ABSTRACT
Objective: Tardive dyskinesia (TD) is a movement disorder that can negatively affect health-related quality of life. However, the impact of TD is not necessarily dependent solely on the objective severity of TD movements. There is currently no easy-to-use, standardized, clinician-rated assessment of the impact of TD on functioning. The aim of this consensus panel was to develop a scale (Impact-TD scale) to assess the impact of TD on patients’ daily functioning in practice settings.
Participants: Nine health care professionals with expertise in TD and clinical scale development met to discuss how TD negatively impacts the functional activities of patients.
Evidence: This panel comprised 7 individuals from a previous panel that developed recommendations on the importance of optimally assessing the functional impact of TD. The previous panel published a narrative literature review that summarized the existing approaches to assess the impact of TD in clinical research and practice.
Consensus Process: A modified Delphi process was used to assess agreement on the format and content of the Impact-TD scale. The panel discussed key features of the Impact-TD scale (ie, simplicity, usability, assessment of frequency of impact versus interference/distress). The scale aimed to describe specific consequences of TD symptoms with which patients may have difficulty.
Conclusions: Consensus was reached on a list of consequences of TD symptoms that have a functional impact and were categorized in 4 functional domains: social, psychological/psychiatric, physical, and vocational/educational/recreational. The Impact-TD scale offers an easy-to-use clinical scale to measure the functional impact of TD in practice settings.
J Clin Psychiatry 2023;84(1):22cs14563
To cite: Jackson R, Brams MN, Carlozzi NE, et al. Impact-Tardive Dyskinesia (Impact-TD) scale: a clinical tool to assess the impact of tardive dyskinesia. J Clin Psychiatry. 2023;84(1):22cs14563.
To share: https://doi.org/10.4088/JCP.22cs14563
© 2022 Physicians Postgraduate Press, Inc.
aDepartment of Psychiatry, University of Michigan School of Medicine, Ann Arbor, Michigan
bDepartment of Psychiatry & Behavioral Sciences, Baylor College of Medicine, Houston, Texas
cDepartment of Physical Medicine & Rehabilitation, University of Michigan School of Medicine, Ann Arbor, Michigan
dDepartment of Psychiatry and Behavioral Sciences, New York Medical College, Valhalla, New York
eDepartment of Physical Therapy, Wayne State University, Detroit, Michigan
fGeroPsych Associates of Central Texas, Austin, Texas
gParkinson’s Disease and Movement Disorders Center of Boca Raton, Boca Raton, Florida
hDepartment of Psychiatry, The Zucker Hillside Hospital, Northwell Health, Glen Oaks, New York
iDepartment of Psychiatry, Donald and Barbara Zucker School of Medicine at Hofstra/Northwell, Hempstead, New York
jInstitute of Behavioral Science, Feinstein Institutes for Medical Research, Manhasset, New York
kRocky Mountain Movement Disorders Center, Englewood, Colorado
*Corresponding author: Richard Jackson, MD, 4111 Andover Rd, Ste W100, Bloomfield Hills, MI 48302 ([email protected]).
Tardive dyskinesia (TD) is a movement disorder that results from exposure to dopamine receptor antagonists, most frequently antipsychotic agents.1 The prevalence of TD in people exposed to first- or second-generation antipsychotics among patients naïve to first-generation antipsychotics is estimated to be approximately 30% and 7%, respectively.2 Patients with TD often experience involuntary movements in orofacial musculature, but movements also commonly occur in the extremities and trunk.3,4 The physical symptoms of TD can negatively affect many aspects of a patient’s health-related quality of life and/or functioning, including social interaction, employment and education, psychological condition, and self-care/instrumental activities of daily living.4–7
The impact of TD symptoms on a patient’s daily life is variable and is not solely dependent on the severity of the movements. Mental health and wellness may be impacted by TD, and TD could potentially exacerbate existing psychiatric comorbidities. In addition, social and occupational interactions may be negatively impacted by TD, often leading to embarrassment, isolation, and stigma. Performance at school and in recreational activities may also be impacted by TD because of physical limitations and/or concerns about how TD movements may be perceived by peers. Therefore, the impact of TD on daily functioning must be a critical part of the routine assessment by health care professionals, including psychiatrists, neurologists, nurse practitioners, physician assistants, clinical psychologists, physical therapists, social workers, and other health care providers. A comprehensive assessment of the impact of TD on the patient, along with follow-up assessments, would provide health care professionals with important feedback on the clinical management of TD. Although previous work has been performed to define and measure the functional impact of TD on patients,4,7 there is currently no standardized clinician-rated assessment of the impact of TD symptoms on functioning.8
As part of a routine clinical assessment, overall impressions of a patient’s health-related quality of life related to the functional impact of TD are important to monitoring disease progression. Based on the input of the patient and/or informed individuals, a formalized assessment of the clinician’s impression of the functional impact of TD in an easy-to-use tool would provide simplicity for this evaluation, as the impact is inherently multifactorial and extends across many domains.
