Abstract
Importance: Increasing evidence suggests a potential role of immune-modulatory drugs for treatment-resistant depression. This scoping review explores the emerging evidence regarding the antidepressant effects of monoclonal antibodies (mAbs), a relatively newer class of immune therapeutics with favorable safety profile.
Observations: PubMed was searched up to November 2023 for English publications addressing the antidepressant effects of mAbs, including meta-analyses, randomized controlled trials, open-label, single-arm studies, and case series. Several mAbs have shown potential antidepressant effects, but most studies in primary inflammatory disorders included patients with mild depression. Only infliximab and sirukumab were directly examined in individuals with primary depression. mAbs that do not require laboratory monitoring, such as ixekizumab and dupilumab, could hold potential promise if future studies establish their safety profile regarding suicide risk.
Conclusions and Relevance: The use of several mAbs for the treatment of primary inflammatory disorders has been associated with improvement of comorbid depressive symptoms. Given their unique mechanisms of action, mAbs may offer a new hope for depressed patients who do not respond to currently available antidepressants. Further research addressing individuals with more severe depressive symptoms is essential. Direct examination of antidepressant effects of mAbs in people with primary depressive disorders is also crucial to refine their clinical use in the treatment of depression.
J Clin Psychiatry 2024;85(3):23nr15243
Author affiliations are listed at the end of this article.
Major depressive disorder (MDD) remains a major public health problem affecting about 20.6% of US individuals within their lifetime.1 The prevalence of MDD has climbed by 12.9% between 2010 and 2018 in the United States2 and continues to increase.3 MDD is associated with significant financial burden.2 Two-thirds of individuals with MDD experience suicidal thoughts and 13.6% attempt suicide.1 Successful treatment of MDD may help reduce disease burden, minimize suicide risk, and improve functional outcomes.
LIMITATIONS OF CURRENT ANTIDEPRESSANT MEDICATIONS
Current treatment options for MDD have limitations. Presently available antidepressants, such as those targeting monoamine neurotransmitters, take weeks to work fully and do not lead to a significant benefit in about half of the patients who adhere to them. Studies like the Sequenced Treatment Alternatives to Relieve Depression trial,4 the largest prospective study of antidepressant efficacy, found that even after several months of sequential treatment trials, one-third of patients with MDD remained depressed.5 More recent evidence indicates that only 43.5% of adults who take antidepressant medications achieve remission after at least 3 months.6 Depression that does not respond to 2 antidepressants at an adequate dose and duration is classified as treatment-resistant.7 Treatment-resistant depression (TRD) is associated with elevated risk of all cause mortality (including suicide), greater utilization of health care resources, lost workdays, and psychiatric comorbidities such as anxiety and stress.8,9 Identifying drugs with novel antidepressant mechanisms could help provide relief for the significant portion of people whose depression does not respond to current medications.
Immune Dysregulation and MDD
Much evidence indicates that immune dysregulation is associated with depression.10,11 Immune abnormalities in MDD include elevated levels of proinflammatory cytokines and acute phase proteins in blood and cerebrospinal fluid, a dysregulated adaptive immune response, changes in the proportions of specific immune cell types, primary humoral immunodeficiencies, a tendency toward autoimmunity, and activation of microglia.12,13 Some of these immune changes have been shown to contribute to treatment resistance.14–16 Elevated levels of the proinflammatory interleukin (IL)- 17A predict nonresponse to antidepressants at 6 weeks in people with MDD.15 Higher baseline IL-6 is more common in patients with TRD, compared with those whose depression responds to antidepressant medications.16 Individuals with MDD and elevated inflammatory and metabolic markers are more likely to remain depressed for 2 years despite taking antidepressants.14
Drugs that modulate dysregulated immune pathways could thus be particularly beneficial for TRD. In fact, increasing evidence supports the role of immune modulation in the treatment of depression. A meta analysis of 18 randomized controlled trials (RCTs) on more than 10,000 patients with primary inflammatory disorders shows that the use of immune-modulatory drugs is associated with significant reduction of depressive symptoms in the subgroup of patients with high baseline depression scores, even after controlling for the physical health benefits of these treatments.17 In addition, previous review articles have commented on some anti-inflammatory and immune-modulating agents as potential treatments for depression.12,18 A meta analysis of 14 RCTs on various nonsteroidal anti inflammatory drugs (NSAIDs) and cytokine inhibitors suggested that these interventions reduced depressive symptoms compared with placebo, in both patients with depression and those with primary inflammatory disorders and comorbid depressive symptoms.19 However, there was a significant heterogeneity among included studies. Another meta-analysis of 30 RCTs suggested that anti-inflammatory agents, including NSAIDs, omega 3 fatty acids, statins, and minocyclines, reduced depressive symptoms and resulted in higher response and remission rates compared with placebo, an effect that was more pronounced when the anti-inflammatory agents were used as adjunctive treatments to antidepressants, rather than monotherapies.20
In recent years, there has been a translational revolution in our understanding of the biomarkers underlying the pathogenesis of immune and inflammatory disorders across skin, gastrointestinal, and other compartments.21,22 This has led to the development of specific anti-inflammatory agents that target these markers, improving patient outcomes. Prior to these advancements, systemic immunosuppressant drugs were used to treat these disorders, but they often resulted in significant side effects. Monoclonal antibodies (mAbs) are a relatively newer class of drugs that target specific cytokines and have shown remarkable efficacy in treating inflammatory immune disorders with high precision and fewer side effects.23 As researchers began to explore the link between immune dysregulation and depression, the potential of mAbs to treat depression, particularly TRD, became an area of interest. A meta-analysis of 7 RCTs involving more than 2,000 participants showed a significant antidepressant effect of anticytokine treatments, including etanercept, infliximab, adalimumab, and tocilizumab, compared with placebo.24 Another analysis of 2 RCTs of adjunctive treatment with anticytokine therapy and 8 nonrandomized and/or nonplacebo studies yielded similar small-to-medium effect estimates favoring those anticytokine therapy for depressive symptoms.24 In this scoping review, we will focus on the newer emerging evidence of the potential antidepressant effects of mAbs.
METHODS
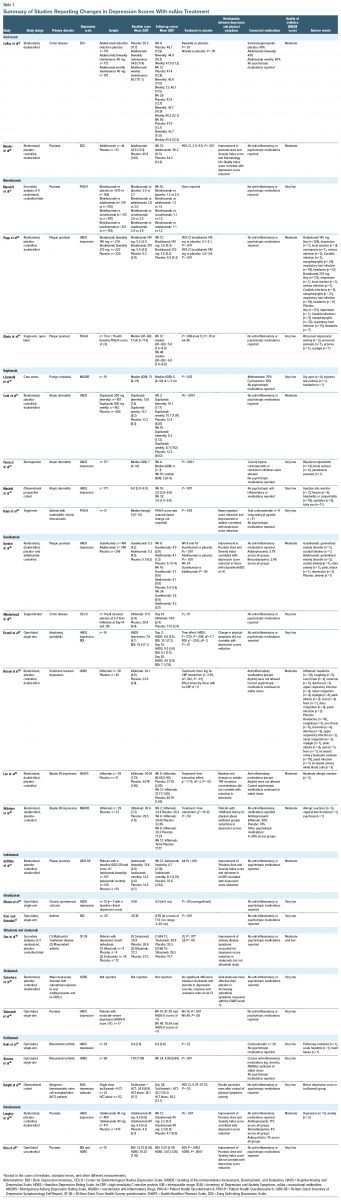
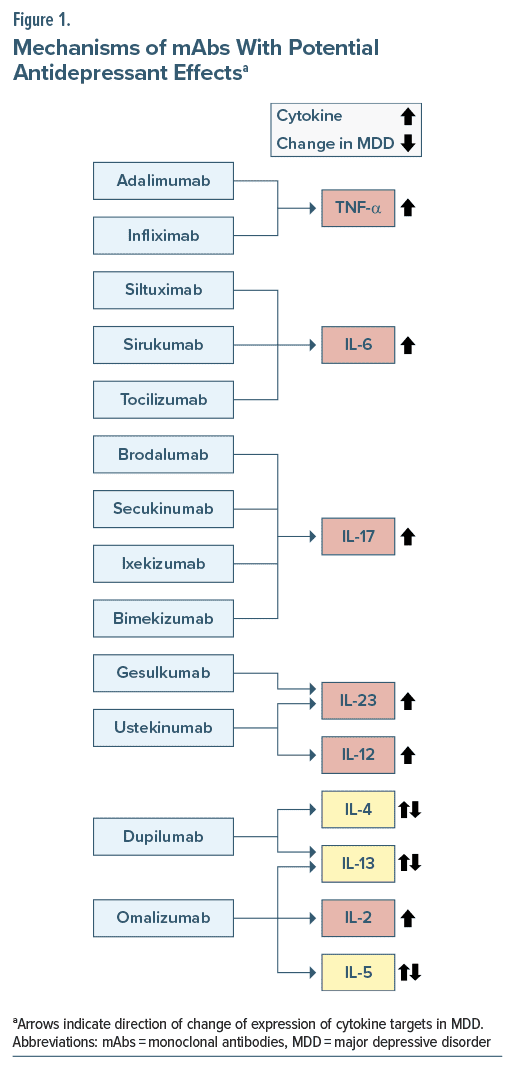
We searched PubMed (up to November 2023) for studies published, using the key terms “depression,” “depressive,” “depressive disorder,” and “major depressive disorder,” with specifiers “monoclonal antibodies,” “immune,” “inflammation,” “inflammatory,” “anti-inflammatory,” as well as “suicide,” “mechanism,” and “safe(ty),” and chose the articles we deemed relevant. We also searched the reference lists of articles identified with the use of this search strategy and included papers that we judged to be applicable. We restricted the search to English-language publications. Table 1 shows details about the studies on potential antidepressant effects of mAbs included in this review. Figure 1 shows the mechanisms of action of these mAbs and the reported changes in their cytokine targets in people with depression.
