker/consultation fees from Lundbeck, Pfizer, AstraZeneca, Eli Lilly, JanssenOrtho, Purdue, Johnson & Johnson, Moksha8, Sunovion, Mitsubishi, Takeda, Forest, Otsuka, Bristol-Myers Squibb, and Shire. Within the last 2 years (2014-2016), Dr Suppes has received research funding from the National Institute of Mental Health, US Department of Veterans Affairs (VA) Cooperative Studies Program, Pathway Genomics, Elan Pharma, and Stanley Medical Research Institute; has received consulting and honorarium fees from Lundbeck, Sunovion, Medscape Education, Global Medical Education, AstraZeneca, CMEology, and Merck; and has received royalties from Jones and Bartlett and UpToDate. Dr Ostacher has been a consultant for Sunovion and Acadia. Dr Tandon has no personal affiliations or financial relationships with any commercial interest to disclose relative to the article.
Funding/support: The work was funded in part by Florida Medicaid Drug Therapy Management Program for Behavioral Health through the Florida Agency for Health Care Administration.
Role of the sponsor: The Florida Medicaid Drug Therapy Management Program for Behavioral Health through the Florida Agency for Health Care Administration had no role in data analysis, data interpretation, or writing of the report. Dr McIntyre had final responsibility for the decision to submit for publication.
Disclaimer: The contents of this article do not represent the views of the VA or the US government. The 2015 Florida Best Practice Psychotherapeutic Medication Guidelines (FPG) for adults with major depressive disorder (MDD) reflect the current state of knowledge at the time of publication on effective and appropriate care, as well as consensus judgements when research is lacking. It is anticipated that changes in scientific evidence and technology would warrant further updating and revisions, which are planned for the future. The FPG for MDD do not apply to all patients; therefore, each guideline must be adapted and tailored to each individual patient. The proper use, adaptation, modifications, or decisions to disregard these or other guidelines are the sole responsibility of the clinician who uses the guidelines.
Acknowledgments: The authors would like to acknowledge the assistance of the following people who participated in the Expert Consensus Panel that developed these guidelines: Nicholas Abid, DO, MBA, FACN—WellCare; Xenia Aponte, MD—Citrus Health Network; John Bailey, DO—Tallahassee, Florida; Don Baracskay, MD, MBA, MSCIS—SalusCare; Edmund Becker, PhD—Rollins School of Public Health at Emory University; Timothy Boaz, PhD—University of South Florida; Daniel Castellanos, MD—FIU Herbert Wertheim College of Medicine; Annie Cherian, MD—Lakeview Center, Inc; Robert Constantine, PhD—University of South Florida; Matlin Gilman—Rollins School of Public Health at Emory University; Randolph Hemsath, MD—Boley Centers; Rona Hu, MD—Stanford University School of Medicine; Mary (Beth) Jones, RPh—Agency for Healthcare Administration; Mohammad Latif-Jangda, MD—Mohammad J Latif Jangda, MD, PA; Beom Lee, PhD—University of South Florida; Rahul Mehra, MD—MehraVista Health; Gnaneswara Midathala, MD—BayCare Health System Behavioral Health Administration; Thea Moore, PharmD, BCPP—University of South Florida; David Mowere, MD—Lake Mary, Florida; Henry Nasrallah, MD (speaker)—Saint Louis University; Narendra Patel, MD, JD—Cenpatico; Bhagirathy Sahasranaman, MD—Henderson Behavioral Health; Bruce Shephard, MD, PA—Tampa, Florida; Rajiv Tandon, MD (speaker)—University of Florida; and James Zenel, MD—James A Haley Veterans Hospital. The acknowledged individuals report no conflict of interest relevant to the subject of this article.
