A recent study of the relationship between tamsulosin and dementia found that, in propensity score-matched analyses that adjusted for measured risk factors for dementia, the use of tamsulosin to treat benign prostatic hyperplasia (BPH) was associated with a small but significant increase in the risk of incident dementia relative to untreated BPH, BPH treated with the 5α-reductase inhibitors dutasteride and finasteride, and BPH treated with the α adrenoceptor blockers doxazosin, terazosin, and alfuzosin. The choice of control groups addressed confounding by indication, confounding by disease severity, and confounding by pharmacologic drug class. The authors of the study provided a wealth of detail in their main paper and in supplementary materials, allowing an almost forensic examination of the findings. This article discusses the study from the perspective of whether the hypothesis relating tamsulosin to dementia was set a priori or emerged after an exploratory exercise; whether tamsulosin crosses the blood-brain barrier for dementia as the event of interest to be attributable to treatment; whether the action of tamsulosin is plausibly related to dementia as a possible outcome; whether confounding was adequately addressed in the analyses; whether the duration of follow-up was sufficient for the event of interest to be attributable to tamsulosin; whether the absolute increase in the rate of incident dementia was clinically significant; and what the curiosities in the dose-dependence and subgroup analysis findings could imply. These matters provide considerable food for thought. Thus, this article is intended to serve as an example of how to read a paper critically and how to think about what the findings might suggest.

ABSTRACT
A recent study of the relationship between tamsulosin and dementia found that, in propensity score-matched analyses that adjusted for measured risk factors for dementia, the use of tamsulosin to treat benign prostatic hyperplasia (BPH) was associated with a small but significant increase in the risk of incident dementia relative to untreated BPH, BPH treated with the 5α-reductase inhibitors dutasteride and finasteride, and BPH treated with the α adrenoceptor blockers doxazosin, terazosin, and alfuzosin. The choice of control groups addressed confounding by indication, confounding by disease severity, and confounding by pharmacologic drug class. The authors of the study provided a wealth of detail in their main paper and in supplementary materials, allowing an almost forensic examination of the findings. This article discusses the study from the perspective of whether the hypothesis relating tamsulosin to dementia was set a priori or emerged after an exploratory exercise; whether tamsulosin crosses the blood-brain barrier for dementia as the event of interest to be attributable to treatment; whether the action of tamsulosin is plausibly related to dementia as a possible outcome; whether confounding was adequately addressed in the analyses; whether the duration of follow-up was sufficient for the event of interest to be attributable to tamsulosin; whether the absolute increase in the rate of incident dementia was clinically significant; and what the curiosities in the dose-dependence and subgroup analysis findings could imply. These matters provide considerable food for thought. Thus, this article is intended to serve as an example of how to read a paper critically and how to think about what the findings might suggest.
J Clin Psychiatry 2018;79(6):18f12660
To cite: Andrade C. How to read a research paper: an exercise in critical thinking in the context of an epidemiologic study on tamsulosin and the risk of dementia. J Clin Psychiatry. 2018;79(6):18f12660.
To share: https://doi.org/10.4088/JCP.18f12660
© Copyright 2018 Physicians Postgraduate Press, Inc.
Benign prostatic hyperplasia (BPH) is a common condition in the aging male. Pathological evidence of BPH is present in 8% of men in the fourth decade of life and in 50% of those in the sixth decade.1 The prevalence of BPH with lower urinary tract symptoms (LUTS) varies widely, depending on the operational definition of the disorder and on the risk factors in the sample.2-4
A peak urinary flow rate of 20 mL/s and above is generally considered normal. In this context, a community-based study found that 6% of men aged 40-44 years had peak urinary flow rates that were < 10 mL/s; this figure was 35% in men aged 75-79 years.5 In the same study, 13% of men aged 40-49 years and 28% of men aged > 70 years had moderate to severe LUTS, as assessed using a validated urinary symptom scale.6 A recent review concluded that the prevalence of BPH/LUTS was 50%-75% in men aged 50 years and above and 80% in men aged 70 years and above.3
Six classes of drugs are used to treat BPH7; the commonest are the α blockers and the 5α-reductase (5-ARI) inhibitors.8 One among these, tamsulosin, was recently associated with an increased risk of incident (new onset) dementia.9,10
Medical Treatment of Benign Prostatic Hyperplasia and the Risk of Incident Dementia
Duan et al9 described an epidemiologic investigation of tamsulosin and the risk of dementia in older men with BPH. They hypothesized that, because tamsulosin is an α1-adrenoceptor antagonist and because these receptors are found in the brain and are involved in cognition, tamsulosin might adversely affect cognitive outcomes.
