Alcohol use disorders (AUD) are common and impairing. Many pharmacologic interventions have been approved for AUD; baclofen is one among these. More than a dozen randomized controlled trials (RCTs) have examined the safety and efficacy of baclofen in AUD; these RCTs have been pooled in 4 recent meta-analyses, each with different study selection criteria, different objectives, different methods, and different results. The general impression from these meta-analyses is that the benefits with baclofen are unimpressive in patients with AUD. With the background that individualized, rather than fixed, high dosing with baclofen could be critical for success, a large (n = 320), industry-independent, 62-center French RCT (the Bacloville trial) examined whether individually uptitrated, high-dose baclofen could reduce alcohol consumption in heavy drinkers across a year of treatment. The study design, the high dropout rate, and the statistical methods of this trial threw up several complexities; in consequence, the main primary and secondary outcomes could not be satisfactorily interpreted. However, there were many other secondary outcomes that seemed to favor baclofen over placebo, though certain concerns remained. This RCT is explained and its findings are carefully dissected and interpreted. It is concluded that individualized treatment with high-dose baclofen (30-300 mg/d) may be a useful second-line approach in heavy drinkers who wish to reduce levels of alcohol intake; baclofen may be particularly useful in patients with liver disease, for whom certain other pharmacologic interventions are relatively contraindicated. However, the risk of serious adverse events with high-dose baclofen, and its pharmacodynamic interaction with alcohol, must both be kept in mind. Practical issues are discussed.
See letter by Braillon and Naudet

ABSTRACT
Alcohol use disorders (AUD) are common and impairing. Many pharmacologic interventions have been approved for AUD; baclofen is one among these. More than a dozen randomized controlled trials (RCTs) have examined the safety and efficacy of baclofen in AUD; these RCTs have been pooled in 4 recent meta-analyses, each with different study selection criteria, different objectives, different methods, and different results. The general impression from these meta-analyses is that the benefits with baclofen are unimpressive in patients with AUD. With the background that individualized, rather than fixed, high dosing with baclofen could be critical for success, a large (n = 320), industry-independent, 62-center French RCT (the Bacloville trial) examined whether individually uptitrated, high-dose baclofen could reduce alcohol consumption in heavy drinkers across a year of treatment. The study design, the high dropout rate, and the statistical methods of this trial threw up several complexities; in consequence, the main primary and secondary outcomes could not be satisfactorily interpreted. However, there were many other secondary outcomes that seemed to favor baclofen over placebo, though certain concerns remained. This RCT is explained and its findings are carefully dissected and interpreted. It is concluded that individualized treatment with high-dose baclofen (30-300 mg/d) may be a useful second-line approach in heavy drinkers who wish to reduce levels of alcohol intake; baclofen may be particularly useful in patients with liver disease, for whom certain other pharmacologic interventions are relatively contraindicated. However, the risk of serious adverse events with high-dose baclofen, and its pharmacodynamic interaction with alcohol, must both be kept in mind. Practical issues are discussed.
J Clin Psychiatry 2020;81(4):20f13606
To cite: Andrade C. Individualized, high-dose baclofen for reduction in alcohol intake in persons with high levels of consumption. J Clin Psychiatry. 2020;81(4):20f13606.
To share: https://doi.org/10.4088/JCP.20f13606
© Copyright 2020 Physicians Postgraduate Press, Inc.
Alcohol use disorders (AUD) are common in the general population; findings from the World Mental Health Surveys suggest a mean lifetime prevalence of 8.6%.1 AUD are associated with substantial economic burden in terms of disability-adjusted life-years, they are a risk factor for noncommunicable and communicable diseases, and they increase the risk of morbidity and mortality.2,3 The management of AUD is therefore a health care priority, worldwide.
In different countries, medications approved for AUD include disulfiram, calcium carbamide, acamprosate, naltrexone, nalmefene, and, recently, baclofen. Many other medications have also been trialled for AUD with varying degrees of success. These include selective serotonin reuptake inhibitors (SSRIs), ondansetron, topiramate, gabapentin, and others.4,5 However, outcomes with all treatments have been modest and relapse rates have been high.6 Better approaches to treatment are therefore necessary.