Find more articles on this and other psychiatry and CNS topics:
The Journal of Clinical Psychiatry
The Primary Care Companion for CNS Disorders
REFERENCES
1. Judd LL, Schettler PJ, Rush AJ, et al. A new empirical definition of major depressive episode recovery and its positive impact on future course of illness. J Clin Psychiatry. 2016;77(8):1065-1073. PubMed doi:10.4088/JCP.15m09918
2. Ferrari AJ, Charlson FJ, Norman RE, et al. Burden of depressive disorders by country, sex, age, and year: findings from the global burden of disease study 2010. PLoS Med. 2013;10(11):e1001547. PubMed doi:10.1371/journal.pmed.1001547
3. Vos T, Barber RM, Bell B, et al; Global Burden of Disease Study 2013 Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 301 acute and chronic diseases and injuries in 188 countries, 1990-2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2015;386(9995):743-800. PubMed doi:10.1016/S0140-6736(15)60692-4
4. Vancampfort D, Mitchell AJ, De Hert M, et al. Type 2 diabetes in patients with major depressive disorder: a meta’ analysis of prevalence estimates and predictors. Depress Anxiety. 2015;32(10):763-773. PubMed doi:10.1002/da.22387
5. Goldstein BI, Carnethon MR, Matthews KA, et al; American Heart Association Atherosclerosis; Hypertension and Obesity in Youth Committee of the Council on Cardiovascular Disease in the Young. Major depressive disorder and bipolar disorder predispose youth to accelerated atherosclerosis and early cardiovascular disease: a scientific statement from the American Heart Association. Circulation. 2015;132(10):965-986. PubMed doi:10.1161/CIR.0000000000000229
6. Rush AJ. Isn’ t it about time to employ measurement-based care in practice? Am J Psychiatry. 2015;172(10):934-936. PubMed doi:10.1176/appi.ajp.2015.15070928
7. Harding KJK, Rush AJ, Arbuckle M, et al. Measurement-based care in psychiatric practice: a policy framework for implementation. J Clin Psychiatry. 2011;72(8):1136-1143. PubMed doi:10.4088/JCP.10r06282whi
8. Malhi GS, Bassett D, Boyce P, et al. Royal Australian and New Zealand College of Psychiatrists clinical practice guidelines for mood disorders. Aust N Z J Psychiatry. 2015;49(12):1087-1206. PubMed doi:10.1177/0004867415617657
9. Bauer M, Severus E, Köhler S, et al; WFSBP Task Force on Treatment Guidelines for Unipolar Depressive Disorders. World Federation of Societies of Biological Psychiatry (WFSBP) guidelines for biological treatment of unipolar depressive disorders, part 2: maintenance treatment of major depressive disorder-update 2015. World J Biol Psychiatry. 2015;16(2):76-95. PubMed doi:10.3109/15622975.2014.1001786
10. Cleare A, Pariante CM, Young AH, et al; Members of the Consensus Meeting. Evidence-based guidelines for treating depressive disorders with antidepressants: a revision of the 2008 British Association for Psychopharmacology guidelines. J Psychopharmacol. 2015;29(5):459-525. PubMed doi:10.1177/0269881115581093
11. Gelenberg AJ. The American Psychiatric Association treatment guideline for major depressive disorder: process and content. Ann Gen Psychiatry. 2010;9(suppl 1):s46. doi:10.1186/1744-859x-9-s1-s46
12. Zimmerman M, Clark HL, Multach MD, et al. Have treatment studies of depression become even less generalizable? a review of the inclusion and exclusion criteria used in placebo-controlled antidepressant efficacy trials published during the past 20 years. Mayo Clin Proc. 2015;90(9):1180-1186. PubMed doi:10.1016/j.mayocp.2015.06.016
13. Driessen E, Hollon SD, Bockting CL, et al. Does publication bias inflate the apparent efficacy of psychological treatment for major depressive disorder? a systematic review and meta-analysis of US. PLoS One. 2015;10(9):e0137864. PubMed doi:10.1371/journal.pone.0137864
14. Fiedorowicz JG, Endicott J, Leon AC, et al. Subthreshold hypomanic symptoms in progression from unipolar major depression to bipolar disorder. Am J Psychiatry. 2011;168(1):40-48. PubMed doi:10.1176/appi.ajp.2010.10030328
15. Zimmerman M, McGlinchey JB, Posternak MA, et al. How should remission from depression be defined? the depressed patient’s perspective. Am J Psychiatry. 2006;163(1):148-150. PubMed doi:10.1176/appi.ajp.163.1.148
