ABSTRACT
Objective: To summarize the breadth of data exploring the relationship between major depressive disorder (MDD) and both the incidence and the disease course of a range of comorbidities.
Data Sources: The authors searched MEDLINE, Embase, PsycINFO, Cochrane Database of Systematic Reviews, and several prespecified congresses. Searches included terms related to MDD and several comorbidity categories, restricted to those published in the English language from 2005 onward.
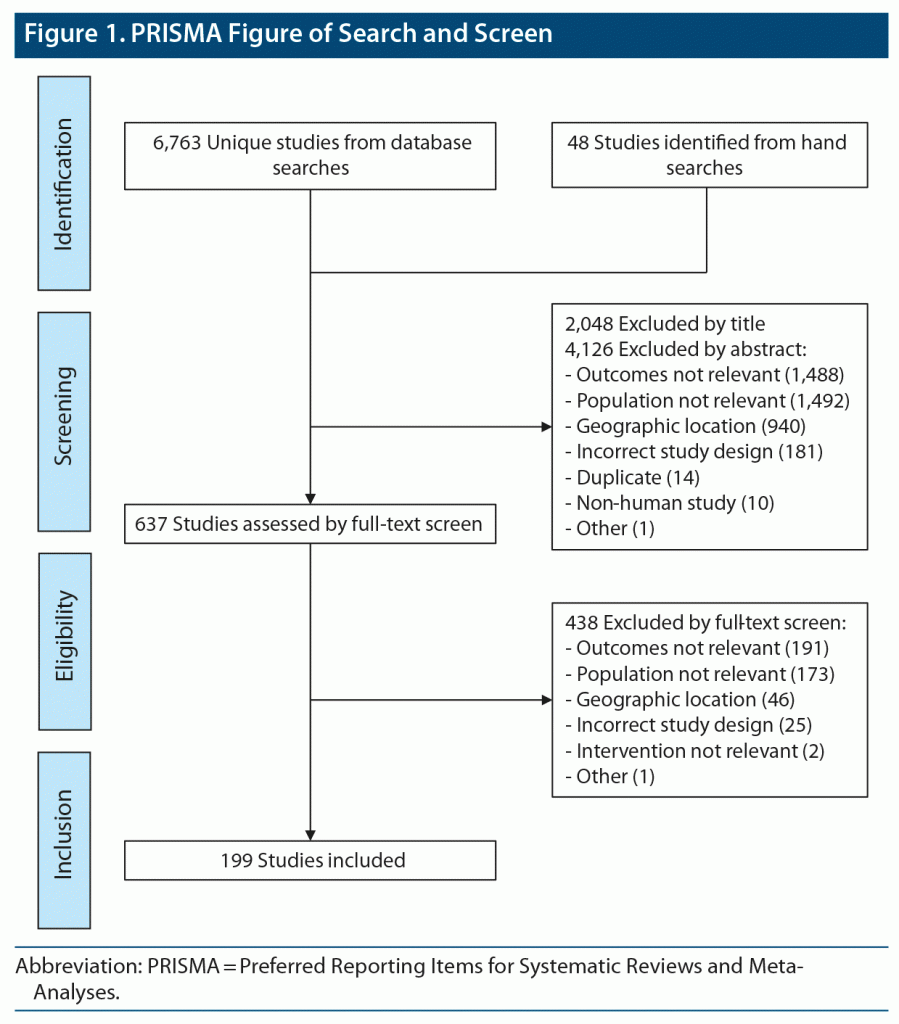
Study Selection: Eligibility criteria included observational studies within North America and Europe that examined the covariate-adjusted impact of MDD on the risk and/or severity of comorbidities. A total of 6,811 articles were initially identified for screening.
Data Extraction: Two investigators extracted data and assessed study quality.
Results: In total, 199 articles were included. Depression was significantly (P < .05) associated with an increased incidence of dementia and Alzheimer’s disease as well as cognitive decline in individuals with existing disease; increased incidence and worsening of cardiovascular disease/events (although mixed results were found for stroke); worsening of metabolic syndrome; increased incidence of diabetes, particularly among men, and worsening of existing diabetes; increased incidence of obesity, particularly among women; increased incidence and worsening of certain autoimmune diseases; increased incidence and severity of HIV/AIDS; and increased incidence of drug abuse and severity of both alcohol and drug abuse.
Conclusions: The presence of MDD was identified as a risk factor for both the development and the worsening of a range of comorbidities. These results highlight the importance of addressing depression early in its course and the need for integrating mental and general health care.
J Clin Psychiatry 2022;83(6):21r14328
To cite: Arnaud AM, Brister TS, Duckworth K, et al. Impact of major depressive disorder on comorbidities: a systematic literature review. J Clin Psychiatry. 2022;83(6):21r14328.
To share: https://doi.org/10.4088/JCP.21r14328
© 2022 Physicians Postgraduate Press, Inc.
aSage Therapeutics, Inc., Cambridge, Massachusetts
bNational Alliance on Mental Illness (NAMI), Arlington, Virginia
cDepression and Bipolar Support Alliance, Chicago, Illinois
dMental Health America of Ohio, Columbus, Ohio
eTantalus Medical Communications Ltd., Victoria, BC, Canada
*Corresponding author: Ellison D. Suthoff, MBA, 215 First St, Cambridge, MA 02142 ([email protected]).
See commentary by Weir and Rapaport
Major depressive disorder (MDD) is defined as a depressed mood and/or loss of interest or pleasure in daily activities along with symptoms such as weight loss or gain, insomnia or hypersomnia, psychomotor agitation or retardation, fatigue, feelings of worthlessness, diminished ability to think or concentrate, and thoughts of death or suicidal ideation (minimum 5 symptoms total), which have collectively been present during the same 2-week period and represent a change from previous functioning.1 The episode is considered MDD if it is not attributable to the physiologic effects of a substance or not better explained by another medical condition (such as schizophrenia) and a manic episode or a hypomanic episode has never been observed.1 MDD affects over 160 million people globally2 and is one of the most common mental health disorders in several countries. Notably, depression is recognized by the World Health Organization (WHO) as a major contributor to the overall global burden of disease.3
Complicating our understanding of MDD and its treatment is the presence of several physical and psychological comorbidities among individuals with depression, demonstrating an apparent relationship between physical and mental health. The interaction between depression and many of these comorbidities is not fully understood but, in several cases, appears to be complex and potentially bidirectional. The presence of comorbidities adds to both the humanistic and the economic burden associated with depression; notably, 63% of total MDD-associated costs in the United States in 2018 were attributed to an increased cost of treating comorbid conditions rather than to MDD itself.4,5 Furthermore, individuals with MDD have been shown to have a decreased life expectancy compared with those without depression6; it is therefore possible that the worsening of associated comorbidities could be a contributing factor to earlier mortality.
Although the associations between depression and several comorbidities have been studied individually, not all have been explored systematically. The objective of this review was to qualitatively identify and summarize the breadth of observational data that examine the potential causality of MDD on multiple comorbidities, including the risk of developing new diseases and the impact on the disease course of preexisting comorbidities. Of note, this review explores several comorbidity categories simultaneously to provide a broader illustration of the collective comorbidity risks among people with depression.
METHODS
A systematic literature search for studies that examined the association between MDD and comorbidities was conducted with methods consistent with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) reporting guidelines.7
Categories of comorbidities were first determined from preliminary literature research, review of national agency and patients’ advocacy group reports, review of US medical claims data, and expert opinion. The final list of comorbidity categories included cancer, central nervous system (CNS) disorders, cardiovascular disease (CVD), metabolic and endocrine diseases, autoimmune and gastrointestinal (GI) diseases, pain-related conditions, respiratory disorders, and substance abuse disorders. This review focused on comorbidities related to physical and substance abuse disorders; anxiety and other psychiatric disorders were not included.