An advisory panel was previously convened with the goal of establishing consensus recommendations on the importance of key elements in assessing the impact symptoms of TD on patients’ functioning.7 An easy-to-use assessment tool to facilitate evaluation of the impact of TD on a patient’s life that could support the monitoring of disease progression and assessment of treatment response in clinical practice was determined to be needed. To address this lack of tools to evaluate the impact of TD on functioning, the consensus panel was reassembled to propose an impact scale that can be used in routine clinical practice.
CONSENSUS PROCESS
Consensus Panel
The previous multidisciplinary consensus panel of 7 health care professionals with expertise in TD and clinical scale development was reconvened (with some modifications based on availability and expertise) and included 4 psychiatrists, 2 movement disorder neurologists, 1 psychiatric nurse practitioner, 1 clinical psychologist, and 1 physical therapist. The goal was to develop a scale to assess the impact of TD in clinical practice (hereinafter called “Impact-TD scale”).
During the consensus panel meetings described here, panelists reviewed the negative impact that TD could have on function and quality of life and discussed which functional domains are most often impacted by TD. In addition, the panel deliberated about which patient characteristics would assist a clinician in evaluating the impact of TD in each domain. The initial discussion emphasized key features that should be considered when developing the Impact-TD scale (ie, simplicity, usability), as well as specific functional domains and consequences of TD. The panelists further discussed how to format the scale to effectively guide clinicians on the collection of relevant information to derive a global assessment for each functional domain. The summary scores were intended to capture both frequency and intensity of the impact stemming from TD movements.
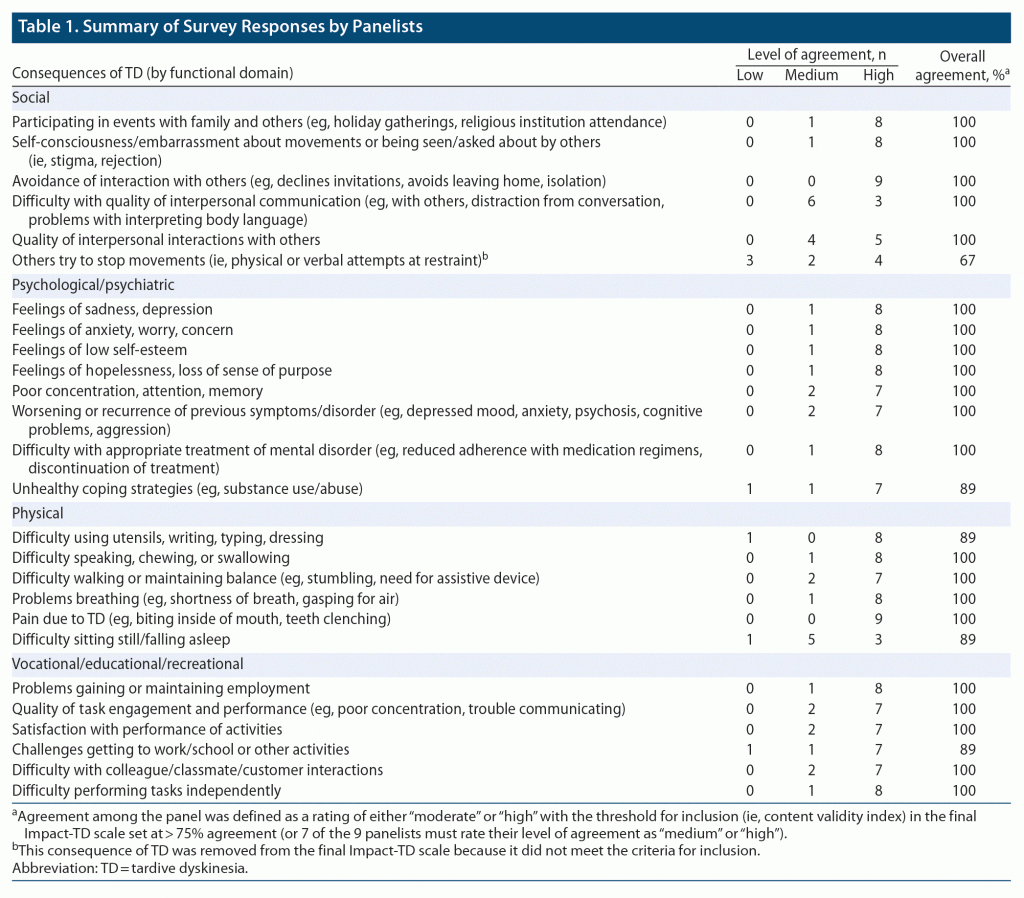
Through group dialogue, panelists reached agreement on the inclusion of specific functional domains and a list of patient characteristics that may be impacted by TD. Each patient characteristic was the basis for establishing specific real-world examples (ie, consequences of TD symptoms) in which a patient might have difficulty functioning in daily life because of TD. Each consequence of TD derived from patient characteristics was developed through an iterative process without consideration of functional domain. The group evaluated the list of consequences to consolidate similar consequences and categorize each into the appropriate functional domain. A draft Impact-TD scale was developed from this group dialogue and initial feedback from the panelists was solicited (Table 1).