Baclofen for Alcohol Use Disorders
Baclofen is a selective GABA-B receptor agonist that, in different countries, is approved for the treatment of spasticity in neurologic disorders and alcohol dependence associated with high-risk alcohol intake. Baclofen is used for off-label indications, such as neuropathic pain, as well.5,7
Baclofen is effective in animal models of alcoholism.8 For example, baclofen was shown to dose-dependently reduce the severity of alcohol withdrawal in alcohol-dependent rats, to protect against evoked seizures in rats withdrawn from alcohol, and to dose-dependently decrease voluntary alcohol intake in alcohol-dependent rats.9
Baclofen was first studied in AUD around the turn of the century.10 More than a dozen randomized controlled trials (RCTs) were subsequently conducted and published. These trials were recently pooled in meta-analyses, 4 of which were published by different teams of authors during the same year, 2018. The different meta-analyses11-14 examined different RCTs in specified analyses, studied different outcomes, were performed in different ways, and did not reach the same conclusions. These meta-analyses are therefore individually examined in the next sections.
Results From Meta-Analyses: 1
Rose and Jones11 described a systematic review and meta-analysis of 12 RCTs that examined whether baclofen reduced measures of drinking behavior, craving, and mood symptoms, relative to placebo, in patients with AUD. They found that baclofen improved abstinence rates, though with a wide and imprecise confidence interval (CI) (odds ratio [OR], 2.67; 95% CI, 1.03 to 6.93; number needed to treat, 8; 6 RCTs; N = 590). However, baclofen did not reduce heavy drinking days (standardized mean difference [SMD], −0.26; 95% CI, −0.68 to 0.15; 6 RCTs) or ratings of craving (SMD, −0.13; 95% CI, −0.36 to 0.09; 11 RCTs). Baclofen also did not significantly increase abstinent days (SMD, 0.03; 95% CI, −0.10 to 0.15; 6 RCTs) or reduce ratings of anxiety (SMD, −0.03; 95% CI, −0.24 to 0.18; 8 RCTs) and depression (SMD, 0.06; 95% CI, −0.22 to 0.34; 8 RCTs).
All analyses were characterized by substantial heterogeneity. Given the identification of only 1 significant pooled outcome (abstinence rates), the authors11 concluded that it is premature to consider baclofen as a treatment for AUD. This conclusion seems reasonable, given the number of outcomes examined and the risk of a type 1 (false positive) error.
Results From Meta-Analyses: 2
Bschor et al12 described a systematic review and meta-analysis of 14 RCTs (pooled N = 1,522) that examined the long-term efficacy and tolerability of baclofen in AUD. The primary outcome was whatever the individual RCTs stated as the primary outcome, even if the outcome was different in different studies; where no primary outcome was stated, a hierarchy was constructed for selection of a variable as the primary outcome. This means, for example, that cumulative days abstinent from one study could be combined with time to relapse in a second study with amount of alcohol consumed per day in a third study, and so on. Thus, the meta-analysis did not examine whether baclofen outperformed placebo on a specific variable; rather, it examined whether baclofen outperformed placebo on primary outcomes, where “primary outcomes” was a heterogeneous construct. Because effect sizes, across RCTs, were converted into SMDs. the pooling of effect sizes was mathematically and conceptually feasible.
In this meta-analysis, the authors12 found that baclofen did not significantly outperform placebo on primary outcomes (SMD, 0.22; 95% CI, −0.03 to 0.47; 14 RCTs); however, the 95% CIs were compatible with an advantage for baclofen. The (nonsignificant) results were consistent in a leave-one-out sensitivity analysis. The authors further estimated that 25 new RCTs would be required, each with an SMD of 0.5, to result in a pooled SMD of 0.4 that favored baclofen.
Meta-analysis of combined outcomes related to abstinence also suggested no advantage for baclofen (SMD, 0.20; 95% CI, −0.08 to 0.49; 13 RCTs). Meta-analysis of outcomes related to amount of drinking suggested an advantage for baclofen that was of borderline significance; however, the effect size was small (SMD, 0.28; 95% CI, 0.00 to 0.56).
In RCTs that dosed baclofen above 80 mg/d, baclofen was associated with an effect size that was compatible with an advantage for baclofen that did not attain statistical significance (OR, 1.63; 95% CI, 0.90 to 2.96; 4 RCTs).