Databases searched included Embase, MEDLINE (including MEDLINE In-Process), Cochrane Database of Systematic Reviews, and PsycINFO, and the search used terms for MDD, comorbidity categories, and observational studies (the full search strategy is provided in Supplementary Table 1). In addition, abstracts from several relevant congresses were reviewed and hand searches of referenced publications were undertaken. Searches were conducted in November 2019 and were restricted to the previous 15 years for database searches and the previous 2 years for conference proceedings.
Included studies assessed the relationship between MDD and comorbidities and were required to use a covariate-adjusted analysis to identify the association of MDD with downstream comorbidities (affecting either the risk of developing a comorbidity or the disease course of an existing comorbidity). Geographically, included studies were restricted to those undertaken in Europe and North America. Covariate-adjusted analyses included those adjusted for at least one relevant covariate to minimize their impact on the association with depression and the comorbidity; examples include demographics such as age and sex, other diseases, and behaviors such as smoking and alcohol consumption. Studies that assessed only associations in the opposite direction (ie, the impact of comorbidities on the development or disease course of depression) were excluded. Included studies were required to be observational in design (including meta-analyses of observational data), and only English-language records were searched; a complete list of criteria is provided in Supplementary Table 2. Although the aim of this review was to identify studies of people with MDD, the heterogeneous way that these individuals are described and identified in scientific literature (such as through symptom rating scales or retrospectively from medical records) did not allow for a strict criterion of physician diagnosis of MDD. Indeed, scales and questionnaires are often used in clinical practice to screen, diagnose, and monitor depression. This review therefore included studies of people with MDD or depression otherwise defined by the authors that was not clearly a diagnosis for a different type or severity level of depression (such as dysthymia).
Search results were screened by 2 separate reviewers initially by titles and abstracts, followed by a review of the full text; any disputes were resolved through discussion between reviewers or consultation with a third reviewer. Data from included studies were extracted by 2 independent reviewers, and any discrepancies between extractions were verified for accuracy by an independent third reviewer. Data describing the study methodology, participant demographic and clinical characteristics, and associations between MDD and comorbidities were extracted. The quality of included studies was assessed by reviewers using the Newcastle-Ottawa Scale for observational studies and the checklist recommended by the National Institute for Health and Care Excellence (NICE) for meta-analyses (see Supplementary Tables 3–6).8,9
RESULTS
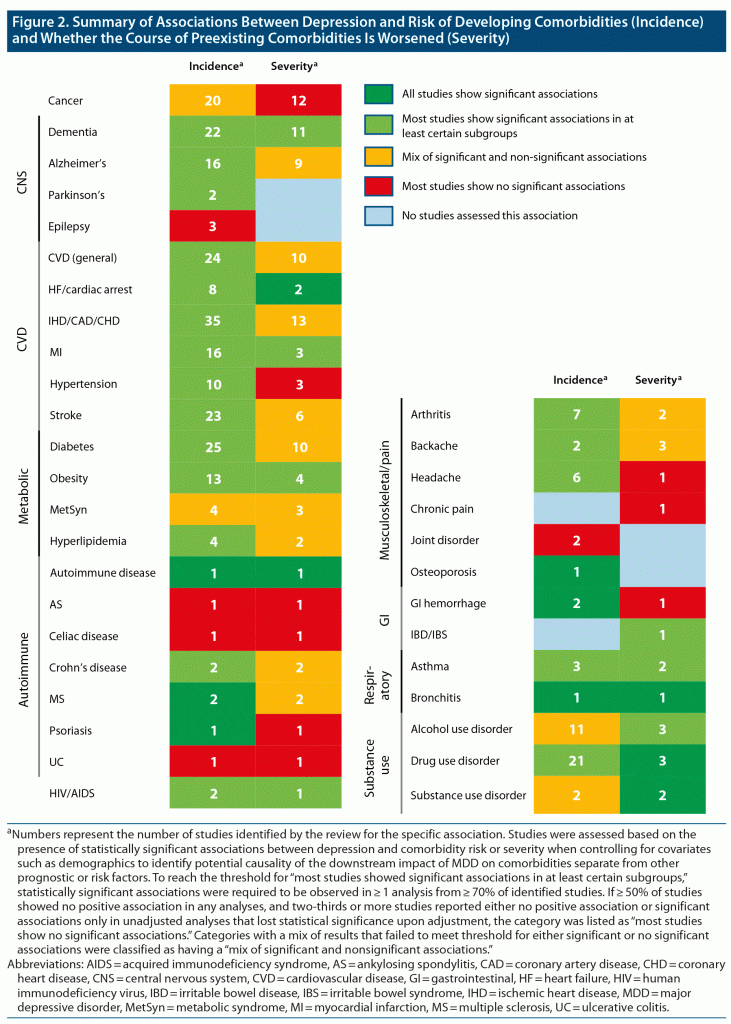
A total of 6,763 articles were identified by database searches for initial screening, and these were combined with another 48 relevant articles identified from conference abstracts and hand searches of references lists from other reviews. Overall, 199 articles met the inclusion criteria (Figure 1); these included 142 cohort studies, 15 cross-sectional studies, 15 case-control studies, 26 meta-analyses of observational data, and 1 ancillary study from a clinical trial. Studies ranged broadly in size from fewer than 50 to nearly 5 million participants (see Supplementary Table 7 for a complete list). A summary of the strength of associations found between depression and the incidence and severity of several comorbidities is provided in Figure 2. Findings from all studies are summarized in the following sections; for brevity, comorbidities with findings of note are reported in tables with all other depression-associated outcomes presented in Supplementary Tables 8–31.
Cancer
A total of 12 observational studies (13 publications) and 2 meta-analyses were identified through this systematic review. One meta-analysis10 of 32 studies showed that although an analysis of studies that included patients with cancer and depressive symptoms demonstrated a significant association between depression and mortality (hazard ratio [HR] = 1.09; 95% confidence interval [CI]: 1.03 to 1.15; P = .003), when a clinical depression diagnosis was required this association had a wider confidence interval and statistical significance was no longer demonstrated (HR = 1.67; 95% CI, 0.96 to 2.90; P = .07). Furthermore, the meta-analysis also showed no significant association between depressive symptoms and cancer recurrence (risk ratio [RR] = 1.23; 95% CI, 0.85 to 1.77; P = .275).10 Among individual studies identified by this systematic review, we did not find a consistent and significant impact of depression on the new incidence of cancer nor on assessments of cancer severity/mortality of patients with preexisting cancer (see Supplementary Tables 8 and 9 for complete information). For example, in the Baltimore Epidemiologic Catchment Area study11 that had the longest duration of follow-up of all identified studies (24 years), a lifetime history of major depressive episodes (MDE) was associated with the risk of any incident cancer (adjusted HR = 1.87; 95% CI, 1.16 to 3.01); however, the adjusted analysis lost significance in a subgroup that excluded 145 respondents who rated their health status as poor at baseline.
CNS Disorders
This review identified a total of 29 observational studies (in 30 publications) and 3 meta-analyses assessing the relationship between depression and CNS disorders. Consistently, depression was demonstrated to be significantly associated with an increased incidence of new dementia and Alzheimer’s disease or cognitive decline in people with existing disease, and with the incidence of Parkinson’s disease.
Incident dementia and Alzheimer’s disease. Two meta-analyses12,13 (in 23 and 20 studies, respectively) that assessed the association of depression with incident dementia (pooled odds ratio [OR] = 1.96; 95% CI, 1.64 to 2.34; P < .0001; 23-study meta-analysis only) and/or Alzheimer’s disease (pooled ORs for Alzheimer’s disease = 1.85; 95% CI, 1.45 to 2.37; P < .0001 and 1.98; 95% CI, 1.76 to 2.24; P < .001, respectively) showed significant associations overall and across all subgroups. Among another 22 studies (in 23 publications) identified by the review for this comparison, most showed a significant association between depression and the incidence of dementia and many also showed a significant association between depression and the incidence of Alzheimer’s disease (see Supplementary Table 10 for complete information).