Modified Delphi Method
In addition to providing detailed feedback about the draft Impact-TD scale throughout the consensus panel meetings, a modified Delphi method was used to assess the agreement among panelists and ensure the content validity for each consequence of TD.9 Panelists anonymously rated their overall level of satisfaction with the format and content of each domain in the draft Impact-TD scale on a scale from 0 (“not at all satisfied”) to 4 (“very satisfied”) (Table 1). Furthermore, panelists rated their level of agreement with each of the consequences of TD as 1 (“low”), 2 (“medium”), or 3 (“high”). The threshold for inclusion (ie, content validity index) in the final Impact-TD scale was set at > 75% agreement (or 7 of the 9 panelists must rate their level of agreement as “medium” or “high”). The panelists were also allowed to provide additional free-form comments for each domain and consequence of TD to facilitate the next round of the Delphi process, if needed.
Development of the Impact-TD Scale
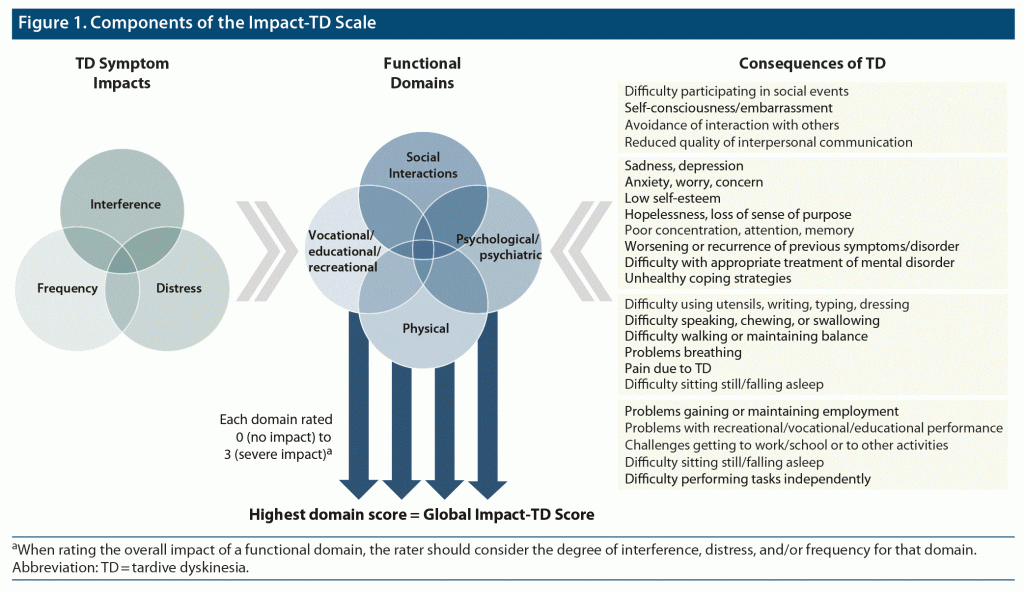
In a part of the previous iteration of this panel, 5 domains were established to describe the impact of TD symptoms: social, physical, vocational, psychological, and psychiatric.7 Here, these domains were re-examined by the panelists and modifications were made to align with a more pragmatic approach to capturing the functional impact of TD during a routine clinical visit. There is an inherent overlap of domains that impact TD; therefore, the panelists aimed to consolidate the previous list of domains. The psychological and psychiatric domains were combined because it can be difficult to differentiate between these domains when assessing the impact of TD symptoms. In addition, educational and recreational components were added to the vocational domain to better capture the impact of TD on the entire population of patients because many patients are likely underemployed or not working. The identified domains are not mutually exclusive; therefore, a proper evaluation with the Impact-TD scale should aim to capture overall impact rather than be limited to a specific domain. Functional impacts of TD that negatively affect patients’ quality of life will often overlap multiple domains, and the functional impact should be considered through the lens of each domain. Based on this, and as the impact of TD can be multidimensional and vary from patient to patient based on a variety of factors, a concise, easy-to-use tool was determined by the panel to be valuable for the assessment of the impact of TD symptoms in these broad-ranging functional domains. Because of the complexity of evaluating these concepts, an overall general score was chosen to allow clinicians to apply their clinical judgment.