Dropout due to any reason and dropout due to adverse effects did not differ significantly between baclofen and placebo groups. Most of the analyses were characterized by moderate to high heterogeneity. However, the 14 RCTs did not show evidence of publication bias.
This meta-analysis was notable for the unconventional manner in which individual RCT outcomes were pooled. The authors12 drew a reasonable conclusion: that benefits with baclofen are probably only slightly above placebo level, so both usual and high doses of baclofen are of questionable value in the long-term treatment of AUD.
Results From Meta-Analyses: 3
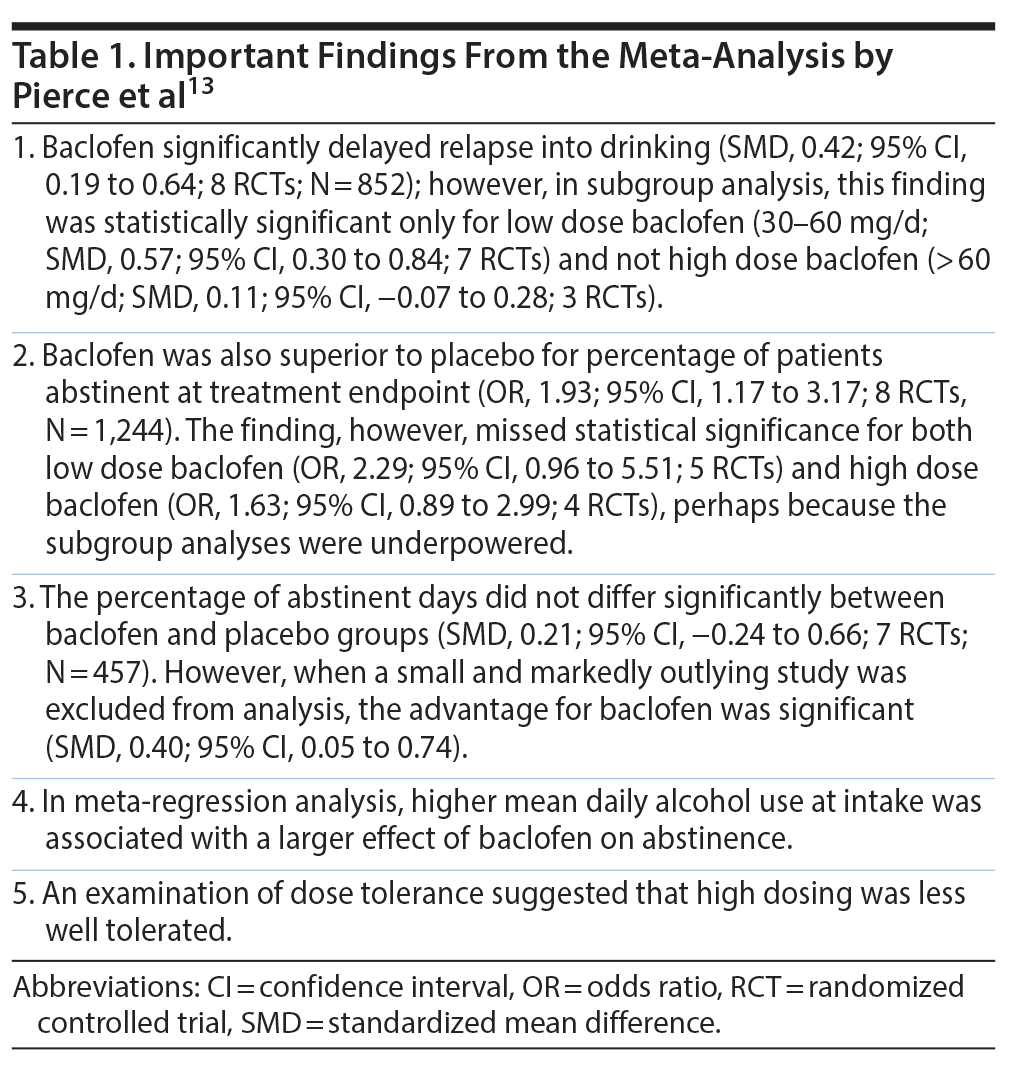
Pierce et al13 described a systematic review and meta-analysis of the efficacy of baclofen in AUD, with special emphasis on the examination of the effects of the baclofen dose. They identified 13 relevant RCTs (pooled N = 1,492). Most studies in most regards were rated to be at low risk of bias.