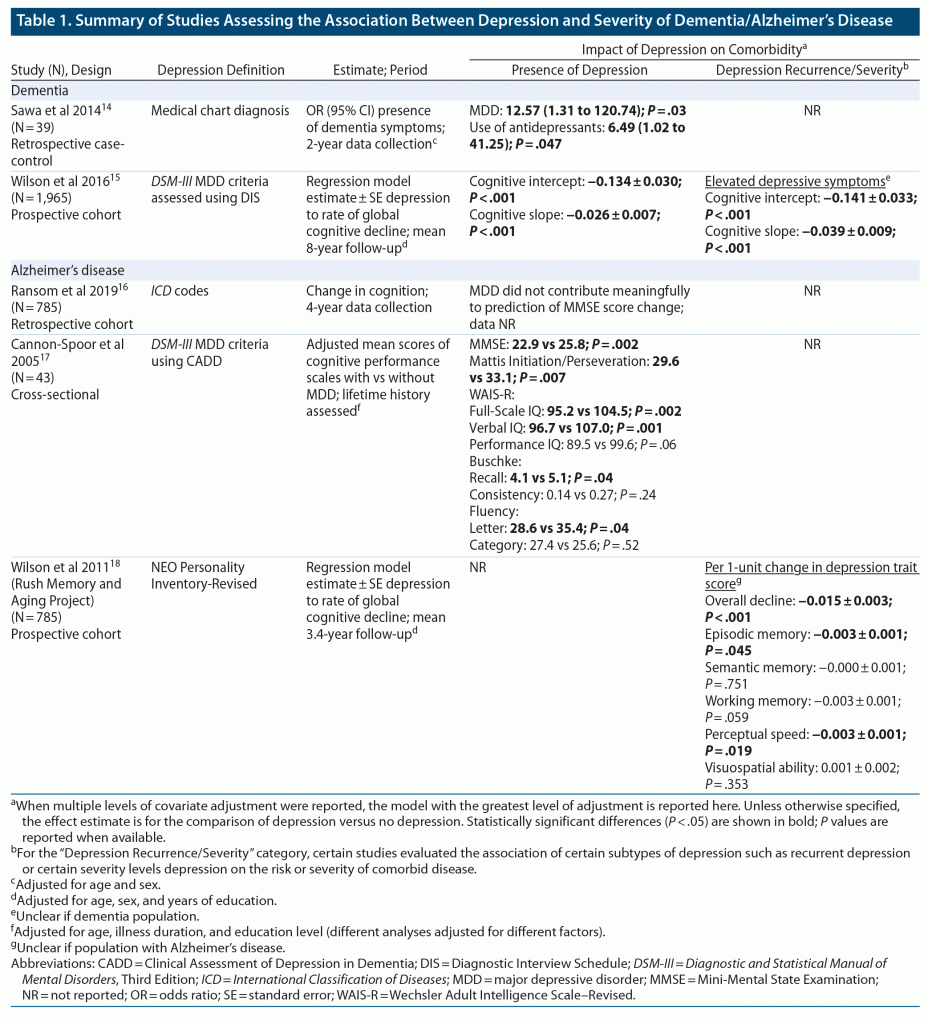
Dementia and Alzheimer’s disease severity. Five studies assessed the impact of depression on the severity of preexisting dementia and/or Alzheimer’s disease and nearly all (4 of 5) showed significant and positive associations between presence/severity of depression and cognitive decline/worsening of symptoms in these cohorts (Table 1). Additional details for associations between depression and CNS disorders, including findings in people with epilepsy and Parkinson’s disease, can be found in or subsequent to Supplementary Table 10.
Cardiovascular Disease
The association between MDD and CVD has been studied widely, and the review identified 67 observational studies and 14 meta-analyses that assessed the association between depression and incidence or worsening of CVD. Most studies identified an association between MDD and the subsequent incidence or worsening of CVD across several disease categories, including general CVD (or combined endpoints), heart failure, hypertension, ischemic heart disease/coronary artery disease, and myocardial infarction (MI; see Supplementary Tables 11–21 for complete information). In this systematic literature review, measures of disease worsening included subsequent cardiovascular events among individuals with prior events/existing chronic CVD as well as cardiac mortality in both the general populations and populations of people with preexisting CVD; the association between depression and these various measures of disease worsening was consistently significant across most analyses (see Supplementary Tables 11–23 for complete information). Among studies that took depression severity into account, these associations often became stronger as depression severity increased (see Supplementary Tables 11–23 for complete information). Furthermore, studies that assessed the duration of MDD and/or number of MDD episodes also found significant associations between greater durations or episode numbers and ischemic heart disease mortality, incidence of hypertension and heart disease, incidence of major coronary heart disease events, and incidence of acute MI (see Supplementary Tables 14–17 and 20 for complete information).19–22
Stroke incidence and severity. Of the many CVD categories, the relationship between depression and stroke incidence had the greatest variability. The review identified 3 meta-analyses (assessing 28 studies [2 meta-analyses each] and 30 studies, respectively), which consistently demonstrated significant associations between depression and incident stroke.23–25 Findings from the individual studies identified by the review (15 in total), however, were mixed, with some showing a significant association for this relationship and others showing mixed results across different analyses, while another group of studies showed no significant associations (see Supplementary Table 22 for complete information). Among 3 studies and 1 meta-analysis of 6 studies included in this review that assessed the association between depression and stroke severity (generally measured as poststroke function or recovery), the meta-analysis reported a significant association between depression and severe long-term disability among people with stroke (OR = 2.16; 95% CI, 1.70 to 2.77),26 and results from individual studies generally reflected these findings, with 2 of 3 studies that assessed poststroke function/recovery reporting a greater likelihood of disability in patients with depression (see Supplementary Table 23 for complete information).
Metabolic and Endocrine Disorders
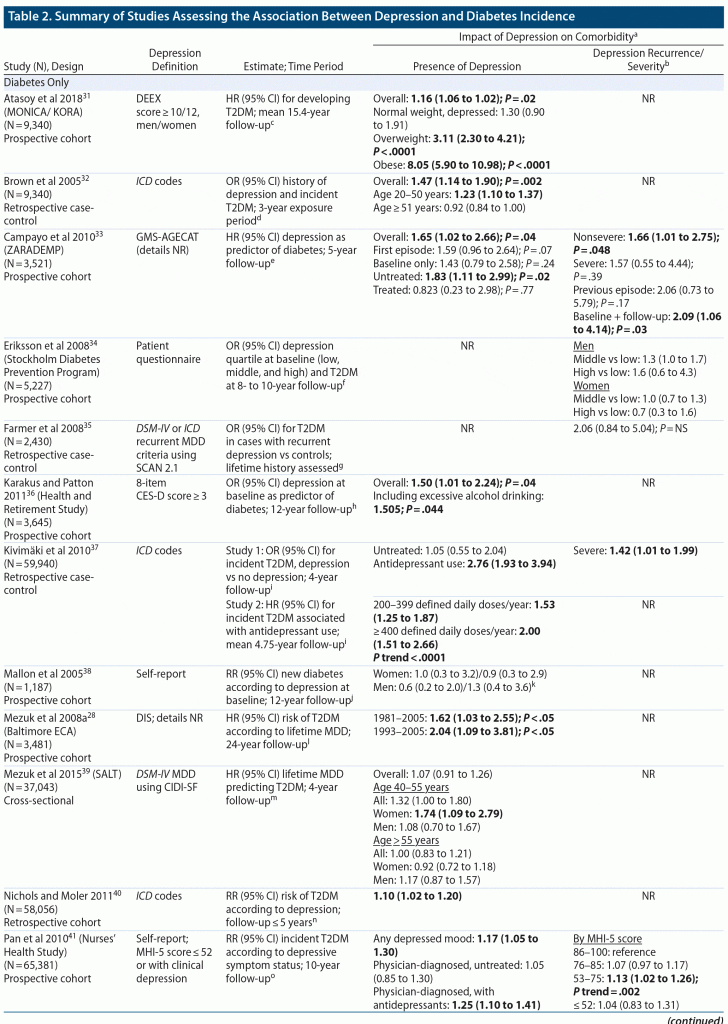
Incident diabetes. In total, 17 studies (including 2 analyses from metabolic syndrome studies) and 4 meta-analyses assessed the relationship between depression and a subsequent diagnosis of diabetes with follow-up times ranging from 3 to 17 years. The meta-analyses showed a consistently significant association between depression and incident diabetes in the follow-up period both overall and across several subgroup and sensitivity analyses.27,29,30,45 Six individual study analyses focused on type 2 diabetes only, 1 study each focused on type 1 diabetes and gestational diabetes only, and the remaining studies either did not clarify diabetes type or included both types 1 and 2. Several individual studies reflected the significant associations found in the meta-analyses (either overall or in 1 or more subgroups of participants; Table 2).