They used US Medicare data for the years 2006-2012 to identify and follow men aged 65 years and above who had been prescribed α blockers and 5-ARIs for BPH. No subject had dementia at baseline. Propensity score matching (1:1) was used to compare men who had filled prescriptions for tamsulosin (n = 253,136) with men who had received no BPH medication (n = 180,926); men who had filled prescriptions for the α blockers doxazosin (n = 28,581), terazosin (n = 23,858), or alfuzosin (n = 17,934); and men who had filled prescriptions for the 5-ARIs dutasteride (n = 34,027) or finasteride (n = 38,767). Men in all cohorts had a diagnosis of BPH, including the men who had received no BPH treatment. The median follow-up for all cohorts was 19.8 months; follow-up ended if dementia was diagnosed, and follow-up was censored if the subject switched treatments for BPH, died, lost Medicare coverage, or reached the end of the study period without event.
Cox proportional hazard regressions were conducted to compare outcomes in tamsulosin vs each of the propensity score-matched comparison cohorts. The analyses were adjusted for sociodemographic variables and risk factors for dementia, including age, sex, medical comorbidities, other medication use, health care service utilization, and other potential confounds.
The main findings from the study are presented in Table 1. In summary, the risk of incident dementia (based on ICD-9 codes) was higher in men with BPH who had received tamsulosin than in men with BPH who had not received any medication for BPH. Furthermore, tamsulosin was associated with higher risks of dementia than each of the other 5 treatments for BPH. In all cases, the increase in risk was small but statistically significant. The significance of these findings persisted in a sensitivity analysis, where dementia was more stringently diagnosed, and when dose-dependent effects were examined. The authors9 concluded that “tamsulosin may increase the risk of dementia in older men with BPH.”
Note to the Reader
While reading the rest of this article, the reader is strongly encouraged to keep the paper by Duan et al9 and the supplementary materials at hand in order to better follow the discussion. However, the discussion is understandable even if the reader does not have access to the original materials. The discussion is intended to cue readers toward critical thought. There is no intention in this article to diminish the intelligent and painstaking efforts of the authors.
With the exception of the important discussion on confounding, this article will not address standard criticisms of studies of this nature. As examples of common limitations of such studies, an ICD-9 diagnosis of dementia that is extracted from records may not be accurate; other important variables may also have been inaccurately recorded; variables of importance, including those related to dementia risk, may not have been available for extraction; and subjects who filled prescriptions for a drug may not have actually taken the drug.
Thinking Critically: 1
Now here are some big questions. There are plenty of neurotransmitters and neuroreceptors that are implicated in cognition and plenty of drugs for medical conditions that also act on neurotransmitter systems in the brain. The curious reader will therefore want to know what made the authors choose to study tamsulosin with dementia as a possible outcome when there wasn’ t even a whiff of previous evidence to suggest that they might hit pay dirt. Was the hypothesis set a priori? Did the authors take a huge gamble? Or did the finding emerge in a general “let’s look and see what we find” exercise?
The authors explicitly stated in the paper that their study had been approved by an institutional review board. This implies the existence of preset hypotheses and possibly even a detailed plan of analysis. They also acknowledged a colleague for his research that inspired the study. However, they provided no further details of what this research was and how it pointed them to tamsulosin and dementia.
Correspondence with the lead and corresponding authors yielded the information that the research team was interested in α1-blockers and that they planned to study this drug in the context of a heterogeneous group of 4 psychiatric disorders: posttraumatic stress disorder, depression, substance use disorders, and dementia. Effectively, therefore, this was an exploratory or hypothesis-generating study rather than a hypothesis-testing or hypothesis-confirming study, something that did not emerge clearly in the paper.