Important findings from the meta-analysis are presented in Table 1. The authors concluded that low dose baclofen showed better efficacy than high dose baclofen; that high dose baclofen was poorly tolerated; and that baclofen appears effective in AUD, especially among heavy drinkers.
The findings of this meta-analysis need to be viewed with much caution for at least 3 reasons. One is that although subgroup analyses were performed for low and high dose baclofen, patients did not necessarily reach the target dose in the high dose group, nor did they maintain the target dose after attaining it. Another is that, in one forest plot that examined low and high dosing, a high dose treatment arm was misclassified among and pooled with the low dose treatment arms. A third and potentially fatal issue is that in all of the main analyses, there were several instances where different active arms were compared with the same placebo arm in the same (pooled) analysis; effectively, therefore, in all of these analyses, the placebo group was duplicated for all the RCTs that had more than 1 baclofen dosing arm. Counting subjects twice in the same analysis is not permitted.
Results From Meta-Analyses: 4
Minozzi et al14 described a systematic review and meta-analysis of baclofen in currently drinking persons with AUD who wished to reduce or discontinue alcohol use. The authors identified 12 RCTs (pooled N = 1,128). One RCT compared baclofen with acamprosate; the rest were placebo-controlled. In these RCTs, baclofen was dosed at 10-150 mg/d. The risk of bias was considered to be low in most of the RCTs.
Important findings from the meta-analysis are presented in Table 2. Many of the analyses were associated with high heterogeneity. The authors concluded that baclofen was no more effective than placebo on any outcome, either primary or secondary. This meta-analysis was unusual in that for 2 efficacy outcomes (alcohol drinks per drinking day [2 RCTs] and ratings of depression [3 RCTs]) outcomes with baclofen were significantly worse than those with placebo. Also, curiously, the risk of muscle spasms/rigidity was nearly doubled with baclofen.
Taking Stock of the Findings
Four meta-analyses published in the same year looked at much the same research but in different ways, so it’s not surprising that their findings differed. Baclofen was found superior to placebo for abstinence rates,11 to be borderline superior to placebo for amount of drinking,12 to outperform placebo with regard to both time to relapse and endpoint abstinence rates (but, clearly so, only in doses of 60 mg/d or lower),13 and to be no better than placebo or even worse than placebo.14 The only meta-analysis to find a clear advantage for baclofen was a meta-analysis that was also methodologically flawed.13 Only 1 meta-analysis stated a single primary outcome, and all meta-analyses tested multiple hypotheses without correcting for a type 1 statistical error (because such correction is not customary in meta-analysis). These findings discourage the consideration of baclofen for patients with AUD.
About 15 years ago, a physician in France published a self-report of his struggles with alcohol dependence and comorbid anxiety.15 He described how he uptitrated baclofen to 270 mg/d, at which point, for the first time in his alcoholic life, he had no craving or even desire for alcohol. This heavily cited report is historically important because it suggested that the effective dose of baclofen may need to be individually discovered. Observational studies with long-term follow-up have also suggested that doses for patients may need to be individually discovered because they can lie within a wide range.16-18 The recently published Bacloville study19 assumes importance in this regard. This RCT is particularly important because of the large sample size (n = 320) and the long follow-up (1 year).
The Bacloville Study
The 62-center Bacloville double-blind RCT19 was an industry-independent investigation that was conducted in France during 2012-2014. This study examined the 1-year efficacy of individually titrated baclofen in the reduction of levels of alcohol intake in patients with heavy drinking.
The sample comprised 320 adult outpatients with high-risk alcohol consumption, defined as > 60 g/d in men and > 40 g/d in women. All patients wished to decrease or stop drinking. No patient had previously received baclofen and no patient was on treatment with other medications approved for AUD.
The median age of the sample was about 46 years. The sample was 70% male. The mean alcohol intake was 129 g/d at baseline. These patients were randomized to receive baclofen (n = 162) or placebo (n = 158) for 1 year. With the exception of use of medications approved for AUD, patients were permitted treatment as usual; however, there was no standardized psychosocial intervention offered.
