See more Academic Highlights in this series: Part 1 | Part 2
This Academic Highlights section of The Journal of Clinical Psychiatry presents the highlights of the virtual roundtable “Patient Functioning and Life Engagement: Unmet Needs in MDD and Schizophrenia,” which was held May 10, 2022.
The roundtable was chaired by Christoph U. Correll, MD, Zucker School of Medicine at Hofstra/Northwell, Hempstead, New York, and Charité Universitätsmedizin, Berlin, Germany. The faculty were Zahinoor Ismail, MD, Department of Psychiatry, Hotchkiss Brain Institute, Cumming School of Medicine, University of Calgary, Calgary, Alberta, Canada; Roger S. McIntyre, MD, Mood Disorder Psychopharmacology Unit, University Health Network, Department of Psychiatry, University of Toronto; Institute of Medical Science, University of Toronto; and Departments of Psychiatry and Pharmacology, University of Toronto, Toronto, Ontario, Canada; Roueen Rafeyan, MD, Department of Psychiatry, Feinberg School of Medicine, Northwestern University, Chicago, Illinois; and Michael E. Thase, MD, Department of Psychiatry, Perelman School of Medicine of the University of Pennsylvania, and the Corporal Michael J. Crescenz Veterans Affairs Medical Center, Philadelphia, Pennsylvania.
Financial disclosures: Please refer to the first Academic Highlights in this series: Correll CU, Ismail Z, McIntyre RS, et al. Patient functioning and life engagement: unmet needs in major depressive disorder and schizophrenia. J Clin Psychiatry. 2022;83(4):LU21112AH1 (https://doi.org/10.4088/JCP.LU21112AH1)
This evidence-based peer-reviewed Academic Highlights was prepared by Healthcare Global Village, Inc. Financial support for preparation and dissemination of this Academic Highlights was provided by H. Lundbeck A/S and Otsuka Product Development and Commercialization. The faculty acknowledges Sarah Brownd, MA, ELS, for editorial assistance in developing the manuscript. The opinions expressed herein are those of the faculty and do not necessarily reflect the views of Healthcare Global Village, Inc., the publisher, or the commercial supporters. This article is distributed by H. Lundbeck A/S and Otsuka Product Development and Commercialization for educational purposes only.
J Clin Psychiatry 2022;83(5):LU21112AH3
To cite: Correll CU, Ismail Z, McIntyre RS, et al. Patient functioning, life engagement, and treatment goals in major depressive disorder. J Clin Psychiatry. 2022;83(5):LU21112AH3.
To share: https://doi.org/10.4088/JCP.LU21112AH3
© 2022 Physicians Postgraduate Press, Inc.
Outcome measures in major depressive disorder have traditionally focused on characteristic symptoms included in the DSM-51 definition of major depressive disorder (MDD): depressed mood, lack of interest or pleasure in activities, fatigue, guilt, and change in weight/appetite. Addressing these symptoms is clearly a treatment priority, especially in the acute stages of the treatment process. However, patients express that, beyond symptom relief, they have additional goals: restoration of functioning and of the feeling that one can participate in, and engage with, their own life. Life engagement encompasses aspects of life experience relating to cognition (including cognition colored by emotion), vitality, motivation and reward, and the ability to feel pleasure.2
In a recent roundtable meeting, a panel of 5 experts discussed life engagement and its relationship to symptoms and functioning in patients with major depressive disorder and schizophrenia. This Academic Highlights, part 3 in a series, summarizes the experts’ discussion of how life engagement can be integrated into patient-centered discussions of treatment goals, as well as how it can inform treatment selection for those with MDD.
INCORPORATING THE PATIENT’S PERSPECTIVE WHEN PLANNING TREATMENT
According to Dr McIntyre, clinicians should involve patients with MDD in the treatment planning process; first, because they want to be involved, and second, because the patient is the one living with the illness. The patient should be the one prioritizing the therapeutic objectives, and it is not for the clinician to impose their objectives on the person living with the condition. Dr Ismail agreed:
Dr Thase pointed out that such discussions can provide valuable context regarding the patient’s past level of engagement and functioning. Further, involving the patient in planning can augment the sense of agency that is often compromised in people with MDD. Shared decision making in patients with depression has been shown to improve treatment adherence and patient satisfaction, with no increase in consultation time.3
A survey of patients with MDD found that 42% had goals for treatment.4 Frequently cited goals included aspects of life engagement such as cognitive function (60%), especially memory, and interpersonal aspects of life (57.8%), particularly social engagement. Dr McIntyre encouraged clinicians to set concrete SMART (Specific, Measurable, Attainable, Relevant, and Time-bound) goals with their patients, saying that they “are time-savers, and they don’t disappoint.” He relayed that in his practice, such conversations have provided fertile ground to hear, in the patient’s own language, what engagement means to them and how it fits into their objectives.