This narrative review aims to provide a comprehensive summary of the association between changes in depressive symptoms and mAbs treatment. To achieve this, we included 2 types of studies: (1) studies focusing on the efficacy of mAbs for primary inflammatory disorders, where depression assessment served as a secondary outcome measure; (2) studies investigating the antidepressant effects of mAbs in patients with a primary diagnosis of depression, including MDD, major depressive episodes of bipolar disorder, or TRD.
We included studies with various designs such as RCTs, observational studies, and uncontrolled studies. While we acknowledge the limitations of including uncontrolled studies, it was necessary to consider them due to the absence of RCTs for some mAbs included in our review. The Grading of Recommendations Assessment, Development, and Evaluation approach was utilized to assess the quality of publications included in this review. RCTs were initially rated as “high quality” and then downgraded by 1 level for serious concerns (or by 2 levels for very serious concerns) about risk of bias, inconsistency, indirectness, imprecision, or publication bias. Similarly, observational nonrandomized studies were rated as “low quality” and then downgraded based on the same factors/concerns. Two coauthors independently assessed the quality of the articles. Given the relative scarcity of publications on the topic of this review, we included all levels of evidence.
TARGETS, MECHANISMS, AND ANTIDEPRESSANT EFFECTS OF MONOCLONAL ANTIBODIES
Tumor Necrosis Factor-α Inhibitors
Tumor necrosis factor-α (TNF-α) is a multifunctional cytokine that induces proinflammatory responses.52,53 TNF-α is associated with an impaired ability to recover from a state of learned helplessness in animals, likely through disruption of the blood-brain barrier (BBB).54 Elevated TNF-α levels are also reported in depressed patients55 and correlate with more severe suicidal ideation.56
Adalimumab. Adalimumab is a TNF-α inhibitor approved for the treatment of moderate-to-severe plaque psoriasis and psoriatic arthritis,57 moderate-to-severe hidradenitis suppurativa,58 rheumatoid arthritis, ankylosing spondylitis, Crohn disease, and ulcerative colitis.59
Two RCTs examined changes in depressive symptoms in association with adalimumab treatment for patients with inflammatory disorders. Loftus et al25 noted that patients with moderate-to-severe Crohn’s disease experienced greater improvement of their comorbid depressive symptoms with adalimumab treatment, compared with placebo. Menter et al26 reported that, in patients with moderate-to-severe psoriasis, depressive symptoms significantly decreased in the adalimumab treated group, compared with placebo. It is worth noting, however, that improvement in depressive symptoms correlated with a reduction in the physical symptoms of psoriasis.26 Also of note, the baseline scores for both the treatment and placebo groups fell in the normal to minimal depression range. In contrast, a case series on the impact of adalimumab in complex regional pain syndrome reported no significant improvements in comorbid depression.60
Infliximab. Infliximab is another TNF-α inhibitor mAb approved for the treatment of moderate-to-severe Crohn’s disease, ulcerative colitis, rheumatoid arthritis, ankylosing spondylitis, psoriatic arthritis, and severe plaque psoriasis.53
A mouse study of inflammation and depression with chronic, mild stress found that infliximab prevented TNF α increases and depressive symptoms brought on by the stress.61 Further, depressed patients treated with infliximab experienced improvement in depressive symptoms related to reduction in TNF-α receptor levels.62
In a single-blinded study of patients with Crohn’s disease, placebo was administered to all patients at baseline, followed by 2 infliximab doses 2 weeks apart.36 Improvement was observed in depressive symptoms 2 and 4 weeks following the start of infliximab, but not with placebo. Further, Ertenli et al37 reported improvements in mild depression scores among patients with ankylosing spondylitis treated with infliximab.
Infliximab has also been tested directly in patients with primary mood disorders, but the findings have been inconclusive. Raison et al38 found that, compared with placebo, infliximab did not reduce depressive symptoms in patients with TRD whose depression was moderate-to severe, but it did improve depression in those with evidence of inflammation [ie, high-sensitivity C-reactive protein (hs-CRP) of greater than 5 mg/L]. Patients treated with infliximab in this study tended to have more improvement in their symptoms of psychomotor retardation, psychic anxiety, anhedonia, depressed mood, and suicidal ideation. Further, in a 12-week RCT on the effectiveness of adjunctive infliximab for the treatment of bipolar depression, McIntyre et al40 reported no difference in depressive symptoms between infliximab and placebo groups. However, a subgroup of patients with a history of childhood trauma showed a reduction in depressive symptoms and higher response rates with infliximab, compared with placebo. In a secondary analysis of data from the same study, Lee et al39 observed an improvement in anhedonia among those treated with infliximab compared with placebo at week 6, but not week 12.
To explain variability in the antidepressant effects of infliximab, Mehta et al63 examined if certain transcriptional genetic changes were related to such effects. They found that genomic indices related to glucose and lipid metabolism predicted treatment response to infliximab in patients with TRD. Another study suggested that patients with TRD and high levels of inflammation along with high levels of lipids and cholesterols were more responsive to infliximab’s antidepressant properties.64 It is worth noting however that a meta-analysis of both studies failed to find that infliximab reduced depressive symptoms when used as an adjuvant treatment for those with TRD.65
IL-6 Inhibitors
IL-6 is a multifunctional proinflammatory cytokine that plays a central role in host defense. IL-6 has been most consistently elevated in patients with MDD66,67 and has been linked to depression severity,68 treatment resistance,69,70 and suicidal behavior.71 IL-6 is also linked to the development of a susceptible behavioral phenotype in mice following chronic stress.70 Further, mice treated with a systemic IL-6 mAb are resilient to social stress.70
Siltuximab and Sirukumab. Siltuximab and sirukumab are anti-IL-6 mAbs.72,73 Siltuximab is approved to treat multicentric Castleman disease.74 Sirukumab has been considered as a treatment for rheumatoid arthritis but has not yet been approved by the Food and Drug Administration (FDA).75
In their secondary analyses of data from 2 controlled trials, Sun et al44 reported reduction in depressive symptoms with sirukumab and siltuximab treatment for multicentric Castleman disease and rheumatoid arthritis, respectively, compared with placebo. Improvement in physical symptoms of the primary disease accounted for depressive symptoms reduction in the group treated with sirukumab, but not siltuximab. In addition, sirukumab was tested as an adjunctive treatment in patients with TRD and plasma CRP levels ≥3 mg/L.45 Although depression severity at week 12 did not differ significantly between sirukumab and placebo, anhedonia rating improved with sirukumab, compared with placebo.45 Moreover, patients with baseline CRP values ≥8 mg/L had greater reduction of depressive symptoms with sirukumab versus placebo.45
Tocilizumab. Tocilizumab is an IL-6 inhibitor approved to treat moderate-to-severe rheumatoid arthritis,76 giant cell arteritis,77 systemic sclerosis-associated interstitial lung disease,78 polyarticular juvenile idiopathic arthritis,79 systemic juvenile idiopathic arthritis,80 cytokine release syndrome,81 and COVID-19.82
Research on the impact of tocilizumab on symptoms of depression has been mixed. Although an open-label study on patients with rheumatoid arthritis reported improvement in depression scores following treatment with tocilizumab,47 another study in similar population found no change in depression with tocilizumab.48 Furthermore, worsening depressive symptoms have been noted with single tocilizumab treatment for individuals undergoing hematopoietic cell transplantation.49
T Helper-17 Blockers
T helper (Th)-17 cells, and the cytokines they produce, play an integral role in host defense as well as the pathogenesis of several autoimmune and inflammatory disorders.83 IL-17A influences disorders such as psoriasis and Crohn’s disease, among others.84 IL-17A is one of the proinflammatory cytokines that may disrupt the BBB85 and thus is hypothesized to promote depression by allowing circulating proinflammatory factors to gain access to mood-relevant brain circuits. IL-17A may also contribute to treatment resistance in MDD, as it has been found to predict nonresponse to antidepressants.15 IL-17F has overlapping functionality with IL-17A.86 IL-17E is a barrier cytokine that alarms the immune cells, among other functions.87
Brodalumab. Brodalumab blocks IL-17 receptor and inhibits IL-17A, IL-17F, IL-17A/F, and IL-17E.88 Brodalumab is an FDA-approved mAb for moderate-to severe plaque psoriasis.89
In their RCT on moderate-to-severe plaque psoriasis, Papp et al90 noted an improvement in depressive symptoms with brodalumab, compared with placebo, although the baseline scores for the treatment and placebo groups fell in the normal range. Further, Ohata et al29 noted that depressive symptoms decreased with brodalumab treatment, but only in those with baseline Patient Health Questionnaire-8 scores of 5 or more (ie, at least mildly depressed). Of note, 2 patients in this study had worsening depressive and anxiety symptoms after the administration of brodalumab. Along similar lines, Rivera-Oyola et al91 reported that although brodalumab treatment was associated with improvements in depressive symptoms in 2 cases of patients with psoriasis and comorbid depression, another patient, who had a history of bipolar I disorder, later developed mania, psychosis, and suicidal ideation during treatment.