Thinking Critically: 2
Drugs do not need to cross the blood-brain barrier (BBB) to harm the brain. For example, a drug can cause metabolic and hormonal disturbances that can result in secondary central nervous system effects. However, tamsulosin does not have peripheral actions that could obviously affect the brain. So, in order to cause dementia, such as by acting on α1 receptors in brain as suggested by Duan et al,9 tamsulosin should cross the BBB.
Systemically administered tamsulosin inhibited centrally driven bulbospongiosus muscle contractions in a rat model,11,12 suggesting that tamsulosin has CNS activity. However, there was negligible binding of radiolabeled tamsulosin in the cerebral cortex after systemic administration, again in a rat model.13 Thus, results are conflicting in animal studies. Whether tamsulosin crosses the BBB in humans is presently unknown. If tamsulosin does not cross the BBB in humans, it is unlikely to be causally responsible for dementia that occurs after treatment initiation.
Thinking Critically: 3
What could the reason be for dementia caused by tamsulosin? The authors9 offered the explanation that, unlike the other α blockers that are used to treat BPH, tamsulosin has a high affinity for the α1a receptor; this receptor is (also) found in the brain and has been implicated in cognition. Because tamsulosin is associated with an increased risk of the intraoperative floppy iris syndrome, over and above that related to other α1-receptor blockers,14 it may also be associated with unique adrenoceptor-related effects, such as cognitive adverse effects.
However, tamsulosin has so far not been shown to produce cognitive disturbances. In fact, it has been shown to improve learning and memory in 2 different animal models.15 It is also not clear why affinity for α1a receptors should result in the neuropathological changes that result in dementia. These considerations suggest that the explanatory mechanisms are weak. If there is no biological plausibility, by the Bradford Hill criteria it is unlikely that a demonstrated association illustrates a cause-and-effect relationship.16
Thinking Critically: 4
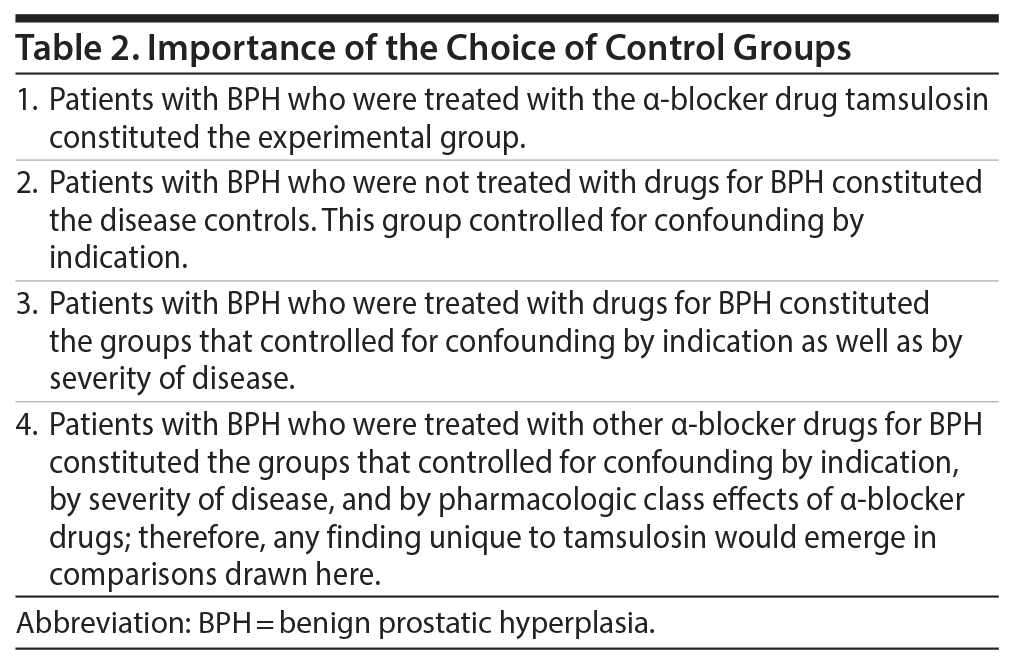
On the surface, the findings in the study appear impressive. Tamsulosin was associated with a higher risk of incident dementia than untreated disease controls. Tamsulosin was also associated with a higher risk than treated disease controls, including those who received treatment with other agents belonging to the same pharmacologic category, that is, α blockers (Table 2). Furthermore, these findings were obtained in propensity score-matched analyses that adjusted for risk factors for dementia. Importantly, as shown in the supplementary materials, none of the other BPH medications, including the α blockers, were associated with an increased risk of dementia in propensity score-matched analyses. Thus, confounding by indication may have been substantially addressed by excellent choices of control groups and well thought out analyses, suggesting that tamsulosin may have been causally responsible for the dementia.