ASSESSING LIFE ENGAGEMENT IN PATIENTS WITH MDD
The movement in clinical research toward outcomes that focus on the patient’s perspective has created a need for valid, reliable patient-reported outcome measures5 that assess outcomes valued highly by patients, such as life engagement. Life engagement reflects an outcome that overlaps with, but is not fully captured by, other outcomes in mental health. It entails the improvement of anhedonia and apathy, but these alone do not fully capture aspects of life engagement relating to attention and alertness.6,7 Likewise, quality of life measures do not capture the energy and alertness aspects of life engagement.7 Dr Thase explained,
Dr Correll summarized the importance of developing a measure specific to life engagement: “We only recognize what we see, and we only see what we look for, and to look for something we need to conceptualize it.” A systematic review7 identified only 2 existing validated measures described by their authors as assessing life engagement: the Engaged Living Scale8 and Life Engagement Test.9 However, these scales do not capture all of the essential areas of life engagement according to a validated framework developed with expert psychiatrists and validated via interviews with patients with MDD.6
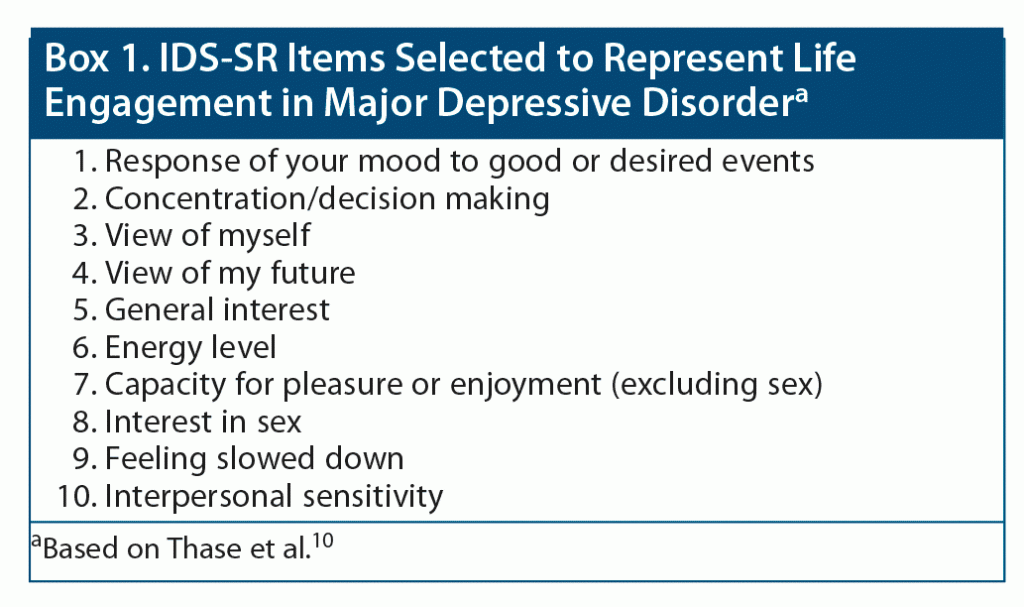
In the absence of a scale to assess life engagement in patients with MDD, an expert panel10 selected 10 items that represent patient well-being and life engagement from the Inventory of Depressive Symptomatology Self-Report (IDS-SR),11 a patient-reported outcome measure that evaluates core depressive symptoms and atypical and melancholic symptom features of MDD. The IDS-SR was included in the phase 3 randomized controlled trials of adjunctive brexpiprazole in MDD, and the expert panelists’ selection of the life engagement items was inspired by feedback from patients who participated in those trials.12–14 Items selected from the IDS-SR to represent life engagement are listed in Box 1.
To confirm that the 10 items in the IDS-SR10 Life Engagement subscale reflected patients’ views of life engagement, and to support the use of the scale in clinical research, interviews were conducted of 20 patients with MDD.15 They found life engagement, as described in the interview, to be an important outcome, and 19 of 20 agreed that all selected IDS-SR items were relevant to life engagement.