Ixekizumab. Ixekizumab is an IL-17A antagonist approved to treat psoriatic arthritis, moderate-to-severe plaque psoriasis,92 and ankylosing spondylitis.93
An analysis of 3 phase III RCTs of ixekizumab treatment for psoriasis noted a significant improvement in depressive symptoms.41 Patients in these studies had moderate depression scores at baseline, and a greater percentage of those treated with ixekizumab met criteria for response and remission criteria at week 12, compared with the placebo. An RCT is being currently conducted to examine the antidepressant effects of ixekuzimab in adults with TRD (NCT04979910).
Secukinumab. Secukinumab has a similar mechanism of action to ixekizumab, specifically targeting IL-17A, thereby blocking its binding with IL-17 receptor.94 Secukinumab is approved for the treatment of moderate-to-severe hidradenitis suppurativa and plaque psoriasis.95
In their secondary analysis of a multicenter open label, single-arm study on secukinumab for patients with moderate-to-severe psoriasis, Talamonti et al46 reported that more than 80% of patients who had moderate-to severe depression at baseline experienced improvement in their depression at week 16 and week 48.
Bimekizumab. Bimekizumab is a monoclonal immunoglobulin G1 (IgG1) antibody that selectively inhibits IL-17A and IL-17F and has resulted in better outcomes in moderate-to-severe psoriasis, compared with IL-17A-only antagonists.96
A secondary analysis of 9 randomized, placebo-, and active comparator-controlled trials showed that following 16 weeks of treatment with bimekizumab, mean Patient Health Questionnaire-9 scores were numerically lower than placebo and similar to active comparators (including adalimumab, secukinumab, and ustekinumab).27
IL-23 Inhibitors
IL-23 is proinflammatory cytokine that belongs to the IL-12 family of cytokines and plays a crucial role in the maintenance and expansion of Th-17 pathway.97 IL-23 gene expression is elevated in people with recurrent depressive disorders.98
Guselkumab. Guselkumab is an IL-23 inhibitor99 approved for the treatment of moderate-to-severe plaque psoriasis100 and psoriatic arthritis.101
Gordon et al35 conducted a 24-week, phase 3, randomized, double-blind, placebo- and adalimumab controlled study to determine the changes in depression and anxiety with guselkumab treatment for more than 900 patients with moderate-to-severe psoriasis. They reported that the number of patients in the guselkumab group with clinical depression decreased significantly at week 16 and week 24, compared with placebo and adalimumab, respectively. However, reduction of depression scores correlated with improvement of psoriasis symptom severity.
Ustekinumab. Ustekinumab is a mAb approved for the treatment of moderate-to-severe plaque psoriasis and psoriatic arthritis,102 as well as moderate-to-severe Crohn’s disease and ulcerative colitis.103 It inhibits the p40 subunit of IL-23 and IL-12,104,105 which have been found to be elevated in people with depression.106
In an RCT of ustekinumab for moderate-to-severe psoriasis, Langley et al50 noted improvement in depression scores, compared with placebo. However, baseline scores were below the cutoff for clinical depression. Additionally, in their open-label, uncontrolled ustekinumab trial, Kim et al51 noted that patients with moderate-to-severe psoriasis and pretreatment mild depressive symptoms experienced a reduction in depression.
Th-2 Blockers
IL-4 and IL-13 are key cytokines of Th-2 inflammatory response with distinct as well as overlapping effects. Specifically, IL-4 drives immunoglobulin class switching to IgG1 and immunoglobulin E (IgE) and induces Th-2 cell differentiation, while IL-13 is involved in B-cell differentiation and eosinophil chemotaxis. Both IL-4 and IL-13 also play a role in inducing alternative macrophages activation and are thus relevant to the pathogenesis of allergic disorders.107
Abnormalities of IL-4 and IL-13 have been linked to depression in animal and human studies, but the exact mechanism and direction of these abnormalities are largely unknown. IL-4 may influence depressive-like behaviors in animals, but there is some debate about this claim. In an interferon-α mouse model, there seems to be an association between depressive symptoms and decreased IL-4 responses of microglia.108 However, Moon et al109 observed more anxiety behaviors in IL-4 knockout mice than in wild-type mice in the elevated zero maze but did not find a significant association with depressive symptoms. Park et al110 noted that the action of IL-4 seemed to prevent increases in prostaglandin E2 and corticosterone levels caused by IL-1β. It also seemed to inhibit tryptophan hydroxylase mRNA and activate the serotonin transporter in hippocampus, thus decreasing the level of IL-1β–induced serotonin. Further, in response to antidepressants given to treatment-naïve depressed patients, plasma IL-4 decreased while IL-13 increased.111 Elevated levels of IL-13 are associated with MDD112 and higher likelihood of suicide attempt history113 and are noted in men who have died by suicide.114 Elevated IL-4 levels are reported in females who have died by suicide.114
Dupilumab. Dupilumab is an FDA-approved mAb for the treatment of moderate-to-severe atopic dermatitis and moderate-to-severe asthma,115 chronic rhinosinusitis with nasal polyposis,116 eosinophilic esophagitis,117 and prurigo nodularis.118 By binding to type I and II IL-4-α receptors, dupilumab inhibits the responses of cytokines IL-4 and IL 13119 and downregulates the Th-2 immune response.
In their case series on patients with prurigo nodularis, Lönndahl et al30 reported that in addition to decrease in pruritus scores, patients with mild depression scores at baseline experienced improvements in depressive symptoms with dupilumab treatment. In a phase 3 RCT on patients with moderate-to-severe atopic dermatitis and moderate depression, Cork et al31 noted an improvement in depressive symptoms by week 2 through week 16 with dupilumab treatment, compared with placebo. Two uncontrolled studies on patients with atopic dermatitis noted a decrease in depression scores32,33—although baseline scores were below the cutoff for mild depression in both studies. Finally, in an uncontrolled study on patients with asthma with eosinophilic chronic rhinosinusitis and comorbid mild depression scores, a significant reduction in depressive symptoms was noted, more prominently in patients who had a nasal symptom score reduction in 50% or greater.34 It is notable, however, that in addition to dupilumab, the patients in this study were also given corticosteroids and long-acting β-agonists.
Omalizumab. Omalizumab is a mAb that is approved to treat moderate-to-severe asthma120 and chronic spontaneous urticaria.121 It is also used as an add-on treatment for nasal polyps.122 Omalizumab binds to IgE,123 hence its role in allergic disorders.124 It also reduces the number of lymphocytes producing IL-2 and IL-13125 and decreases the levels of IL-5126; all are components of Th-2 pathway. Research suggests that allergen-specific IgE positive people tend to have worse depression scores.127
In their study of omalizumab treatment for chronic spontaneous urticaria, Diluvio et al42 reported that 71% of participants who had mild depressive symptoms at baseline experienced improvement of their depression at 6 months. Further, in an uncontrolled study of omalizumab treatment for asthma, Uzer and Ozbudak43 noted an improvement of comorbid depression in 9 of 12 patients who had moderate-to-severe depression before treatment initiation.
