Abstract
Objective: Repetitive transcranial magnetic stimulation (rTMS) is a standard treatment approach for major depressive disorder. There is growing clinical experience to support the use of high-frequency left-sided rTMS in bipolar depression. This study collected open-label safety and effectiveness data in a sample of patients with bipolar depression.
Methods: Thirty-one adults (13 male/ 18 female; mean age: 42.2 [14.3] years) with bipolar (I or II) depression verified by DSM-5 criteria were recruited at Sheppard Pratt and Mayo Clinic between August 2017 and February 2020 for rTMS. Standardized treatment protocols employed 6 weeks of 10-Hz rTMS to the left dorsolateral prefrontal cortex at 120% of motor threshold with 3,000 pulses per session in 4-second trains with intertrain intervals of 26 seconds. All patients were treated concurrently with a mood stabilizer. The primary outcome measure was the Montgomery-Asberg Depression Rating Scale (MADRS). Response and remission were defined as MADRS score reductions of ≥50% or score <10, respectively. We examined response, remission, and potential contributing factors with multivariate and logistic regression models.
Results: The majority of patients with bipolar depression reached response (n = 27; 87.1%) and remission (n = 23; 74.2%). Older age and concurrent treatment with lithium were associated with higher MADRS scores throughout the treatment course (0.1 ± 0.05, P =.05; 4.05 ± 1.27, P = .003, respectively). Concurrent treatment with lamotrigine was associated with lower MADRS scores (−3.48 ± 1.26, P = .01). Treatment with rTMS was safe and well tolerated. There were no completed suicides, induced manic episodes, or other serious adverse events.
Conclusion: Although preliminary, the present findings are encouraging regarding the safety and effectiveness of 10-Hz rTMS for bipolar depression.
Trial Registration: ClinicalTrials.gov identifier: NCT02640950.
J Clin Psychiatry 2024;85(2):23m15056
Author affiliations are listed at the end of this article.
With the approval of repetitive transcranial magnetic stimulation (rTMS) for use in unipolar depression following a 2007 clinical trial,1 the hope for new treatment options in patients with severe forms of depressive psychopathology was bolstered. Recently expanded with indications for obsessive compulsive disorder,2 anxious depression,3 and smoking cessation,4 rTMS allows a broadened approach for difficult-to-treat5 symptoms, and there is increased interest in exploring applications in bipolar disorder.6–8 Perhaps most importantly, rTMS carries virtually none of the systemic long-term adverse effects seen from pharmacologic agents.
Affecting over 50 million individuals globally and accounting for roughly 1% of total lifetime prevalence,9 bipolar disorder is listed among the top causes of disability-adjusted life years,10 a measure of disease burden. The need for novel and safe interventions is further compounded by the increased health risks for the bipolar population from cardiometabolic, vascular, inflammatory, and other conditions both as a known side effect of medications currently offered and as pathophysiologic consequences of the disease itself.11
As opposed to unipolar depression, activated mood states differentiate bipolar disorder type I (BDI) and bipolar disorder type II (BDII), strictly dependent on the presence of a manic or a hypomanic episode, respectively. Strategies to better treat the more severe forms of bipolar depression are desperately needed, especially given the absolute risk for suicide is higher for bipolar disorder among all primary psychiatric conditions by most accounts.12 Indeed, BDI and BDII share similar rates of suicide completion,13 again illustrating the importance of developing new protocols to alleviate their distress.
To address the paucity of treatment options and strategies available for bipolar disorder, the Systematic Treatment Enhancement Program for Bipolar Disorder (STEP-BD) was commissioned in 2003.14 Currently, Food and Drug Administration (FDA) recommendations for bipolar depression are limited to 5 atypical antipsychotics, one of which is coupled with an antidepressant.15–17 Pivotal results from the STEP-BD studies demonstrated that when used alongside mood stabilizers, antidepressants provided negligible improvements and even carried risk of triggering a mixed or manic episode.18–20 This devastating adverse event, known as treatment-emergent affective switching, occurs at rates reported up to 25% depending on the antidepressant used and population21 in question, posing significant barriers to treatment in bipolar depression and underscoring the need for novel ways to deliver therapeutic benefits.
Preliminary reports of rTMS in bipolar depression have garnered growing optimism with an evidence base suggesting safety and feasibility.22,23 The acute risk of triggering a manic or hypomanic mood state appears better controlled when lamotrigine24,25 or lithium26,27 is used. To mitigate this risk, it is advised that patients are provided with mood-stabilizing medications concurrently with rTMS.28 Significant limitations in moving efforts forward, however, exist in the widely disparate treatment protocols used among rTMS providers and clinical studies, employing varied pulse frequencies, target sites, treatment time, and other stimulation parameters.7 Protocols used for unipolar depression have undergone adaptations and improvements,29 yet bipolar depression protocols continue without a baseline to work from given its current lack of FDA indication. Indeed, several studies performed found equivocal, or otherwise negative, results with respect to treatment outcomes in bipolar depression, albeit using varied approach to TMS administration protocols. In the highest profile rTMS clinical trial in bipolar depression to date,8 intermittent theta burst rTMS was delivered over 20 sessions, and the study was terminated for futility after 37 participants were treated, one of whom experiencing treatment emergent hypomania. Although discouraging, a key factor remained regarding the parameter of rTMS frequency used, that is, the intermittent theta burst stimulation, as distinct from other forms of standard rTMS. Other notable randomized clinical trials have been conducted with similar underperformances from a TMS responsiveness perspective, where no added efficacy was afforded when unilateral rTMS augmentation was combined with quetiapine use in BDII30 or sequential bilateral rTMS.31 An interpretation of these studies may be that quetiapine blunts the effects of rTMS, such that antipsychotics as a class may act as a moderator toward treatment responsiveness as suggested by others32; meanwhile, mood stabilizers and antiepileptic drugs commonly used in difficult-to-treat mood disorders, including bipolar disorder, are not suggested to impinge on treatment effect of rTMS.33 Moreover, sequential bilateral rTMS fails to achieve superiority to unilateral treatment, and given the established efficacy unilateral stimulation offers, efforts to optimize unilateral rTMS serve as a useful heuristic platform to begin to build from.
Our group recently published outcomes from a retrospective analysis of rTMS for BDI and BDII.34 We showed that rTMS offered a 77% response rate and a 41% remission rate, strikingly outperforming typical efficacy in unipolar depression despite using identical rTMS protocols approved for unipolar depression. No treatment-emergent affective switching occurred, and similar effectiveness appeared for the depressive episode of both forms of bipolar disorder. These results are largely in agreement with other “non-iTBS” studies in effectiveness, safety, and side effect profile.7,22 Given the optimism that rTMS may present as an additional treatment strategy to bipolar depression, we conducted an open-label, prospective trial to further study its utility.