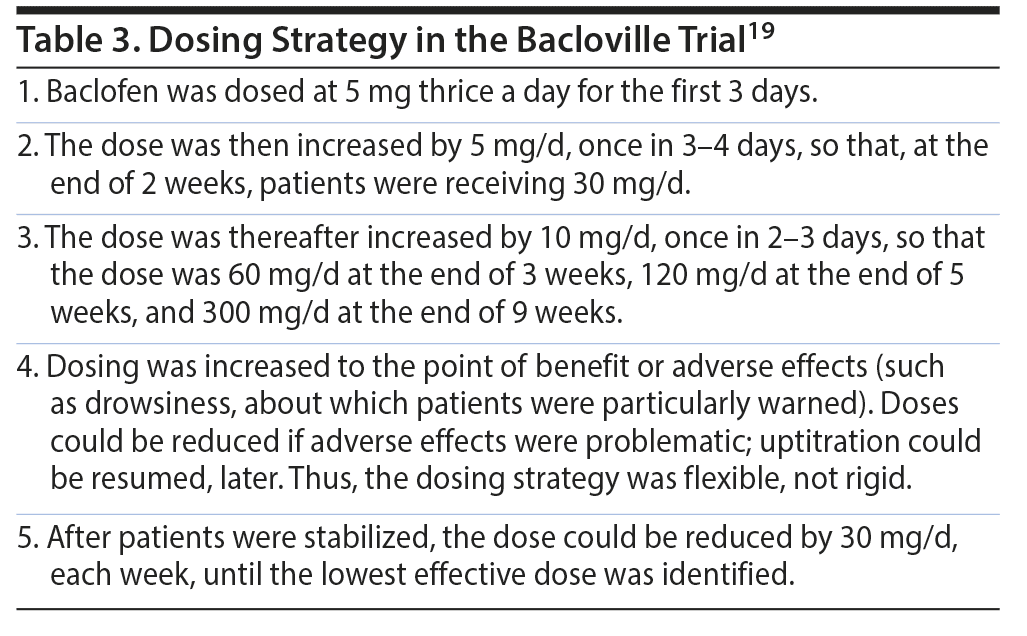
The baclofen dosing strategy is presented in Table 3. The median (interquartile range) maximum dose of treatment received was 180 (100-270) mg/d in the baclofen arm and 210 (138-290) mg/d in the placebo arm. If patients and physicians jointly considered the assigned, blinded treatment ineffective, patients could switch to open-label baclofen, without unblinding, while remaining in the RCT.
Drinking parameters and baclofen use were recorded in daily diaries. Craving was rated using a visual analog scale (VAS). Other assessments included ratings on the Hospital Anxiety and Depression Scale (HADS), the Obsessive Compulsive Drinking Scale, and the Medical Outcomes Study Short Form 36 (SF-36) quality of life questionnaire.
By the 1-year study endpoint, significantly fewer baclofen than placebo patients had switched to open-label baclofen treatment (12% vs 38%, respectively), and significantly fewer baclofen than placebo patients had dropped out of the trial (59% vs 80%, respectively). The median treatment duration was 34 vs 20 weeks in baclofen vs placebo arms. Last-month diaries were returned by only 42% vs 34% of baclofen vs placebo patients, and the diaries were completed by only 23% vs 22% of the patients. Missing data were filled by multiple imputation, based on patient and group trajectories.
The Bacloville Results: Primary Outcome
The primary outcome in the Bacloville study19 was success in baclofen vs placebo patients. Success was defined as abstinence, or reduction in drinking to low risk levels, as assessed during the last month of the study. In this context, low risk was defined as consumption that was < 40 g/d in men and < 20 g/d in women. Importantly, patients were classified as per their randomization assignment in the different analyses, and patients (regardless of treatment group) who switched to open-label baclofen were considered to be treatment failures.
Baclofen outperformed placebo on the primary outcome (success rates, 57% vs 36%; RR, 1.59; 95% CI, 1.17 to 2.15). In one sensitivity analysis, baclofen was superior to placebo even when patients who had > 25% missing data in the last month were regarded as treatment failures (success rates, 25% vs 10%; RR, 2.49; 95% CI, 1.44 to 4.31). In the other sensitivity analysis, baclofen was superior to placebo even when patients with 100% missing data in the last month were regarded as treatment failures (success rates, 28% vs 12%; RR, 2.28; 95% CI, 1.42 to 3.66).