Among studies that presented stratified analyses by age and sex, no clear and consistent trends were observed,32,38,39 although it was notable that a meta-analysis showed a significant association between incident type 2 diabetes in men and depression (RR = 1.57; 95% CI, 1.24 to 1.99), whereas this association was not significant among women (RR = 1.26; 95% CI, 0.95 to 1.67).45 Three studies evaluated the impact of depression severity on the increased risk of incident diabetes. In one analysis, people with severe depression had greater odds of developing type 2 diabetes compared with those who had no depression (OR = 1.42; 95% CI, 1.01 to 1.99), and at the same time antidepressant use (regardless of depression severity) was also significantly associated with incident diabetes (OR = 2.76; 95% CI, 1.93 to 3.94), suggesting that medication use could be indicative of a unique depression-diabetes association.37 By contrast, the Zaragoza Dementia and Depression (ZARADEMP) study, which adjusted for antidepressant use as a covariate, found an association between nonsevere depression and incident diabetes (HR = 1.66; 95% CI, 1.01 to 2.75; P = .048), but this did not remain significant in people with severe depression.33 A separate analysis of the Nurses’ Health Study restricted to participants without depression at baseline found that women had a higher risk of developing type 2 diabetes as their depressive symptoms, measured by the 5-item Mental Health Index (MHI-5) score (categorized into 4 groups in order from best to worst mental health: 86–100 [reference case], 76–85, 53–75, and 52 or lower), worsened over time to 53–75 compared with those who had scores that remained between 86 and 100 (RR = 1.13; 95% CI, 1.02 to 1.26), but this association was no longer significant when scores decreased to a threshold of 52 and below, which was considered “depressed mood.”41
Diabetes severity. Six studies, including 3 separate analyses of the Pathways Epidemiologic Study, assessed the association between depression and diabetes severity (type 2 diabetes only in 5 of 6 studies; 1 study included both type 1 and type 2 diabetes). A consistent association between presence/severity of depression with diabetes severity, measured by glycemic control, macrovascular and microvascular events, and diabetic retinopathy, was demonstrated across most analyses, with all 6 studies reporting at least one positive association between depression and diabetes severity (see Supplementary Table 25 for complete information).
Incident metabolic syndrome. The association between depression and a subsequent diagnosis of metabolic syndrome was reported in 3 studies. In general, most analyses did not show a significant association between depression presence or recurrence and the incidence of metabolic syndrome. In one exception, a subgroup of men in the cross-sectional Study of Health In Pomerania (SHIP-0; but not the similar SHIP-TREND-0) study had a significant association between a history of depression at the syndromal level and metabolic syndrome (OR = 1.53; 95% CI, 1.06 to 2.21; P ≤ .05), but other subgroups of men (those with lifetime MDD and those from the SHIP-TREND-0 study) and all parallel subgroups of women did not report similar findings.43 People with recurrent depression in the Study of Women’s Health Across the Nation (SWAN) study also demonstrated nonsignificant associations between history of or current MDE as a predictor of metabolic syndrome (HR = 1.83; 95% CI, 0.99 to 4.76), and when this was no longer restricted to recurrent depression only the association weakened further (HR = 1.54; 95% CI, 0.93 to 3.40).44 In the prospective Cohorte Lausannoise (CoLaus)/Psychiatric arm of the CoLaus Study (PsyCoLaus) population cohort that assessed the relationship by MDD subtypes, only atypical MDD (OR = 2.49; 95% CI, 1.30 to 4.77; P < .01), and not melancholic (OR = 1.45; 95% CI, 0.78 to 2.69) or unspecified MDD (OR = 1.44; 95% CI, 0.83 to 2.49), was associated with incident metabolic syndrome over approximately 5 years.46
Incident obesity. The review identified 10 studies (including 2 analyses of abdominal obesity from metabolic syndrome studies) and 2 meta-analyses that assessed the association between depression and incident obesity (see Supplementary Table 26 for complete details). Findings varied across studies; however, there was a notable trend of a stronger association between depression and incident obesity among women compared with men. In particular, both meta-analyses demonstrated a significant association between depression and incident obesity among all participants and among women-only subgroups but not among men-only subgroups.47,48 The use of antidepressants has a known association with obesity; the Canadian National Population Health Survey (NPHS) demonstrated that both use of venlafaxine (a serotonin and norepinephrine reuptake inhibitor; HR = 4.9; 95% CI, 1.8 to 13.0; P < .001) and use of selective serotonin reuptake inhibitors (SSRIs; HR = 1.9; 95% CI, 1.2 to 3.2; P < .01) were significantly associated with incident obesity, whereas a diagnosis of MDE was not (with or without covariate adjustment for sex, age, overweight body mass index at baseline, level of activity, and antidepressant use).49 The meta-analyses did not indicate any adjustment for medication use; thus, the potential impact of antidepressants on the findings among men and women subgroups should be taken into account.47 Of the 10 individual studies identified, only 3 mentioned an adjustment for antidepressant medication; these studies still reported significant associations between depression and obesity in at least some subgroups analyzed (see Supplementary Table 26 for complete details).
