Because this piece does not have an abstract, we have provided for your benefit the first 3 sentences of the full text.
To the Editor: Minor depression (MinD) is a subclinical depressive disorder affecting nearly 10% of the elderly population. According to the fourth edition of the Diagnostic and Statistical Manual of Mental Disorders (DSM-IV), a minor depressive episode requires 2 to 4 depressive symptoms that are present for at least 2 weeks. Minor depressive disorder is diagnosed when a lifetime history of major depressive disorder (MDD) is excluded.
No Changes in Gray Matter Density or Cortical Thickness in Late-Life Minor Depression
To the Editor: Minor depression (MinD) is a subclinical depressive disorder affecting nearly 10% of the elderly population.1 According to the fourth edition of the Diagnostic and Statistical Manual of Mental Disorders (DSM-IV), a minor depressive episode requires 2 to 4 depressive symptoms that are present for at least 2 weeks. Minor depressive disorder is diagnosed when a lifetime history of major depressive disorder (MDD) is excluded.2 Although MinD may seem transient, its consequences are often severe.3,4 The pathophysiology of MinD remains largely unexplored.1
We analyzed structural magnetic resonance images (MRI) of 38 subjects with MinD and 80 healthy controls (aged 60-79 years) using voxel-based morphometry (VBM) and region-of-interest (ROI) analyses of gray matter density and cortical thickness.
According to meta-analyses5-10 of structural brain changes in MDD, we hypothesized disease-specific decreases of gray matter density in the bilateral anterior cingulate cortex (ACC), hippocampus, and amygdalae and the right dorsomedial frontal cortex. In the ROI analysis, we expected decreased gray matter density within the meta-analytically derived11 mask (Supplementary eFigure 1) (the largest cluster: left insula, temporal pole, inferior frontal, superior temporal gyrus) and cortical thinning bilaterally in the medial orbitofrontal cortex, fusiform gyrus, insula, rostral anterior, and posterior cingulate cortex and unilaterally in the left middle temporal, right inferior temporal gyrus, and right caudal ACC.12
Methods. We included 38 subjects with a MinD episode and 80 healthy subjects (aged 60-79 years) from the community-based LIFE-Adult study13 (see Supplementary Methods). The study was conducted in accordance with the Declaration of Helsinki and was approved by the Ethics Committee of the University of Leipzig. Subjects provided written informed consent prior to participation.
Participants underwent the Structured Clinical Interview for DSM-IV (SCID),14 cognitive testing, and MRI. MinD episode was diagnosed on the basis of DSM-IV criteria.2 High-resolution structural brain images were obtained with a 3T MAGNETOM Verio (Siemens; Erlangen, Germany) scanner using standard Alzheimer’s Disease Neuroimaging Initiative protocol.15
Structural images were preprocessed using VBM in SPM8 (www.fil.ion.ucl.ac.uk/spm) as previously described.16 Gray matter density across the whole brain was compared between MinD and control groups using 2-sample t test corrected for gender, age, and degree of white matter hyperintensities, measured on the Fazekas scale.17 In a second analysis, we used a mask for reduced gray matter density in MDD, obtained from the recent meta-analysis.11 Further analysis was performed using ROIs based on the meta-analysis of cortical thickness in MDD.12 Structural images were preprocessed using Freesurfer segmentation (http://surfer.nmr.mgh.harvard.edu) and compared between the groups using analysis of covariance in SPSS version 21 (IBM; Chicago, Illinois).