METHODS
Eligibility Criteria
All study procedures were approved by the appropriate institutional review board (IRB) prior to any subject recruitment, enrollment, interventional treatment, or other research activities. The trial was registered on ClinicalTrials.gov (NCT02640950). All patients signed an IRB-approved informed consent form prior to study screening activities and ensuing study participation. Eligibility criteria were confirmed with a clinical psychiatric interview and completion of the Mini International Neuropsychiatric Interview. Eligible patients were at least 18 years of age, met DSM-5 criteria for bipolar disorder (either I or II) with a current depressive episode ≥4 weeks but <3 years in duration, Montgomery-Asberg Depression Rating Scale (MADRS) score ≥20, Young Mania Rating Scale (YMRS) score <12, and a Clinical Global Impression (CGI) Severity Scale of ≥4 at the screening visit. All patients were required to be on a suitable mood stabilizer (or/including) antipsychotic medication clinically used for bipolar disorder, as determined by the study clinician, for at least 4 days prior to the commencement of rTMS treatment. Female patients of childbearing potential had a negative pregnancy test at screening, had no plans to become pregnant or nurse, and used a medically acceptable form of birth control.
Participants were recruited between August 2017 and February 2020 from outpatient services at Sheppard Pratt Health System (n = 26) and Mayo Clinic (n = 5); psychiatry department employees were not eligible for participation. Patients who had another primary Axis I diagnosis or an Axis II disorder (based on the primary investigator’s assessment) that would interfere with study participation were not eligible for study participation. Patients who were taking or discontinued an antidepressant <2 weeks prior to enrollment were not eligible. A history of psychotic symptoms, substance abuse, and substance dependence within the 6 months prior to screening was also exclusionary. A lifetime history of nonresponse to electroconvulsive therapy, vagus nerve stimulation, or rTMS was exclusionary; participants did not have any prior attempts with rTMS. A positive urine drug screen for substances with the exception of benzodiazepines was exclusionary. Current suicide risk based on the investigator’s judgment, screening “yes” on item 4 or 5 of the screening Columbia Suicide Severity Rating Scale (C-SSRS), or a suicide attempt within the last 12 months was exclusionary. Patients with safety concerns relevant to rTMS were not eligible to participate, including past head injuries, seizure disorder, or nonremovable metallic implants in or around the head.
Primary Outcome
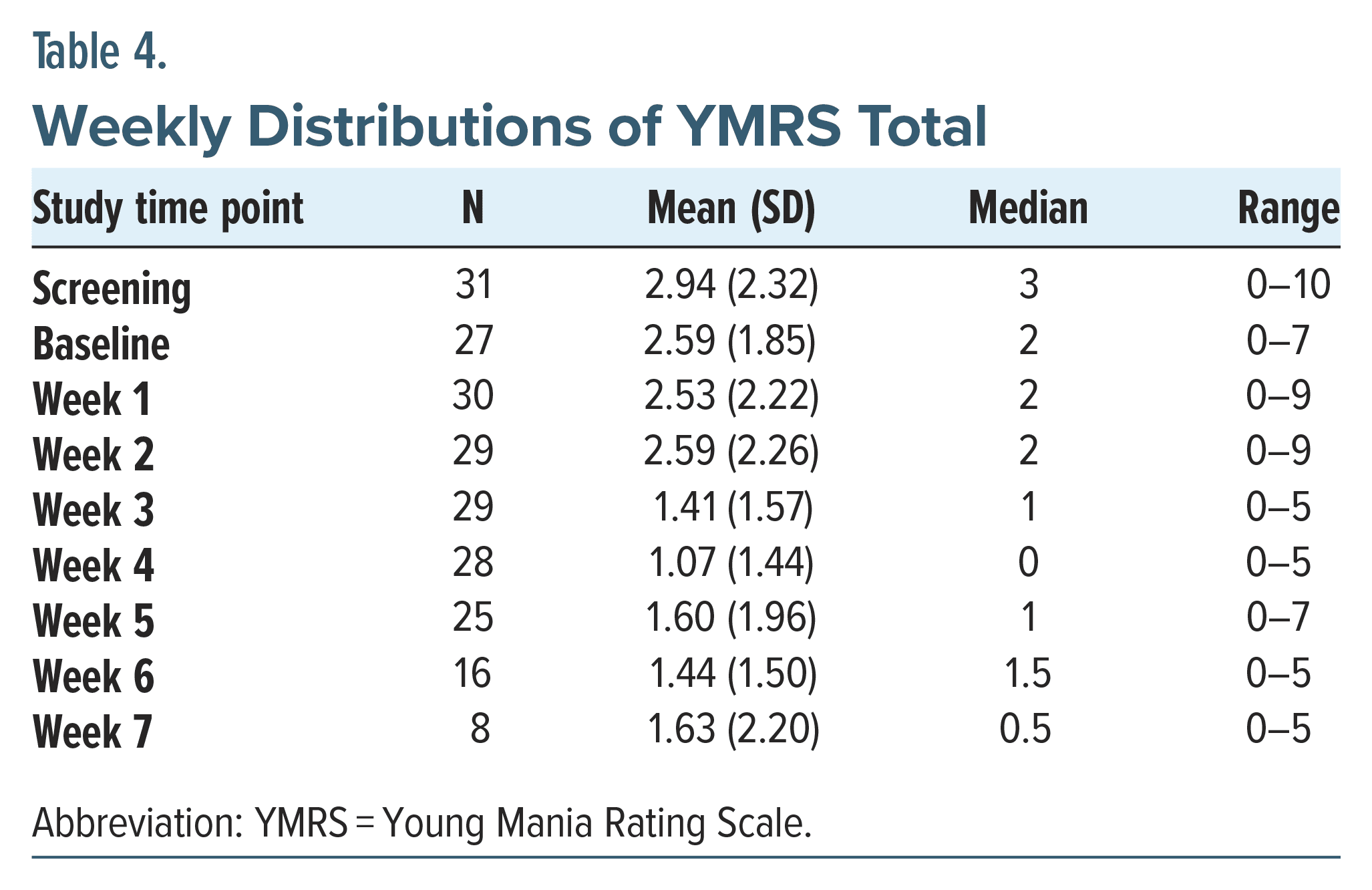
The change from baseline to end point MADRS total score was the primary efficacy measure.35 The scale consists of 10 items and ranges from 0 to 60. Higher scores denote more severe symptoms. Scores are sensitive to detect changes in the emotional functioning rather than physical symptoms of depression.36
Secondary Outcome Measures
The 17-item Hamilton Depression Rating Scale (HDRS) was a secondary rating scale, which captures physical symptoms of depression more than emotional ones.37 The Quick Inventory of Depressive Symptomatology-Self Report (QIDS) is a 16-item scale in which patients evaluate the severity of their depression.38 The C-SSRS is a clinician-administered instrument that assesses suicidal ideation.39 The “baseline” version of the instrument was administered at the screening visit. The “since-last-visit” version was administered at all other visits. The YMRS is the most often used scale to assess the degree of a patient’s manic symptoms.40 This scale was used to identify patients in mixed or manic states at enrollment and was used during the trial to assess for any concerns of rTMS-induced hypomania or mania. The CGI-S and CGI-I, collected at baseline and throughout the trial, are global assessment scales of the severity of a patient’s illness on a 7-point scale and degree of change from baseline on a 7-point scale.41 The CGI improvement scale is often the most sensitive marker to recognize status change in patients.
rTMS Protocol
All rTMS was delivered using the NeuroStar (Neuronetics, Malvern, PA) machine. Resting motor threshold (RMT) was determined using visual left abductor brevis pollicis (thumb) contraction, and the left dorsolateral prefrontal cortex (DLPFC) was located by moving the coil 5.5 cm anteriorly from the thumb contraction. Treatments were given at 120% of the subject’s RMT, at 10 Hz for a 4-second train, with a 26- second intertrain interval. Repositioning of the coil, per the NeuroStar User Manual, and prophylactic use of acetaminophen or ibuprofen were allowed for stimulation site discomfort or pain. Dose intensity was allowed to be adjusted to 110% RMT for intolerability during the first week of treatment only. Treatment sessions lasted 37.5 minutes (75 trains) to provide a total of 3,000 pulses per session. All qualified study patients received open-label rTMS treatment, 5 times a week for a total of 35 treatments or until they met remission criteria. Those who finished at least 30 sessions or met remission criteria before 30 sessions were considered completers.