These arguments notwithstanding, the conservative reader will immediately realize that, no matter how cleverly chosen the control groups,17 causality cannot be concluded from nonrandomized observational studies; thus, all that this study establishes is that there is an association between tamsulosin treatment and incident dementia. The association can be mediated by confounding variables; cause vs association in nonrandomized observational studies was discussed and explained in an earlier article in this column18 and elsewhere.19,20 Supporting such a conservative interpretation is the authors’ acknowledgment that the tamsulosin cohort differed from the other cohorts in several regards. At baseline, the tamsulosin cohort had higher rates of congestive cardiac failure, ischemic heart disease, cerebrovascular disease, peripheral vascular disease, Parkinson’s disease, traumatic brain injury, and depression. The tamsulosin cohort also had higher rates of other medication use and use of health care services. The data were presented in the supplementary materials.
Propensity score matching ironed out these differences. Thus, the pairs of subgroups (tamsulosin vs no treatment; tamsulosin vs each α blocker; tamsulosin vs each 5-ARI) that were carved out of the main cohorts were closely similar with regard to the baseline characteristics that were risk factors for dementia. However, this does not mean that confounding was fully accounted for. All that propensity score matching does is match for measured variables. Propensity score matching cannot compensate for inadequately or inaccurately measured, unmeasured, and unknown confounds.21 Given the extent to which the tamsulosin cohort differed from the other cohorts on important dementia risk factors at baseline, it is reasonable to consider that residual confounding may have explained the observed association between tamsulosin and incident dementia.
Thinking Critically: 5
The median duration of follow-up was just 19.8 months. Is such a short duration of exposure and follow-up sufficient for a drug to produce dementia? It seems unlikely. This suggests that tamsulosin may have been preferentially prescribed to patients who had early symptoms of dementia.
In order to avoid confounding by indication related to the presence of early, undiagnosed disease, many authors exclude from analysis events that occur during the initial year of follow-up. As an example of a slightly different strategy, in an observational study of benzodiazepine use and the risk of incident dementia or cognitive decline, Gray et al22 excluded dementia diagnosed during the most recent year because of possible use of the drugs for the treatment of prodromal symptoms.
Whereas Duan et al9 did not provide the data in their paper, they did present cumulative incidence (of dementia) plots in the supplementary data file. In these plots, there was a linear increase in the cumulative incidence of dementia across time and the separation between tamsulosin and comparison cohorts became visually distinct only at around 48-72 months of follow-up. Thus, the median follow-up of 19.8 months does not limit the conclusion that the authors drew from their findings.
In a separate context related to follow-up duration, the risk of dementia increases with age, so case detection would increase in study participants who became older while being followed for longer periods. Although the authors did not present the mean follow-up durations for the cohorts in individual propensity score-matched analyses, these could be calculated (from the information provided in the tables) by dividing person-years of follow-up by the number of subjects in the cohort in each analysis. Eyeballing the values, it did appear that, in many of the tamsulosin vs other drug (but not vs no treatment) analyses, the follow-up was longer in the tamsulosin cohort. It is not clear to what extent this difference in the duration of follow-up influenced outcomes.
Thinking Critically: 6
If tamsulosin is indeed associated with an increased risk of incident dementia, the risk is very small. Consider: the incidence of dementia was 31.3 per 1,000 person-years in the tamsulosin cohort and 25.9 per 1,000 person-years in the untreated BPH cohort. Thus, tamsulosin was associated with a risk of (31.3-25.9), that is, 5.4 extra cases per 1,000 person-years of follow-up, or 1 extra case per 185 years of follow-up. This risk appears low.