Panelists discussed aspects of a patient’s life in which they might experience the impact of TD and initially agreed on providing 6 to 8 common consequences of TD for each domain to provide guidance on common topics to discuss with patients. However, the list of consequences of TD symptoms provided is not exhaustive of all potential impacts of TD. The panelists decided that the scale anchors (ie, patient characteristics/consequences of TD) in each domain should be connected to the level of functional impact caused by TD, rather than absolute or weighted measures of frequency and/or severity (ie, interference, distress) of symptoms. For example, a patient may have difficulty getting dressed on a frequent basis, but if they have a caregiver who helps, this difficulty may not cause them distress or interfere with their daily life. Furthermore, some panelists who tested the scale in the clinical setting emphasized the importance of the patient’s and caregiver’s perspectives regarding frequency and importance to the patient of the interference and/or distress experienced in determining level of TD impact. However, the Impact-TD scale is intended to assess the impact of TD on patients, not on family and/or caregivers, and hence only the impact of TD on the patient is assessed.
Clinician-reported outcomes, by design, should allow clinicians to rate objective symptoms in context of the overall patient experience. The ability of clinicians to prioritize the functional impact of TD and subjectively interpret feedback from individuals who have considerable exposure to the patients (ie, family, caregivers) is also important to maximize. Symptoms of TD can fluctuate from day to day and within the day, and family and caregivers who are around the patients most often are frequently the best sources to provide insight on the impact of TD.10 Because the impact of TD is multifactorial, it is unlikely that one source would be exposed to a broad spectrum of a patient’s behavior, activities, difficulties, and/or frustrations. Likewise, it is recommended that a multidisciplinary clinical team is used to ensure the comprehensive management of TD.11,12 The clinician assessing the impact of TD also may want to consider these sources when determining the level of impact of each consequence of TD. Based on this, panelists determined that during the assessment of the functional impact of TD using the Impact-TD scale, multiple sources, if available, should be probed and considered, including the patient, family, caregiver, other health care professionals, and/or direct observations. Therefore, the Impact-TD scale was developed to be a comprehensive view of a patient’s daily life and difficulties that they may have because of TD. In addition, the Impact-TD scale will potentially provide awareness of the functional impact of TD beyond movements to a wider range of clinicians.
For the Impact-TD scale, a 4-point Likert scale was developed by the panelists to rate each functional domain: “Impact scores should range from 0 to 3, where 0 = no impact, 1 = mild impact (impact is present, but minimal), 2 = moderate impact (exceeds minimal impact, but is not severe), and 3 = severe impact (significant and detrimental impact).”
CONSENSUS SUMMARY
Impact-TD Scale
The panelists developed a draft of the Impact-TD scale for which the panelists anonymously rated their overall satisfaction with the scale and agreement with the consequences of TD (Table 1). The initial review of the Impact-TD scale resulted in all panelists being either “satisfied” or “very satisfied” with the format and content of the scale. The range of mean agreement (ie, 1 = “low,” 2 = “medium,” and 3 = “high”) for each consequence of TD indicated at least medium agreement for all consequences of TD and is as follows: social domain (2.1–3.0), psychological/psychiatric domain (2.7–2.9), physical domain (2.2–3.0), and vocational/educational/recreational domain (2.7–2.9), except for 1 consequence, “Others try to stop movements (ie, physical or verbal attempts at restraint),” in the social domain that did not meet the predefined criteria for inclusion in the final Impact-TD scale (ie, > 75% agreement) and was removed.