On the surface, these findings are impressive. Given how heavily patients were drinking at baseline (mean, 129 g/d), to achieve success, as defined in this study, was no mean feat, and statistical significance was obtained for the primary outcome in main as well as in sensitivity analyses. Nevertheless, there are 3 important caveats. First, given how strongly sedating baclofen is (especially in high doses), it would have been next to impossible for patients to remain blinded to their treatment assignment, so placebo mechanisms related to unblinding could have improved response to baclofen and diminished response to placebo. Next, more placebo patients may have switched to open-label baclofen merely because of unblinding, and these switchers were, by default, classified as treatment failures regardless of whether or not they actually failed; thus, the placebo group was again disadvantaged. Third, a large number of patients dropped out of the RCT, compromising the internal validity of the study; imputation of missing data in such a situation is a poor solution.
A saving grace is that, in the sensitivity analyses, whereas the first 2 caveats stand, the third caveat does not apply.
The Bacloville Results: The “First” Secondary Outcome
The main secondary outcome of the Bacloville study19 was identical to the primary outcome, except that patients who switched to open-label baclofen were considered as treatment failures only if they actually failed, that is, if they did not meet the study-specified criteria for success (described in the previous section) during the last month of the study. On this outcome, baclofen did not significantly outperform placebo (success rates, 62% vs 55%; RR, 1.12; 95% CI, 0.88 to 1.41).
On the one hand, this analysis reduces the bias associated with placebo patients being misclassified merely because they were unblinded and consequently opted for open-label baclofen. On the other hand, this analysis is heavily biased against baclofen because, if baclofen is truly effective, then placebo patients who benefited from open-label baclofen (but who continued to be classified under “placebo” in the analyses) would have increased the placebo group success rate, thereby narrowing the gap between baclofen and placebo successes.
Interestingly, when the secondary outcome was examined at 3, 6, and 9 months, baclofen outperformed placebo, but the advantage for baclofen narrowly missed statistical significance on each occasion.
The Bacloville Results: Other Secondary Outcomes
Curiously, other secondary outcomes in the Bacloville study19 were probably more meaningful than the primary and the main secondary outcomes. Between months 6 and 12, in each month the mean consumption of alcohol per day was about 10 g (1 drink) less in the baclofen arm. A reduction in drinking by 10 g/d may seem to be only a small advantage, but the advantage could actually be greater because, during these months, a large number of placebo patients opted for open-label baclofen but were still classified as placebo patients in the analysis. Thus, switching to open-label baclofen would have falsely magnified actual or imputed benefits in the placebo group.
The mean number of abstinent days was also significantly greater with baclofen than with placebo; the advantage was 3 more days of abstinence, each month, from the third month onward. Again, this small advantage could have been larger had the placebo patients who switched to open-label baclofen not falsely inflated outcomes in the placebo group.
Next, there were several outcomes for which baclofen did not significantly outperform placebo, but for which the 95% CI were compatible with an advantage for baclofen. These included a mean difference of 2 (95% CI, −0.8 to 4.8) days in the number of heavy drinking days during the last month; a mean difference of 10% (95% CI, −2% to 21%) in the percentage of patients with a DSM-IV diagnosis of alcohol dependence in the last month; and a mean difference of 1.9 (95% CI, −0.6 to 4.4) on Obsessive Compulsive Drinking Scale ratings. Again, each of these differences could have been larger, and statistically significant, had placebo patients who switched to open-label baclofen not falsely inflated the benefits in the placebo group.
The comments stated above assume that baclofen was truly associated with benefit and that, therefore, placebo group patients who switched to open-label baclofen would show better outcomes than if they had continued receiving placebo. The suggested advantage for baclofen would be valid only if the imputations for missing data were valid; in this context, the authors19 observed that their imputation strategy was probably reasonable because there were no wide week-to-week variations in the alcohol consumption reported. A further limitation of the findings that favored baclofen is that there was no adjustment of P values for multiple hypothesis testing, so it is possible that some of the significant findings could have been false positives.
Finally, there were many secondary outcomes during the last month that did not differ between the 2 groups. These included heavy drinking days, VAS craving scores, SF-36 physical and mental functioning scores, and HADS anxiety and depression scores (which were however low, even at baseline).