The authors presented sufficient information in their tables for an inquisitive reader to calculate the tamsulosin-related risks in other contexts. By way of example, when incident dementia was more stringently diagnosed, relative to no treatment of BPH tamsulosin was associated with only 1 extra case per 400 person-years of follow-up. This means that even if tamsulosin truly increases the risk of incident dementia, the increase in risk is very small.
A note is made here that the calculations are based on person-years of follow-up and not person-years of treatment. The latter were not presented. Whereas the former was not explicitly stated in the article, it was clarified in a personal communication with the lead and corresponding authors.
Thinking Critically: 7
Duan et al9 examined whether the association between tamsulosin and dementia was dose-dependent. The defined daily dose (DDD) for tamsulosin was 0.4 mg. One to 30 DDDs was categorized as the reference group, 31-90 DDDs as low exposure, 91-360 DDDs as medium exposure, and > 360 DDDs as high exposure. In different analyses, the authors found that medium and high exposure were reasonably consistently associated with a slightly higher risk of incident dementia relative to the reference group; the HRs, where significant, were in the 1.14-1.42 range. Furthermore, higher levels of exposure to tamsulosin were associated with significantly higher risk of incident dementia when compared with equivalently higher levels of exposure to the other BPH medications. One of the Bradford Hill criteria is the presence of a biological gradient; thus, the identification of a dose-dependent relationship suggests the possibility that an association may be cause-and-effect in nature.16
Relative to the reference level of exposure, low exposure to tamsulosin was not associated with higher dementia risk in any of 5 analyses; medium exposure was associated with significantly higher risk in 4 of 5 analyses, and high exposure in all of 5 analyses. Relative to equivalent levels of exposure to other BPH drugs, medium and high exposure were associated with significantly higher risk of dementia in all of 5 and 4 of 5 analyses, respectively, and low level of exposure was associated with higher risk in 2 of 5 analyses.
As a quick aside, perhaps subjects in the higher exposure group were older and were followed for longer periods, both of which could explain an increased opportunity for the occurrence and detection of incident dementia. However, if this were true, the bias would apply to all treatments and not to tamsulosin alone.
Returning to the discussion, low exposure means that the participant had received 31-90 DDDs. Thus, exposure to just 1-3 months of treatment with tamsulosin (0.4 mg/d) was sufficient to significantly raise the risk of dementia in comparison with 1-3 months of exposure to dutasteride (0.5 mg/d) or finasteride (5 mg/d). Similarly, exposure to just 7-8 months (taking the median of the class interval) of tamsulosin (0.4 mg/d) was sufficient to significantly raise the risk of dementia in comparison with 7-8 months of exposure to doxazosin (4 mg/d), terazosin (5 mg/d), alfuzosin (10 mg/d), dutasteride (0.5 mg/d), or finasteride (5 mg/d).
Now here is a matter that is subtle but compelling. How many common and long-available treatments can the reader name that consistently raise the risk of dementia in patients after administration in standard doses for 7-8 months? Or perhaps for just 1-3 months?
For a drug to increase the risk of dementia after just 1-3 months of use, the neurotoxicity potential of the drug must be high. If so, and this is a critical point, the drug should be associated with a high risk of dementia, and not a risk that requires 185-400 person-years of follow-up for 1 extra case to be observed. Stated otherwise, if just 1-3 months of use suffices for the drug to be associated with a significantly increased risk of dementia, then, given the global popularity of tamsulosin, there should be an epidemic of dementia associated with the drug in patients with BPH, and not a risk that requires 185-400 person-years of follow-up for 1 extra case to be observed. Arguing backward, if the neurotoxicity of a drug requires 185-400 person-years of follow-up for 1 extra case to be observed, then it is unlikely that exposure to this drug for just 1-3 months would be neurotoxic.