Dr McIntyre stated that a scale measuring life engagement should be sensitive to changes in illness severity and that the IDS-SR subscale accomplishes this. He noted that he includes measures of depression, anxiety, functioning, and engagement into his practice and that the IDS-SR10 subscale (as well as a life engagement subscale developed from the Positive and Negative Syndrome Scale for use in schizophrenia16) would be brief, actionable, and cost-efficient to implement at the point of care.
PATIENT FACTORS AFFECTING LIFE ENGAGEMENT
Dr Correll asked if there are any caveats to including life engagement as a treatment goal. Dr McIntyre said that in his experience, engagement is “compartmentalized” for some patients. They would like to engage with a particular part of their life but may not be able to do so at the moment, owing perhaps to psychodynamic reasons or trauma; however, engagement in that area might be revisited later. For these people, a wholesale “engagement” objective might not be appropriate.
Baseline level of engagement could also impact achievement of engagement goals. Dr Thase noted, “If you’ve never been a joyous, libidinous, engaged person, your chances to become one at age 30 or 35 are diminished. It’s harder to be better than your premorbid self, unless you’ve identified another functional characteristic of your premorbid self that might be treatable. Given that caveat though…very few people will tell us that they meet that restrictive category. Most people have experienced a better life before they became ill, and we should clarify what that better life entails.”
LIFE ENGAGEMENT AND CURRENT TREATMENT OPTIONS
Life Engagement and Treatment Selection in MDD
Dr Thase said that he believes “the pathways to pleasure involve dopamine, and to a lesser extent norepinephrine” and so, from one perspective, the antidepressant with the most life-affirming therapeutic profile might be bupropion. The positive effect of dopaminergic agents on quality of life is quite likely driven by improvement of interest, pleasure, energy, and sexual and cognitive functioning and reduction of fatigue.17 Dr Thase emphasized, though, that the first order of business is to get the person better and out of the current episode. If a particular medication has helped in the past, then that should drive the medication choice, and treatment can be fine-tuned later. Dr McIntyre picked up the focus on individual patients’ needs:
Augmentation Strategies
Many patients have inadequate response to antidepressant monotherapy,18 and augmentation strategies appear to be more beneficial than switching within or between classes of antidepressants.19 Dr Thase said that among patients with inadequate response to antidepressants, lack of motivation is a common non-core symptom and that, for such patients, improving life engagement “has less to do with the first choice of treatment than perhaps the second or third choice of treatment.” Symptoms of low life engagement, such as anhedonia, are frequent drivers of adjunctive antipsychotic prescription in MDD.20
Dr Thase noted that not all antidepressants or adjunctive strategies are equal at the individual-patient level. Further, he said, of the available augmentation therapies, dopamine partial agonists have the most life-affirming effects for people with difficult-to-treat depression. Of the 3 medications in this class, aripiprazole and brexpiprazole are FDA-approved for augmentation in MDD. Dr McIntyre noted that although the evidence is mixed regarding efficacy of cariprazine as augmentation in MDD,21,22 its effects on hedonic tone and possibly cognition are of interest in terms of life engagement.
Brexpiprazole is the dopamine partial agonist that has been studied most with regard to life engagement. Data from clinical trial exit interviews suggested that patients were more engaged with life and calmer after treatment with adjunctive brexpiprazole compared to the pretreatment baseline, with 88.6% of patients describing improvements consistent with at least 1, and most commonly 2 or 3, of the 4 domains of life engagement (emotional, physical, social, and cognitive).6 Analyses of clinical trials data based on the IDS-SR10 Life Engagement subscale also indicated a benefit for adjunctive brexpiprazole, with improvement on items reflecting “hot” cognition, motivation, energy, capacity for pleasure, and subjective well-being.10
 PATIENT PERSPECTIVES
PATIENT PERSPECTIVES
Improved engagement with life after brexpiprazole treatment in patients with MDD or schizophrenia was reflected in spontaneous calls made to the drug manufacturer’s call center10:
“I felt like myself again”
“Like a light switch turned on and I had my old life back”
“It’s like sunshine“
“I’ve been feeling pretty awesome; I’ve completed more projects in the last two weeks than I have in the last 5 years because I have been feeling better”
Level of patient functioning. For some antidepressants, patients with MDD who have better functioning at baseline have better outcomes, with low functioning predicting diminished antidepressant response.23,24 However, Dr McIntyre said, that might not be true for other treatments, especially in people who have the ability to improve aspects of engagement during treatment. Data from 3 studies revealed that improvement in life engagement seen with adjunctive brexpiprazole was greatest among patients with MDD who have lower functioning at baseline.25 Dr McIntyre noted that other atypical antipsychotics could be investigated in this regard as well.
