ABSTRACT
Two recent cohort studies, one from Norway and the other from Taiwan, examined for perhaps the first time whether gestational exposure to benzodiazepines and to z-hypnotics was associated with a clinical diagnosis of autism spectrum disorder (ASD) and attention-deficit/hyperactivity disorder (ADHD) in offspring. The studies had important methodological strengths that are not often seen; these included actual assessment during pregnancy of whether drugs prescribed were used, and, if so, when; adjustment of analyses for postbaseline time-varying covariates; inclusion of discordant sibling pair and paternal exposure analyses; inclusion of pre-pregnancy vs intrapregnancy analyses; and others. The studies also had important limitations; these included inadequate statistical power resulting in a failure to identify potential associations in even unadjusted analyses; lack of information on intermittent use vs daily dosing; and others. The strengths and limitations are identified and explained to empower readers to identify similar issues in other studies. Important findings apparent in these studies are that benzodiazepine exposure may be associated with an increased risk of both ASD and ADHD, regardless of the trimester of exposure. The magnitude of increased risk is small and diminishes to statistical nonsignificance in adjusted analyses. The risks appear elevated in association with paternal exposure. In discordant sibling pair analyses, risks do not appear to be significantly higher in the exposed sib relative to the unexposed sib. These findings imply that observed associations, if any, between gestational exposure to benzodiazepines and ASD or ADHD in offspring may be due to maternal and paternal genetic factors, to family environmental variables, and to confounding by indication, rather than to benzodiazepine exposure itself. Nevertheless, decision-making should be tailored to individual context and shared between prescriber and patient. Finally, no conclusions can be drawn regarding the neurodevelopmental safety of z-drug exposure during pregnancy.
J Clin Psychiatry 2023;84(2):23f14863
To cite: Andrade C. Gestational exposure to benzodiazepines and z-hypnotics and the risk of autism spectrum disorder and attention-deficit/hyperactivity disorder in offspring. J Clin Psychiatry. 2023;84(2):23f14863.
To share: https://doi.org/10.4088/JCP.23f14863
© 2023 Physicians Postgraduate Press, Inc.
Maternal use of medications during pregnancy is often discouraged because of a possibly increased risk of a large number of adverse gestational outcomes, ranging from spontaneous abortion and preterm birth to major congenital malformations and poor neonatal adaptation syndrome. Very many studies have examined risks associated with psychotropic drug use during pregnancy, including the risk of impaired offspring neurodevelopment following gestational exposure to sodium valproate, antidepressant drugs, and antipsychotic drugs. Earlier articles in this column discussed gestational exposure to antidepressants and antipsychotics and the risk of neurodevelopmental disorders in offspring1–4 as well as gestational exposure to benzodiazepines and the risk of spontaneous abortion and congenital malformations.5–7 The present article examines the risk of gestational exposure to benzodiazepines and z-hypnotics (zopiclone, zolpidem) and the risk of neurodevelopmental disorders in offspring.
Background
Pregnancy is associated with psychological, physiological, and even physical stress, and so pregnant women may be as or more vulnerable to mental health disturbances, such as anxiety, as women in the general population. Sleep may be disturbed during pregnancy, either as an isolated symptom or in conjunction with a mental health disorder. Benzodiazepines and z-drugs are used to treat anxiety and sleep disturbances; so, these drugs may be prescribed to women who need them during pregnancy. These drugs may be used either daily, if symptoms reach diagnostic thresholds, or intermittently, for occasional anxiety and difficulties with falling or staying asleep.
In a systematic review and meta-analysis of 32 studies with data from 28 countries (pooled N = 7,343,571 pregnancies), the pooled prevalence of benzodiazepine use (or prescription) during pregnancy was 1.9%; this value was 3.1% in the third trimester.8 In recently published studies, the exposure rate during pregnancy was 0.8% for benzodiazepines and z-drugs in Norway9 and 5.0% for benzodiazepines in Taiwan.10
Systematic Reviews
In a systematic review, Wang et al11 identified 19 cohort studies of neurodevelopmental outcomes following benzodiazepine and z-drug exposure during pregnancy. These studies had examined neurodevelopment in cognitive, emotional, behavioral, and motor domains. Almost all studies assessed outcomes using rating instruments rather than clinical diagnoses. Some studies found significant associations between medication exposure and impaired neurodevelopment in some domains; most studies, however, found no significant associations. These reassuring findings were supported by the possibility that the significant associations were driven by confounding by indication; that is, the maternal condition that necessitated treatment with benzodiazepines or z-drugs may have predisposed to the adverse neurodevelopmental outcomes rather than the medications themselves.