Based on free-form comments by panelists during the review process, modifications were made to improve the comprehensibility and consistency of the instructions and consequences of TD provided. As a result, some consequences of TD were combined to reduce redundancies and improve clarity. The consequences “Difficulty with quality of interpersonal communication (eg, with others, distraction from conversation, problems with interpreting body language)” and “Quality of interpersonal interactions with others” were combined to “Reduced quality of interpersonal communication (eg, distraction from conversation, problems interpreting body language)” in the social domain. The consequence of TD in psychiatric/psychological domain, “Worsening or recurrence of previous symptoms/disorder (eg, depressed mood, anxiety, psychosis, cognitive problems, aggression),” was revised to exclude “cognitive problems” as it was redundant with the previous example, “Poor concentration, attention, memory.” Vocational/educational/recreational domain consequences of TD, “Quality of task engagement and performance (eg, poor concentration, trouble communicating)” and “Satisfaction with performance of activities,” were combined to the new consequence, “Problems with recreational or vocational/educational performance (eg, poor concentration, trouble communicating, physical limitations),” which incorporated both aspects of poor performance and/or low satisfaction with performance.
Instructions for Use
A diagram of the Impact-TD scale components (Figure 1) briefly summarizes how the rating of each functional domain and Global Impact-TD score should be determined. The final Impact-TD scale for clinical use is provided as Figure 2. Attention should be given to severe functional impairment caused by TD symptoms, and clinicians should provide a domain impact score based on the highest level of frequency, interference, and/or distress for each domain. For patients who report impact for multiple consequences of TD within a domain, the domain score should reflect the highest degree of impact among these consequences. Under certain circumstances, infrequent symptoms can cause more severe distress and consequently more functional impact. Similar to the Abnormal Involuntary Movement Scale (AIMS), a total score that appears low may be masking a more severe score in a single muscle group.13 For example, choking may have more impact on a patient’s life than a more frequent but potentially less distressing symptom such as clenching the teeth. A total Impact-TD score may be difficult to interpret, but a single domain score may be more helpful for clinical decision-making, because even if one domain is severe, the functional impact of TD may still be profound. Therefore, additional guidance is included in the instructions of the Impact-TD scale to encourage denoting a global score (ie, Global Impact-TD Score) based on the highest impact score in any functional domain.
Although not mandatory, fields for recording the source(s) that provided information (ie, family, caregiver, other clinician, direct observations) to assist in the completion of the Impact-TD scale are also included in the Impact-TD scale form.
DISCUSSION
The association of TD movements with limitations in a patient’s daily activities is poorly understood.8 This disconnect might be because previously available TD scales only considered the severity of movements without regard for the impact of these movements on function. The AIMS items regarding global judgment for incapacitation and awareness/distress because of abnormal movements do not consistently capture the elements included in the Impact-TD scale. The Impact-TD scale can better assist with a more comprehensive understanding and assessment of the severity of TD in the context of a patient’s ability to function and quality of life.
To the best of the authors’ knowledge, the Impact-TD scale is the first standardized clinician-rated instrument designed specifically to assess the impact of TD on a patient’s day-to-day life and functioning. This tool can aid clinicians in determining when and how to manage TD and has the potential to improve clinical care by improving patient outcomes. Most importantly, the Impact-TD scale may raise awareness of the functional effects of TD on patients’ daily lives may otherwise be underappreciated or ignored.
The American Psychiatric Association recommends that patients who have moderate to severe or disabling TD associated with antipsychotic agent use should be treated with a vesicular monoamine transporter 2 (VMAT2) inhibitor.14 The guidelines also state that treatment with a VMAT2 inhibitor can be considered for patients with mild TD on the basis of such factors as patient preference, associated impairment, or effect on psychosocial functioning.14 Patients should have access to approved medications, including VMAT2 inhibitors, but sometimes they may be limited for a variety of reasons including formulary exclusion, step therapy, out-of-pocket cost, and patient’s unwillingness to take another medication.