Side effects. Augmentation of antidepressants with atypical antipsychotics is more effective versus placebo for remission (odds ratio = 2.00),26 but it is also associated with discontinuation due to adverse events.27 Patients should be made aware of potential side effects and be encouraged to report them. With regard to life engagement, Dr Thase commented, “It’s hard to be engaged when you’re sleepy, and it’s hard to be amorous when you can’t have an orgasm…the side effect profiles of some medications do affect what might be possible with that medication.”
Of the agents approved by the FDA as adjunctive treatments for MDD, quetiapine extended release and olanzapine-fluoxetine combination are associated with weight gain and sedating side effects (which, the experts noted, could interfere with life engagement), and aripiprazole is associated with activating side effects.28 Aside from apathy and amotivation, symptoms of overactivation (agitation, hostility, irritability) are also key drivers for antipsychotic use in MDD,20 so clinicians should keep activation-related side effects in mind when considering treatment options.
Psychosocial and Other Nonpharmacologic Interventions
Dr Rafeyan emphasized the importance of encouraging patients to follow appropriate diets and exercise as much as possible. That by itself, he noted, can have a tremendous impact on improvement of symptoms. Picking up this point, Dr McIntyre stated that building exercise into the treatment plan may enhance social function and combat the “pandemic” of loneliness seen in recent years. Normalizing sleep hygiene is important too; he pointed out that “it’s hard to be engaged if you’re exhausted.”
The panelists endorsed combining psychotherapy with medication. As Dr McIntyre put it,
He summarized the strategy: “We should aim for rational multimodality treatments that not only bring about remission on a rating scale but also achieve the engagement goals that patients prioritize.”
Published online: September 7, 2022.
References (28)

- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders: DSM-5. Fifth Edition. American Psychiatric Association; 2013.
- Bartrés-Faz D, Cattaneo G, Solana J, et al. Meaning in life: resilience beyond reserve. Alzheimers Res Ther. 2018;10(1):47. PubMed CrossRef
- Loh A, Simon D, Wills CE, et al. The effects of a shared decision-making intervention in primary care of depression: a cluster-randomized controlled trial. Patient Educ Couns. 2007;67(3):324–332. PubMed CrossRef
- McNaughton EC, Curran C, Granskie J, et al. Patient attitudes toward and goals for MDD treatment: a survey study. Patient Prefer Adherence. 2019;13:959–967. PubMed CrossRef
- Weldring T, Smith SMS. Patient-reported outcomes (PROs) and patient-reported outcome measures (PROMs). Health Serv Insights. 2013;6:61–68. PubMed CrossRef
- Weiss C, Meehan SR, Brown TM, et al. Effects of adjunctive brexpiprazole on calmness and life engagement in major depressive disorder: post hoc analysis of patient-reported outcomes from clinical trial exit interviews. J Patient Rep Outcomes. 2021;5(1):128. PubMed CrossRef
- McIntyre RS, Ismail Z, Watling CP, et al. Patient-reported outcome measures for life engagement in mental health: a systematic review. J Patient Rep Outcomes. 2022;6(1):62. PubMed CrossRef
- Trompetter HR, Ten Klooster PM, Schreurs KMG, et al. Measuring values and committed action with the Engaged Living Scale (ELS): psychometric evaluation in a nonclinical sample and a chronic pain sample. Psychol Assess. 2013;25(4):1235–1246. PubMed CrossRef
- Scheier MF, Wrosch C, Baum A, et al. The Life Engagement Test: assessing purpose in life. J Behav Med. 2006;29(3):291–298. PubMed CrossRef
- Thase ME, Pedersen A, Ismail Z, et al. Efficacy of adjunctive brexpiprazole in adults with MDD: improvement of patient engagement based on selected items from the Inventory of Depressive Symptomatology Self-Report (IDS-SR) scale. Presented at the 32nd Annual Psych Congress. October 3, 2019; San Diego, CA. https://www.hmpgloballearningnetwork.com/site/pcn/posters/efficacy-adjunctive-brexpiprazole-adults-mdd-improvement-patient-engagement-based-selected. Accessed March 11, 2022.