In another systematic review of much the same data, Jensen et al12 identified 13 cohort studies of psychological, social, motor, and other neurodevelopmental outcomes in children who had had prenatal exposure to benzodiazepines or z-drugs. They found some evidence that exposure was associated with an increased risk of internalizing problems, gross motor skill impairments, academic underachievement, and attention-deficit/hyperactivity disorder (ADHD) traits in exposed children. However, they noted that the findings were inconsistent and of uncertain clinical relevance, that the persistence of the findings in later childhood remained to be demonstrated, and that confounding by indication and selection bias may have been responsible for significant associations.
Two new cohort studies have now been published.9,10 Both studies had important methodological strengths (Table 1 and Table 2) and are considered in the present article.
The Study From Norway9
Sundbakk et al9 examined the risk of ADHD in children who had been gestationally exposed to benzodiazepines or z-drugs. The data were extracted from the population-based Norwegian Mother, Father, and Child Cohort Study. Subjects in the study were recruited during 1999–2008 and were assessed during pregnancy using questionnaires. The final sample comprised 82,201 mother-child dyads among whom 19,585 had received treatment for mental health indications. The mean age of the women was about 30 years. Slightly more than half of the sample was primiparous. In the full sample, 681 (0.8%) children had been gestationally exposed to benzodiazepines or to z-drugs (or to both). In the sample of mothers with mental health conditions, 468 (2.4%) had been exposed.
Women who reported benzodiazepine or z-drug exposure had higher rates of smoking, alcohol use, and illicit drug use during pregnancy; their pregnancies were less likely to be planned. They were more likely to report sleeping problems and a history of major depression. They were more likely to have used psychotropic and nonpsychotropic medications during pregnancy. Interestingly, their husbands were also more likely to have a history of major depression.
Exposure to benzodiazepines or to z-drugs during early pregnancy (weeks 0–16) was more common than exposure in middle or late pregnancy, and exposure in a single 4-week window was more common than exposure in more than one 4-week window.
The sample was followed until 2016; the mean duration of follow-up of the children was 11 years. A diagnosis of ADHD was recorded in 2.7% of children (3.9% in the women with mental health conditions). The risk of ADHD was assessed in the children after adjusting for baseline and time-varying covariates (Table 1).
Important findings from the study are presented in Table 3. In summary, in none of the crude or weighted main, subgroup, and sensitivity analyses was exposure to benzodiazepines or z-drugs during pregnancy associated with a statistically significant risk of ADHD diagnosis in the offspring.
Despite its strengths, the study had many limitations. Important limitations are listed in Table 4. The most important limitation, one that was not acknowledged either by the authors of the study9 or by the authors of a commentary13 on the study, is subtle: the study failed to identify a statistically significant association between gestational drug exposure and ADHD in the crude (unadjusted) analyses not only in the main sample but even in the subsample of women with mental health conditions. Readers who are familiar with the research will be aware that there is an increased prevalence of genetic, environmental, behavioral, and other risk factors for adverse gestational outcomes among women who use psychotropic drugs during pregnancy and that, for this reason (confounding by indication), crude analyses almost always show relationships between gestational exposure to psychotropic drugs and adverse gestational outcomes. So, in the study by Sundbakk et al,9 if the number of exposed pregnancies was too small to detect a combined confounding plus drug effect, it was too small to detect a drug effect, if any existed; this means that the adjusted analyses were perhaps unnecessary. Furthermore, if the main analysis was underpowered, then all subgroup and sensitivity analyses were also underpowered, and the study therefore cannot provide reassurances about the safety of benzodiazepine and z-drug exposure during pregnancy.