Data Analysis
Demographic and clinical characteristics were summarized using mean (SD) or N (%). Response and remission were defined as MADRS score reductions of ≥50% from baseline or score <10, respectively. Paired t-tests were used to assess pre- vs posttreatment completion clinical scales and scores. Univariate and multivariable longitudinal repeated-measure mixed models were used to examine predictors of the MADRS scores over the entire course of treatments, adjusted for treatment week. Gender, age, screening diagnosis, and concurrent medication were included as predictors. Concurrent medications were lamotrigine, lithium, anticonvulsant (excluding lamotrigine), or antipsychotic. Pairwise interactions were considered but were nonsignificant and not included in the results. A post hoc multivariable model included effects of week of treatment, age, screening diagnosis, lamotrigine, and lithium. Kaplan–Meier curves were generated to show the relationship between baseline and remission (including follow-up time). A standard unadjusted level of significance was used (P value < .05). Data management and statistical analysis were conducted using SAS version 9.4 (SAS Institute Inc, Cary, NC) and R version 4.1.2 (RStudio Team 2021, Boston, MA).
RESULTS
Participant Demographic and Clinical Outcome Measures
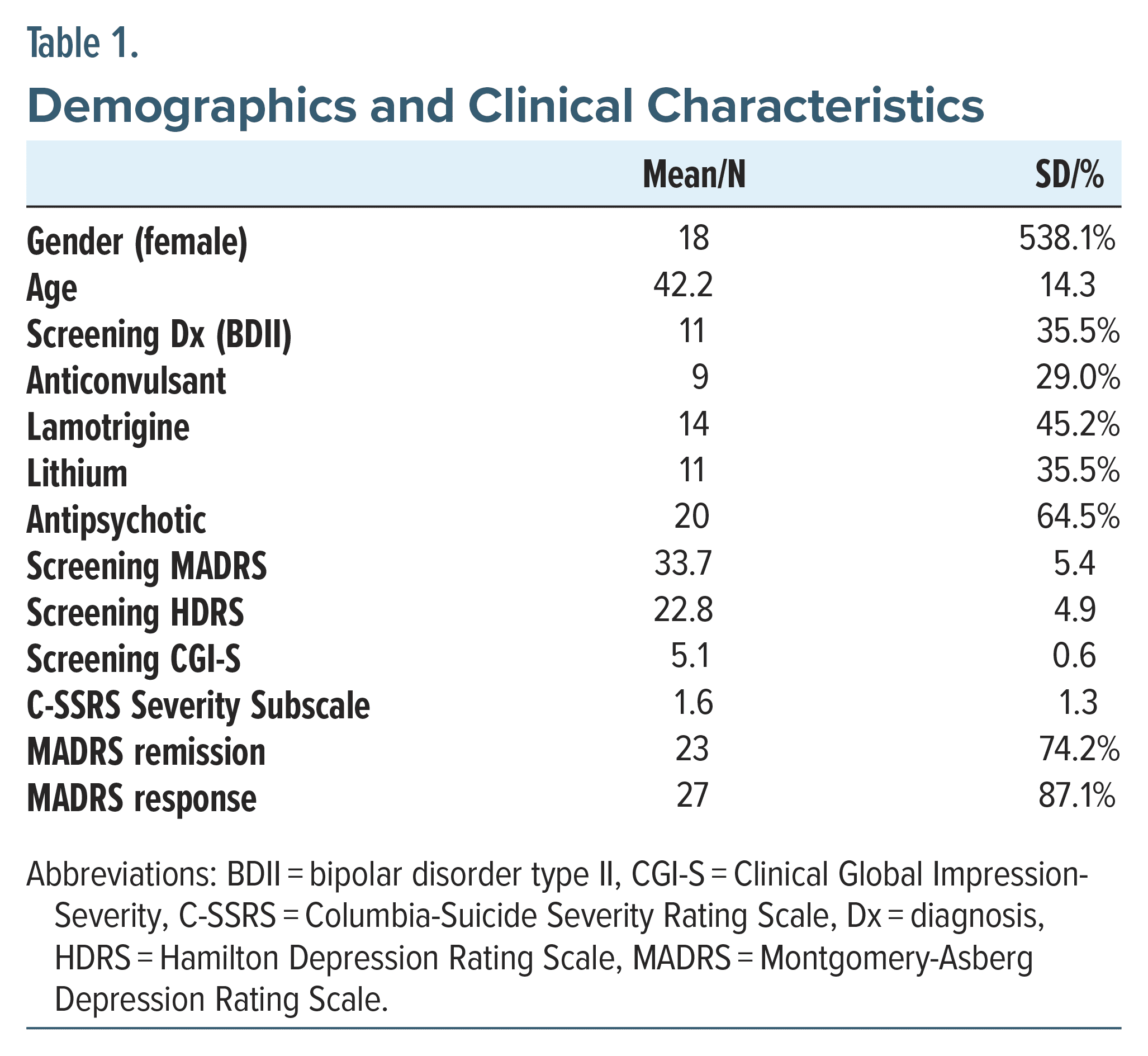
Demographic and clinical features (Table 1) included participants [N = 31; 18 female; age 42.2 (14.3)] recruited from 2 large outpatient psychiatric clinics. Eleven patients (35.5%) met diagnostic criteria for BDII, and the remaining were BDI.
