Gabapentin, available as gabapentin and as the prodrug gabapentin enacarbil, is an approved treatment for partial seizures, postherpetic neuralgia, and the restless legs syndrome. Gabapentin has been studied for diverse off-label indications, including alcohol use disorder (AUD). Meta-analyses of randomized controlled trials (RCTs) suggest that gabapentin reduces the severity of alcohol withdrawal symptoms (AWS) as well as the percentage of heavy drinking days in persons with AUD; however, the magnitude of benefit is small, and no benefits are apparent for other drinking outcomes. Furthermore, a recent, large RCT found an extended-release formulation of gabapentin enacarbil ineffective for a wide range of drinking and other outcomes in patients with AUD. Some research suggests that gabapentin may improve drinking outcomes specifically in AUD patients with higher levels of AWS; this may be a result of gabapentin-associated reduction in AWS, precluding AWS-triggered continued drinking. In this context, a recent, large RCT found that gabapentin reduced heavy drinking and increased abstinence, and that these findings were apparent only in patients with higher levels of AWS during the 2 weeks before randomization; disconcertingly, gabapentin appeared to worsen drinking outcomes in the patients with low AWS. Whereas these findings support the conjecture that gabapentin could be considered indicated in AUD patients with high AWS, problems with this RCT and with its findings limit the applicability of the findings to everyday clinical practice. These problems are discussed in detail. It is concluded that, in line with the recommendations of a recent treatment guideline, gabapentin may be considered for patients with AUD only if first line drugs such as naltrexone and acamprosate cannot be used. It may also be worth examining benefits with gabapentin in AUD associated with chronic pain, anxiety, and chronic insomnia because gabapentin is suggested to attenuate these syndromes.

ABSTRACT
Gabapentin, available as gabapentin and as the prodrug gabapentin enacarbil, is an approved treatment for partial seizures, postherpetic neuralgia, and the restless legs syndrome. Gabapentin has been studied for diverse off-label indications, including alcohol use disorder (AUD). Meta-analyses of randomized controlled trials (RCTs) suggest that gabapentin reduces the severity of alcohol withdrawal symptoms (AWS) as well as the percentage of heavy drinking days in persons with AUD; however, the magnitude of benefit is small, and no benefits are apparent for other drinking outcomes. Furthermore, a recent, large RCT found an extended-release formulation of gabapentin enacarbil ineffective for a wide range of drinking and other outcomes in patients with AUD. Some research suggests that gabapentin may improve drinking outcomes specifically in AUD patients with higher levels of AWS; this may be a result of gabapentin-associated reduction in AWS, precluding AWS-triggered continued drinking. In this context, a recent, large RCT found that gabapentin reduced heavy drinking and increased abstinence, and that these findings were apparent only in patients with higher levels of AWS during the 2 weeks before randomization; disconcertingly, gabapentin appeared to worsen drinking outcomes in the patients with low AWS. Whereas these findings support the conjecture that gabapentin could be considered indicated in AUD patients with high AWS, problems with this RCT and with its findings limit the applicability of the findings to everyday clinical practice. These problems are discussed in detail. It is concluded that, in line with the recommendations of a recent treatment guideline, gabapentin may be considered for patients with AUD only if first line drugs such as naltrexone and acamprosate cannot be used. It may also be worth examining benefits with gabapentin in AUD associated with chronic pain, anxiety, and chronic insomnia because gabapentin is suggested to attenuate these syndromes.
J Clin Psychiatry 2020;81(6):20f13775
To cite: Andrade C. Gabapentin for alcohol-related disorders: critical appraisal of the symptom-driven approach. J Clin Psychiatry. 2020;81(6):20f13775.
To share: https://doi.org/10.4088/JCP.20f13775
© Copyright 2020 Physicians Postgraduate Press, Inc.
An earlier article in this column examined a possible role for individualized, high-dose baclofen for reduction in alcohol consumption in a specific population of persons with alcohol use disorder (AUD): those with high levels of alcohol intake.1 This article examines a possible role for gabapentin for reduction in alcohol consumption in an even more specific population of persons with AUD: those with high levels of alcohol intake and who also display high levels of alcohol withdrawal symptoms.