The Study From Taiwan10
Chen et al10 examined the risk of autism spectrum disorder (ASD) and ADHD in children who had been gestationally exposed to benzodiazepines. The data were extracted from the Taiwan National Health Insurance Research Database for the period 2004–2017. The study sample comprised 1,138,732 mothers and 1,134,320 fathers of 1,516,846 children aged < 14 years who had been followed for a mean of 8.5 years.
Five percent of the children (n = 76,411) had been exposed to benzodiazepines during pregnancy. Exposure was classified by trimester, with prescriptions for the previous 90 days plus prescriptions during the trimester qualifying for exposure during that trimester. Whereas this inevitably created overlap, it was intended to include the possibility that, because medications were typically prescribed for 28–90 days, women might use the medications distal from the date of prescription. Exposure was recorded as 4.6%, 2.2%, and 0.6% for the first, second, and third trimesters, respectively.
Exposed mothers were more likely to have used opioids and were far more likely to have had anxiety, major depressive disorder, or any mental disorder than unexposed mothers. Data were unavailable for other covariates of importance (Table 5).
Among exposed vs unexposed children, ASD was diagnosed in 1.1% vs 0.9% and ADHD in 4.9% vs 3.9% of children, respectively. Diagnoses were based on at least 1 inpatient record or at least 3 outpatient records in order to improve the likelihood of valid diagnoses. Strengths and limitations of the study are presented in Table 2 and Table 5.
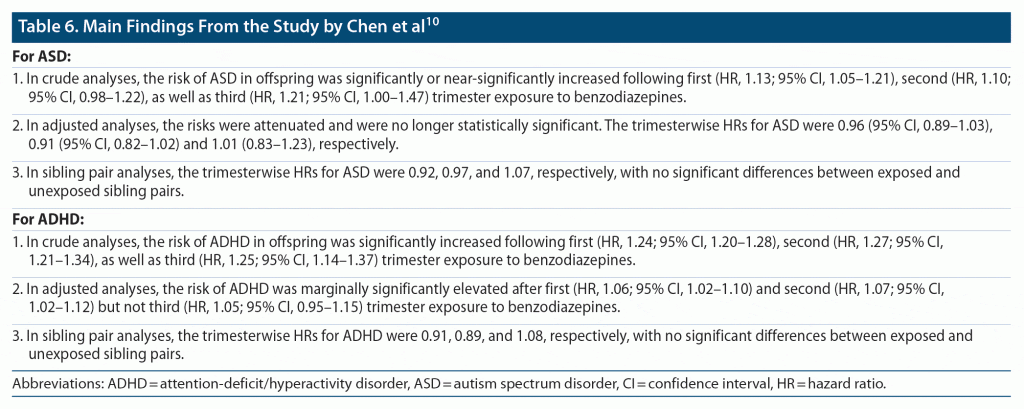
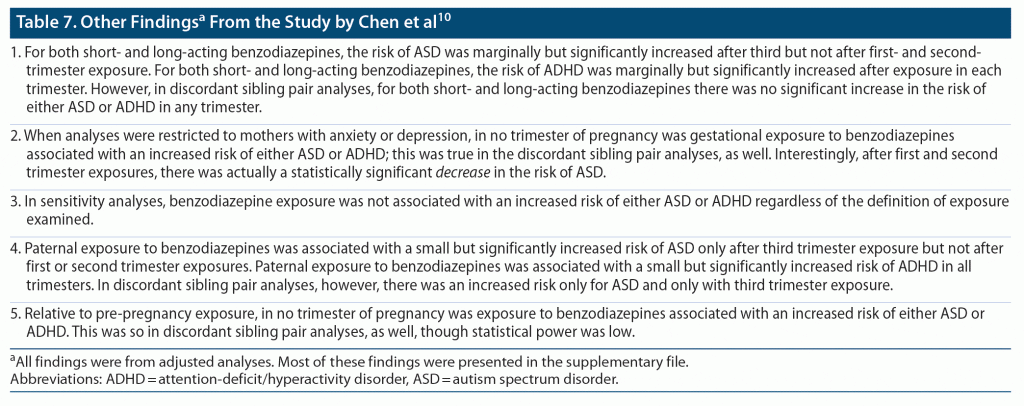
Important findings from the study10 are presented in Table 6 and Table 7. In summary, in almost all of the crude analyses, gestational exposure to benzodiazepines was associated with a small but significantly increased risk of ASD and ADHD regardless of trimester of exposure; these findings attenuated and even lost significance in adjusted analyses. There was no significant increase in risks in discordant sibling pair analyses or in analyses of exposure during pregnancy vs exposure before pregnancy. Risks were very slightly but significantly increased in association with paternal exposure to benzodiazepines during maternal pregnancy. These findings suggest that there is a small association between gestational exposure to benzodiazepines and ASD or ADHD and that the risk is probably driven not by benzodiazepine exposure but by maternal genetic factors, paternal genetic factors, confounding by indication, and/or shared family risk factors. The possible contribution of confounding by indication is underlined by the absence of adjustment for important risk variables (Table 5).