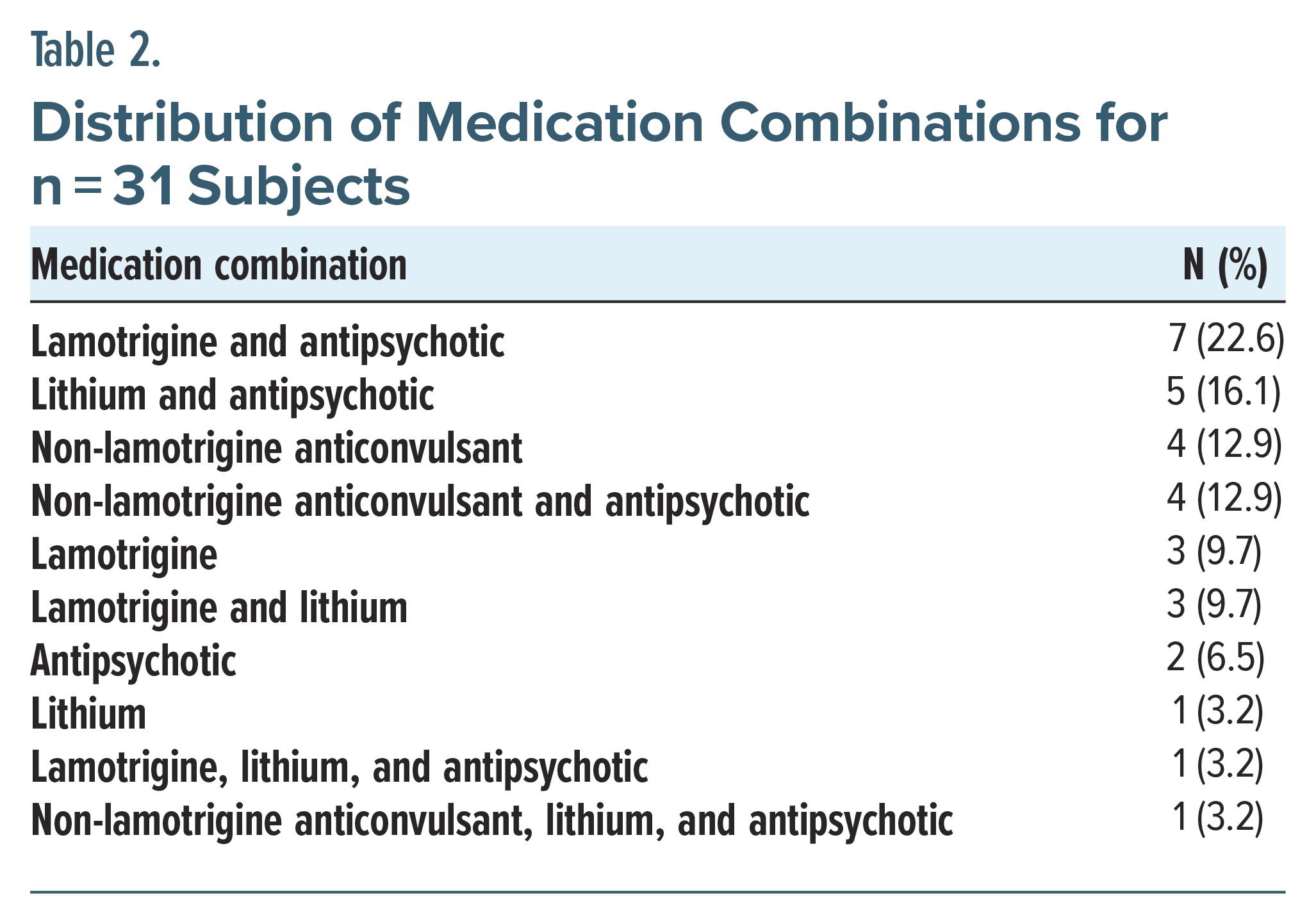
Psychopharmacologic use included N = 9 (29%) participants on an anticonvulsant, N = 14 (45.2%) on lamotrigine, N = 11 (35.5%) on lithium, and N = 20 (64.5%) on an antipsychotic regimen, maintained prior to treatment intervention. Details of the medication combinations are shown in Table 2.
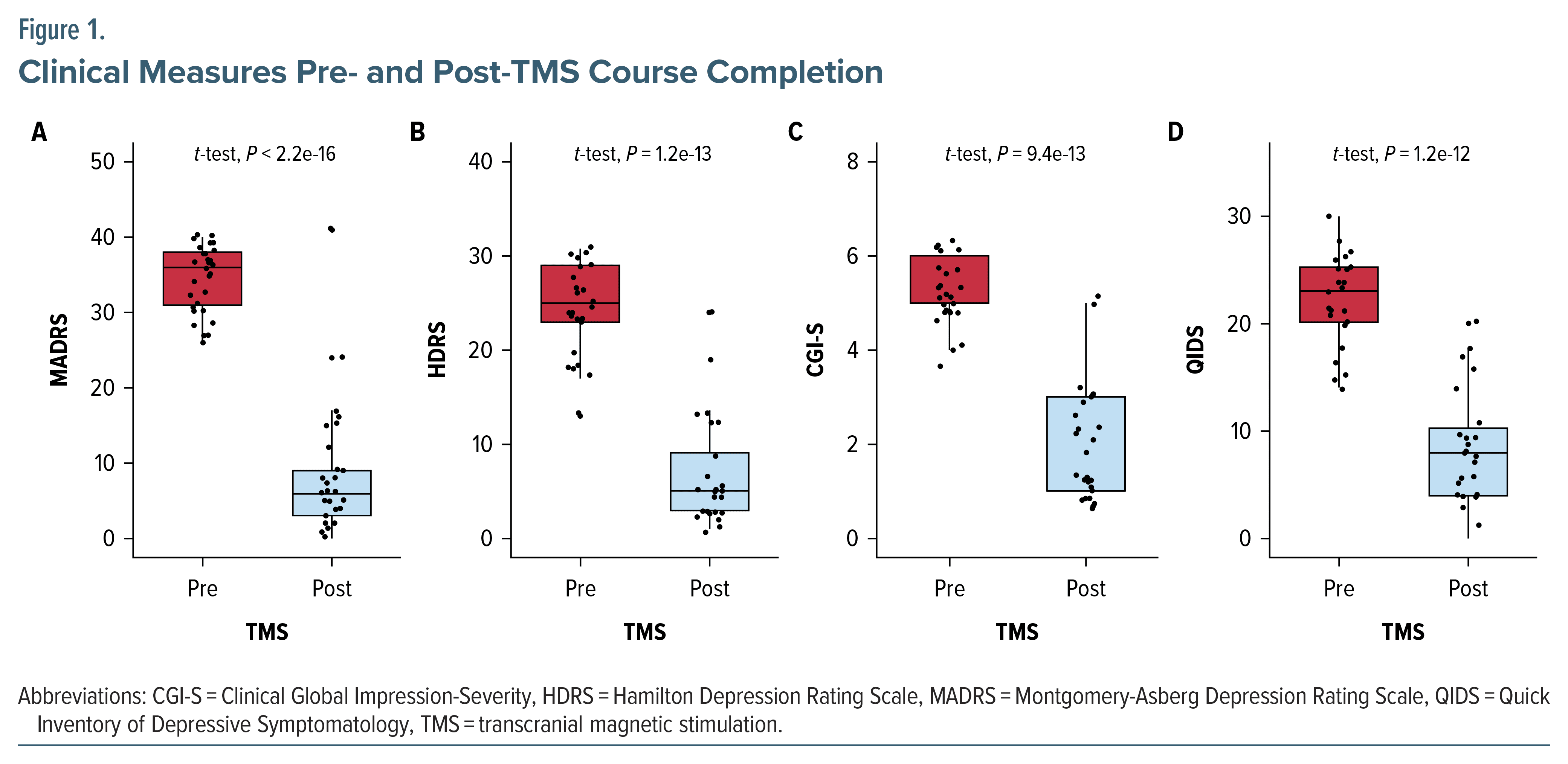
Remission and response rates to rTMS based on MADRS score were 74.2% and 87.1%, respectively, across the entire cohort. Figure 1 represents the pre- vs posttreatment outcomes to time (in number of rTMS sessions) to completion from the MADRS (Figure 1A), HDRS (Figure 1B), CGI-S (Figure 1C), and QIDS (Figure 1D), all P < .001. The timing of response across the entire cohort (Supplementary Figure 1A) and grouped by gender (Supplementary Figure 1B) are also presented, showing the largest reductions toward response and remission to occur after 4 weeks from baseline.
rTMS Effectiveness and Psychopharmacologic Considerations
Of the 31 patients who began treatment, 29 completed treatment. One patient dropped out due to feeling uncomfortably stimulated but was not manic or mixed, and the other due to not being able to continue with daily treatments.