ADVERSE EFFECTS AND SAFETY PROFILES
The most common reported adverse effects with mAbs are injection site reactions, infusion reactions (with intravenous mAbs), headaches, nausea, and increased risk of infections (eg, conjunctivitis and upper or lower respiratory tract infections). Rare serious side effects include increased risk of malignancy (with guselkumab,128 infliximab,129–131 and ixekizumab),132 tuberculosis (with infliximab),129–131 and cardiovascular events (with fremanezumab133,134 and ixekizumab)132 as well as hepatic, pulmonary, and pancreatic reactions (with tocilizumab).135 Dupilumab has one of the best safety profiles among all mAbs. It has been approved by the FDA for use in children as young as 6 months old. Unlike most other immune-modulating drugs, dupilumab has not been shown to suppress the immune system’s ability to fight infections, as shown in a meta-analysis of 8 RCTs.136 In contrast, sirukumab has been denied approval by the FDA due to concerns of increased all cause mortality due to markedly increased risk of serious infections.137,138
Review of mAbs clinical trials and package inserts shows that suicidal ideation and behavior were reported during the course of treatment with a number of mAbs although a causal link has not been found in these instances.139–141 Two patients with psoriasis receiving adalimumab reportedly died by suicide.142 Higher rates of suicidal ideation were reported in bimekizumab treated patients than in placebo-treated patients in the psoriasis clinical trials. One patient receiving open-label bimekizumab without prior psychiatric history completed suicide. Brodalumab treatment was associated with 6 cases of suicide across all psoriasis clinical trials.143 This led the FDA to issue a black box warning about worsening of suicidal ideation and behavior during treatment with brodalumab. It is worth noting, however, that brodalumab users with a history of suicidality or depression had an increased incidence of suicidal ideation and behavior as compared to users without such a history. Further, in a 2022 4-year pharmacovigilance report, brodalumab was not associated with any increase in suicide risk.144 One suicide attempt and 1 death by suicide have been reported in dupilumab treatment studies,140,141 as well as with guselkumab.145 An adolescent female developed depression and attempted suicide during infliximab treatment for Crohn disease.146 One patient developed suicidal ideation during ixekizumab treatment.132 Undercounts of suicidal ideation and behavior remain a possibility given that most mAbs clinical studies relied on patient’s reporting of suicidality rather than using suicide-specific assessment scales.
CONCLUSIONS AND FUTURE DIRECTIONS
Improvement in depressive symptoms has been reported in association with several mAbs used for the treatment of a wide variety of primary inflammatory skin, joint, and gastrointestinal disorders. Given their specific mechanisms of action, mAbs may offer a new option for the treatment of depression in many patients who do not respond to currently available antidepressants. Ixekizumab and dupilumab (only mAbs that do not require any monitoring)147,148 seem to be the 2 mAbs that offer the best benefit-risk balance, but further research is needed to refine their use in depression clinical practice. It should be noted that it remains unclear whether improvements in depressive symptoms are due to direct effects of the drugs or secondary to improvements in physical symptoms associated with the primary inflammatory disorders and related positive impact on quality of life. This question would be only addressed by studies examining the antidepressant effects of mAbs in patients with primary depressive disorders and no inflammatory conditions. Few studies such as Raison et al38 and those analyzed in Bavaresco et al65 mentioned above have examined this question, but otherwise, research is lacking. Additionally, the effect of concurrent use of psychotropic medications on the changes of depressive symptoms across the duration of mAbs studies should be considered. Participants in the infliximab RCTs in MDD and bipolar depression were allowed to continue using their psychotropic medications provided that doses remained stable during the infliximab trials. A small percentage of patients in the guselkumab and ustekinumab trials were using a wide variety of psychotropic medications. Other studies did not report information about concurrent use of psychiatric medications.
With the exception of a few studies, patients included in most studies examining the changes in depression scores with mAb treatment had mild depression. The lower the depression score, the more likely it is that the symptoms reported are related to the primary inflammatory disorder (eg, vegetative symptoms). Thus, any reported improvements in depression scores could be attributed to improvements in the physical health condition. Conversely, higher depression severity score may mean it is more likely that patients were in fact depressed. Future studies need to examine the effect of mAbs in patients with more severe depressive symptoms as well as those with TRD. These studies could also determine whether the benefits of mAbs outweigh their side effects and/or favor their use for depression over other treatment options. Most of the mAbs showed better safety profiles compared to conventional immune modulating drugs. For example, dupilumab has been approved for children as young with atopic dermatitis as 6 months and does not require any monitoring.149,150 It remains unclear whether reported suicidal ideation and behavior with several mAbs are causally related to the drug administration or to the primary disease. Again, these questions could only be answered with RCTs that directly examine the antidepressant effects of mAbs in patients with primary depressive disorders.
While our review primarily focuses on summarizing the existing evidence on changes in depressive symptoms associated with mAb treatment, we recognize the importance of considering the underlying mechanisms of action and potential limitations of this therapeutic approach. The mechanism by which cytokine antagonists may exert their antidepressant effects remains largely unknown, and it is crucial to critically evaluate whether targeting a single cytokine can effectively counteract the multifaceted alterations in inflammatory markers observed in MDD. Future research should aim to elucidate the specific mechanisms underlying the antidepressant effects of mAbs and explore potential synergistic or compensatory mechanisms involved in MDD pathophysiology.
In light of the potential benefits of mAbs in treating depression, it is crucial to consider their practical application in clinical settings. Currently, it is not recommended that psychiatrists consider prescribing these immune therapies for patients who have not responded to traditional treatments because there is not enough evidence to support such use. Much research is needed on the efficacy and safety of mAbs as monotherapy or adjunctive medication to currently available antidepressants. The placement of these immune therapies within treatment hierarchies and guidelines would depend on a variety of factors, including the severity of the patient’s symptoms, their overall health status, and their response to other treatments. It is also important to consider the cost of these medications, which can be quite high and will only be covered by insurance for individuals with mood disorders when the FDA approves them for this indication.
Finally, the study of the antidepressant properties of mAbs prompts discussion of the need for immune screening methods in clinical practice. Recent advances in immunology research could help identify the similarities in immune profiles between MDD and primary inflammatory disorders, an important step toward helping select the mAbs that could produce the most positive impact on depressive symptoms. Future studies could also be directed toward developing methods to refine the measurement of dysregulated immune pathways in depression and link those findings to the mechanism of antidepressant action of mAbs.
Article Information
Published Online: June 24, 2024. https://doi.org/10.4088/JCP.23nr15243
© 2024 Physicians Postgraduate Press, Inc.
Submitted: January 3, 2024; accepted April 12, 2024.
To Cite: Rizk MM, Bolton L, Cathomas F, et al. Immune-targeted therapies for depression: current evidence for antidepressant effects of monoclonal antibodies.
J Clin Psychiatry. 2024;85(3):23nr15243.
Author Affiliations: Department of Psychiatry, Icahn School of Medicine at Mount Sinai, New York, New York (Rizk, Russo, Murrough); Molecular Imaging and Neuropathology Division, New York State Psychiatric Institute, New York (Rizk, Bolton, Mann); Nash Family Department of Neuroscience, Icahn School of Medicine at Mount Sinai, New York, New York (Cathomas, Russo, Murrough); Friedman Brain Institute, Icahn School of Medicine at Mount Sinai, New York, New York (Cathomas, Russo, Murrough); Department of Dermatology, Icahn School of Medicine at Mount Sinai, New York, New York (He, Guttman-Yassky); Department of Psychiatry, Columbia University Irving Medical Center, New York, New York (Mann); Department of Radiology, Columbia University Irving Medical Center, New York, New York (Mann).
Corresponding Author: Mina M. Rizk, MD, Department of Psychiatry, Icahn School of Medicine at Mount Sinai, 1 Gustave Levy Place, Box #1230, New York, NY 10029 ([email protected]).
Dr Rizk and Ms Bolton are co-first authors.
Relevant Financial Relationships: Dr Guttman-Yassky has received research grants (paid to the institution) from Boehringer Ingelheim, Leo Pharma, Pfizer, Cara Therapeutics, UCB, Kyowa Kirin, RAPT, Amgen, GSK, Incyte, Sanofi, Bristol-Myers Squibb, Aslan, Regeneron, Anaptysbio, Concert, and Janssen. She is a consultant for AbbVie, Almirall, Amgen, AnaptysBio, Apogee Therapeutics, Apollo Therapeutics Limited, Artax Biopharma Inc, AstraZeneca, Bristol-Myers Squibb, Boehringer Ingelheim, Cara Therapeutics, Centrexion Therapeutics Corporation, Connect Biopharm, Eli Lilly, Enveda Biosciences, Escient Pharmaceuticals, Inc, Fairmount Funds Management LLC, FL2022-001, Inc, Galderma, Gate Bio, Google Ventures (GV), GSK Immunology, Horizon Therapeutics USA, Inc, Incyte, Inmagene, Janssen Biotech, Japan Tobacco, Jasper Therapeutics, Kyowa Kirin, Leo Pharma, Merck, Nektar Therapeutics, Novartis Pharmaceuticals Corporation, NUMAB Therapeutics AG, OrbiMed Advisors LLC, Otsuka, Pfizer, Pharmaxis Ltd, Pioneering Medicine VII, Inc, Proteologix US Inc, RAPT, Regeneron Pharmaceuticals, RibonTherapeutics, Inc, Sanofi, SATO, Schrödinger, Inc, Sun Pharma Advanced Research Company (SPARC), Teva Branded Pharmaceutical Products R&D, Inc, and UCB. In the past 24 months, Dr Mann receives royalties for commercial use of the Columbia-Suicide Severity Rating Scale from the Research Foundation for Mental Hygiene. Dr Murrough has provided consultation services and/or served on advisory boards for LivaNova, KetaMed, Inc, Merk, Cliniclabs, Inc, Biohaven Pharmaceuticals, Inc, Compass Pathfinder, Xenon Pharmaceuticals, and Clexio Biosciences. The other authors have no conflicts of interest to declare.