While a patient may have mild TD as defined by movements, the functional impact of TD may profoundly affect the patient’s daily life. The Impact-TD scale was designed to provide insight into the functional disability associated with TD (eg, social, vocational), which can be extensive and go potentially undetected by assessments such as the AIMS. Furthermore, the Impact-TD scale could support the monitoring of disease progression and possibly assist in assessing response to treatments such as with a VMAT2 inhibitor.
The Impact-TD scale was designed to serve as an easy-to-use, clinician-rated tool to evaluate the multidimensional impact of TD on patients and guide TD treatment decisions. Because the patient perspective of TD is inherently subjective and often skewed, the inclusion of multiple sources (ie, family, caregivers, other clinicians) can provide a more comprehensive understanding of the real-world impact of TD on the patient. Likewise, by assessing multiple functional domains, the highest domain score (ie, Global Impact-TD Score) can help clinicians identify the overall impact of TD on their patients. The scale was purposely developed for use by a broad range of health care professionals and can potentially serve to increase the appreciation among these clinicians for the need to assess the impact of TD. This will help to establish the assessment of impact from TD as part of the standard of care when evaluating and treating patients with TD. Furthermore, when it is necessary to involve additional resources in the treatment and management of TD, the Impact-TD scale could assist clinicians in ensuring appropriate and timely referrals are made to other health care professionals as needed.
While the Impact-TD scale was designed to assess the impact of TD movements on the patient, a recent study suggests that abnormal movements associated with TD also impact the ability of caregivers (of persons with TD) to continue their own usual activities, be productive, socialize, or take care of themselves.15 Future work may include modification of the Impact-TD scale to assess caregiver burden with regard to their own daily functioning. Furthermore, the involvement of patients with TD and their families/caregivers in modification of the current scale will increase the comprehensiveness of assessing the impact of TD on patients’ daily functioning.
In addition to providing guidance in the assessment of TD, providing specific consequences of TD symptoms should increase awareness of TD impact and improve dialogue between patients and clinicians, making the scale a valuable teaching tool for all types of health care professionals.
Limitations
There were several limitations in this consensus panel process. There are no standards for the number of rounds of agreement or the number of individuals on a Delphi panel. This panel reached agreement after 1 round, and the number of participants was smaller than what is typical or suggested.16–19 Furthermore, although some panelists piloted the Impact-TD scale in their practice to assess the impact of TD on their own patients, the assessment tool has not been validated with respect to psychometric properties. Further assessment of validity and reliability is necessary for the scale to be used for quantitative research. Additional research should also explore the predictive validity of the Impact-TD scale to identify correlates with long-term outcomes of function and quality of life with or without treatment. However, in the meantime, this does not preclude use of the scale in routine clinical practice for informational or descriptive purposes. Lastly, it should be noted that this scale is a product of a consensus panel funded by Teva Branded Pharmaceutical Products R&D, Inc, and although Teva was not directly involved in the development of the Impact-TD scale, the company has commercialized a treatment for TD.
CONCLUSIONS
The impact of TD should be assessed routinely in clinical practice to guide disease management decisions. However, there is not a standard scale to assess the impact of TD on patients’ functional daily life. Therefore, a consensus panel of experts in TD management and measurement met to develop an easy-to-use clinical scale to assess the functional impact of TD: the Impact-TD scale.
Submitted: June 29, 2022; accepted October 3, 2022.
Published online: November 28, 2022.