Gabapentin is an approved treatment for partial seizures and for postherpetic neuralgia.2 Gabapentin enacarbil, a prodrug of gabapentin, is better absorbed than gabapentin and has a longer duration of action3; this prodrug drug has been approved for the treatment of restless legs syndrome and postherpetic neuralgia.2 Gabapentin has also been studied for a number of off-label indications, including anxiety disorders,4,5 acute mania,6 bipolar disorder (as maintenance therapy),7 migraine prophylaxis,8 nausea and vomiting,9 fibromyalgia,10 chronic neuropathic pain including painful diabetic peripheral neuropathy,11 postoperative pain,12 and alcohol-related disorders.13
Gabapentin for Alcohol-Related Disorders
Gabapentin acts on the α-2 δ-1 subunit of voltage-gated calcium channels; there are downstream effects on GABAergic and glutamatergic signaling.14-16 These actions may be therapeutically beneficial in alcohol-related disorders, especially during alcohol withdrawal. However, the research findings have generally been disappointing. In this context, a systematic review and meta-analysis of randomized controlled trials (RCTs) found that gabapentin significantly reduced the percentage of heavy drinking days (7 RCTs) but not the abstinence rate (6 RCTs), rate of relapse into heavy drinking (6 RCTs), percentage of days abstinent (4 RCTs), number of drinks per day (5 RCTs), and γ-glutamyltransferase concentration (4 RCTs).17 Another meta-analysis18 found that gabapentin significantly reduced alcohol withdrawal symptoms (AWS), but the effect size was small (Hedges g, 0.25).
In one of the largest studies on the subject, Falk et al19 examined the safety and efficacy of gabapentin enacarbil extended-release (GEXR) in patients with AUD. The sample comprised 346 subjects with AUD that was at least moderately severe, recruited at 10 sites in the US. These subjects were randomized to receive GEXR (600 mg twice daily) or placebo for 6 months. All subjects also received a computerized behavioral intervention. The primary outcome was the percentage of subjects with no heavy drinking days during the last 4 weeks of the study. The authors found that this outcome did not differ significantly between GEXR and placebo groups (28.3% vs 21.5%). GEXR did not outperform placebo on other measures, either, including the percentage of subjects abstinent, the percentage of days abstinent, the percentage of heavy drinking days, the number of drinks per week, the number of drinks per drinking day, alcohol craving, alcohol-related consequences, problems with sleeping, smoking, and symptoms of anxiety and depression.
A Symptom-Driven Approach
Genetic information may drive a personalized approach to pharmacotherapy in psychiatry20; however, psychiatrists more commonly use a symptom-driven approach, such as favoring an antidepressant with sedating properties in depressed patients with insomnia. In the context of AUD, studies conducted by a single team of authors suggested that gabapentin might be specifically useful in AUD patients who experience severe withdrawal.
In an early, small (n = 60) study, Anton et al21 showed that AWS in alcohol-dependent subjects were attenuated by gabapentin dosed at up to 1,200 mg nightly for 39 days. Importantly, gabapentin was superior to placebo only in subjects with high levels of AWS; this advantage for gabapentin was observed for both the primary outcomes: the percentage of days abstinent and the time to first heavy drinking during the treatment period.
In a later, larger (n = 150) study, these authors22 found that a combination of gabapentin (up to 1,200 mg/d) and naltrexone (50 mg/d) was superior to naltrexone alone (50 mg/d) and double placebo; the advantage was variously observed for time to heavy drinking, number of heavy drinking days, and drinks per drinking day. However, the advantage did not persist beyond the first 6 weeks. The combined treatment was particularly beneficial in subjects with a history of alcohol withdrawal. The authors concluded that their findings encouraged the study of the benefits of gabapentin monotherapy in alcohol-dependent persons with severe current or past withdrawal symptomatology. This approach is in principle promising because patients who experience AWS during attempts to stop drinking may resume drinking in order to reduce the discomfort of withdrawal; as already reviewed in the previous section, gabapentin may be useful here because it reduces the severity of AWS.