The Bacloville Results: Adverse Events
Unlike efficacy outcomes, adverse effects (AEs) in the Bacloville study19 were (appropriately) analyzed based on the treatment that patients actually received and not based on the group to which they had been randomized. AEs, especially drowsiness and fatigue, were expectedly more common with baclofen; curiously, so too was insomnia. Serious AEs occurred significantly more frequently with baclofen than with placebo (in 85 vs 36 patients, respectively). There were 7 vs 3 deaths in the baclofen vs placebo arms.
Take-Home Message and Practical Points
The results of the Bacloville study19 suggest that baclofen, slowly uptitrated to individualized high doses, may be useful to reduce levels of drinking in persons who are current heavy drinkers, especially when standardized psychosocial intervention is not implemented or unavailable; this treatment strategy can straightaway be implemented in outpatients, and there is no need for formal detoxification in an inpatient setting. Reduced drinking could result in harm reduction in family, social, medical, and other domains.
Dosing could start at 5 mg, administered 2-3 times a day. Uptitration could proceed in 5-10 mg/d steps, once in 2-4 days or as tolerated, to a target dose at which patients report reduced craving, reduced drinking, or problematic AEs. This target dose could be anywhere between 30 and 300 mg/d, and women may require lower doses than men.20 The dose may be reduced once patients stabilize at lower drinking levels; the lowest effective dose, like the target dose, will need to be individually discovered.
Because baclofen is predominantly (80%) eliminated by renal excretion, it could be particularly useful in alcoholic patients with liver disease. However, baclofen should be used with caution or avoided in patients with renal disease, in those with seizure disorders, in those at risk of hypomania or mania, and in those who might overdose on the drug.20,21
Severe sedation and the risk of associated serious AEs are a matter of concern, especially because alcohol itself results in similar impairment. Individualized high-dose baclofen is therefore best considered as a second-line intervention in AUD, with patients being warned about the dose-dependent risks.
These conclusions and recommendations are in line with the Cagliari Statement on baclofen for the treatment of AUD21 as well as with the guidance provided in a recent review,20 both of which are recommended reading.
Afternotes
AUDs are chronic, difficult to treat, and characterized by high short-term relapse rates in patients who are successfully treated. The 1-year study Bacloville study19 was therefore ambitious; treatment dropout and treatment contamination by switching were both inevitable. It is hoped that this commentary provides insights to readers on how to read, understand, and draw conclusions from what was a difficult paper to get a grip on. Interested readers may also wish to consider other criticisms of the Bacloville study22 and the explanations provided, in response, by the authors.23
In France, baclofen (maximum dose, 80 mg/d) is approved for adult patients with high-risk alcohol intake (> 60 g/d in men and > 40 g/d in women) who fail other medical treatments for alcohol dependence.5 With regard to the maximum dose, one retrospective study18 described the use of baclofen in doses of 50-520 mg/d; the success rate in 144 patients was 63%.
Published online: August 4, 2020.
 Each month in his online column, Dr Andrade considers theoretical and practical ideas in clinical psychopharmacology with a view to update the knowledge and skills of medical practitioners who treat patients with psychiatric conditions.
Each month in his online column, Dr Andrade considers theoretical and practical ideas in clinical psychopharmacology with a view to update the knowledge and skills of medical practitioners who treat patients with psychiatric conditions.
Department of Clinical Psychopharmacology and Neurotoxicology, National Institute of Mental Health and Neurosciences, Bangalore, India ([email protected]).
Financial disclosure and more about Dr Andrade.
REFERENCES
1.Glantz MD, Bharat C, Degenhardt L, et al; WHO World Mental Health Survey Collaborators. The epidemiology of alcohol use disorders cross-nationally: findings from the World Mental Health Surveys. Addict Behav. 2020;102:106128. PubMed CrossRef
2.GBD 2016 Alcohol Collaborators. Alcohol use and burden for 195 countries and territories, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet. 2018;392(10152):1015-1035. PubMed CrossRef
3.Shield K, Manthey J, Rylett M, et al. National, regional, and global burdens of disease from 2000 to 2016 attributable to alcohol use: a comparative risk assessment study. Lancet Public Health. 2020;5(1):e51-e61. PubMed CrossRef
4.Witkiewitz K, Litten RZ, Leggio L. Advances in the science and treatment of alcohol use disorder. Sci Adv. 2019;5(9):x4043.