Funding/Support: Dr Rizk is supported by a NARSAD Young Investigator Award from the Brain and Behavior Research Foundation as well as a R25 Grant from NIMH (MH129256).
Role of the Funder/Sponsor: The funding agency has no role in the conduct of the study; design, management, analysis, or interpretation of the data; or preparation, review, or approval of the manuscript.
Clinical Points
- Immune dysregulation is associated with depression and may contribute to treatment resistance.
- Monoclonal antibodies are novel immune therapies that have shown promise in the treatment of depression when used for primary inflammatory disorders.
- Future research is needed to determine the efficacy and safety of the use of monoclonal antibodies for patients with primary depression.
References (150)

- Hasin DS, Sarvet AL, Meyers JL, et al. Epidemiology of adult DSM-5 major depressive disorder and its specifiers in the United States. JAMA Psychiatry. 2018;75(4):336–346. PubMed
- Greenberg PE, Fournier AA, Sisitsky T, et al. The economic burden of adults with major depressive disorder in the United States (2010 and 2018). Pharmacoeconomics. 2021;39(6):653–665. PubMed
- Goodwin RD, Dierker LC, Wu M, et al. Trends in U.S. Depression prevalence from 2015 to 2020: the widening treatment gap. Am J Prev Med. 2022;63(5):726–733. PubMed
- Rush AJ. STAR*D: what have we learned?. Am J Psychiatry. 2007;164(2):201–204. PubMed
- Mann JJ, Rizk MM. Rethinking the medication management of major depression. Expert Rev Neurother. 2023;23(4):331–363. PubMed
- Mojtabai R, Amin-Esmaeili M, Spivak S, et al. Remission and treatment augmentation of depression in the United States. J Clin Psychiatry. 2021;82(6):21m13988. PubMed
- Fava M. Diagnosis and definition of treatment-resistant depression. Biol Psychiatry. 2003;53(8):649–659. PubMed CrossRef
- Brenner P, Reutfors J, Nijs M, et al. Excess deaths in treatment-resistant depression. Ther Adv Psychopharmacol. 2021;11:20451253211006508. PubMed
- Lundberg J, Cars T, Loov SA, et al. Association of treatment-resistant depression with patient outcomes and health care resource utilization in a population-wide study. JAMA Psychiatry. 2023;80(2):167–175. PubMed
- Miller AH, Raison CL. The role of inflammation in depression: from evolutionary imperative to modern treatment target. Nat Rev Immunol. Jan 2016;16(1):22–34. PubMed CrossRef
- Branchi I, Poggini S, Capuron L, et al. Brain-immune crosstalk in the treatment of major depressive disorder. Eur Neuropsychopharmacol. 2021;45:89–107. PubMed
- Drevets WC, Wittenberg GM, Bullmore ET, et al. Immune targets for therapeutic development in depression: towards precision medicine. Nat Rev Drug Discov. 2022;21(3):224–244. PubMed
- Isung J, Williams K, Isomura K, et al. Association of primary humoral immunodeficiencies with psychiatric disorders and suicidal behavior and the role of autoimmune diseases. JAMA Psychiatry. 2020;77(11):1147–1154. PubMed
- Vogelzangs N, Beekman ATF, van Reedt Dortland AKB, et al. Inflammatory and metabolic dysregulation and the 2-year course of depressive disorders in antidepressant users. Neuropsychopharmacology. 2014;39(7):1624–1634. PubMed CrossRef
- Nothdurfter C, Milenkovic VM, Sarubin N, et al. The cytokine IL-17A as a marker of treatment resistance in major depressive disorder?. Eur J Neurosci. 2021;53(1):172–182. PubMed
- Yoshimura R, Hori H, Ikenouchi-Sugita A, et al. Higher plasma interleukin-6 (IL-6) level is associated with SSRI- or SNRI-refractory depression. Prog Neuropsychopharmacol Biol Psychiatry. 2009;33(4):722–726. PubMed CrossRef
- Wittenberg GM, Stylianou A, Zhang Y, et al. Effects of immunomodulatory drugs on depressive symptoms: a mega-analysis of randomized, placebo-controlled clinical trials in inflammatory disorders. Mol Psychiatry. 2020;25(6):1275–1285. PubMed CrossRef
- Roman M, Irwin MR. Novel neuroimmunologic therapeutics in depression: a clinical perspective on what we know so far. Brain Behav Immun. 2020;83:7–21. PubMed CrossRef
- Kohler O, Benros ME, Nordentoft M, et al. Effect of anti-inflammatory treatment on depression, depressive symptoms, and adverse effects: a systematic review and meta-analysis of randomized clinical trials. JAMA Psychiatry. 2014;71(12):1381–1391. PubMed CrossRef
- Bai S, Guo W, Feng Y, et al. Efficacy and safety of anti-inflammatory agents for the treatment of major depressive disorder: a systematic review and meta-analysis of randomised controlled trials. J Neurol Neurosurg Psychiatry. 2020;91(1):21–32. PubMed CrossRef
- Bakker D, de Bruin-Weller M, Drylewicz J, et al. Biomarkers in atopic dermatitis. J Allergy Clin Immunol. 2023;151(5):1163–1168. PubMed
- Wagatsuma K, Yokoyama Y, Nakase H. Role of biomarkers in the diagnosis and treatment of inflammatory bowel disease. Life (Basel). 2021;11(12):1375. PubMed
- Nelson AL, Dhimolea E, Reichert JM. Development trends for human monoclonal antibody therapeutics. Nat Rev Drug Discov. 2010;9(10):767–774. PubMed CrossRef
- Kappelmann N, Lewis G, Dantzer R, et al. Antidepressant activity of anti-cytokine treatment: a systematic review and meta-analysis of clinical trials of chronic inflammatory conditions. Mol Psychiatry. 2018;23(2):335–343. PubMed CrossRef
- Loftus EV, Feagan BG, Colombel JF, et al. Effects of adalimumab maintenance therapy on health-related quality of life of patients with Crohn’s disease: patient-reported outcomes of the CHARM trial. Am J Gastroenterol. 2008;103(12):3132–3141. PubMed CrossRef
- Menter A, Augustin M, Signorovitch J, et al. The effect of adalimumab on reducing depression symptoms in patients with moderate to severe psoriasis: a randomized clinical trial. J Am Acad Dermatol. 2010;62(5):812–818. PubMed CrossRef
- Blauvelt A, Armstrong A, Merola JF, et al. Bimekizumab in patients with moderate to severe plaque psoriasis: analysis of mental health and associated disorders. SKIN J Cutan Med. 2023;7(6):s300.
- Papp KA, Reich K, Paul C, et al. A prospective phase III, randomized, double-blind, placebo-controlled study of brodalumab in patients with moderate-to-severe plaque psoriasis. Br J Dermatol. 2016;175(2):273–286. PubMed CrossRef
- Ohata C, Kanai Y, Murotani K, et al. Effectiveness of long-term treatment with brodalumab on anxiety or depressive symptoms in Japanese patients with psoriasis: the ProLOGUE study. Dermatol Ther. 2023;13(4):1039–1052.