16. Lam RW, Kennedy SH, Grigoriadis S, et al; Canadian Network for Mood and Anxiety Treatments (CANMAT). Canadian Network for Mood and Anxiety Treatments (CANMAT) clinical guidelines for the management of major depressive disorder in adults. III. Pharmacotherapy. J Affect Disord. 2009;117(suppl 1):S26-S43. PubMed doi:10.1016/j.jad.2009.06.041
17. Ostacher MJ, Tandon R, Suppes T. Florida Best Practice Psychotherapeutic Medication Guidelines for Adults With Bipolar Disorder: a novel, practical, patient-centered guide for clinicians. J Clin Psychiatry. 2016;77(7):920-926. PubMed doi:10.4088/JCP.15cs09841
18. Manning JS, Jackson WC. Providing guideline-concordant assessment and monitoring for major depression in primary care. J Clin Psychiatry. 2015;76(1):e3. PubMed
19. Chang SM, Bass EB, Berkman N, et al. Challenges in implementing The Institute of Medicine systematic review standards. Syst Rev. 2013;2:69. PubMed doi:10.1186/2046-4053-2-69
20. Arnow BA, Blasey C, Williams LM, et al. Depression subtypes in predicting antidepressant response: a report from the iSPOT-D Trial. Am J Psychiatry. 2015;172(8):743-750. PubMed doi:10.1176/appi.ajp.2015.14020181
21. Fava M, Rush AJ, Alpert JE, et al. Difference in treatment outcome in outpatients with anxious versus nonanxious depression: a STAR*D report. Am J Psychiatry. 2008;165(3):342-351. PubMed doi:10.1176/appi.ajp.2007.06111868
22. Suppes T, Silva R, Cucchiaro J, et al. Lurasidone for the treatment of major depressive disorder with mixed features: a randomized, double-blind, placebo-controlled study. Am J Psychiatry. 2016;173(4):400-407. PubMed doi:10.1176/appi.ajp.2015.15060770
23. Guo T, Xiang Y-T, Xiao L, et al. Measurement-based care versus standard care for major depression: a randomized controlled trial with blind raters. Am J Psychiatry. 2015;172(10):1004-1013. PubMed doi:10.1176/appi.ajp.2015.14050652
24. Coventry PA, Hudson JL, Kontopantelis E, et al. Characteristics of effective collaborative care for treatment of depression: a systematic review and meta-regression of 74 randomised controlled trials. PLoS One. 2014;9(9):e108114. PubMed doi:10.1371/journal.pone.0108114
25. Trivedi MH, Greer TL, Grannemann BD, et al. TREAD: TReatment with Exercise Augmentation for Depression: study rationale and design. Clin Trials. 2006;3(3):291-305. PubMed doi:10.1191/1740774506cn151oa
26. Mead GE, Morley W, Campbell P, et al. Exercise for depression. Cochrane Database Syst Rev. 2009;(3):CD004366. PubMed
27. McIntyre RS, Cha DS, Soczynska JK, et al. Cognitive deficits and functional outcomes in major depressive disorder: determinants, substrates, and treatment interventions. Depress Anxiety. 2013;30(6):515-527. PubMed doi:10.1002/da.22063
28. Bora E, Berk M. Theory of mind in major depressive disorder: a meta-analysis. J Affect Disord. 2016;191:49-55. PubMed doi:10.1016/j.jad.2015.11.023
29. McIntyre RS, Lee Y. Cognition in major depressive disorder: a ‘ Systemically Important Functional Index’ (SIFI). Curr Opin Psychiatry. 2016;29(1):48-55. PubMed doi:10.1097/YCO.0000000000000221
30. McIntyre RS, Soczynska JZ, Woldeyohannes HO, et al. The impact of cognitive impairment on perceived workforce performance: results from the International Mood Disorders Collaborative Project. Compr Psychiatry. 2015;56:279-282. PubMed doi:10.1016/j.comppsych.2014.08.051
31. Jaeger J, Berns S, Uzelac S, et al. Neurocognitive deficits and disability in major depressive disorder. Psychiatry Res. 2006;145(1):39-48. PubMed doi:10.1016/j.psychres.2005.11.011
32. Baune BT, Miller R, McAfoose J, et al. The role of cognitive impairment in general functioning in major depression. Psychiatry Res. 2010;176(2-3):183-189. PubMed doi:10.1016/j.psychres.2008.12.001
33. Baldessarini RJ, Lau WK, Sim J, et al. Duration of initial antidepressant treatment and subsequent relapse of major depression. J Clin Psychopharmacol. 2015;35(1):75-76. PubMed doi:10.1097/JCP.0000000000000263
34. Galling B, Calsina Ferrer A, Abi Zeid Daou M, et al. Safety and tolerability of antidepressant co-treatment in acute major depressive disorder: results from a systematic review and exploratory meta-analysis. Expert Opin Drug Saf. 2015;14(10):1587-1608. PubMed doi:10.1517/14740338.2015.1085970