Autoimmune/GI Disorders and Musculoskeletal/Pain Conditions
Incident autoimmune diseases. Six studies assessed the impact of depression on the incidence of various autoimmune diseases (see Supplementary Table 27 for complete details). The most comprehensive study was a prospective, population-based analysis of “any” autoimmune disease (including ankylosing spondylitis, autoimmune thyroiditis, celiac disease, Crohn’s disease, idiopathic thrombocytopenic purpura, iridocyclitis, multiple sclerosis, primary adrenocortical insufficiency, psoriasis vulgaris, rheumatoid arthritis, Sjögren’s syndrome, systemic lupus erythematosus, thyrotoxicosis, type 1 diabetes mellitus, and ulcerative colitis) in a series of linked databases assessing a cohort of over 1 million people in Denmark over a 17-year period.50 In this analysis, a history of depression was significantly associated with the incidence of any autoimmune disease (IRR = 1.25; 95% CI, 1.19 to 1.31; P < .01).50 The incidence of certain autoimmune disorders in the same population-based cohort was also shown to be significantly associated with a history of depression, including Crohn’s disease (IRR = 1.36; 95% CI, 1.16 to 1.60; P < .01), systemic lupus erythematosus (IRR = 1.38; 95% CI, 1.00 to 1.91; P < 0.01), and psoriasis (IRR = 1.45; 95% CI, 1.13 to 1.85; P < .01).50 By contrast, a history of depression was not significantly associated with the incidence of ulcerative colitis, celiac disease, or ankylosing spondylitis.50
For the multiple sclerosis comorbidity, the Danish population-based cohort demonstrated a significant association between a history of depression and incident multiple sclerosis (IRR = 1.46; 95% CI, 1.26 to 1.69; P < .01).50 In addition, incident multiple sclerosis was significantly associated with a single episode of depression (IRR = 1.48; 95% CI, 1.27 to 1.74; P < .01) but not multiple episodes of depression (IRR = 1.30; 95% CI, 0.88 to 1.92).50 In alignment with these findings, a study of the Swedish National Patient Register also demonstrated a significant association between depression (overall [HR = 1.86; 95% CI, 1.73 to 2.00; P < .001] and severe depression [HR = 1.46; 95% CI, 1.27 to 1.68; P < .0001]) and multiple sclerosis.51
The impact of depression on incident rheumatoid arthritis was mixed. The Danish population cohort study reported no significant associations between a history of depression and incident rheumatoid arthritis (IRR = 1.01; 95% CI, 0.90 to 1.44),50 whereas an analysis of The Health Improvement Network (THIN) database found a strong significant association between depression and incident rheumatoid arthritis over a nearly 7-year follow-up (HR = 1.38; 95% CI, 1.31 to 1.46; P < .0001).52 A UK-based case-control study showed that, among a group of people with recurrent depression, there were elevated odds of rheumatoid arthritis (OR = 2.72; 95% CI, 1.31 to 5.63).35 However, when corrected for multiple testing, this association was not considered to be significant (P = .10). The Canadian NPHS study reported that MDD at baseline led to a significant association with arthritis/rheumatism over the 8-year follow-up (HR = 1.7; 95% CI, 1.3 to 2.2); when assessed based on duration of past-year depressive episodes, this was significant only for those with a depression episode of 13–52+ weeks (HR = 2.2; 95% CI, 1.5 to 3.3) and not for those with a duration of 2–12 weeks (HR = 1.2; 95% CI, 0.8 to 1.7).19
Incident headache and other chronic pain. Four studies (5 publications) described the association between depression and migraine or headache; most (3 of 4) did not show a significant association in the fully adjusted models for baseline depression and risk of “any” migraine, although some significant associations were reported between depression presence/severity and migraine with aura (see Supplementary Table 28 for complete details). Three studies that assessed other pain-related outcomes (spinal pain, temporomandibular pain, and back problems) generally did not show an association between depression presence or severity and incident pain, with the exception of back problems reported in 1 study (see Supplementary Table 28 for complete details).19 In parallel with the single-study finding, a meta-analysis of 11 studies assessing the association between depression and low-back pain reported a pooled OR of 1.59 (95% CI, 1.26 to 2.01), demonstrating a significant association, and this relationship remained significant across all subgroup and sensitivity analyses (method of depression diagnosis, whether studies adjusted for confounders, and studies restricted to older participants).53 The meta-analysis also showed that this association was affected by depression severity, with a significant association observed for people with the most-severe level of depression (OR = 2.51; 95% CI, 1.58 to 3.99) but not for the lowest level of depression severity (OR = 1.51; 95% CI, 0.89 to 2.56).53
Infectious Diseases
Searches for infectious diseases identified 3 studies assessing the relationship between MDD and HIV infection. A US-based Medicaid-eligible cohort analysis of over 4 million people demonstrated that baseline MDD was associated with increased odds of incident HIV/AIDS in people both with (OR = 3.04; 95% CI, 2.75 to 3.36; P < .001) and without (OR = 1.12; 95% CI, 1.04 to 1.21; P < .01) concurrent substance abuse disorder.54 Furthermore, among HIV-infected individuals, the odds of low HIV RNA levels (ie, maintaining viral suppression) at 12 months were significantly decreased in those with depression who did not use SSRIs compared with those who did not have depression (OR = 0.77; 95% CI, 0.62 to 0.95; P = .02).55 Similar findings were observed in a study that used generalized linear mixed models to show that individuals classified as having “moderate-increasing” depression severity led to significantly greater odds of a low CD4 count (ie, disease worsening) compared with “low-chronic” depression (OR = 1.53; 95% CI, 1.08 to 2.19).56
Respiratory Disorders
The review identified 3 studies assessing the relationship between MDD and respiratory disorders, including asthma and bronchitis. Although several significant associations were identified, directionality was often unclear. For example, in a case-control analysis based in the United Kingdom, people with recurrent depression reported significantly higher lifetime asthma (OR = 2.19; 95% CI, 1.53 to 3.13; P = .00022), but the methods did not permit clear confirmation of whether depression preceded asthma.35 Similarly, an analysis from the cross-sectional National Health and Nutrition Examination Survey (NHANES) study demonstrated a significantly increased prevalence of asthma according to both presence and severity of depression (OR = 3.18; 95% CI, 2.37 to 4.26; P < .01 for moderately severe; OR = 3.95; 95% CI, 2.38 to 6.56; P < .01 for severe depression).57 However, the directionality was again unclear because the cross-sectional study design did not account for the order in which these disorders occurred in participants.57 The Canadian NPHS study showed significant associations with incident asthma and bronchitis in both people who had MDD at baseline (HR = 1.8; 95% CI, 1.3 to 2.5 for asthma; HR = 2.1; 95% CI, 1.5 to 2.9 for bronchitis) and those who had MDD assessed as a time-varying characteristic (HR = 1.7; 95% CI, 1.2 to 2.4 for asthma; HR = 2.6; 95% CI, 1.9 to 3.7 for bronchitis) throughout the 8-year follow-up, regardless of duration of depression and covariates added to the model.19
Substance Abuse
A total of 23 observational study publications that described the association between depression and substance abuse were identified by the review, including multiple analyses from several large studies such as the Canadian NPHS cohort, the National Epidemiologic Survey on Alcohol and Related Conditions (NESARC) cohort, and the cross-sectional annual National Survey on Drug Use and Health (NSDUH) survey in the United States. Results varied widely among studies that assessed the impact of depression on incident alcohol or cannabis abuse or related disorders. In general, a greater proportion of studies did not show a significant association with incident drug or alcohol abuse, although there appeared to be a consistent relationship between MDD severity and these incidences (see Supplementary Table 30 for complete information). Furthermore, studies showed a consistent impact of depression on the severity of alcohol abuse, particularly among the young adult cohorts (see Supplementary Table 31 for complete information).
Quality Assessment
Quality assessment was conducted for 198 of the 199 included studies; 1 study did not undergo quality assessment because it lacked a validated checklist. Most studies were assessed to be truly or somewhat representative of the average population in the community and selected their comparator group (cohort, controls) using sufficient methods from either the same population or the general community. In addition, most studies were deemed to adequately control for the most important factors and often for additional important factors; this was likely a reflection of the covariate adjustment required by the review inclusion criteria. However, several cohort studies lost > 20% of participants to follow-up or provided insufficient information about follow-up rates; approximately half of the case-control studies provided no description of the nonresponse rate (ie, dropouts) among both groups; and all cross-sectional studies either did not describe their statistical test or used one that was incomplete or not appropriate. Further details of the quality assessment for individual studies are reported in Supplementary Tables 4–7.
DISCUSSION
In a systematic review of nearly 200 studies, the presence of MDD was identified as a risk factor for both the development and the worsening of a range of comorbidities in several categories, including CNS disorders (eg, dementia/Alzheimer’s disease and Parkinson’s disease), CVD (eg, general CVD, ischemic heart/coronary artery disease, MI, and heart failure), metabolic and endocrine disorders (particularly diabetes in men and obesity in women), certain autoimmune disorders (such as Crohn’s disease, psoriasis, and multiple sclerosis), and substance use disorders. These associations were observed consistently despite the notable variability in methodology across studies, including population sampled, criteria for depression, length of follow-up, and covariates included in the analysis. Although the association was less consistent between depression and other comorbidity categories, significant associations were often observed for certain subgroups or specific relationships. For other associations, such as those in the cancer category, wide confidence intervals were often observed, which was likely influenced to some extent by the heterogeneity of these comorbid diseases. It should also be noted that within multiple studies, certain associations strengthened with increasing depression severity or with increasing number of depressive episodes.