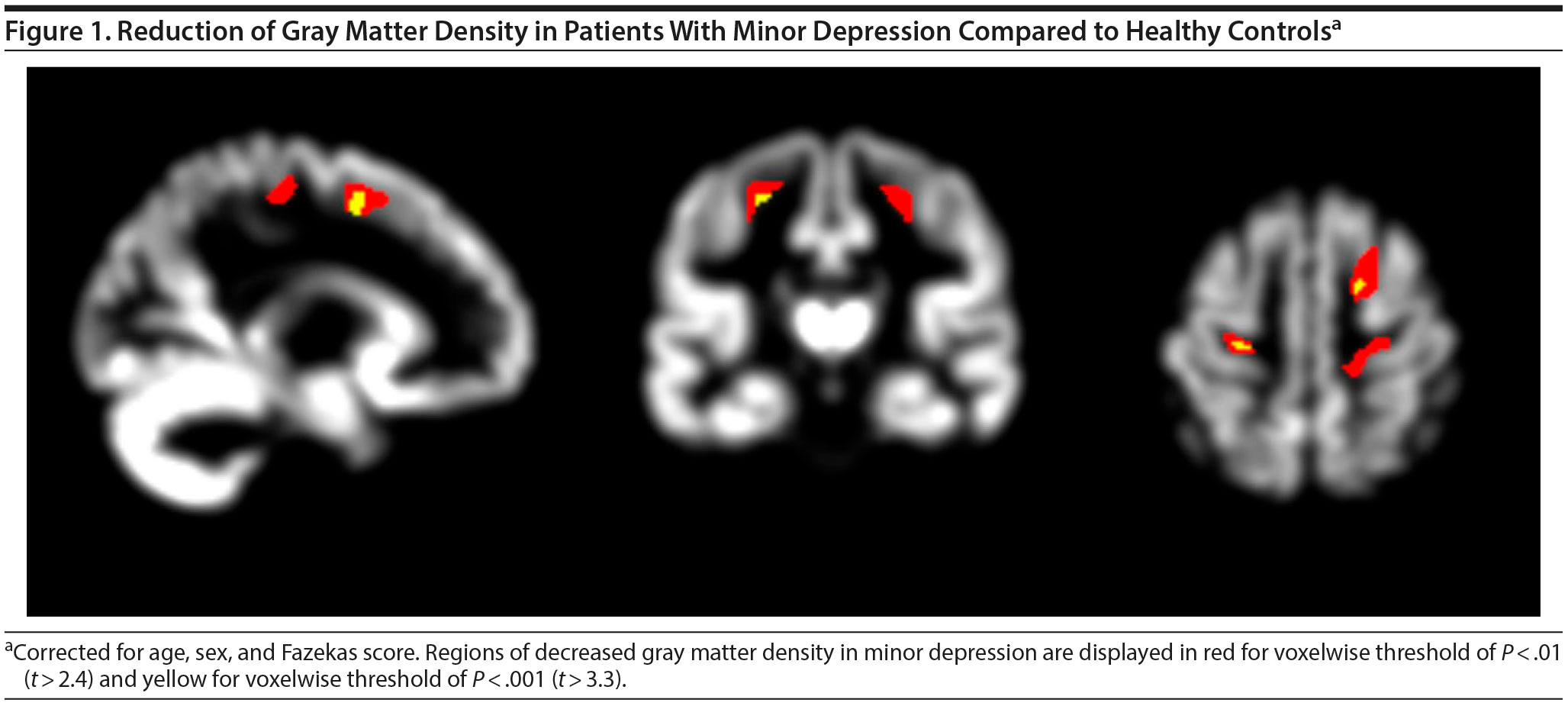
Results. Demographic and clinical data of participants are presented in the Supplementary Results in Supplementary eTable 1. In VBM, we found a reduction of gray matter density in the bilateral precentral gyri, right superior frontal gyrus, and left thalamus using a voxel threshold of P < .001 (Figure 1). However, these differences did not reach statistical significance after correction for multiple comparisons.
Comparing gray matter density within the meta-analytically derived mask did not yield any significant differences between the groups. Reanalyses of the imaging data with sex or age as single covariate, with age- and gender-matched controls, or in subgroups with and without a history of depression compared to controls confirmed nonsignificant effects. The ROI analysis based on Freesurfer segmentation did not show any significant cortical thinning (Supplementary eTable 2).