A puzzling contradiction appeared in the findings and was not discussed by the authors: the risks for ASD and ADHD appeared greater and were more likely to be statistically significant when short- and long-acting benzodiazepine exposures were considered separately in subgroup analyses than when they were considered together in the main analysis.
Concluding Notes
Despite its many strengths (Table 1), no reassurances can be drawn from the study by Sundbakk et al9 because the number of exposed children (n = 681) was too small for even the main analyses to be adequately powered. This means that no conclusions are possible about the neurodevelopmental safety of z-drug exposure during pregnancy. Conclusions about benzodiazepines can be drawn from the study by Chen et al.10
The number of exposed children in the study by Chen et al10 was more than a hundredfold larger (n = 76,411) than that in the study by Sundbakk et al.9 In crude analyses, this study10 found that benzodiazepine exposure was associated with an increased risk of both ASD and ADHD, regardless of the trimester of exposure. The magnitude of increased risk was however small, and the hazard ratios further decreased and were mostly not statistically significant in adjusted analyses. Furthermore, risks were elevated in association with paternal exposure; and in discordant sibling pair analyses, risks were not significantly higher in the exposed sib relative to the unexposed sib. These findings suggest that the observed association between gestational exposure to benzodiazepines and ASD and ADHD in offspring may be more due to maternal and paternal genetic factors, to shared environmental factors, and to confounding by indication, than to benzodiazepine exposure itself.
This reassurance is not testimony that benzodiazepines can be safely used in pregnancy. For one, the neurodevelopmental safety of gestational exposure to benzodiazepines at present rests on the single cohort study by Chen et al.10 For another, other gestational risks associated with benzodiazepines need to be examined in context.14–19 Decision-making should therefore be both tailored to individual context and shared between prescriber and patient.
Published online: March 27, 2023
 Each month in his online column, Dr Andrade considers theoretical and practical ideas in clinical psychopharmacology with a view to update the knowledge and skills of medical practitioners who treat patients with psychiatric conditions.
Each month in his online column, Dr Andrade considers theoretical and practical ideas in clinical psychopharmacology with a view to update the knowledge and skills of medical practitioners who treat patients with psychiatric conditions.Department of Clinical Psychopharmacology and Neurotoxicology, National Institute of Mental Health and Neurosciences, Bangalore, India ([email protected]).
Financial disclosure and more about Dr Andrade.