The effects of depression on comorbidities can arise both directly, through biological pathways, and indirectly, through a reduced ability to care for oneself or other risky health behaviors. Several biological mechanisms have been implicated in the potential relationship between depression and physical comorbidities; many of these involve dysfunction in the hypothalamic-pituitary-adrenal (HPA) axis and its impact on cortisol levels and the immune system. In particular, it has been suggested that elevated cortisol is responsible for activating cancer cell growth pathways in vitro58 and for hippocampal atrophy, accumulation of amyloid-β plaques, inflammatory processes, blood flow alterations, and lack of nerve growth factors in people with dementia and Alzheimer’s disease.59,60 Inflammation, whether activated by HPA axis dysfunction or other mechanisms such as the sympathetic nervous system, has been suggested as a contributing factor to the biological mechanisms underpinning depression, and the association between depression and several inflammation-related disorders (such as Crohn’s disease and coronary heart disease) could be thus explained to some degree by inflammation.61 Elevated cortisol may also be responsible for visceral fat accumulation, insulin resistance (type 2 diabetes), and disturbances in lipid metabolism in people with metabolic and endocrine disorders.62,63 It also remains possible, however, that depression could be a prodromal symptom of certain neurologic disorders, which was acknowledged by authors of included studies that identified associations between depression and dementia,64 Parkinson’s disease,65,66 and stroke.67 However, in a sensitivity analysis of the Pathways Epidemiologic Study that excluded people with an International Classification of Diseases, 9th revision (ICD-9) diagnosis of dementia in the 2 years after baseline, depression remained associated with an increased risk of dementia, suggesting that depression was less likely to be a prodromal symptom or secondary to dementia.68 Depression could be both a causal factor and a prodromal symptom for these neurologic disorders, and additional research could further clarify the directionality of this relationship.
In addition to biological mechanisms, depression may impact a person’s ability to take care of themselves, seek care, and adhere to treatment recommendations, which could lead to an increased risk of developing comorbidities and/or a worsened disease course. For example, many studies acknowledged the role that diet and lifestyle factors could play, in addition to biological mechanisms, for the association between depression and CVD.69–72 In addition, many substance abuse studies focused on the self-medication hypothesis as a primary factor that was likely to be mediating the association between depression and substance abuse.73
The relationship between physical and mental health has several implications for the integration of care and the need for further education among physicians, patients, caregivers, and society. Certain clinical guidelines as well as various government and patient organizations have begun to address comorbidities among people with MDD to varying degrees. For example, both the Canadian Network for Mood and Anxiety Treatments (CANMAT) 2016 guidelines and the US Centers for Disease Control and Prevention (CDC) note that depression is an independent risk factor for several diseases.74,75 The CDC recommends actively addressing mental health disorders early along with providing support to improve healthy behaviors as a strategy to decrease the risk of physical comorbidities such as cardiovascular events76; suggested actions to promote heart disease prevention include integrating mental health into multidisciplinary teams and incorporating mental health screening in the care of other diseases. However, there remains considerable room to further acknowledge and explore the relationship between depression and comorbidities and to update guidelines and other educational tools to incorporate recommendations that are informed by the link between mental and physical health.
Several limitations should be acknowledged when interpreting the results of this review. First, the observational study search and screen were date-limited to studies published between the years 2005 and 2020, and therefore any relevant studies outside of this range were not included in the evidence summary. In addition, based on their design, some studies may not present a true causal relationship in which depression occurred before the comorbidity in all individuals. It should also be noted that some of these findings could be explained to a degree by changes in patient care following a depression diagnosis and not an actual causal relationship between depression and a comorbidity. For example, if a person is diagnosed with depression, he or she may undergo more frequent contact with health care providers, which could in turn lead to the identification of diseases that had previously gone undiagnosed.
In conclusion, the presence of MDD was identified as a statistically significant risk factor for both the development and the worsening of a range of comorbidities. Collectively, these results highlight that depression may impact many comorbidities concurrently and could thus have a considerable negative impact on a person’s whole health. Additional research assessing the combined comorbidity risks may be warranted to further our understanding of the collective burden of depression-associated comorbidities. Furthermore, addressing depression with appropriate services, treatment, and support could have a broad positive impact on physical health, thus renewing the importance of appropriately timed screening and diagnosis of depression and adequate treatment of MDD.
Submitted: November 18, 2021; accepted June 27, 2022.
Published online: October 19, 2022.
Relevant financial relationships: Mr Suthoff is a current employee and Ms Arnaud and Dr Werneburg are former employees of Sage Therapeutics, Inc., and own stock/stock options. Drs Reinhart and Aleksanderek are current or former employees of Tantalus Medical Communications, Ltd, which received financial support from Sage Therapeutics, Inc., and Biogen, Inc., for research and medical writing support. During the peer review process, both Sage Therapeutics, Inc., and their collaboration partner Biogen, Inc., had the opportunity to review and comment on this manuscript; however, the authors had full editorial control of the manuscript and provided final approval on all content. Ms Fulwider has received consulting fees from Sage Therapeutics, Inc. Drs Brister and Duckworth and Ms Foxworth have nothing to disclose.
Funding/support: This work was supported by Sage Therapeutics, Inc., and Biogen, Inc.
Role of the sponsor: Employees of Sage Therapeutics, Inc., contributed to the protocol design and manuscript drafting and review but had no role in the screening and study selection for inclusion in the systematic review.
Previous presentation: Portions of this work were previously presented as an abstract at the International Society for Pharmacoeconomics and Outcomes Research Annual Meeting; May 18–20, 2020 (virtual meeting).
Acknowledgments: The authors thank Katie Stenson, MSc, a former employee of Sage Therapeutics, Inc., for contributions to the design and interpretation of the systematic review and Shawn Eapen, MSc, and Sara Watchko, MPH, both affiliates of Tantalus Medical Communications (Victoria, BC, Canada) for assistance with the systematic review. Mr Eapen and Ms Watchko have no conflicts of interest to declare. The authors thank Tantalus Medical Communications for providing research and medical writing support, which was funded by Sage Therapeutics, Inc., and Biogen, Inc., in accordance with Good Publication Practice (GPP3) guidelines.
Supplementary material: Available at Psychiatrist.com.
Clinical Points
- Although there are many well-studied comorbidities among people with depression, a broader illustration of how the risk and severity of several comorbidities across different disease areas are simultaneously impacted by the presence of depression is less clear.
- Providers should ensure that people have appropriate access to screening, diagnosis, and adequate treatment not only for depression, but also for the comorbidities that could be impacted by depression.
References (76)

- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. Fifth Edition. Washington, DC: American Psychiatric Association; 2013.
- GBD 2017 Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392(10159):1789–1858. PubMed CrossRef
- World Health Organization. Depression: Key Facts. WHO website. www.who.int/news-room/fact-sheets/detail/depression. Published 2020. Accessed October 17, 2020.
- Greenberg PE, Fournier AA, Sisitsky T, et al. The economic burden of adults with major depressive disorder in the United States (2005 and 2010). J Clin Psychiatry. 2015;76(2):155–162. PubMed CrossRef
- Greenberg PE, Fournier AA, Sisitsky T, et al. The economic burden of adults with major depressive disorder in the United States (2010 and 2018). Pharmacoeconomics. 2021;39(6):653–665. PubMed CrossRef
- Laursen TM, Musliner KL, Benros ME, et al. Mortality and life expectancy in persons with severe unipolar depression. J Affect Disord. 2016;193:203–207. PubMed CrossRef
- Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;339:b2700. PubMed CrossRef
- Wells GA, Shea B, O’Connell D, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. The Ottawa Hospital Research Institute website. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp. Published 2019. Accessed February 5, 2020.