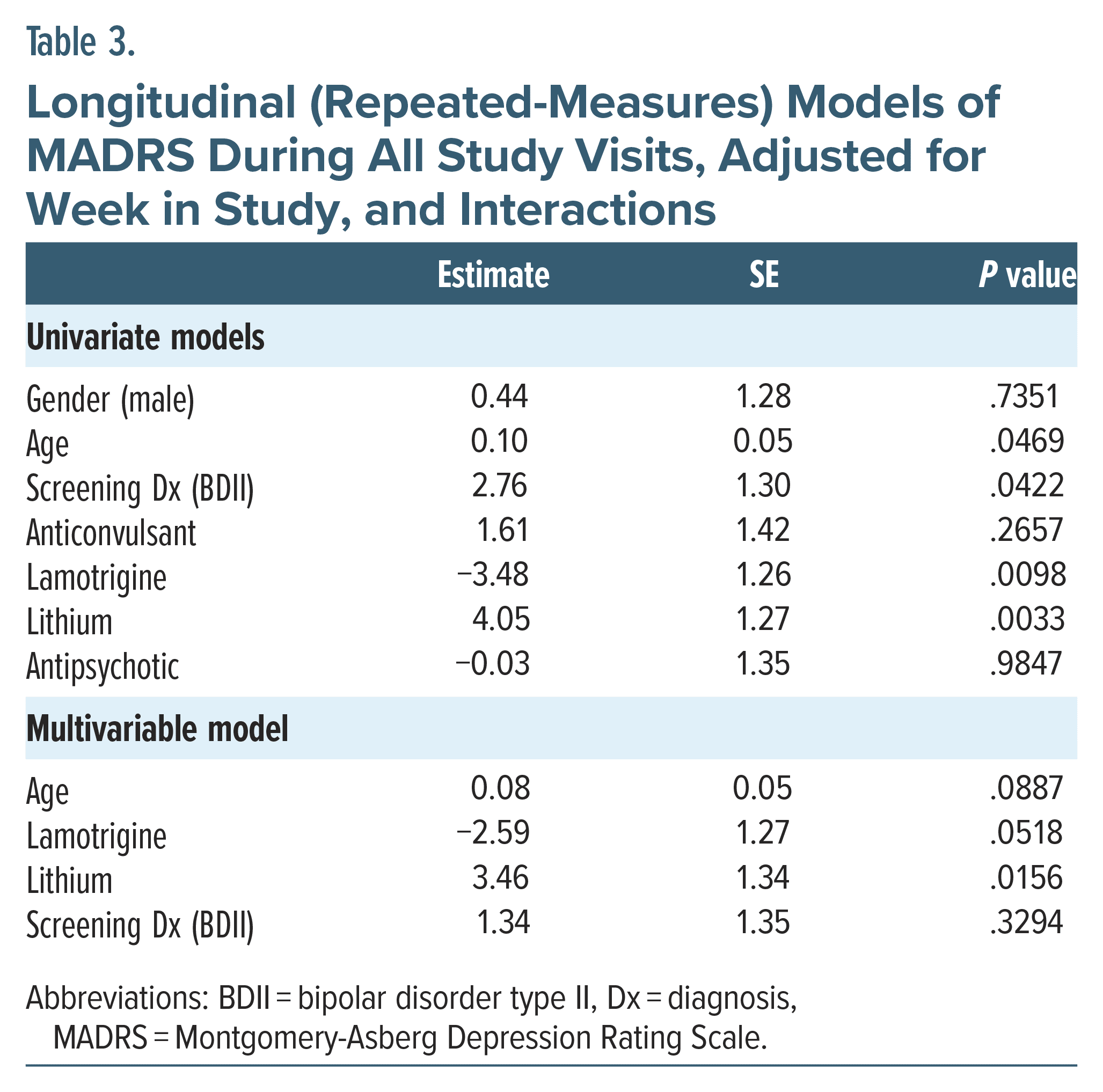
Significant associations were observed on repeated measures across the study longitudinally (Table 3). Age had a weak but appreciable effect (0.1 ± 0.05, P = .05) on treatment outcome. The usage of lamotrigine and lithium was strongly associated with responsiveness to treatment, in both univariate (−3.84 ± 1.26, P = .01; 4.05 ± 1.27, P = .003, respectively) and post hoc multivariate (−2.59 ± 1.27, P = .05; 3.46 ± 1.34, P = .02, respectively) models. No significant associations were observed for gender, anticonvulsant, or antipsychotic usage (P = .27–.98) on univariate modeling. Lastly, BDII diagnosis was significant on univariate analysis to favorably benefit from rTMS (2.76 ± 1.30, P = .04) which did not remain significant on multivariate testing (1.34 ± 1.35, P = .33).
Sustainability, Safety, and Tolerability of rTMS for Bipolar Depression
There were no significant adverse events except for 1 patient with increased agitation who felt uncomfortably stimulated and dropped out after 1 week. Another patient experienced worsening sleep and paused treatment for 1 week to increase her lithium. She completed 7 weeks of treatment and was a responder. Both of these patients had BDI. None of the patients had any increase in YMRS scores to suggest switch into mania, and the scores improved as the treatments continued (Table 4). No differences in response were observed between the 2 study sites.