We used 3 methods to assess structural brain changes in later life MinD. However, we obtained no evidence for structural gray matter abnormalities similar to those in MDD or in previously reported MinD samples.18,19 Major limitations of the study are our exceptional inclusion of subjects in late life, with an age of 60 years or older, and inclusion of subjects having a depressive episode in anamnesis. Nevertheless, our sample was larger than those in previous studies,18,19 deeply phenotyped, and recruited from a representative population-based study. A further reason for the absence of evidence for structural brain alterations in MinD might be its heterogeneity. Before MinD is hypothesized as a functional syndrome or adjustment disorder, it should be further investigated with respect to endophenotypes of MDD, such as a glutamatergic deficit in the ACC,20 or in combination with disease-specific biomarkers.21-23
References
1. Polyakova M, Sonnabend N, Sander C, et al. Prevalence of minor depression in elderly persons with and without mild cognitive impairment: a systematic review. J Affect Disord. 2014;152-154:28-38. PubMed CrossRef
2. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. Fourth Edition. Washington, DC: American Psychiatric Association; 2000.
3. Lyness JM, King DA, Cox C, et al. The importance of subsyndromal depression in older primary care patients: prevalence and associated functional disability. J Am Geriatr Soc. 1999;47(6):647-652. PubMed CrossRef
4. Angst J. The epidemiology of depressive disorders. Eur Neuropsychopharmacol. 1995;5:95-98. PubMed CrossRef
5. Sacher J, Neumann J, Fünfstück T, et al. Mapping the depressed brain: a meta-analysis of structural and functional alterations in major depressive disorder. J Affect Disord. 2012;140(2):142-148. PubMed CrossRef
6. Bora E, Fornito A, Pantelis C, et al. Gray matter abnormalities in Major Depressive Disorder: a meta-analysis of voxel based morphometry studies. J Affect Disord. 2012;138(1-2):9-18. PubMed CrossRef
7. Du MY, Wu QZ, Yue Q, et al. Voxelwise meta-analysis of gray matter reduction in major depressive disorder. Prog Neuropsychopharmacol Biol Psychiatry. 2012;36(1):11-16. PubMed CrossRef
8. Du M, Liu J, Chen Z, et al. Brain grey matter volume alterations in late-life depression. J Psychiatry Neurosci. 2014;39(6):397-406. PubMed CrossRef
9. Zhao YJ, Du MY, Huang XQ, et al. Brain grey matter abnormalities in medication-free patients with major depressive disorder: a meta-analysis. Psychol Med. 2014;44(14):2927-2937. PubMed CrossRef
10. Zhang H, Li L, Wu M, et al. Brain gray matter alterations in first episodes of depression: a meta-analysis of whole-brain studies. Neurosci Biobehav Rev. 2016;60:43-50. PubMed CrossRef
11. Wise T, Radua J, Via E, et al. Common and distinct patterns of grey-matter volume alteration in major depression and bipolar disorder: evidence from voxel-based meta-analysis. Mol Psychiatry. 2017;22(10):1455-1463. PubMed CrossRef
12. Schmaal L, Hibar DP, Sפmann PG, et al. Cortical abnormalities in adults and adolescents with major depression based on brain scans from 20 cohorts worldwide in the ENIGMA Major Depressive Disorder Working Group. Mol Psychiatry. 2017;22(6):900-909. PubMed CrossRef
13. Loeffler M, Engel C, Ahnert P, et al. The LIFE-Adult-Study: objectives and design of a population-based cohort study with 10,000 deeply phenotyped adults in Germany. BMC Public Health. 2015;15(1):691. PubMed CrossRef
14. Demmel R. The SKID (Strukturiertes Klinisches Interview fur DSM IV). Zeitschrift Fur Klinische Psychologie-Forschung Und Praxis. 1999;28(1):68-70.
15. Alzheimer’s Disease Neuroimaging Initiative protocol. Alzheimer’s Disease Neuroimaging Initiative website. http://www.adni-info.org/Scientists/ADNIStudyProcedures.html.