Relevant financial relationships: Dr Jackson reports serving as a consultant for Alkermes, Adlon Therapeutics, BioXcel, Sage Therapeutics, Supernus, and Teva Pharmaceuticals and has received payment or honoraria for lectures, presentations, speaker bureaus, manuscript writing, or educational events from AbbVie, Alkermes, BioXcel, GeneSight, Intra-Cellular Therapies, Lundbeck, Otsuka, Sage Therapeutics, Sunovion, Supernus, Takeda, and Teva Pharmaceuticals. Dr Brams reports no conflicts of interest. Dr Carlozzi has received funding from Neilsen Foundation, Alzheimer’s Association, Teva, United States Food and Drug Administration, Patient-Centered Outcomes Research Institute, National Institute of Nursing Research, National Heart, Lung, and Blood Institute, Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Center for Medical Rehabilitation Research, National Institute of Diabetes and Digestive and Kidney Diseases, National Institute of Neurological Disorders and Stroke, National Institute on Drug Abuse, National Institutes of Health, Department of Centers for Medicare and Medicaid Services, CHDI Foundation, and Tampa VA Research and Education Foundation. Dr Citrome reports serving as a consultant for AbbVie/Allergan, Acadia Pharmaceuticals, Adamas Pharmaceuticals, Alkermes, Angelini Pharma, Astellas, Avanir, Axsome, BioXcel, Boehringer Ingelheim, Cadent Therapeutics, Cerevel, Clinilabs, COMPASS Pathways, Eisai, Enteris BioPharma, HLS Therapeutics, Idorsia, INmune Bio, Impel Pharmaceuticals, Intra-Cellular Therapies, Janssen, Karuna, Lundbeck, Lyndra, Medavante-ProPhase, Meiji, Merck, Mitsubishi-Tanabe Pharma, Neurocrine, Neurelis, Novartis, Noven, Otsuka, Ovid Therapeutics, Praxis Precision Medicines, Recordati, Relmada, Reviva Pharmaceuticals, Sage Therapeutics, Sunovion, Supernus, Teva Pharmaceuticals, and University of Arizona and for individuals/entities conducting one-off ad hoc marketing, commercial, or scientific scoping research; as a speaker for AbbVie/Allergan, Acadia Pharmaceuticals, Alkermes, Angelini Pharma, Axsome, BioXcel, Eisai, Idorsia, Intra-Cellular Therapies, Janssen, Lundbeck, Neurocrine, Noven, Otsuka, Recordati, Sage Therapeutics, Sunovion, Takeda, and Teva Pharmaceuticals, and CME activities organized by medical education companies such as Medscape, NACCME, NEI, Vindico Medical Education, and Universities and Professional Organizations/Societies. He reports that he owns stocks (small number of shares of common stock) from Bristol Myers Squibb, Lilly, Johnson & Johnson, Merck, and Pfizer purchased more than 10 years ago, and stock options from Reviva Pharmaceuticals; and receives royalties and/or publishing income from Taylor & Francis (Editor-in-Chief, Current Medical Research and Opinion, 2022–date), Wiley (Editor-in-Chief, International Journal of Clinical Practice, through 2019), UpToDate (reviewer), Springer Healthcare (book), and Elsevier (Topic Editor, Psychiatry, Clinical Therapeutics). Dr Fritz has received support from Teva, National Institutes of Health, National Multiple Sclerosis Society, Consortium of Multiple Sclerosis Centers, and American Physical Therapy Association—Michigan. Ms Hoberg has received support from Teva, Neurocrine, Acadia, Avanir Pharmaceuticals, and Intracellular Therapies. Dr Isaacson has received support from Teva and Neurocrine. Dr Kane has been a consultant for or received honoraria from Alkermes, EnVivo Pharmaceuticals (Forum), Forest (Allergan), Genentech, Intra-Cellular Therapies, Janssen, Johnson & Johnson, Karuna Therapeutics, LB Pharmaceuticals, Lilly, Lundbeck, Lyndra Therapeutics, Merck, Neurocrine Biosciences, Otsuka, Pierre Fabre, Reviva Pharmaceuticals, Roche, Saladax Biomedical, Sunovion, Takeda, and Teva Pharmaceuticals; has received grant support from Janssen, Lundbeck, and Otsuka; and is a shareholder of LB Pharmaceuticals and Vanguard Research Group. Dr Kumar has received support from Teva Pharmaceuticals and stock/stock options from CenExel, LLC.
Funding/support: The manuscript is a product of a consensus panel organized by Interactive Forums, Inc., through funding by an independent medical grant from Teva Branded Pharmaceutical Products R&D, Inc. Assistance in the preparation of this manuscript has been provided by Ashfield MedComms, an Inizio company, and funded by Teva Branded Pharmaceutical Products R&D, Inc. The sponsor had no direct involvement in the development of these consensus recommendations or in the development of this manuscript.
Role of the sponsor: This research was supported by Teva Branded Pharmaceutical Products R&D, Inc.
Acknowledgments: Medical writing support for the development of this manuscript, under the direction of the authors, was provided by Mark Skopin, PhD, CMPP, and Jennifer C. Jaworski, MS, BCMAS, and editing support by Kelsey Hogan, MS, all of Ashfield MedComms, an Inizio company, and funded by Teva Branded Pharmaceutical Products R&D, Inc.
Clinical Points
- Currently, there is no broadly accepted and published clinician-rated scale that can be used to assess the impact of tardive dyskinesia (TD) on patients’ functional daily life.