Use in Patients With Withdrawal Symptoms
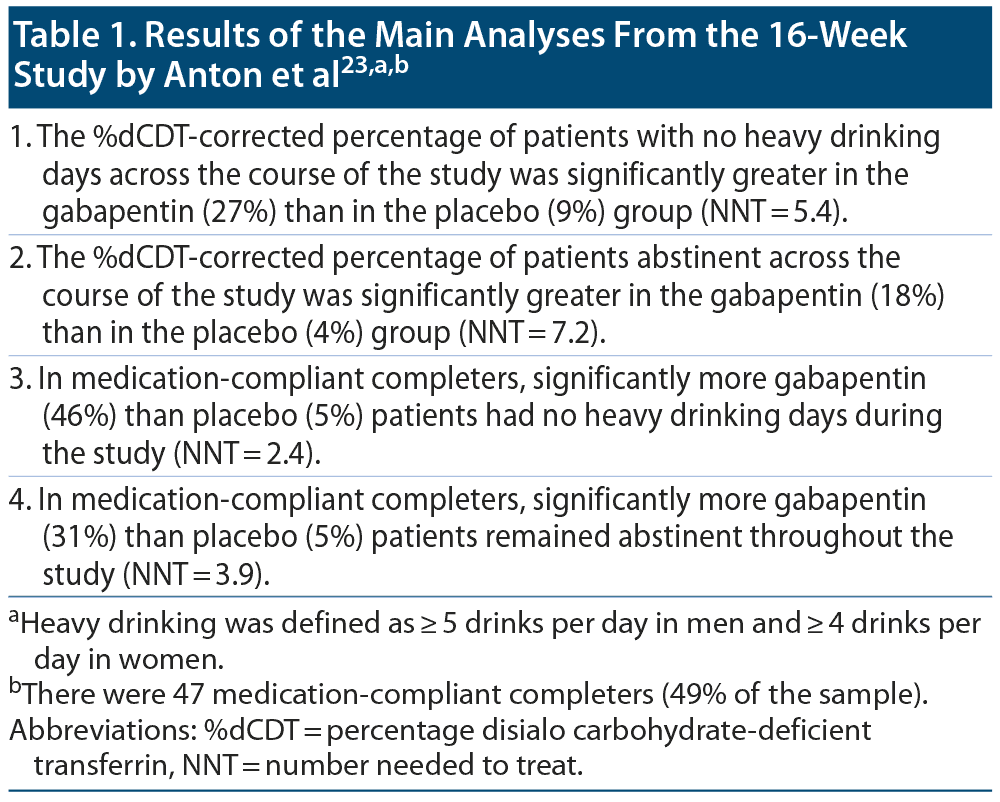
In the most recent study, Anton et al23 recruited 96 adult outpatients with AUD (DSM-5), all of whom also had a present or past history of alcohol withdrawal (DSM-5). These patients were primarily recruited through advertisements and were paid for attending the final assessment. The study was industry-independent and was conducted at a single site in the US. The primary aim of the study was to determine whether gabapentin was superior to placebo in this purposive sample of AUD patients with current or past history of AWS. A secondary aim was to determine whether higher severity of recent, self-reported withdrawal was associated with greater benefit.
All patients were drinking at least 5 drinks a day for the past 90 days; in this context, 1 drink was defined as the equivalent of 14 g of ethanol. All patients were abstinent for at least 3 days prior to randomization, and no patient was currently in mild or more than mild withdrawal. All patients were medically stable. No patient had a current diagnosis of a major psychiatric disorder; however, patients with comorbid posttraumatic stress disorder (PTSD) could be recruited. Antidepressants were the only psychotropics permitted as concurrent medications in the study, provided that the dose was stable for at least the past month. No patient was currently receiving (other) treatment for AUD.
The mean age of the sample was 50 years. The sample was 77% male and 94% white. A quarter of the sample had comorbid PTSD. These patients were drinking a mean of 11 drinks per day during the past 3 months. On average, 4.4 days had passed since their last drink.