Suicidality as a Symptom Outcome
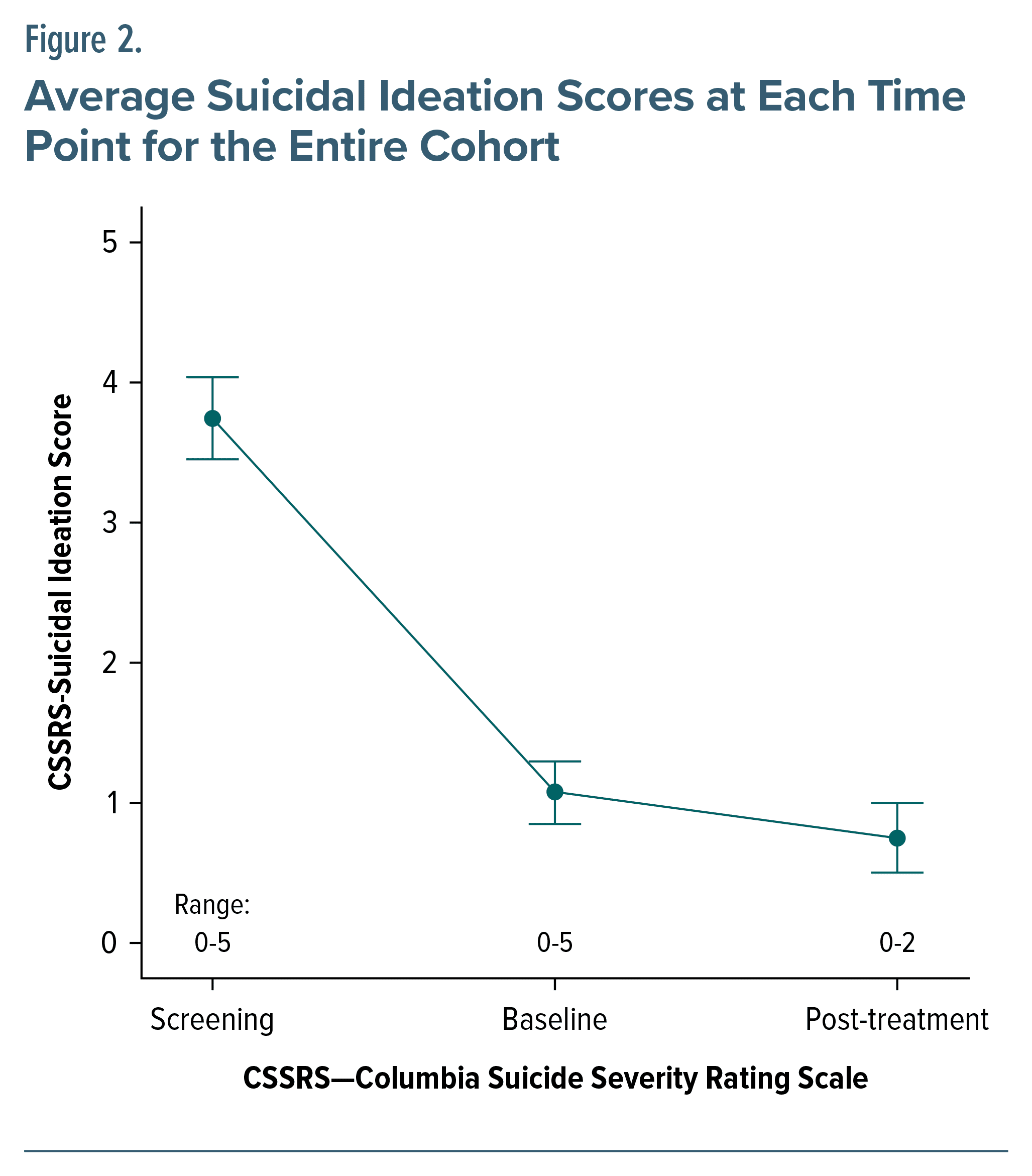
Suicidal ideation was assessed secondarily from the C-SSRS and found to be decreased (3.74 to 0.75) from screening to treatment culmination (Figure 2).
DISCUSSION
rTMS is being incorporated into standard treatment algorithms for unipolar depression at an exciting pace. Despite growing attention from clinical research communities for administration in bipolar depression, evidence is still lacking in similarly rigorous randomized clinical trials like that performed for unipolar depression. For this purpose, our group sought to contribute to the mounting support for formal clinical trials of rTMS for bipolar depression. In our open-label pilot trial, we prospectively treated 31 BDI and BDII patients with high-frequency 10-Hz rTMS to the left dorsolateral prefrontal cortex. Exceptional remission and esponse rates were achieved, 74.2% and 87.1%, respectively, supporting its effectiveness as a treatment. In terms of safety and tolerability, no serious adverse events, including treatment-emergent affective switching to hypomania or mania, were observed at any point in the protocol.
Remission obtained by rTMS for treatment-resistant unipolar depression typically requires 6–7 weeks.1,42 Interestingly, our data suggest remission prior to week 5 in bipolar depression. This remarkable finding underscores the impact that rTMS may have on bipolar depression, not only by providing a new modality but also one that may provide faster symptom relief in a safe and tolerable manner. Moreover, on subanalysis, BDI appeared to clinically improve approximately 5 treatment sessions prior to BDII. Despite not achieving statistical significance, this finding may be clinically relevant in important ways, including pointing to BDII as a closer biological correlate to unipolar depression than BDI, given their more similarly achieved response rates here.
Another important and fascinating result was the differential interaction observed between older age and concurrent treatment with lithium and lamotrigine with MADRS scores throughout the course of treatment. Older age and concurrent treatment with lithium were associated with higher MADRS scores; meanwhile, concurrent treatment with lamotrigine was associated with lower MADRS scores throughout the treatment course. These novel findings leave uncertainty as to the role that mood stabilizers may play in rTMS for bipolar depression, although it may be reasonable to suggest that mood stabilization was protective against the devastating side effect of treatment-emergent affective switching, as none was seen in this study. It is premature and incomplete to offer recommendations based on this finding, as this was not a primary aim of this study, but should be considered in further analysis and trials to optimize all treatment parameters, including psychopharmacology. Of note, the bimodal distribution of high response rates and low response rates is similar to those seen in other rTMS open-label and randomized controlled studies,8,30,31,43 even in those failing to show clinical difference from experimental treatment groups.
This further illustrates the complicated landscape from which to interpret our findings. Nonetheless, our data support a potential biological difference in certain depressive illnesses that are more amenable versus more resistant to effects of rTMS.
Lastly, suicidality improved as well. Although not a primary aim of this study, this encouraging result supports current research targeting suicidal ideation44 in major depression using novel rTMS protocols45 that may be even more efficacious in bipolar depression given the improved outcomes over unipolar depression. Caution is still required when interpreting these findings, especially given the recent attempts at using intermittent theta burst stimulation rather than standard rTMS in the treatment of bipolar depression8 which required cessation of the trial due to futility at 4 weeks of treatment. Several important differences exist between our study and that of McGirr and colleagues,8 which may play a contributing role toward these disparate outcomes. It may be reasonable to consider the method of treatment delivery, that is, intermittent theta burst stimulation versus standard rTMS, as a potentially different mechanism of action, akin to the different mechanisms of action of antidepressants versus mood stabilizers. Indeed, alternative dosing and delivery strategies exist in the field as a whole, and whether the so-called “inhibitory” effects of continuous theta burst stimulation may be a viable alternative to intermittent theta burst stimulation to the left DLPFC remain to be determined as well. More research is needed to fully discern these interesting results.
One of the strengths of this study is that stimulation parameters were set and delivered using the standard rTMS protocols for unipolar depression, so that the results could be compared to extant literature in unipolar depression outcomes. Knowing that there are many different stimulation parameters used even in bipolar disorders,6 the use of standard unipolar depression parameters would be beneficial to the field for comparison and replication.
We previously conducted a retrospective analysis of a separate cohort34 of 44 patients with bipolar depression treated with rTMS. We showed that for bipolar depression overall, 77% and 41% met response and remission criteria, respectively, which surpassed typical outcomes seen in unipolar depression, and also without treatment-emergent affective switching. The present study furthers this optimism with encouraging findings to support the use of high-frequency rTMS to the left DLPFC in bipolar depression.