- A consensus panel of experts in TD and clinical scale development met to develop the Impact-TD scale, an assessment tool that can lead to a better understanding of how TD affects a patient’s quality of life.
References (19)

- Waln O, Jankovic J. An update on tardive dyskinesia: from phenomenology to treatment. Tremor Other Hyperkinet Mov (N Y). 2013;3:tre‐03-161-4138-1. PubMed CrossRef
- Carbon M, Hsieh CH, Kane JM, et al. Tardive dyskinesia prevalence in the period of second-generation antipsychotic use: a meta-analysis. J Clin Psychiatry. 2017;78(3):e264–e278. PubMed CrossRef
- Caroff SN, Citrome L, Meyer J, et al. A modified Delphi consensus study of the screening, diagnosis, and treatment of tardive dyskinesia. J Clin Psychiatry. 2020;81(2):19cs12983. PubMed CrossRef
- Caroff SN, Yeomans K, Lenderking WR, et al. RE-KINECT: a prospective study of the presence and healthcare burden of tardive dyskinesia in clinical practice settings. J Clin Psychopharmacol. 2020;40(3):259–268. PubMed CrossRef
- Caroff SN. Overcoming barriers to effective management of tardive dyskinesia. Neuropsychiatr Dis Treat. 2019;15:785–794. PubMed CrossRef
- Jain R, Correll CU. Tardive dyskinesia: recognition, patient assessment, and differential diagnosis. J Clin Psychiatry. 2018;79(2):nu17034ah1c. PubMed CrossRef
- Jackson R, Brams MN, Citrome L, et al. Assessment of the impact of tardive dyskinesia in clinical practice: consensus panel recommendations. Neuropsychiatr Dis Treat. 2021;17:1589–1597. PubMed CrossRef
- Strassnig M, Rosenfeld A, Harvey PD. Tardive dyskinesia: motor system impairments, cognition and everyday functioning. CNS Spectr. 2018;23(6):370–377. PubMed CrossRef
- Lynn MR. Determination and quantification of content validity. Nurs Res. 1986;35(6):382–386. PubMed CrossRef
- Kremens DE. Earlier diagnosis of tardive dyskinesia. J Clin Psychiatry. 2019;81(1):NU18041BR1C. PubMed CrossRef
- Bakke M, Henriksen T, Biernat HB, et al. Interdisciplinary recognizing and managing of drug-induced tardive oromandibular dystonia: two case reports. Clin Case Rep. 2018;6(11):2150–2155. PubMed CrossRef
- Vasan S, Padhy RK. Tardive Dyskinesia. StatPearls Publishing; 2021.
- Guy W. National Institute of Mental H, Psychopharmacology Research B, Early Clinical Drug Evaluation P. ECDEU Assessment Manual for Psychopharmacology. Dept. of Health, Education, and Welfare, Public Health Service, Alcohol, Drug Abuse, and Mental Health Administration, National Institute of Mental Health, Psychopharmacology Research Branch, Division of Extramural Research Programs; 1976.
- The American Psychiatric Association Practice Guideline for the Treatment of Patients With Schizophrenia. 3rd ed. American Psychiatric Association; 2021. https://psychiatryonline.org/doi/book/10.1176/appi.books.9780890424841
- Cutler AJ, Caroff SN, Tanner CM, et al. Caregiver-reported burden in RE-KINECT: data from a prospective real-world tardive dyskinesia screening study. J Am Psychiatr Nurses Assoc. 2021;10783903211023565. PubMed CrossRef
- Crane D, Henderson EJ, Chadwick DR. Exploring the acceptability of a ‘limited patient consent procedure’ for a proposed blood-borne virus screening programme: a Delphi consensus building technique. BMJ Open. 2017;7(5):e015373. PubMed CrossRef
- Murphy MK, Black NA, Lamping DL, et al. Consensus development methods, and their use in clinical guideline development. Health Technol Assess. 1998;2(3):i–iv, 1–88. PubMed CrossRef
- Wainwright P, Gallagher A, Tompsett H, et al. The use of vignettes within a Delphi exercise: a useful approach in empirical ethics? J Med Ethics. 2010;36(11):656–660. PubMed CrossRef
- Santaguida P, Dolovich L, Oliver D, et al. Protocol for a Delphi consensus exercise to identify a core set of criteria for selecting health related outcome measures (HROM) to be used in primary health care. BMC Fam Pract. 2018;19(1):152. PubMed CrossRef
This PDF is free for all visitors!
Save
Cite