- Lönndahl L, Lundqvist M, Bradley M, et al. Dupilumab significantly reduces symptoms of prurigo nodularis and depression: a case series. Acta Derm Venereol. 2022;102:adv00754. PubMed
- Cork MJ, Eckert L, Simpson EL, et al. Dupilumab improves patient-reported symptoms of atopic dermatitis, symptoms of anxiety and depression, and health-related quality of life in moderate-to-severe atopic dermatitis: analysis of pooled data from the randomized trials SOLO 1 and SOLO 2. J Dermatolog Treat. 2020;31(6):606–614. PubMed CrossRef
- Ferrucci S, Casazza G, Angileri L, et al. Clinical response and quality of life in patients with severe atopic dermatitis treated with dupilumab: a single-center real-life experience. J Clin Med. 2020;9(3):791. PubMed
- Miniotti M, Lazzarin G, Ortoncelli M, et al. Impact on health-related quality of life and symptoms of anxiety and depression after 32 weeks of Dupilumab treatment for moderate-to-severe atopic dermatitis. Dermatol Ther. 2022;35(5):e15407. PubMed
- Koya T, Sakai N, Sasaki T, et al. Effect of dupilumab on depression in asthma with eosinophilic chronic rhinosinusitis in the Japanese population. Int Arch Allergy Immunol. 2022;183(3):289–297. PubMed
- Gordon KB, Armstrong AW, Han C, et al. Anxiety and depression in patients with moderate-to-severe psoriasis and comparison of change from baseline after treatment with guselkumab vs. adalimumab: results from the Phase 3 VOYAGE 2 study. J Eur Acad Dermatol Venereol. 2018;32(11):1940–1949. PubMed CrossRef
- Minderhoud IM, Samsom M, Oldenburg B. Crohn’s disease, fatigue, and infliximab: is there a role for cytokines in the pathogenesis of fatigue?. World J Gastroenterol. 2007;13(14):2089–2093. PubMed CrossRef
- Ertenli I, Ozer S, Kiraz S, et al. Infliximab, a TNF-α antagonist treatment in patients with ankylosing spondylitis: the impact on depression, anxiety and quality of life level. Rheumatol Int. 2012;32(2):323–330. PubMed CrossRef
- Raison CL, Rutherford RE, Woolwine BJ, et al. A randomized controlled trial of the tumor necrosis factor antagonist infliximab for treatment-resistant depression: the role of baseline inflammatory biomarkers. JAMA Psychiatry. 2013;70(1):31–41. PubMed CrossRef
- Lee Y, Mansur RB, Brietzke E, et al. Efficacy of adjunctive infliximab vs. placebo in the treatment of anhedonia in bipolar I/II depression. Brain Behav Immun. 2020;88:631–639. PubMed CrossRef
- McIntyre RS, Subramaniapillai M, Lee Y, et al. Efficacy of adjunctive infliximab vs placebo in the treatment of adults with bipolar I/II depression: a randomized clinical trial. JAMA Psychiatry. 2019;76(8):783–790. PubMed
- Griffiths CEM, Fava M, Miller AH, et al. Impact of ixekizumab treatment on depressive symptoms and systemic inflammation in patients with moderate-to-severe psoriasis: an integrated analysis of three phase 3 clinical studies. Psychother Psychosom. 2017;86(5):260–267. PubMed
- Diluvio L, Piccolo A, Marasco F, et al. Improving of psychological status and inflammatory biomarkers during omalizumab for chronic spontaneous urticaria. Future Sci OA. 2020;6(9):Fso618. PubMed CrossRef
- Uzer F, Ozbudak O. Benefits of omalizumab on anxiety and depression in patients with severe asthma. Caspian J Intern Med. 2018;9(3):228–231. PubMed CrossRef
- Sun Y, Wang D, Salvadore G, et al. The effects of interleukin-6 neutralizing antibodies on symptoms of depressed mood and anhedonia in patients with rheumatoid arthritis and multicentric Castleman’s disease. Brain Behav Immun. 2017;66:156–164. PubMed CrossRef
- Salvadore G, Nash A, Bleys C, et al. A double-blind, placebo-controlled, multicenter study of sirukumab as adjunctive treatment to a monoaminergic antidepressant in adults with major depressive disorder. Neuropsychopharmacology; 2018:S292.
- Talamonti M, Malara G, Natalini Y, et al. Secukinumab improves patient perception of anxiety and depression in patients with moderate to severe psoriasis: a post hoc analysis of the supreme study. Acta Derm Venereol. 2021;101(3):adv00422. PubMed
- Traki L, Rostom S, Tahiri L, et al. Responsiveness of the EuroQol EQ-5D and hospital anxiety and depression scale (HADS) in rheumatoid arthritis patients receiving tocilizumab. Clin Rheumatol. 2014;33(8):1055–1060. PubMed CrossRef
- Tiosano S, Yavne Y, Watad A, et al. The impact of tocilizumab on anxiety and depression in patients with rheumatoid arthritis. Eur J Clin Invest. 2020;50(9):e13268. PubMed CrossRef
- Knight JM, Costanzo ES, Singh S, et al. The IL-6 antagonist tocilizumab is associated with worse depression and related symptoms in the medically ill. Transl Psychiatry. 2021;11(1):58. PubMed
- Langley RG, Feldman SR, Han C, et al. Ustekinumab significantly improves symptoms of anxiety, depression, and skin-related quality of life in patients with moderate-to-severe psoriasis: results from a randomized, double-blind, placebo-controlled phase III trial. J Am Acad Dermatol. 2010;63(3):457–465. PubMed CrossRef
- Kim SJ, Park MY, Pak K, et al. Improvement of depressive symptoms in patients with moderate-to-severe psoriasis treated with ustekinumab: an open label trial validated using beck depression inventory, Hamilton Depression Rating Scale measures and 18fluorodeoxyglucose (FDG) positron emission tomography (PET). J Dermatolog Treat. 2018;29(8):761–768. PubMed CrossRef
- Uzzan S, Azab AN. Anti-TNF-α compounds as a treatment for depression. Molecules. 2021;26(8):2368. PubMed
- Fatima R, Bittar K, Aziz M. Infliximab. StatPearls Publishing; 2022.
- Cheng Y, Desse S, Martinez A, et al. TNFα disrupts blood brain barrier integrity to maintain prolonged depressive-like behavior in mice. Brain Behav Immun. 2018;69:556–567. PubMed CrossRef
- Das R, Emon MPZ, Shahriar M, et al. Higher levels of serum IL-1β and TNF-α are associated with an increased probability of major depressive disorder. Psychiatry Res. 2021;295:113568. PubMed CrossRef
- Choi KW, Jang EH, Kim AY, et al. Predictive inflammatory biomarkers for change in suicidal ideation in major depressive disorder and panic disorder: a 12-week follow-up study. J Psychiatr Res. 2021;133:73–81. PubMed
- Jese R, Perdan-Pirkmajer K, Dolenc-Voljč M, et al. A case of inverse psoriasis successfully treated with adalimumab. Acta Dermatovenerol Alp Pannonica Adriat. 2014;23:21–23. PubMed
- Kyriakou A, Trigoni A, Galanis N, et al. Efficacy of adalimumab in moderate to severe hidradenitis suppurativa: real life data. Dermatol Rep. 2018;10(2):7859. PubMed CrossRef
- Ellis CR, Azmat CE. Adalimumab. In: StatPearls [Internet]. StatPearls Publishing; 2024.
- Eisenberg E, Sandler I, Treister R, et al. Anti tumor necrosis factor – alpha adalimumab for complex regional pain syndrome type 1 (CRPS-I): a case series. Pain Pract. 2013;13(8):649–656. PubMed CrossRef
- Liu YN, Peng YL, Liu L, et al. TNFα mediates stress-induced depression by upregulating indoleamine 2,3-dioxygenase in a mouse model of unpredictable chronic mild stress. Eur Cytokine Netw. 2015;26(1):15–25. PubMed CrossRef
- Mansur RB, Delgado-Peraza F, Subramaniapillai M, et al. Extracellular vesicle biomarkers reveal inhibition of neuroinflammation by infliximab in association with antidepressant response in adults with bipolar depression. Cells. 2020;9(4):895. PubMed
- Mehta D, Raison CL, Woolwine BJ, et al. Transcriptional signatures related to glucose and lipid metabolism predict treatment response to the tumor necrosis factor antagonist infliximab in patients with treatment-resistant depression. Brain Behav Immun. 2013;31:205–215. PubMed CrossRef
- Bekhbat M, Chu K, Le NA, et al. Glucose and lipid-related biomarkers and the antidepressant response to infliximab in patients with treatment-resistant depression. Psychoneuroendocrinology. 2018;98:222–229. PubMed CrossRef
- Bavaresco DV, Uggioni MLR, Ferraz SD, et al. Efficacy of infliximab in treatment-resistant depression: a systematic review and meta-analysis. Pharmacol Biochem Behav. 2020;188:172838. PubMed CrossRef
- Fan N, Luo Y, Ou Y, et al. Altered serum levels of TNF-α, IL-6, and IL-18 in depressive disorder patients. Hum Psychopharmacol. 2017;32(4):e2588.