References (19)

- Andrade C. Antidepressant exposure during pregnancy and risk of autism in the offspring, 1: meta-review of meta-analyses. J Clin Psychiatry. 2017;78(8):e1047–e1051. PubMed CrossRef
- Andrade C. Antidepressant exposure during pregnancy and risk of autism in the offspring, 2: do the new studies add anything new? J Clin Psychiatry. 2017;78(8):e1052–e1056. PubMed CrossRef
- Andrade C. Gestational and neurodevelopmental outcomes associated with antipsychotic drug exposure during pregnancy. J Clin Psychiatry. 2021;82(5):21f14265. PubMed CrossRef
- Andrade C. Attention-deficit/hyperactivity disorder, autism spectrum disorder, and other neurodevelopmental outcomes associated with antipsychotic drug exposure during pregnancy. J Clin Psychiatry. 2022;83(3):22f14529. PubMed CrossRef
- Andrade C. Gestational exposure to benzodiazepines, 1: the risk of spontaneous abortion examined through the prism of research design. J Clin Psychiatry. 2019;80(5):19f13076. PubMed CrossRef
- Andrade C. Gestational exposure to benzodiazepines, 2: The risk of congenital malformations examined through the prism of compatibility intervals. J Clin Psychiatry. 2019;80(5):19f13081. PubMed CrossRef
- Andrade C. Gestational exposure to benzodiazepines, 3: clobazam and major congenital malformations. J Clin Psychiatry. 2019;80(6):19f13151. PubMed CrossRef
- Bais B, Molenaar NM, Bijma HH, et al. Prevalence of benzodiazepines and benzodiazepine-related drugs exposure before, during and after pregnancy: a systematic review and meta-analysis. J Affect Disord. 2020;269:18–27. PubMed CrossRef
- Sundbakk LM, Gran JM, Wood ME, et al. Association of prenatal exposure to benzodiazepines and Z-hypnotics with risk of attention-deficit/hyperactivity disorder in childhood. JAMA Netw Open. 2022;5(12):e2246889. PubMed CrossRef
- Chen VC, Wu SI, Lin CF, et al. Association of prenatal exposure to benzodiazepines with development of autism spectrum and attention-deficit/hyperactivity disorders. JAMA Netw Open. 2022;5(11):e2243282. PubMed CrossRef
- Wang X, Zhang T, Ekheden I, et al. Prenatal exposure to benzodiazepines and Z-drugs in humans and risk of adverse neurodevelopmental outcomes in offspring: A systematic review. Neurosci Biobehav Rev. 2022;137:104647. PubMed CrossRef
- Jensen AG, Knudsen SS, Bech BH. Prenatal exposure to benzodiazepines and the development of the offspring: a systematic review. Neurotoxicol Teratol. 2022;91:107078. PubMed CrossRef
- Suarez EA, Bushnell GA. Association between attention-deficit/hyperactivity disorder and benzodiazepines and Z-hypnotics in pregnancy—questions remain. JAMA Netw Open. 2022;5(12):e2246896. PubMed CrossRef
- Grigoriadis S, Graves L, Peer M, et al. Pregnancy and delivery outcomes following benzodiazepine exposure: a systematic review and meta-analysis. Can J Psychiatry. 2020;65(12):821–834. PubMed CrossRef
- Lee H, Koh JW, Kim YA, et al. Pregnancy and neonatal outcomes after exposure to alprazolam in pregnancy. Front Pharmacol. 2022;13:854562. PubMed CrossRef
- Noh Y, Lee H, Choi A, et al. First-trimester exposure to benzodiazepines and risk of congenital malformations in offspring: a population-based cohort study in South Korea. PLoS Med. 2022;19(3):e1003945. PubMed CrossRef
- Szpunar MJ, Freeman MP, Kobylski LA, et al. Risk of major malformations in infants after first-trimester exposure to benzodiazepines: results from the Massachusetts General Hospital National Pregnancy Registry for Psychiatric Medications. Depress Anxiety. 2022;39(12):751–759. PubMed CrossRef
- Grigoriadis S, Graves L, Peer M, et al. Benzodiazepine use during pregnancy alone or in combination with an antidepressant and congenital malformations: systematic review and meta-analysis. J Clin Psychiatry. 2019;80(4):18r12412. PubMed CrossRef
- Grigoriadis S, Alibrahim A, Mansfield JK, et al. Hypnotic benzodiazepine receptor agonist exposure during pregnancy and the risk of congenital malformations and other adverse pregnancy outcomes: a systematic review and meta-analysis. Acta Psychiatr Scand. 2022;146(4):312–324. PubMed CrossRef
Save
Cite
Advertisement
GAM ID: sidebar-top