Yet another point that must be considered is that in the tamsulosin vs other medication analyses for dose-dependent effects, in 3 of 5 analyses the hazard ratio and significance level for the highest level of exposure were the same as or lower than those for the medium level of exposure. It seems incongruent that the neurotoxic effect of a drug plateaus or drops at higher levels of exposure, unless the analyses were underpowered at the highest level of exposure. An examination of the supplementary data shows that the numbers of subjects at the highest level of exposure were similar to or not substantially different from the numbers at other levels of exposure; this was especially true for the tamsulosin group.
The authors did not state whether the exposure level analyses were also propensity score-matched. All in all, these considerations also throw doubt on the possibility that tamsulosin is causally related to dementia.
Thinking Critically: 8
In most analyses, age did not moderate the effect of tamsulosin. This is an incongruent finding because one would expect older patients to have lower (remaining) cognitive reserve and to therefore be more vulnerable to a neurotoxic drug, especially one that may be neurotoxic after just 1-3 months of exposure.
Summing Up
The wealth of information provided by Duan et al9 in the main paper and in the supplementary materials allowed an almost forensic examination of the study and its findings. In sum, whereas the authors performed an admirable job in selecting their control groups and in performing their analyses, there were too many incongruencies in the findings for a cause-effect relationship between tamsulosin and dementia to be supported. For the present, a conservative conclusion seems merited, which is that tamsulosin use was a marker for dementia risk; that is, patients receiving tamsulosin may already have been predisposed to dementia in ways that were not captured in the propensity-matching exercise because of unknown, unmeasured, or inadequately measured confounds. The data did show that in the Medicare database, clinicians had preferred tamsulosin over other BPH medications for patients who were more incapacitated. As a first follow-up step, therefore, the study needs to be replicated in another database, preferably in another country, such as one where another treatment for BPH is more popular.
It is hoped that this article will help readers toward more critical thought in their interpretation of published research.
Parting Notes
Here is some interesting though unrelated information. Tamsulosin can improve erectile functioning23 but may delay ejaculation, decrease ejaculatory volume, or possibly cause retrograde ejaculation.24-26 Phosphodiesterase-5 inhibitor drugs such as tadalafil, which were introduced for treating erectile dysfunction, are also useful in patients with BPH and LUTS.27
Published online: December 11, 2018.
 Each month in his online column, Dr Andrade considers theoretical and practical ideas in clinical psychopharmacology with a view to update the knowledge and skills of medical practitioners who treat patients with psychiatric conditions.
Each month in his online column, Dr Andrade considers theoretical and practical ideas in clinical psychopharmacology with a view to update the knowledge and skills of medical practitioners who treat patients with psychiatric conditions.
Department of Clinical Psychopharmacology and Neurotoxicology, National Institute of Mental Health and Neurosciences, Bangalore, India ([email protected]).
Financial disclosure and more about Dr Andrade.
REFERENCES
1. Berry SJ, Coffey DS, Walsh PC, et al. The development of human benign prostatic hyperplasia with age. J Urol. 1984;132(3):474-479. PubMed CrossRef
2. Hollingsworth JM, Wilt TJ. Lower urinary tract symptoms in men. BMJ. 2014;349:g4474. PubMed CrossRef
3. Egan KB. The epidemiology of benign prostatic hyperplasia associated with lower urinary tract symptoms: prevalence and incident rates. Urol Clin North Am. 2016;43(3):289-297. PubMed CrossRef