- Hodes GE, Menard C, Russo SJ. Integrating Interleukin-6 into depression diagnosis and treatment. Neurobiol Stress. 2016;4:15–22. PubMed CrossRef
- Lanquillon S, Krieg JC, Bening-Abu-Shach U, et al. Cytokine production and treatment response in major depressive disorder. Neuropsychopharmacology. 2000;22(4):370–379. PubMed CrossRef
- Kiraly DD, Horn SR, Van Dam NT, et al. Altered peripheral immune profiles in treatment-resistant depression: response to ketamine and prediction of treatment outcome. Transl Psychiatry. 2017;7(3):e1065. PubMed CrossRef
- Hodes GE, Pfau ML, Leboeuf M, et al. Individual differences in the peripheral immune system promote resilience versus susceptibility to social stress. Proc Natl Acad Sci U S A. 2014;111(45):16136–16141. PubMed CrossRef
- Neupane SP, Daray FM, Ballard ED, et al. Immune-related biomarkers and suicidal behaviors: a meta-analysis. Eur Neuropsychopharmacol. 2023;75:15–30. PubMed
- Davis CC, Shah KS, Lechowicz MJ. Clinical development of siltuximab. Curr Oncol Rep. 2015;17(7):29. PubMed
- Taylor PC, Schiff MH, Wang Q, et al. Efficacy and safety of monotherapy with sirukumab compared with adalimumab monotherapy in biologic-naive patients with active rheumatoid arthritis (SIRROUND-H): a randomised, double-blind, parallel-group, multinational, 52-week, phase 3 study. Ann Rheum Dis. 2018;77(5):658–666. PubMed CrossRef
- Rehman MEU, Chattaraj A, Neupane K, et al. Efficacy and safety of regimens used for the treatment of multicentric Castleman disease: a systematic review. Eur J Haematol. 2022;109(4):309–320. PubMed
- Smolen JS, Weinblatt ME, Sheng S, et al. Sirukumab, a human anti-interleukin-6 monoclonal antibody: a randomised, 2-part (proof-of-concept and dose-finding), phase II study in patients with active rheumatoid arthritis despite methotrexate therapy. Ann Rheum Dis. 2014;73(9):1616–1625. PubMed CrossRef
- Shetty A, Hanson R, Korsten P, et al. Tocilizumab in the treatment of rheumatoid arthritis and beyond. Drug Des Devel Ther. 2014;8:349–364. PubMed CrossRef
- Schirmer M, Muratore F, Salvarani C. Tocilizumab for the treatment of giant cell arteritis. Expert Rev Clin Immunol. 2018;14(5):339–349. PubMed CrossRef
- Khanna D, Lescoat A, Roofeh D, et al. Systemic sclerosis–associated interstitial lung disease: how to incorporate two food and drug administration–approved therapies in clinical practice. Arthritis Rheumatol. 2022;74(1):13–27. PubMed
- Oberle EJ, Harris JG, Verbsky JW. Polyarticular juvenile idiopathic arthritis – epidemiology and management approaches. Clin Epidemiol. 2014;6:379–393. PubMed CrossRef
- Yokota S, Tanaka T, Kishimoto T. Efficacy, safety and tolerability of tocilizumab in patients with systemic juvenile idiopathic arthritis. Ther Adv Musculoskelet Dis. 2012;4(6):387–397. PubMed CrossRef
- Le RQ, Li L, Yuan W, et al. FDA approval summary: tocilizumab for treatment of chimeric antigen receptor T cell-induced severe or life-threatening cytokine release syndrome. Oncologist. 2018;23(8):943–947. PubMed
- Flisiak R, Flisiak-Jackiewicz M, Rzymski P, et al. Tocilizumab for the treatment of COVID-19. Expert Rev Anti Infect Ther. 2023;21(8):791–797. PubMed
- Tesmer LA, Lundy SK, Sarkar S, et al. Th17 cells in human disease. Immunol Rev. 2008;223:87–113. PubMed
- Chen K, Kolls JK. Interluekin-17a (il17a). Gene. 2017;614:8–14. PubMed
- Kebir H, Kreymborg K, Ifergan I, et al. Human TH17 lymphocytes promote blood-brain barrier disruption and central nervous system inflammation. Nat Med. 2007;13(10):1173–1175. PubMed CrossRef
- Chang SH, Dong C. IL-17F: regulation, signaling and function in inflammation. Cytokine. 2009;46(1):7–11. PubMed CrossRef
- Borowczyk J, Shutova M, Brembilla NC, et al. IL-25 (IL-17E) in epithelial immunology and pathophysiology. J Allergy Clin Immunol. 2021;148(1):40–52. PubMed
- Foulkes AC, Warren RB. Brodalumab in psoriasis: evidence to date and clinical potential. Drugs Context. 2019;8:212570. PubMed CrossRef
- Golbari NM, Basehore BM, Zito PM. Brodalumab. In: StatPearls [Internet]: StatPearls Publishing; 2024.
- Papp KA, Reich K, Paul C, et al. A prospective phase III, randomized, double-blind, placebo-controlled study of brodalumab in patients with moderate-to-severe plaque psoriasis. Br J Dermatol. 2016;175(2):273–286. PubMed CrossRef
- Rivera-Oyola R, Stanger R, Litchman GH, et al. The use of brodalumab in three patients with psoriasis and psychiatric comorbidities. J Clin Aesthet Dermatol. 2020;13(12):44–48.
- Blegvad C, Skov L, Zachariae C. Ixekizumab for the treatment of psoriasis: an update on new data since first approval. Expert Rev Clin Immunol. 2019;15(2):111–121. PubMed CrossRef
- Huang JX, Lee YH, Wei JCC. Ixekizumab for the treatment of ankylosing spondylitis. Expert Rev Clin Immunol. 2020;16(8):745–750. PubMed CrossRef
- Frieder J, Kivelevitch D, Menter A. Secukinumab: a review of the anti-IL-17A biologic for the treatment of psoriasis. Ther Adv Chronic Dis. 2018;9(1):5–21. PubMed CrossRef
- Fala L. Cosentyx (secukinumab): first IL-17a antagonist receives FDA approval for moderate-to-severe plaque psoriasis. Am Health Drug Benefits. 2016;9(Spec Feature):60–63. PubMed
- McInnes IB, Asahina A, Coates LC, et al. Bimekizumab in patients with psoriatic arthritis, naive to biologic treatment: a randomised, double-blind, placebo-controlled, phase 3 trial (BE OPTIMAL). Lancet. 2023;401(10370):25–37. PubMed
- Iwakura Y, Ishigame H. The IL-23/IL-17 axis in inflammation. J Clin Invest. 2006;116(5):1218–1222. PubMed
- Gałecka M, Bliźniewska-Kowalska K, Orzechowska A, et al. Inflammatory versus anti-inflammatory profiles in major depressive disorders–the role of IL-17, IL-21, IL-23, IL-35 and Foxp3. J Pers Med. 2021;11(2):66. PubMed
- Yang EJ, Smith MP, Ly K, et al. Evaluating guselkumab: an anti-IL-23 antibody for the treatment of plaque psoriasis. Drug Des Devel Ther. 2019;13:1993–2000. PubMed CrossRef
- Megna M, Balato A, Raimondo A, et al. Guselkumab for the treatment of psoriasis. Expert Opin Biol Ther. 2018;18(4):459–468. PubMed
- Boehncke WH, Brembilla NC, Nissen MJ. Guselkumab: the first selective IL-23 inhibitor for active psoriatic arthritis in adults. Expert Rev Clin Immunol. 2021;17(1):5–13. PubMed
- Wofford J, Menter A. Ustekinumab for the treatment of psoriatic arthritis. Expert Rev Clin Immunol. 2014;10(2):189–202. PubMed
- Meserve J, Ma C, Dulai PS, et al. Effectiveness of reinduction and/or dose escalation of ustekinumab in Crohn’s disease: a systematic review and meta-analysis. Clin Gastroenterol Hepatol. 2022;20(12):2728–2740.e1. PubMed
- Koutruba N, Emer J, Lebwohl M. Review of ustekinumab, an interleukin-12 and interleukin-23 inhibitor used for the treatment of plaque psoriasis. Ther Clin Risk Manag. 2010;6:123–141. PubMed CrossRef
- Vignali DAA, Kuchroo VK. IL-12 family cytokines: immunological playmakers. Nat Immunol. 2012;13(8):722–728. PubMed CrossRef
- Osimo EF, Pillinger T, Rodriguez IM, et al. Inflammatory markers in depression: a meta-analysis of mean differences and variability in 5,166 patients and 5,083 controls. Brain Behav Immun. 2020;87:901–909. PubMed CrossRef
- Junttila IS. Tuning the cytokine responses: an update on interleukin (IL)-4 and IL-13 receptor complexes. Front Immunol. 2018;9:888. PubMed
- Wachholz S, Knorr A, Mengert L, et al. Interleukin-4 is a participant in the regulation of depressive-like behavior. Behav Brain Res. 2017;326:165–172. PubMed CrossRef
- Moon ML, Joesting JJ, Blevins NA, et al. IL-4 knock out mice display anxiety-like behavior. Behav Genet. 2015;45(4):451–460. PubMed
- Park HJ, Shim HS, An K, et al. IL-4 inhibits IL-1β-induced depressive-like behavior and central neurotransmitter alterations. Mediators Inflamm. 2015;2015:941413. PubMed
- Syed SA, Beurel E, Loewenstein DA, et al. Defective inflammatory pathways in never-treated depressed patients are associated with poor treatment response. Neuron. 2018;99(5):914–924.e3. PubMed
- Pavón L, Sandoval-López G, Eugenia Hernández M, et al. Th2 cytokine response in major depressive disorder patients before treatment. J Neuroimmunol. 2006;172(1–2):156–165. PubMed
- Vai B, Mazza MG, Cazzetta S, et al. Higher Interleukin 13 differentiates patients with a positive history of suicide attempts in major depressive disorder. J Affect Disord Rep. 2021;6:100254.