These patients were randomized to receive gabapentin or placebo. The dose of gabapentin was up-titrated from 300 mg at night on the first day to 300 mg in the morning, 300 mg in the afternoon, and 600 mg at night on the fifth day; this dose was continued until the end of the 16-week study. All patients also received an educational and supportive medical management intervention. The dropout rate was 30% vs 39% in gabapentin vs placebo groups, respectively; the dropout rate due to adverse events was not explicitly stated.
Important results from the main and additional analyses are presented in Table 1 and Table 2. In summary, gabapentin reduced heavy drinking and increased abstinence, and these findings were apparent only in patients with higher levels of AWS during the 2 weeks before randomization. More gabapentin (n = 25) than placebo (n = 15) patients reported mild to moderate dizziness. Other adverse events were not presented and compared between the 2 groups.
Critical Appraisal
The study23 suffered from several limitations. The study was based on a convenience sample; patients were recruited primarily through advertisements and so may have been more motivated to stop drinking. It was a purposive sample; patients were recruited only if they had a present or past history of AWS. Whereas most patients with AUD will have AWS if they stop drinking, the patients in this sample were additionally motivated by the promise of payment if they attended the last follow-up. A limitation of convenience and purposive samples is that findings from such samples may not generalize well to patients in everyday clinical practice24; in this context, to patients who have to be motivated to stop drinking and who are not paid to attend follow-up. This limits the external validity of the study.
Next, as part of the consenting process, it would have been necessary to warn patients about the strongly sedating effects of gabapentin. This means that many if not most patients may have been unblinded by the experience of sedation in the gabapentin group and by the absence of sedation in the placebo group. So the placebo response could have been magnified in patients who received gabapentin and diminished in those who received placebo. This compromises the internal validity of the study.
Although the number-needed-to-treat values were impressive in the main results (Table 1), the absolute benefits were small: just 27% vs 9% for gabapentin vs placebo for the outcome "no heavy drinking days" during the study and just 18% vs 4% for gabapentin vs placebo for the outcome "abstinence" during the study. These numbers are possibly acceptable in usual clinical practice because AUDs are difficult to treat but are perhaps disappointing for a sample that was sufficiently motivated to respond to advertisements and further motivated by a financial incentive.
The elephant in the room, not commented upon by the authors of the study,23 is the result of the additional analyses performed in patients with high vs low AWS. In short, as evident from the data for the continuous variables in Table 2, for mean number of heavy drinking days and mean number of days abstinent, gabapentin patients fared only marginally better than placebo patients if AWS were high, and worse or substantially worse than placebo patients if AWS were low. For the categorical outcomes "percentage of patients with no relapse into heavy drinking" and "percentage of patients abstinent," the more striking advantage for gabapentin in the high AWS subgroup suggests that gabapentin may have had a particularly detrimental effect in the low AWS group to explain the unimpressive results in the analysis of the continuous variables and to explain the absence of benefit for the outcomes "mean number of drinks per day" and "mean number of drinks per drinking day."
There are 2 ways of interpreting these findings. One interpretation is that the marginal but statistically significant benefits in patients with high AWS are spurious because they were driven by anomalously worse outcomes in the low AWS patients. If so, an appropriate conclusion is that, regardless of the level of AWS, gabapentin improves the drinking outcomes addressed in the main analysis (Table 1); there is no differential effect driven by the level of AWS. The other interpretation is troubling because it suggests that the drug may actually cause harm. If gabapentin paradoxically worsens indices of drinking in AUD patients with low AWS, the problem may not be resolved by recommending that gabapentin be prescribed only to patients with high AWS. This is because, in this study, AWS were categorized as low and high by a median split of the AWS scores obtained in the sample. There is no assurance that the median split value applied here will generalize to other samples. A further problem is that if patients are detoxified as inpatients, they will, for clinical and ethical reasons, routinely receive medications such as benzodiazepines to prevent severe withdrawal; so severity of withdrawal may not be assessable, and historical assessments may be inaccurate. Thus, there is no good way of knowing in advance who may or may not be harmed by the drug because the level of AWS is not sufficiently high.