16. Mueller K, Horstmann A, Möller H, et al. Obesity associated cerebral gray and white matter alterations are interrelated in the female brain. PLoS One. 2014;9(12):e114206. PubMed CrossRef
17. Fazekas F, Chawluk JB, Alavi A, et al. MR signal abnormalities at 1.5 T in Alzheimer’s dementia and normal aging. AJR Am J Roentgenol. 1987;149(2):351-356. PubMed CrossRef
18. Kumar A, Schweizer E, Jin Z, et al. Neuroanatomical substrates of late-life minor depression: a quantitative magnetic resonance imaging study. Arch Neurol. 1997;54(5):613-617. PubMed CrossRef
19. Kumar A, Ajilore O, Zhang A, et al. Cortical thinning in patients with late-life minor depression. Am J Geriatr Psychiatry. 2014;22(5):459-464. PubMed CrossRef
20. Demenescu LR, Colic L, Li M, et al. A spectroscopic approach toward depression diagnosis: local metabolism meets functional connectivity. Eur Arch Psychiatry Clin Neurosci. 2017;267(2):95-105. PubMed CrossRef
21. Gos T, Schroeter ML, Lessel W, et al. S100B-immunopositive astrocytes and oligodendrocytes in the hippocampus are differentially afflicted in unipolar and bipolar depression: a postmortem study. J Psychiatr Res. 2013;47(11):1694-1699. PubMed CrossRef
22. Schroeter ML, Sacher J, Steiner J, et al. Serum S100B represents a new biomarker for mood disorders. Curr Drug Targets. 2013;14(11):1237-1248. PubMed CrossRef
23. Schroeter ML, Steiner J, Mueller K. Glial pathology is modified by age in mood disorders: a systematic meta-analysis of serum S100B in vivo studies. J Affect Disord. 2011;134(1-3):32-38. PubMed CrossRef
aDepartment of Neurology, Max Planck Institute for Human Cognitive and Brain Sciences, Leipzig, Germany
bClinic for Psychiatry and Psychotherapy, University of Leipzig, Leipzig, Germany
cLIFE—Leipzig Research Center for Civilization Diseases, University of Leipzig, Germany
dInstitute of Social Medicine, Occupational Health and Public Health (ISAP), Leipzig University, Leipzig, Germany
eDepartment of Neuroradiology, Leipzig University, Leipzig, Germany
fBerlin School of Mind and Brain and the Mind-Brain Institute, Humboldt-University of Berlin, Berlin, Germany
gClinic for Cognitive Neurology, University of Leipzig, Germany
hAcademic State Hospital Arnsdorf, Arnsdorf, Germany
Potential conflicts of interest: None.
Funding/support: This study was supported by the International Max Planck Research School on Neuroscience of Communication (IMPRS NeuroCom; Ms Polyakova); by LIFE (Leipzig Research Centre for Civilization Diseases at the University of Leipzig), funded by the European Union, the European Regional Development Fund, and the Free State of Saxony within the framework of the excellence initiative (Drs Riedel-Heller, Villringer, Schoenknecht, and Schroeter); by the German Consortium for Frontotemporal Lobar Degeneration, funded by the German Federal Ministry of Education and Research (Dr Schroeter); by the Parkinson’s Disease Foundation (Dr Schroeter; grant no. PDF-IRG-1307); and by the Michael J. Fox Foundation (Dr Schroeter; grant no. 11362). Dr Then has also been supported by LIFE—Leipzig Research Center for Civilization Diseases, University of Leipzig. Her collaboration with LIFE was funded by the European Social Fund and the Free State of Saxony.
Role of the sponsor: The sponsors had no role in the design, analysis, interpretation, preparation, review, or publication of this letter.
Supplementary material: See accompanying pages.
J Clin Psychiatry 2018;79(2):17l11604
To cite: Polyakova M, Mueller K, Beyer F, et al. No changes in gray matter density or cortical thickness in late-life minor depression. J Clin Psychiatry. 2018;79(2):17l11604.
To share: https://doi.org/10.4088/JCP.17l11604
© Copyright 2018 Physi Postgradciansuate Press, Inc.
Save
Cite
Advertisement
GAM ID: sidebar-top