- National Institute for Health and Care Excellence. The social care guidance manual Appendix B Methodology checklist: systematic reviews and meta-analyses. NICE website. https://www.nice.org.uk/process/pmg10/chapter/appendix-b-methodology-checklist-systematic-reviews-and-meta-analyses. Published 2013. Updated 2018. Accessed February 5, 2020.
- Satin JR, Linden W, Phillips MJ. Depression as a predictor of disease progression and mortality in cancer patients: a meta-analysis. Cancer. 2009;115(22):5349–5361. PubMed CrossRef
- Gross AL, Gallo JJ, Eaton WW. Depression and cancer risk: 24 years of follow-up of the Baltimore Epidemiologic Catchment Area sample. Cancer Causes Control. 2010;21(2):191–199. PubMed CrossRef
- Diniz BS, Butters MA, Albert SM, et al. Late-life depression and risk of vascular dementia and Alzheimer’s disease: systematic review and meta-analysis of community-based cohort studies. Br J Psychiatry. 2013;202(5):329–335. PubMed CrossRef
- Ownby RL, Crocco E, Acevedo A, et al. Depression and risk for Alzheimer disease: systematic review, meta-analysis, and metaregression analysis. Arch Gen Psychiatry. 2006;63(5):530–538. PubMed CrossRef
- Sawa M, Chan P, Donnelly M, et al. A case-control study regarding relative factors for behavioural and psychological symptoms of dementia at a Canadian regional long-term extended care facility: a preliminary report. Psychogeriatrics. 2014;14(1):25–30. PubMed CrossRef
- Wilson RS, Boyle PA, Capuano AW, et al. Late-life depression is not associated with dementia-related pathology. Neuropsychology. 2016;30(2):135–142. PubMed CrossRef
- Ransom J, Shilnikova A, Rusli E, et al. Patterns and prediction for cognitive decline in Alzheimer’s patients as assessed by the mini-mental status exam in an ambulatory electronic medical record. Value Health. 2019;22(suppl 3):S755. CrossRef
- Cannon-Spoor HE, Levy JA, Zubenko GS, et al. Effects of previous major depressive illness on cognition in Alzheimer disease patients. Am J Geriatr Psychiatry. 2005;13(4):312–318. PubMed CrossRef
- Wilson RS, Begeny CT, Boyle PA, et al. Vulnerability to stress, anxiety, and development of dementia in old age. Am J Geriatr Psychiatry. 2011;19(4):327–334. PubMed CrossRef
- Patten SB, Williams JV, Lavorato DH, et al. Major depression as a risk factor for chronic disease incidence: longitudinal analyses in a general population cohort. Gen Hosp Psychiatry. 2008;30(5):407–413. PubMed CrossRef
- Brunner EJ, Shipley MJ, Britton AR, et al. Depressive disorder, coronary heart disease, and stroke: dose-response and reverse causation effects in the Whitehall II cohort study. Eur J Prev Cardiol. 2014;21(3):340–346. PubMed CrossRef
- Janszky I, Ahlbom A, Hallqvist J, et al. Hospitalization for depression is associated with an increased risk for myocardial infarction not explained by lifestyle, lipids, coagulation, and inflammation: the SHEEP Study. Biol Psychiatry. 2007;62(1):25–32. PubMed CrossRef
- Surtees PG, Wainwright NW, Luben RN, et al. Depression and ischemic heart disease mortality: evidence from the EPIC-Norfolk United Kingdom prospective cohort study. Am J Psychiatry. 2008;165(4):515–523. PubMed CrossRef
- Barlinn K, Kepplinger J, Puetz V, et al. Exploring the risk-factor association between depression and incident stroke: a systematic review and meta-analysis. Neuropsychiatr Dis Treat. 2014;11:1–14. PubMed
- Correll CU, Solmi M, Veronese N, et al. Prevalence, incidence and mortality from cardiovascular disease in patients with pooled and specific severe mental illness: a large-scale meta-analysis of 3,211,768 patients and 113,383,368 controls. World Psychiatry. 2017;16(2):163–180. PubMed CrossRef
- Van der Kooy K, van Hout H, Marwijk H, et al. Depression and the risk for cardiovascular diseases: systematic review and meta analysis. Int J Geriatr Psychiatry. 2007;22(7):613–626. PubMed CrossRef
- Blöchl M, Meissner S, Nestler S. Does depression after stroke negatively influence physical disability? a systematic review and meta-analysis of longitudinal studies. J Affect Disord. 2019;247:45–56. PubMed CrossRef
- Cosgrove MP, Sargeant LA, Griffin SJ. Does depression increase the risk of developing type 2 diabetes? Occup Med (Lond). 2008;58(1):7–14. PubMed CrossRef
- Mezuk B, Eaton WW, Golden SH, et al. The influence of educational attainment on depression and risk of type 2 diabetes. Am J Public Health. 2008;98(8):1480–1485. PubMed CrossRef
- Rotella F, Mannucci E. Depression as a risk factor for diabetes: a meta-analysis of longitudinal studies. J Clin Psychiatry. 2013;74(1):31–37. PubMed CrossRef
- Vancampfort D, Mitchell AJ, De Hert M, et al. Type 2 diabetes in patients with major depressive disorder: a meta-analysis of prevalence estimates and predictors. Depress Anxiety. 2015;32(10):763–773. PubMed CrossRef
- Atasoy S, Johar H, Fang XY, et al. Cumulative effect of depressed mood and obesity on type II diabetes incidence: findings from the MONICA/KORA cohort study. J Psychosom Res. 2018;115:66–70. PubMed CrossRef
- Brown LC, Majumdar SR, Newman SC, et al. History of depression increases risk of type 2 diabetes in younger adults. Diabetes Care. 2005;28(5):1063–1067. PubMed CrossRef
- Campayo A, de Jonge P, Roy JF, et al; ZARADEMP Project. Depressive disorder and incident diabetes mellitus: the effect of characteristics of depression. Am J Psychiatry. 2010;167(5):580–588. PubMed CrossRef
- Eriksson AK, Ekbom A, Granath F, et al. Psychological distress and risk of pre-diabetes and Type 2 diabetes in a prospective study of Swedish middle-aged men and women. Diabet Med. 2008;25(7):834–842. PubMed CrossRef
- Farmer A, Korszun A, Owen MJ, et al. Medical disorders in people with recurrent depression. Br J Psychiatry. 2008;192(5):351–355. PubMed CrossRef
- Karakus MC, Patton LC. Depression and the onset of chronic illness in older adults: a 12-year prospective study. J Behav Health Serv Res. 2011;38(3):373–382. PubMed CrossRef
- Kivimäki M, Hamer M, Batty GD, et al. Antidepressant medication use, weight gain, and risk of type 2 diabetes: a population-based study. Diabetes Care. 2010;33(12):2611–2616. PubMed CrossRef
- Mallon L, Broman JE, Hetta J. High incidence of diabetes in men with sleep complaints or short sleep duration: a 12-year follow-up study of a middle-aged population. Diabetes Care. 2005;28(11):2762–2767. PubMed CrossRef
- Mezuk B, Heh V, Prom-Wormley E, et al. Association between major depression and type 2 diabetes in midlife: findings from the Screening Across the Lifespan Twin Study. Psychosom Med. 2015;77(5):559–566. PubMed CrossRef
- Nichols GA, Moler EJ. Cardiovascular disease, heart failure, chronic kidney disease and depression independently increase the risk of incident diabetes. Diabetologia. 2011;54(3):523–526. PubMed CrossRef
- Pan A, Lucas M, Sun Q, et al. Bidirectional association between depression and type 2 diabetes mellitus in women. Arch Intern Med. 2010;170(21):1884–1891. PubMed CrossRef