35. Amick HR, Gartlehner G, Gaynes BN, et al. Comparative benefits and harms of second generation antidepressants and cognitive behavioral therapies in initial treatment of major depressive disorder: systematic review and meta-analysis. BMJ. 2015;351:h6019. PubMed doi:10.1136/bmj.h6019
36. Bowie CR, Gupta M, Holshausen K, et al. Cognitive remediation for treatment-resistant depression: effects on cognition and functioning and the role of online homework. J Nerv Ment Dis. 2013;201(8):680-685. PubMed doi:10.1097/NMD.0b013e31829c5030
37. Weitz ES, Hollon SD, Twisk J, et al. Baseline depression severity as moderator of depression outcomes between cognitive behavioral therapy vs pharmacotherapy: an individual patient data meta-analysis. JAMA Psychiatry. 2015;72(11):1102-1109. PubMed doi:10.1001/jamapsychiatry.2015.1516
38. Farahani A, Correll CU. Are antipsychotics or antidepressants needed for psychotic depression? a systematic review and meta-analysis of trials comparing antidepressant or antipsychotic monotherapy with combination treatment. J Clin Psychiatry. 2012;73(4):486-496. PubMed doi:10.4088/JCP.11r07324
39. Kudlow PA, Cha DS, McIntyre RS. Predicting treatment response in major depressive disorder: the impact of early symptomatic improvement. Can J Psychiatry. 2012;57(12):782-788. PubMed
40. Szegedi A, Jansen WT, van Willigenburg APP, et al. Early improvement in the first 2 weeks as a predictor of treatment outcome in patients with major depressive disorder: a meta-analysis including 6562 patients. J Clin Psychiatry. 2009;70(3):344-353. PubMed doi:10.4088/JCP.07m03780
41. Thase ME, Youakim JM, Skuban A, et al. Efficacy and safety of adjunctive brexpiprazole 2 mg in major depressive disorder: a phase 3, randomized, placebo-controlled study in patients with inadequate response to antidepressants. J Clin Psychiatry. 2015;76(9):1224-1231. PubMed doi:10.4088/JCP.14m09688
42. Thase ME, Youakim JM, Skuban A, et al. Adjunctive brexpiprazole 1 and 3 mg for patients with major depressive disorder following inadequate response to antidepressants: a phase 3, randomized, double-blind study. J Clin Psychiatry. 2015;76(9):1232-1240. PubMed doi:10.4088/JCP.14m09689
43. McIntyre RS, Gorwood P, Thase ME, et al. Early symptom improvement as a predictor of response to extended release quetiapine in major depressive disorder. J Clin Psychopharmacol. 2015;35(6):706-710. PubMed doi:10.1097/JCP.0000000000000416
44. Muzina DJ, Chambers JS, Camacho TA, et al. Adjunctive aripiprazole for depression: predictive value of early assessment. Am J Manag Care. 2011;17(12):793-801. PubMed
45. Zhou X, Keitner GI, Qin B, et al. Atypical antipsychotic augmentation for treatment-resistant depression: a systematic review and network meta-analysis. Int J Neuropsychopharmacol. 2015;18(11):pyv060. PubMed doi:10.1093/ijnp/pyv060
46. Wang HR, Woo YS, Ahn HS, et al. Can atypical antipsychotic augmentation reduce subsequent treatment failure more effectively among depressed patients with a higher degree of treatment resistance. Int J Neuropsychopharmacol. 2015;18(8):pyv023. PubMed doi:10.1093/ijnp/pyv023
47. Goldberg JF, Freeman MP, Balon R, et al. The American Society of Clinical Psychopharmacology survey of psychopharmacologists’ practice patterns for the treatment of mood disorders. Depress Anxiety. 2015;32(8):605-613. PubMed doi:10.1002/da.22378
48. Rush AJ, Trivedi MH, Stewart JW, et al. Combining medications to enhance depression outcomes (CO-MED): acute and long-term outcomes of a single-blind randomized study. Am J Psychiatry. 2011;168(7):689-701. PubMed doi:10.1176/appi.ajp.2011.10111645
49. Kedzior KK, Reitz SK, Azorina V, et al. Durability of the antidepressant effect of the high-frequency repetitive transcranial magnetic stimulation (rTMS) in the absence of maintenance treatment in major depression: a systematic review and meta-analysis of 16 double-blind, randomized, sham-controlled trials. Depress Anxiety. 2015;32(3):193-203. PubMed doi:10.1002/da.22339
50. Gaynes BN, Lloyd SW, Lux L, et al. Repetitive transcranial magnetic stimulation for treatment-resistant depression: a systematic review and meta-analysis. J Clin Psychiatry. 2014;75(5):477-489, quiz 489. PubMed doi:10.4088/JCP.13r08815