LIMITATIONS
The small sample size limited abilities to perform proper subgroup analyses, despite the encouraging results in BDI and BDII, gender, and age. Concurrent mood stabilizers in bipolar depression have not been clearly established in terms of well-sourced guidelines, and the differential responses from lamotrigine and lithium presented here are not readily explainable. Without control groups inherent in the design, our interpretations remain largely speculative, even though consistently reassuring improvements in symptom and benefits from rTMS have been found. Another caveat is that extrapolations from rTMS paradigms used in unipolar depression such as deep rTMS with an H1 coil or intermittent theta burst stimulation protocols may not generate the same degree of response as the more standard rTMS protocol that was used here. With future studies fully powdered and amply sized, dropout rates and adverse events can be more properly assessed. Despite this limitation, the dropout rate due to adverse events in our study was 1 in 31 patients, roughly 3%.
CONCLUSIONS
The remarkably positive results at 2 sites suggest that rTMS may well be an effective treatment for bipolar depression. The higher response rates than seen in unipolar depression suggest that bipolar disorder is more likely to be a better biological target. Combined with the current data, we again demonstrate that rTMS in bipolar depression may be a highly effective and safe treatment strategy and requires further investigations in a fully powered, blinded, sham-controlled, randomized clinical trial.
Article Information
Published Online: May 20, 2024. https://doi.org/10.4088/JCP.23m15056
© 2024 Physicians Postgraduate Press, Inc.
Submitted: August 8, 2023; accepted January 25, 2024.
To Cite: Aaronson ST, Goldwaser EL, Croarkin PE, et al. A pilot study of high-frequency transcranial magnetic stimulation for bipolar depression.
J Clin Psychiatry. 2024;85(2):23m15056.
Author Affiliations: Institute for Advanced Diagnostics and Therapeutics, Sheppard Pratt Health System, Baltimore, Maryland (Aaronson, Sklar); Department of Psychiatry, Interventional Psychiatry Program, Weill Cornell Medicine, New York, New York (Goldwaser); Department of Psychiatry and Psychology, Mayo Clinic, Rochester, Minnesota (Croarkin, Kung, Geske, LeMahieu).
Corresponding Author: Scott T. Aaronson, MD, Institute for Advanced Diagnostics and Therapeutics, Sheppard Pratt Health System, 6501 N Charles St, Baltimore, MD 21204 ([email protected]).
Drs Aaronson and Goldwaser are co-first authors.
Relevant Financial Relationships: Dr Aaronson has received research support from Compass Pathways and Neuronetics and serves as a consultant for Neuronetics, LivaNova, Sage Therapeutics, Janssen, and Genomind. Dr Croarkin has received equipment support from Neuronetics, Inc, and received supplies and genotyping services from Assurex Health, Inc, for investigator-initiated studies. He was the primary investigator for a multicenter study funded by Neuronetics, Inc, and a site primary investigator for a study funded by NeoSync, Inc. Dr Croarkin served as a paid consultant for Procter & Gamble Company and Myriad Neuroscience. Drs Goldwaser and Kung and Mss Geske, LeMahieu, and Sklar have no disclosures to report.
Funding/Support: The study was funded by Sheppard Pratt Philanthropic Funds. Neuronetics provided equipment support through the investigator-initiated trial program. Dr Croarkin was supported under NIH grant R01MH113700.
Role of the Sponsor: The supporters of the study had no role in the design, analysis, interpretation, or publication of the manuscript.
Disclaimer: The content of this publication is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Previous Presentation: Presented in part at the annual meeting of the American College of Neuropsychopharmacology, Orlando, Florida, December 11, 2019.
Supplementary Material: Available at Psychiatrist.com.
Clinical Points
- Few treatment options exist for patients with bipolar depression type I or II, despite the accompanying high levels of morbidity and mortality.
- In this open-label trial, repetitive transcranial magnetic stimulation (rTMS) demonstrated remarkably positive results on depressive symptoms with few adverse events.
- rTMS should be further investigated as a treatment for bipolar depression in a fully powered, blinded, sham controlled, randomized trial.
References (45)

- O’Reardon JP, Solvason HB, Janicak PG, et al. Efficacy and safety of transcranial magnetic stimulation in the acute treatment of major depression: a multisite randomized controlled trial. Biol Psychiatry. 2007;62(11):1208–1216. PubMed
- Roth Y, Barnea-Ygael N, Carmi L, et al. Deep transcranial magnetic stimulation for obsessive-compulsive disorder is efficacious even in patients who failed multiple medications and CBT. Psychiatry Res. 2020;290:113179. PubMed CrossRef
- Pell GS, Harmelech T, Zibman S, et al. Efficacy of deep TMS with the H1 coil for anxious depression. J Clin Med. 2022;11(4):1015. PubMed
- Li X, Hartwell KJ, Henderson S, et al. Two weeks of image-guided left dorsolateral prefrontal cortex repetitive transcranial magnetic stimulation improves smoking cessation: a double-blind, sham-controlled, randomized clinical trial. Brain Stimul. 2020;13(5):1271–1279. PubMed CrossRef
- Rush AJ, Aaronson ST, Demyttenaere K. Difficult-to-treat depression: a clinical and research roadmap for when remission is elusive. Aust N Z J Psychiatry. 2019;53(2):109–118. PubMed CrossRef
- Gold AK, Ornelas AC, Cirillo P, et al. Clinical applications of transcranial magnetic stimulation in bipolar disorder. Brain Behav. 2019;9(10):e01419. PubMed CrossRef
- McGirr A, Karmani S, Arsappa R, et al. Clinical efficacy and safety of repetitive transcranial magnetic stimulation in acute bipolar depression. World Psychiatry. 2016;15(1):85–86. PubMed CrossRef
- McGirr A, Vila-Rodriguez F, Cole J, et al. Efficacy of active vs sham intermittent theta burst transcranial magnetic stimulation for patients with bipolar depression: a randomized clinical trial. JAMA Netw Open. 2021;4(3):e210963. PubMed CrossRef
- Merikangas KR, Jin R, He JP, et al. Prevalence and correlates of bipolar spectrum disorder in the world mental health survey initiative. Arch Gen Psychiatry. 2011;68(3):241–251. PubMed CrossRef
- Ferrari AJ, Stockings E, Khoo JP, et al. The prevalence and burden of bipolar disorder: findings from the Global Burden of Disease Study 2013. Bipolar Disord. 2016;18(5):440–450. PubMed CrossRef
- Crump C, Sundquist K, Winkleby MA, et al. Comorbidities and mortality in bipolar disorder: a Swedish national cohort study. JAMA Psychiatry. 2013;70(9):931–939. PubMed CrossRef
- Nordentoft M, Mortensen PB, Pedersen CB. Absolute risk of suicide after first hospital contact in mental disorder. Arch Gen Psychiatry. 2011;68(10):1058–1064. PubMed CrossRef