- Windle M, Windle RC. Recurrent depression, cardiovascular disease, and diabetes among middle-aged and older adult women. J Affect Disord. 2013;150(3):895–902. PubMed CrossRef
- Block A, Schipf S, Van der Auwera S, et al. Sex- and age-specific associations between major depressive disorder and metabolic syndrome in two general population samples in Germany. Nord J Psychiatry. 2016;70(8):611–620. PubMed CrossRef
- Goldbacher EM, Bromberger J, Matthews KA. Lifetime history of major depression predicts the development of the metabolic syndrome in middle-aged women. Psychosom Med. 2009;71(3):266–272. PubMed CrossRef
- Mezuk B, Eaton WW, Albrecht S, et al. Depression and type 2 diabetes over the lifespan: a meta-analysis. Diabetes Care. 2008;31(12):2383–2390. PubMed CrossRef
- Lasserre AM, Strippoli MF, Glaus J, et al. Prospective associations of depression subtypes with cardio-metabolic risk factors in the general population. Mol Psychiatry. 2017;22(7):1026–1034. PubMed CrossRef
- de Wit L, Luppino F, van Straten A, et al. Depression and obesity: a meta-analysis of community-based studies. Psychiatry Res. 2010;178(2):230–235. PubMed CrossRef
- Luppino FS, de Wit LM, Bouvy PF, et al. Overweight, obesity, and depression: a systematic review and meta-analysis of longitudinal studies. Arch Gen Psychiatry. 2010;67(3):220–229. PubMed CrossRef
- Patten SB, Williams JV, Lavorato DH, et al. Major depression, antidepressant medication and the risk of obesity. Psychother Psychosom. 2009;78(3):182–186. PubMed CrossRef
- Andersson NW, Gustafsson LN, Okkels N, et al. Depression and the risk of autoimmune disease: a nationally representative, prospective longitudinal study. Psychol Med. 2015;45(16):3559–3569. PubMed CrossRef
- Johansson V, Lundholm C, Hillert J, et al. Multiple sclerosis and psychiatric disorders: comorbidity and sibling risk in a nationwide Swedish cohort. Mult Scler. 2014;20(14):1881–1891. PubMed CrossRef
- Vallerand IA, Lewinson RT, Frolkis AD, et al. Depression as a risk factor for the development of rheumatoid arthritis: a population-based cohort study. RMD Open. 2018;4(2):e000670. PubMed CrossRef
- Pinheiro MB, Ferreira ML, Refshauge K, et al. Symptoms of depression and risk of new episodes of low back pain: a systematic review and meta-analysis. Arthritis Care Res (Hoboken). 2015;67(11):1591–1603. PubMed CrossRef
- Prince JD, Walkup J, Akincigil A, et al. Serious mental illness and risk of new HIV/AIDS diagnoses: an analysis of Medicaid beneficiaries in eight states. Psychiatr Serv. 2012;63(10):1032–1038. PubMed CrossRef
- Horberg MA, Silverberg MJ, Hurley LB, et al. Effects of depression and selective serotonin reuptake inhibitor use on adherence to highly active antiretroviral therapy and on clinical outcomes in HIV-infected patients. J Acquir Immune Defic Syndr. 2008;47(3):384–390. PubMed CrossRef
- Owora AH. Major depression disorder trajectories and HIV disease progression: results from a 6-year outpatient clinic cohort. Medicine (Baltimore). 2018;97(12):e0252. PubMed CrossRef
- Han YY, Forno E, Marsland AL, et al. Depression, asthma, and bronchodilator response in a nationwide study of US adults. J Allergy Clin Immunol Pract. 2016;4(1):68–73.e1. PubMed CrossRef
- Lutgendorf SK, Cole S, Costanzo E, et al. Stress-related mediators stimulate vascular endothelial growth factor secretion by two ovarian cancer cell lines. Clin Cancer Res. 2003;9(12):4514–4521. PubMed
- Sapolsky RM. Glucocorticoids and hippocampal atrophy in neuropsychiatric disorders. Arch Gen Psychiatry. 2000;57(10):925–935. PubMed CrossRef
- Butters MA, Young JB, Lopez O, et al. Pathways linking late-life depression to persistent cognitive impairment and dementia. Dialogues Clin Neurosci. 2008;10(3):345–357. PubMed CrossRef
- Slavich GM, Irwin MR. From stress to inflammation and major depressive disorder: a social signal transduction theory of depression. Psychol Bull. 2014;140(3):774–815. PubMed CrossRef
- Milaneschi Y, Simmons WK, van Rossum EFC, et al. Depression and obesity: evidence of shared biological mechanisms. Mol Psychiatry. 2019;24(1):18–33. PubMed CrossRef
- Björntorp P. Abdominal obesity and the development of noninsulin-dependent diabetes mellitus. Diabetes Metab Rev. 1988;4(6):615–622. PubMed CrossRef
- Luppa M, Luck T, Ritschel F, et al. Depression and incident dementia: an 8-year population-based prospective study. PLoS One. 2013;8(3):e59246. PubMed CrossRef
- Fang F, Xu Q, Park Y, et al. Depression and the subsequent risk of Parkinson’s disease in the NIH-AARP Diet and Health Study. Mov Disord. 2010;25(9):1157–1162. PubMed CrossRef
- Wang S, Mao S, Xiang D, et al. Association between depression and the subsequent risk of Parkinson’s disease: a meta-analysis. Prog Neuropsychopharmacol Biol Psychiatry. 2018;86:186–192. PubMed CrossRef
- Surtees PG, Wainwright NW, Luben RN, et al. Psychological distress, major depressive disorder, and risk of stroke. Neurology. 2008;70(10):788–794. PubMed CrossRef
- Katon WJ, Lin EH, Williams LH, et al. Comorbid depression is associated with an increased risk of dementia diagnosis in patients with diabetes: a prospective cohort study. J Gen Intern Med. 2010;25(5):423–429. PubMed CrossRef
- Hamano T, Li X, Lönn SL, et al. Depression, stroke and gender: evidence of a stronger association in men. J Neurol Neurosurg Psychiatry. 2015;86(3):319–323. PubMed CrossRef
- Köhler S, Verhey F, Weyerer S, et al. Depression, non-fatal stroke and all-cause mortality in old age: a prospective cohort study of primary care patients. J Affect Disord. 2013;150(1):63–69. PubMed CrossRef
- Nabi H, Kivimäki M, Suominen S, et al. Does depression predict coronary heart disease and cerebrovascular disease equally well? the Health and Social Support Prospective Cohort Study. Int J Epidemiol. 2010;39(4):1016–1024. PubMed CrossRef
- Pan A, Okereke OI, Sun Q, et al. Depression and incident stroke in women. Stroke. 2011;42(10):2770–2775. PubMed CrossRef
- Markou A, Kosten TR, Koob GF. Neurobiological similarities in depression and drug dependence: a self-medication hypothesis. Neuropsychopharmacology. 1998;18(3):135–174. PubMed CrossRef
- Lam RW, McIntosh D, Wang J, et al; CANMAT Depression Work Group. Canadian Network for Mood and Anxiety Treatments (CANMAT) 2016 Clinical Guidelines for the Management of Adults with Major Depressive Disorder: Section 1. Disease Burden and Principles of Care. Can J Psychiatry. 2016;61(9):510–523. PubMed CrossRef
- Centers for Disease Control and Prevention. Mental Health. CDC website. https://www.cdc.gov/mentalhealth/learn/index.htm#:~:text=Mental. Published 2018. Accessed October 6, 2020.
- Centers for Disease Control and Prevention. Heart Disease and Mental Health Disorders. CDC website.https://www.cdc.gov/heartdisease/mentalhealth.htm. Published 2020. Accessed December 15, 2020.
Save
Cite
Advertisement
GAM ID: sidebar-top