Concluding Notes
Gabapentin is negligibly metabolized in the liver.25 It does not induce or inhibit liver enzymes.26 It is therefore a reasonably safe choice in patients with alcoholic liver disease. However, whereas gabapentin does seem to reduce heavy drinking days and promote abstinence, overall, if the additional analyses by Anton et al23 are credible, gabapentin is effective only in patients with high AWS and may paradoxically worsen drinking outcomes in patients with low AWS. It could be hard to accurately and prospectively classify patients into high and low AWS categories. For these reasons, it appears that the new data do not recommend a change in the stance of the American Psychiatric Association27 Practice Guideline for the Pharmacological Treatment of Patients With Alcohol Use Disorders. In this guideline, gabapentin is suggested for patients with moderate to severe AUD who wish to reduce alcohol consumption or achieve abstinence, who prefer gabapentin or do not tolerate or respond to naltrexone and acamprosate, and for whom gabapentin is not contraindicated. The guideline notes that gabapentin moderately improves abstinence rates from drinking and from heavy drinking but that the strength of research evidence is low.
On a final note, patients with neuropsychiatric disorders such as chronic pain syndromes, anxiety, and chronic insomnia may be vulnerable to AUD. Gabapentin may be of value in such patients because, besides the suggested benefits for AUD, studies have shown that it reduces pain, anxiety, and sleep disturbance. One awaits the results of studies of gabapentin for AUD in such specific contexts. A particular advantage of gabapentin over benzodiazepines in such patients is that patients will not develop drug dependence to the treatment.
Published online: November 24, 2020.
 Each month in his online column, Dr Andrade considers theoretical and practical ideas in clinical psychopharmacology with a view to update the knowledge and skills of medical practitioners who treat patients with psychiatric conditions.
Each month in his online column, Dr Andrade considers theoretical and practical ideas in clinical psychopharmacology with a view to update the knowledge and skills of medical practitioners who treat patients with psychiatric conditions.
Department of Clinical Psychopharmacology and Neurotoxicology, National Institute of Mental Health and Neurosciences, Bangalore, India ([email protected]).
Financial disclosure and more about Dr Andrade.
REFERENCES
1.Andrade C. Individualized, high-dose baclofen for reduction in alcohol intake in persons with high levels of consumption. J Clin Psychiatry. 2020;81(4):20f13606. PubMed CrossRef
2.Wallach JD, Ross JS. Gabapentin approvals, off-label use, and lessons for postmarketing evaluation efforts. JAMA. 2018;319(8):776-778. PubMed CrossRef
3.Lal R, Sukbuntherng J, Luo W, et al. Population pharmacokinetics and pharmacodynamics of gabapentin after administration of gabapentin enacarbil. J Clin Pharmacol. 2013;53(1):29-40. PubMed CrossRef
4.Pande AC, Davidson JR, Jefferson JW, et al. Treatment of social phobia with gabapentin: a placebo-controlled study. J Clin Psychopharmacol. 1999;19(4):341-348. PubMed CrossRef
5.Lavigne JE, Heckler C, Mathews JL, et al. A randomized, controlled, double-blinded clinical trial of gabapentin 300 versus 900 mg versus placebo for anxiety symptoms in breast cancer survivors. Breast Cancer Res Treat. 2012;136(2):479-486. PubMed CrossRef
6.Cipriani A, Barbui C, Salanti G, et al. Comparative efficacy and acceptability of antimanic drugs in acute mania: a multiple-treatments meta-analysis. Lancet. 2011;378(9799):1306-1315. PubMed CrossRef
7.Vieta E, Manuel Goikolea J, Mart×nez-Arán A, et al. A double-blind, randomized, placebo-controlled, prophylaxis study of adjunctive gabapentin for bipolar disorder. J Clin Psychiatry. 2006;67(3):473-477. PubMed CrossRef