- Tonelli LH, Stiller J, Rujescu D, et al. Elevated cytokine expression in the orbitofrontal cortex of victims of suicide. Acta Psychiatr Scand. 2008;117(3):198–206. PubMed CrossRef
- Thibodeaux Q, Smith MP, Ly K, et al. A review of dupilumab in the treatment of atopic diseases. Hum Vaccin Immunother. 2019;15(9):2129–2139. PubMed CrossRef
- Morse JC, Miller C, Senior B. Management of chronic rhinosinusitis with nasal polyposis in the era of biologics. J Asthma Allergy. 2021;14:873–882. PubMed
- Nhu QM, Aceves SS. Current state of biologics in treating eosinophilic esophagitis. Ann Allergy Asthma Immunol. 2023;130(1):15–20. PubMed
- Cao P, Xu W, Jiang S, et al. Dupilumab for the treatment of prurigo nodularis: a systematic review. Front Immunol. 2023;14:1092685. PubMed
- Del Rosso JQ. MONOCLONAL ANTIBODY THERAPIES for atopic dermatitis: where are we now in the spectrum of disease management?. J Clin Aesthet Dermatol. 2019;12(2):39–41.
- Sattler C, Garcia G, Humbert M. Novel targets of omalizumab in asthma. Curr Opin Pulm Med. 2017;23(1):56–61. PubMed CrossRef
- Licari A, Marseglia G, Castagnoli R, et al. The discovery and development of omalizumab for the treatment of asthma. Expert Opin Drug Discov. 2015;10(9):1033–1042. PubMed CrossRef
- Kumar C, Zito PM. Omalizumab. StatPearls [Internet]. StatPearls Publishing; 2022.
- Easthope S, Jarvis B. Omalizumab. Drugs. 2001;61:253–261. PubMed
- Kelly BT, Grayson MH. Immunoglobulin E, what is it good for?. Ann Allergy Asthma Immunol. 2016;116(3):183–187. PubMed CrossRef
- Noga O, Hanf G, Brachmann I, et al. Effect of omalizumab treatment on peripheral eosinophil and T-lymphocyte function in patients with allergic asthma. J Allergy Clin Immunol. 2006;117(6):1493–1499. PubMed CrossRef
- Takaku Y, Soma T, Nishihara F, et al. Omalizumab attenuates airway inflammation and interleukin-5 production by mononuclear cells in patients with severe allergic asthma. Int Arch Allergy Immunol. 2013;161(suppl 2):107–117. PubMed CrossRef
- Manalai P, Hamilton RG, Langenberg P, et al. Pollen-specific immunoglobulin E positivity is associated with worsening of depression scores in bipolar disorder patients during high pollen season. Bipolar Disord. 2012;14(1):90–98. PubMed CrossRef
- Xiang DC, Chen W, Fu ZW, et al. Adverse events of guselkumab in the real world: emerging signals to target preventive strategies from the FDA adverse event reporting system. Expert Opin Drug Saf. 2023;22(10):943–955. PubMed
- Li J, Zhang Z, Wu X, et al. Risk of adverse events after anti-TNF treatment for inflammatory rheumatological disease. A meta-analysis. Front Pharmacol. 2021;12:746396. PubMed
- Miehsler W, Novacek G, Wenzl H, et al. A decade of infliximab: the Austrian evidence based consensus on the safe use of infliximab in inflammatory bowel disease. J Crohns Colitis. 2010;4(3):221–256. PubMed CrossRef
- Subedi S, Gong Y, Chen Y, et al. Infliximab and biosimilar infliximab in psoriasis: efficacy, loss of efficacy, and adverse events. Drug Des Devel Ther. 2019;13:2491–2502. PubMed CrossRef
- Deodhar AA, Combe B, Accioly AP, et al. Safety of ixekizumab in patients with psoriatic arthritis: data from four clinical trials with over 2000 patient-years of exposure. Ann Rheum Dis. 2022;81(7):944–950. PubMed
- Silberstein SD, McAllister P, Ning X, et al. Safety and tolerability of fremanezumab for the prevention of migraine: a pooled analysis of phases 2b and 3 clinical trials. Headache J Head Face Pain. 2019;59(6):880–890.
- Gklinos P, Mitsikostas DD. Galcanezumab in migraine prevention: a systematic review and meta-analysis of randomized controlled trials. Ther Adv Neurol Disord. 2020;13:1756286420918088. PubMed CrossRef
- Gatti M, Fusaroli M, Caraceni P, et al. Serious adverse events with tocilizumab: pharmacovigilance as an aid to prioritize monitoring in COVID-19. Br J Clin Pharmacol. 2021;87(3):1533–1540. PubMed
- Ou Z, Chen C, Chen A, et al. Adverse events of dupilumab in adults with moderate-to-severe atopic dermatitis: a meta-analysis. Int Immunopharmacol. 2018;54:303–310. PubMed CrossRef
- Choy EH, De Benedetti F, Takeuchi T, et al. Translating IL-6 biology into effective treatments. Nat Rev Rheumatol. 2020;16(6):335–345. PubMed CrossRef
- Rovin BH, van Vollenhoven RF, Aranow C, et al. A multicenter, randomized, double-blind, placebo-controlled study to evaluate the efficacy and safety of treatment with sirukumab (CNTO 136) in patients with active lupus nephritis. Arthritis Rheumatol. 2016;68(9):2174–2183. PubMed
- Lebwohl MG, Papp KA, Marangell LB, et al. Psychiatric adverse events during treatment with brodalumab: analysis of psoriasis clinical trials. J Am Acad Dermatol. 2018;78(1):81–89.e5. PubMed CrossRef
- Sears AV, Woolf RT, Gribaleva E, et al. Real-world effectiveness and tolerability of dupilumab in adult atopic dermatitis: a single-centre, prospective 1-year observational cohort study of the first 100 patients treated. Br J Dermatol. 2021;184(4):755–757. PubMed
- Simpson EL, Bieber T, Guttman-Yassky E, et al. Two phase 3 trials of dupilumab versus placebo in atopic dermatitis. N Engl J Med. 2016;375(24):2335–2348. PubMed
- Ellard R, Ahmed A, Shah R, et al. Suicide and depression in a patient with psoriasis receiving adalimumab: the role of the dermatologist. Clin Exp Dermatol. 2014;39(5):624–627. PubMed CrossRef
- Rusta-Sallehy S, Gooderham M, Papp K. Brodalumab: a review of safety. Skin Ther Lett. 2018;23(2):1–3.
- Lebwohl M, Koo J, Leonardi C, et al. Brodalumab: 4-year US pharmacovigilance report. J Drugs Dermatol. 2023;22(4):419–422. PubMed
- Blauvelt A, Tsai TF, Langley RG, et al. Consistent safety profile with up to 5 years of continuous treatment with guselkumab: pooled analyses from the phase 3 VOYAGE 1 and VOYAGE 2 trials of patients with moderate-to-severe psoriasis. J Am Acad Dermatol. 2022;86(4):827–834. PubMed
- Shayowitz M, Bressler M, Ricardo AP, et al. Infliximab-induced depression and suicidal behavior in adolescent with Crohn’s disease: case report and review of literature. Pediatr Qual Saf. 2019;4(6):e229. PubMed
- Wollenberg A, Beck LA, Blauvelt A, et al. Laboratory safety of dupilumab in moderate-to-severe atopic dermatitis: results from three phase III trials (LIBERTY AD SOLO 1, LIBERTY AD SOLO 2, LIBERTY AD CHRONOS). Br J Dermatol. 2020;182(5):1120–1135. PubMed CrossRef
- Genovese MC, Mysler E, Tomita T, et al. Safety of ixekizumab in adult patients with plaque psoriasis, psoriatic arthritis and axial spondyloarthritis: data from 21 clinical trials. Rheumatol Oxf. 2020;59(12):3834–3844. PubMed CrossRef
- Paller AS, Wollenberg A, Siegfried E, et al. Laboratory safety of dupilumab in patients aged 6–11 years with severe atopic dermatitis: results from a phase III clinical trial. Paediatr Drugs. 2021;23(5):515–527. PubMed
- Paller AS, Simpson EL, Siegfried EC, et al. Dupilumab in children aged 6 months to younger than 6 years with uncontrolled atopic dermatitis: a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2022;400(10356):908–919. PubMed
This PDF is free for all visitors!
Save
Cite