51. Vallejo-Torres L, Castilla I, González N, et al. Cost-effectiveness of electroconvulsive therapy compared to repetitive transcranial magnetic stimulation for treatment-resistant severe depression: a decision model. Psychol Med. 2015;45(7):1459-1470. PubMed doi:10.1017/S0033291714002554
52. Guidi J, Tomba E, Fava GA. The sequential integration of pharmacotherapy and psychotherapy in the treatment of major depressive disorder: a meta-analysis of the sequential model and a critical review of the literature. Am J Psychiatry. 2016;173(2):128-137. PubMed doi:10.1176/appi.ajp.2015.15040476
53. Meadows GN, Shawyer F, Enticott JC, et al. Mindfulness-based cognitive therapy for recurrent depression: a translational research study with 2-year follow-up. Aust N Z J Psychiatry. 2014;48(8):743-755. PubMed doi:10.1177/0004867414525841
54. Xu Y, Hackett M, Carter G, et al. Effects of low-dose and very low-dose ketamine among patients with major depression: a systematic review and meta-analysis. Int J Neuropsychopharmacol. 2016;19(4):pyv124. PubMed doi:10.1093/ijnp/pyv124
55. Newport DJ, Carpenter LL, McDonald WM, et al; APA Council of Research Task Force on Novel Biomarkers and Treatments. Ketamine and other NMDA antagonists: early clinical trials and possible mechanisms in depression. Am J Psychiatry. 2015;172(10):950-966. PubMed doi:10.1176/appi.ajp.2015.15040465
56. Schatzberg AF. A word to the wise about ketamine. Am J Psychiatry. 2014;171(3):262-264. PubMed doi:10.1176/appi.ajp.2014.13101434
57. Fond G, Loundou A, Rabu C, et al. Ketamine administration in depressive disorders: a systematic review and meta-analysis. Psychopharmacology (Berl). 2014;231(18):3663-3676. PubMed doi:10.1007/s00213-014-3664-5
58. Perugi G, Angst J, Azorin J-M, et al; BRIDGE-II-Mix Study Group. Mixed features in patients with a major depressive episode: the BRIDGE-II-MIX study. J Clin Psychiatry. 2015;76(3):e351-e358. PubMed doi:10.4088/JCP.14m09092
59. Akiskal HS, Benazzi F, Perugi G, et al. Agitated “unipolar” depression re-conceptualized as a depressive mixed state: implications for the antidepressant-suicide controversy. J Affect Disord. 2005;85(3):245-258. PubMed doi:10.1016/j.jad.2004.12.004
60. Benazzi F, Akiskal HS. Psychometric delineation of the most discriminant symptoms of depressive mixed states. Psychiatry Res. 2006;141(1):81-88. PubMed doi:10.1016/j.psychres.2005.07.024
61. Malhi GS, Lampe L, Coulston CM, et al. Mixed state discrimination: a DSM problem that won’ t go away? J Affect Disord. 2014;158:8-10. PubMed doi:10.1016/j.jad.2014.01.008
62. Patkar A, Gilmer W, Pae C-U, et al. A 6 week randomized double-blind placebo-controlled trial of ziprasidone for the acute depressive mixed state. PLoS One. 2012;7(4):e34757. PubMed doi:10.1371/journal.pone.0034757
63. Mitchell PB, Wilhelm K, Parker G, et al. The clinical features of bipolar depression: a comparison with matched major depressive disorder patients. J Clin Psychiatry. 2001;62(3):212-216, quiz 217. PubMed doi:10.4088/JCP.v62n0314a
64. Meyers BS, Flint AJ, Rothschild AJ, et al; STOP-PD Group. A double-blind randomized controlled trial of olanzapine plus sertraline vs olanzapine plus placebo for psychotic depression: the study of pharmacotherapy of psychotic depression (STOP-PD). Arch Gen Psychiatry. 2009;66(8):838-847. PubMed doi:10.1001/archgenpsychiatry.2009.79
65. Etkin A, Patenaude B, Song YJC, et al. A cognitive-emotional biomarker for predicting remission with antidepressant medications: a report from the iSPOT-D trial. Neuropsychopharmacology. 2015;40(6):1332-1342. PubMed doi:10.1038/npp.2014.333
66. Schatzberg AF, DeBattista C, Lazzeroni LC, et al. ABCB1 genetic effects on antidepressant outcomes: a report from the iSPOT-D Trial. Am J Psychiatry. 2015;172(8):751-759. PubMed doi:10.1176/appi.ajp.2015.14050680
67. Dubovsky SL. The usefulness of genotyping cytochrome P450 enzymes in the treatment of depression. Expert Opin Drug Metab Toxicol. 2015;11(3):369-379. PubMed doi:10.1517/17425255.2015.998996
68. Berm EJJ, Hak E, Postma M, et al. Effects and cost-effectiveness of pharmacogenetic screening for CYP2D6 among older adults starting therapy with nortriptyline or venlafaxine: study protocol for a pragmatic randomized controlled trial (CYSCEtrial). Trials. 2015;16:37. PubMed doi:10.1186/s13063-015-0561-0
This PDF is free for all visitors!
Save
Cite