- Rush AJ, Gullion CM, Basco MR, et al. The Inventory of Depressive Symptomatology (IDS): psychometric properties. Psychol Med. 1996;26(3):477–486. PubMed CrossRef
- Thase ME, Youakim JM, Skuban A, et al. Efficacy and safety of adjunctive brexpiprazole 2 mg in major depressive disorder: a phase 3, randomized, placebo-controlled study in patients with inadequate response to antidepressants. J Clin Psychiatry. 2015;76(9):1224–1231. PubMed CrossRef
- Hobart M, Skuban A, Zhang P, et al. A randomized, placebo-controlled study of the efficacy and safety of fixed-dose brexpiprazole 2 mg/d as adjunctive treatment of adults with major depressive disorder. Psychiatrist.com website. https://www.psychiatrist.com/JCP/article/Pages/2018/v79/17m12058.aspx. Published online May 22, 2018. Accessed March 11, 2022.
- Thase ME, Youakim JM, Skuban A, et al. Adjunctive brexpiprazole 1 and 3 mg for patients with major depressive disorder following inadequate response to antidepressants: a phase 3, randomized, double-blind study. J Clin Psychiatry. 2015;76(9):1232–1240. PubMed CrossRef
- Therrien F, MacKenzie E, Brown M. Patient confirmation of a self-reported assessment of life engagement in major depressive disorder. Presented at the 17th International Society for CNS Clinical Trials and Methodology (ISCTM). April 6, 2021.
- Ismail Z, Pedersen AM, Thase ME, et al. Effect of brexpiprazole on engagement in patients with schizophrenia: post-hoc analysis of three studies. Schizophr Bull. 2020;46(suppl 1):S208–S209. CrossRef
- IsHak WW, Davis M, Jeffrey J, et al. The role of dopaminergic agents in improving quality of life in major depressive disorder. Curr Psychiatry Rep. 2009;11(6):503–508. PubMed CrossRef
- Möller HJ. Outcomes in major depressive disorder: the evolving concept of remission and its implications for treatment. World J Biol Psychiatry. 2008;9(2):102–114. PubMed CrossRef
Philip NS, Carpenter LL, Tyrka AR, et al. Pharmacologic approaches to treatment resistant depression: a re-examination for the modern era. Expert Opin Pharmacother. 2010;11(5):709–722. PubMed CrossRef
- McIntyre RS, Weiller E. Real-world determinants of adjunctive antipsychotic prescribing for patients with major depressive disorder and inadequate response to antidepressants: a case review study. Adv Ther. 2015;32(5):429–444. PubMed CrossRef
Earley WR, Guo H, Németh G, et al. Cariprazine augmentation to antidepressant therapy in major depressive disorder: results of a randomized, double-blind, placebo-controlled trial. Psychopharmacol Bull. 2018;48(4):62–80. PubMed
- Durgam S, Earley W, Guo H, et al. Efficacy and safety of adjunctive cariprazine in inadequate responders to antidepressants: a randomized, double-blind, placebo-controlled study in adult patients with major depressive disorder. J Clin Psychiatry. 2016;77(3):371–378. PubMed CrossRef
- McIntyre RS, Florea I, Tonnoir B, et al. Efficacy of vortioxetine on cognitive functioning in working patients with major depressive disorder. J Clin Psychiatry. 2017;78(1):115–121. PubMed CrossRef
- Trivedi MH, Rush AJ, Wisniewski SR, et al; STAR*D Study Team. Evaluation of outcomes with citalopram for depression using measurement-based care in STAR*D: implications for clinical practice. Am J Psychiatry. 2006;163(1):28–40. PubMed CrossRef
- McIntyre RS, Wang F, Chen D, et al. The influence of baseline functioning on life engagement outcomes: post hoc analysis of three brexpiprazole studies in major depressive disorder. Presented at the 34th ECNP Congress. October 2, 2021.
- Nelson JC, Papakostas GI. Atypical antipsychotic augmentation in major depressive disorder: a meta-analysis of placebo-controlled randomized trials. Focus. 2010;8(4):570–582. CrossRef
- Fleurence R, Williamson R, Jing Y, et al. A systematic review of augmentation strategies for patients with major depressive disorder. Psychopharmacol Bull. 2009;42(3):57–90. PubMed
- Citrome L. Activating and sedating adverse effects of second-generation antipsychotics in the treatment of schizophrenia and major depressive disorder: absolute risk increase and number needed to harm. J Clin Psychopharmacol. 2017;37(2):138–147. PubMed CrossRef
This PDF is free for all visitors!
Save
Cite