8.Mathew NT, Rapoport A, Saper J, et al. Efficacy of gabapentin in migraine prophylaxis. Headache. 2001;41(2):119-128. PubMed CrossRef
9.Guttuso T Jr. Gabapentin’s anti-nausea and anti-emetic effects: a review. Exp Brain Res. 2014;232(8):2535-2539. PubMed CrossRef
10.Arnold LM, Goldenberg DL, Stanford SB, et al. Gabapentin in the treatment of fibromyalgia: a randomized, double-blind, placebo-controlled, multicenter trial. Arthritis Rheum. 2007;56(4):1336-1344. PubMed CrossRef
11.Moore A, Derry S, Wiffen P. Gabapentin for chronic neuropathic pain. JAMA. 2018;319(8):818-819. PubMed CrossRef
12.Verret M, Lauzier F, Zarychanski R, et al; Canadian Perioperative Anesthesia Clinical Trials (PACT) Group. Perioperative use of gabapentinoids for the management of postoperative acute pain: a systematic review and meta-analysis. Anesthesiology. 2020;133(2):265-279. PubMed CrossRef
13.Furieri FA, Nakamura-Palacios EM. Gabapentin reduces alcohol consumption and craving: a randomized, double-blind, placebo-controlled trial. J Clin Psychiatry. 2007;68(11):1691-1700. PubMed CrossRef
14.Stahl SM. Anticonvulsants as anxiolytics, part 2: pregabalin and gabapentin as alpha(2)delta ligands at voltage-gated calcium channels. J Clin Psychiatry. 2004;65(4):460-461. PubMed CrossRef
15.Stahl SM. Mechanism of action of alpha2delta ligands: voltage sensitive calcium channel (VSCC) modulators. J Clin Psychiatry. 2004;65(8):1033-1034. PubMed CrossRef
16.Sills GJ. The mechanisms of action of gabapentin and pregabalin. Curr Opin Pharmacol. 2006;6(1):108-113. PubMed CrossRef
17.Kranzler HR, Feinn R, Morris P, et al. A meta-analysis of the efficacy of gabapentin for treating alcohol use disorder. Addiction. 2019;114(9):1547-1555. PubMed CrossRef
18.Cheng YC, Huang YC, Huang WL. Gabapentinoids for treatment of alcohol use disorder: a systematic review and meta-analysis. Hum Psychopharmacol. 2020;35(6):1-11. PubMed
19.Falk DE, Ryan ML, Fertig JB, et al; National Institute on Alcohol Abuse and Alcoholism Clinical Investigations Group (NCIG) Study Group. Gabapentin enacarbil extended-release for alcohol use disorder: a randomized, double-blind, placebo-controlled, multisite trial assessing efficacy and safety. Alcohol Clin Exp Res. 2019;43(1):158-169. PubMed
20.Rosenblat JD, Lee Y, McIntyre RS. Does pharmacogenomic testing improve clinical outcomes for major depressive disorder? a systematic review of clinical trials and cost-effectiveness studies. J Clin Psychiatry. 2017;78(6):720-729. PubMed CrossRef
21.Anton RF, Myrick H, Baros AM, et al. Efficacy of a combination of flumazenil and gabapentin in the treatment of alcohol dependence: relationship to alcohol withdrawal symptoms. J Clin Psychopharmacol. 2009;29(4):334-342. PubMed CrossRef
22.Anton RF, Myrick H, Wright TM, et al. Gabapentin combined with naltrexone for the treatment of alcohol dependence. Am J Psychiatry. 2011;168(7):709-717. PubMed CrossRef
23.Anton RF, Latham P, Voronin K, et al. Efficacy of gabapentin for the treatment of alcohol use disorder in patients with alcohol withdrawal symptoms: a randomized clinical trial. JAMA Intern Med. 2020;180(5):728-736. PubMed CrossRef
24.Andrade C. The inconvenient truth about convenience and purposive samples. Indian J Psychol Med. 2020; In Press.
25.Andrade C. Drugs that escape hepatic metabolism. J Clin Psychiatry. 2012;73(7):e889-e890. PubMed CrossRef
26.McLean MJ. Clinical pharmacokinetics of gabapentin. Neurology. 1994;44(suppl 5):S17-S22, discussion S31-S32. PubMed
27.American Psychiatric Association. Practice Guideline for the Pharmacological Treatment of Patients With Alcohol Use Disorder. American Psychiatric Association; 2018. Accessed November 9, 2020. CrossRef
Save
Cite
Advertisement
GAM ID: sidebar-top