- Schaffer A, Isometsä ET, Tondo L, et al. International Society for Bipolar Disorders Task Force on Suicide: meta-analyses and meta-regression of correlates of suicide attempts and suicide deaths in bipolar disorder. Bipolar Disord. 2015;17(1):1–16. PubMed CrossRef
- Sachs GS, Thase ME, Otto MW, et al. Rationale, design, and methods of the systematic treatment enhancement program for bipolar disorder (STEP-BD). Biol Psychiatry. 2003;53(11):1028–1042. PubMed CrossRef
- Earley W, Burgess MV, Rekeda L, et al. Cariprazine treatment of bipolar depression: a randomized double-blind placebo-controlled Phase 3 study. Am J Psychiatry. 2019;176(6):439–448. PubMed CrossRef
- Nierenberg AA, McIntyre RS, Sachs GS. Improving outcomes in patients with bipolar depression: a comprehensive review. J Clin Psychiatry. 2015;76(3):e10. PubMed CrossRef
- Calabrese JR, Durgam S, Satlin A, et al. Efficacy and safety of lumateperone for major depressive episodes associated with bipolar I or bipolar II disorder: a phase 3 randomized placebo-controlled trial. Am J Psychiatry. 2021;178(12):1098–1106. PubMed CrossRef
- Sachs GS, Nierenberg AA, Calabrese JR, et al. Effectiveness of adjunctive antidepressant treatment for bipolar depression. N Engl J Med. 2007;356(17):1711–1722. PubMed CrossRef
- Goldberg JF, Perlis RH, Ghaemi SN, et al. Adjunctive antidepressant use and symptomatic recovery among bipolar depressed patients with concomitant manic symptoms: findings from the STEP-BD. Am J Psychiatry. 2007;164(9):1348–1355. PubMed CrossRef
- El-Mallakh RS, Vöhringer PA, Ostacher MM, et al. Antidepressants worsen rapid cycling course in bipolar depression: a STEP-BD randomized clinical trial. J Affect Disord. 2015;184:318–321. PubMed CrossRef
- Bottlender R, Rudolf D, Strauss A, et al. Antidepressant-associated maniform states in acute treatment of patients with bipolar-I depression. Eur Arch Psychiatry Clin Neurosci. 1998;248(6):296–300. PubMed CrossRef
- Harel EV, Zangen A, Roth Y, et al. H-coil repetitive transcranial magnetic stimulation for the treatment of bipolar depression: an add-on, safety and feasibility study. World J Biol Psychiatry. 2011;12(2):119–126. PubMed CrossRef
- Dolberg OT, Dannon PN, Schreiber SS, et al. Transcranial magnetic stimulation in patients with bipolar depression: a double blind, controlled study. Bipolar Disord. 2002;4(supp 1):94–95. PubMed CrossRef
- Calabrese JR, Bowden CL, Sachs GS, et al. A double-blind placebo-controlled study of lamotrigine monotherapy in outpatients with bipolar I depression. Lamictal 602 Study Group. J Clin Psychiatry. 1999;60(2):79–88. PubMed CrossRef
- Calabrese JR, Bowden CL, Sachs G, et al. A placebo-controlled 18-month trial of lamotrigine and lithium maintenance treatment in recently depressed patients with bipolar I disorder. J Clin Psychiatry. 2003;64(9):1013–1024. PubMed CrossRef
- Thase ME. Bipolar depression: issues in diagnosis and treatment. Harv Rev Psychiatry. 2005;13(5):257–271. PubMed CrossRef
- Nemeroff CB, Evans DL, Gyulai L, et al. Double-blind, placebo-controlled comparison of imipramine and paroxetine in the treatment of bipolar depression. Am J Psychiatry. 2001;158(6):906–912. PubMed CrossRef
- Xia G, Gajwani P, Muzina DJ, et al. Treatment-emergent mania in unipolar and bipolar depression: focus on repetitive transcranial magnetic stimulation. Int J Neuropsychopharmacol. 2008;11(1):119–130. PubMed CrossRef
- Blumberger DM, Vila-Rodriguez F, Thorpe KE, et al. Effectiveness of theta burst versus high-frequency repetitive transcranial magnetic stimulation in patients with depression (THREE-D): a randomised non-inferiority trial. Lancet. 2018;391(10131):1683–1692. PubMed CrossRef
- Hu SH, Lai JB, Xu DR, et al. Efficacy of repetitive transcranial magnetic stimulation with quetiapine in treating bipolar II depression: a randomized, double-blinded, control study. Sci Rep. 2016;6:30537. PubMed CrossRef
- Fitzgerald PB, Hoy KE, Elliot D, et al. A negative double-blind controlled trial of sequential bilateral rTMS in the treatment of bipolar depression. J Affect Disord. 2016;198:158–162. PubMed CrossRef
- Hebel T, Abdelnaim M, Deppe M, et al. Attenuation of antidepressive effects of transcranial magnetic stimulation in patients whose medication includes drugs for psychosis. J Psychopharmacol. 2020;34(10):1119–1124. PubMed CrossRef
- Hebel T, Abdelnaim MA, Deppe M, et al. Antidepressant effect of repetitive transcranial magnetic stimulation is not impaired by intake of lithium or antiepileptic drugs. Eur Arch Psychiatry Clin Neurosci. 2021;271(7):1245–1253. PubMed CrossRef
- Goldwaser EL, Daddario K, Aaronson ST. A retrospective analysis of bipolar depression treated with transcranial magnetic stimulation. Brain Behav. 2020;10:e01805. PubMed CrossRef
- Montgomery SA, Åsberg M. A new depression scale designed to be sensitive to change. Br J Psychiatry. 1979;134:382–389. PubMed CrossRef
- Carmody TJ, Rush AJ, Bernstein I, et al. The Montgomery Asberg and the Hamilton ratings of depression: a comparison of measures. Eur Neuropsychopharmacol. 2006;16(8):601–611. PubMed CrossRef
- Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23(1):56–62. PubMed CrossRef
- Rush AJ, Trivedi MH, Ibrahim HM, et al. The 16-Item Quick Inventory of Depressive Symptomatology (QIDS), clinician rating (QIDS-C), and self-report (QIDS-SR): a psychometric evaluation in patients with chronic major depression. Biol Psychiatry. 2003;54(5):573–583. PubMed CrossRef
- Posner K, Brown GK, Stanley B, et al. The Columbia-Suicide Severity Rating Scale: initial validity and internal consistency findings from three multisite studies with adolescents and adults. Am J Psychiatry. 2011;168(12):1266–1277. PubMed CrossRef
- Young RC, Biggs JT, Ziegler VE, et al. A rating scale for mania: reliability, validity and sensitivity. Br J Psychiatry. 1978;133:429–435. PubMed CrossRef
- Guy W. ECDEU Assessment Manual for Psychopharmacology. US Department of Health, Education, and Welfare Publication (ADM). National Institute of Mental Health; 1976.
- Manzardo AM, Ely B, Davila MC. Time to remission analysis for major depressive disorder after repetitive transcranial magnetic stimulation (rTMS). Ment Illn. 2019;11(1):8141. PubMed CrossRef
- Bulteau S, Beynel L, Marendaz C, et al. Twice-daily neuronavigated intermittent theta burst stimulation for bipolar depression: a Randomized Sham-Controlled Pilot Study. Neurophysiol Clin. 2019;49(5):371–375. PubMed CrossRef
- Croarkin PE, Nakonezny PA, Deng ZD, et al. High-frequency repetitive TMS for suicidal ideation in adolescents with depression. J Affect Disord. 2018;239:282–290. PubMed CrossRef
- Williams NR, Sudheimer KD, Bentzley BS, et al. High-dose spaced theta-burst TMS as a rapid-acting antidepressant in highly refractory depression. Brain. 2018;141(3):e18. PubMed CrossRef
This PDF is free for all visitors!
Save
Cite