5.Garbutt JC. Approval of baclofen for alcohol use disorders in France: a perspective from the United States. Alcohol Alcohol. 2020;55(1):48. PubMed CrossRef
6.Sliedrecht W, de Waart R, Witkiewitz K, et al. Alcohol use disorder relapse factors: a systematic review. Psychiatry Res. 2019;278:97-115. PubMed CrossRef
7.Kent CN, Park C, Lindsley CW. Classics and chemical neuroscience: baclofen. ACS Chem Neurosci. 2020;11(12):1740-1755. PubMed CrossRef
8.Colombo G, Gessa GL. Suppressing effect of baclofen on multiple alcohol-related behaviors in laboratory animals. Front Psychiatry. 2018;9:475. PubMed CrossRef
9.Colombo G, Agabio R, Carai MA, et al. Ability of baclofen in reducing alcohol intake and withdrawal severity, 1: preclinical evidence. Alcohol Clin Exp Res. 2000;24(1):58-66. PubMed
10.Addolorato G, Caputo F, Capristo E, et al. Ability of baclofen in reducing alcohol craving and intake, 2: preliminary clinical evidence. Alcohol Clin Exp Res. 2000;24(1):67-71. PubMed
11.Rose AK, Jones A. Baclofen: its effectiveness in reducing harmful drinking, craving, and negative mood: a meta-analysis. Addiction. 2018;113(8):1396-1406. PubMed CrossRef
12.Bschor T, Henssler J, Müller M, et al. Baclofen for alcohol use disorder: a systematic meta-analysis. Acta Psychiatr Scand. 2018;138(3):232-242. PubMed CrossRef
13.Pierce M, Sutterland A, Beraha EM, et al. Efficacy, tolerability, and safety of low-dose and high-dose baclofen in the treatment of alcohol dependence: a systematic review and meta-analysis. Eur Neuropsychopharmacol. 2018;28(7):795-806. PubMed CrossRef
14.Minozzi S, Saulle R, Rösner S. Baclofen for alcohol use disorder. Cochrane Database Syst Rev. 2018;11(11):CD012557. PubMed
15.Ameisen O. Complete and prolonged suppression of symptoms and consequences of alcohol-dependence using high-dose baclofen: a self-case report of a physician. Alcohol Alcohol. 2005;40(2):147-150. PubMed CrossRef
16.de Beaurepaire R. Suppression of alcohol dependence using baclofen: a 2-year observational study of 100 patients. Front Psychiatry. 2012;3:103. PubMed CrossRef
17.Rigal L, Alexandre-Dubroeucq C, de Beaurepaire R, et al. Abstinence and “low-risk” consumption 1 year after the initiation of high-dose baclofen: a retrospective study among “high-risk” drinkers. Alcohol Alcohol. 2012;47(4):439-442. PubMed CrossRef
18.Pinot J, Rigal L, Granger B, et al. Tailored-dose baclofen in the management of alcoholism: a retrospective study of 144 outpatients followed for 3 years in a French general practice. Front Psychiatry. 2018;9:486. PubMed CrossRef
19.Rigal L, Sidorkiewicz S, Tréluyer J-M, et al. Titrated baclofen for high-risk alcohol consumption: a randomized placebo-controlled trial in out-patients with 1-year follow-up. Addiction. 2020;115(7):1265-1276. PubMed CrossRef
20.de Beaurepaire R, Sinclair JMA, Heydtmann M, et al. The use of baclofen as a treatment for alcohol use disorder: a clinical practice perspective. Front Psychiatry. 2019;9:708. PubMed CrossRef
21.Agabio R, Sinclair JM, Addolorato G, et al. Baclofen for the treatment of alcohol use disorder: the Cagliari Statement. Lancet Psychiatry. 2018;5(12):957-960. PubMed CrossRef
22.Naudet F, Braillon A, Cristea IA, et al. Restoring the Bacloville trial: efficacy and harms [published online ahead of print May 4, 2020]. Addiction. PubMed
23.Rigal L, Sidorkiewicz S, Le Jeunne C, et al. Reply to comments on the Rigal et al (2019) Bacloville trial [published online ahead of print May 4, 2020]. Addiction. PubMed CrossRef
Save
Cite
Advertisement
GAM ID: sidebar-top