4. Lim KB. Epidemiology of clinical benign prostatic hyperplasia. Asian J Urol. 2017;4(3):148-151. PubMed CrossRef
5. Girman CJ, Panser LA, Chute CG, et al. Natural history of prostatism: urinary flow rates in a community-based study. J Urol. 1993;150(3):887-892. PubMed CrossRef
6. Chute CG, Panser LA, Girman CJ, et al. The prevalence of prostatism: a population-based survey of urinary symptoms. J Urol. 1993;150(1):85-89. PubMed CrossRef
7. Vuichoud C, Loughlin KR. Benign prostatic hyperplasia: epidemiology, economics and evaluation. Can J Urol. 2015;22(suppl 1):1-6. PubMed
8. Silva J, Silva CM, Cruz F. Current medical treatment of lower urinary tract symptoms/BPH: do we have a standard? Curr Opin Urol. 2014;24(1):21-28. PubMed CrossRef
9. Duan Y, Grady JJ, Albertsen PC, et al. Tamsulosin and the risk of dementia in older men with benign prostatic hyperplasia. Pharmacoepidemiol Drug Saf. 2018;27(3):340-348. PubMed CrossRef
10. Frankel JK, Duan Y, Albertsen PC. Is tamsulosin linked to dementia in the elderly? Curr Urol Rep. 2018;19(9):69. PubMed CrossRef
11. Giuliano F, Bernabi J, Laurin M, et al. Tamsulosin impairs bulbospongiosus muscle contractions induced by central injection of 8-OH-DPAT in anaesthetised rats while alfuzosin does not. J Urol. 2005;173(4 suppl):391. CrossRef
12. Giuliano F. Impact of medical treatments for benign prostatic hyperplasia on sexual function. BJU Int. 2006;97(suppl 2):34-38, discussion 44-45. PubMed CrossRef
13. Yamada S, Ohkura T, Deguchi Y, et al. In vivo measurement by [3H]tamsulosin of alpha1 adrenoceptors in rat tissues in relation to the pharmacokinetics. J Pharmacol Exp Ther. 1999;289(3):1575-1583. PubMed
14. Enright JM, Karacal H, Tsai LM. Floppy iris syndrome and cataract surgery. Curr Opin Ophthalmol. 2017;28(1):29-34. PubMed CrossRef
15. Kim CH, Ko IG, Kim SE, et al. Alpha1-adrenoceptor antagonists improve memory by activating N-methyl-d-aspartate-induced ion currents in the rat hippocampus. Int Neurourol J. 2015;19(4):228-236. PubMed CrossRef
16. Grimes DA, Schulz KF. Bias and causal associations in observational research. Lancet. 2002;359(9302):248-252. PubMed CrossRef
17. Andrade C. Offspring outcomes in studies of antidepressant-treated pregnancies depend on the choice of control group. J Clin Psychiatry. 2017;78(3):e294-e297. PubMed CrossRef
18. Andrade C. Cause versus association in observational studies in psychopharmacology. J Clin Psychiatry. 2014;75(8):e781-e784. PubMed CrossRef
19. Andrade C. Confounding. Indian J Psychiatry. 2007;49(2):129-131. PubMed CrossRef
20. Andrade C. Prenatal depression and infant health: the importance of inadequately measured, unmeasured, and unknown confounds. Indian J Psychol Med. 2018;40(4):395-397. PubMed CrossRef
21. Andrade C. Propensity score matching in nonrandomized studies: a concept simply explained using antidepressant treatment during pregnancy as an example. J Clin Psychiatry. 2017;78(2):e162-e165. PubMed CrossRef
22. Gray SL, Dublin S, Yu O, et al. Benzodiazepine use and risk of incident dementia or cognitive decline: prospective population based study. BMJ. 2016;352:i90. PubMed CrossRef
23. Shelbaia A, Elsaied WM, Elghamrawy H, et al. Effect of selective alpha-blocker tamsulosin on erectile function in patients with lower urinary tract symptoms due to benign prostatic hyperplasia. Urology. 2013;82(1):130-135. PubMed CrossRef
24. Lee M. Tamsulosin for the treatment of benign prostatic hypertrophy. Ann Pharmacother. 2000;34(2):188-199. PubMed CrossRef
25. Hellstrom WJ, Sikka SC. Effects of acute treatment with tamsulosin versus alfuzosin on ejaculatory function in normal volunteers. J Urol. 2006;176(4 pt 1):1529-1533. PubMed CrossRef
26. Kaplan SA. Side effects of a-blocker use: retrograde ejaculation. Rev Urol. 2009;11(suppl 1):S14-S18. PubMed
27. Cantrell MA, Baye J, Vouri SM. Tadalafil: a phosphodiesterase-5 inhibitor for benign prostatic hyperplasia. Pharmacotherapy. 2013;33(6):639-649. PubMed CrossRef
This PDF is free for all visitors!
Save
Cite