ABSTRACT
Objective: Long-acting injectable antipsychotic agents (LAIs) are effective in schizophrenia relapse prevention but are often underutilized. This study aims to understand treatment patterns leading to a successful LAI implementation following schizophrenia diagnosis in a large dataset that included commercially insured patients in the United States.
Methods: Patients aged 18–40 years with a first schizophrenia diagnosis (per ICD-9 or ICD-10 criteria), successful second-generation LAI implementation (defined a priori as ≥ 90 consecutive days of use), and ≥ 1 second-generation oral antipsychotic agent (OA) were identified from IBM MarketScan Commercial and Medicare Supplemental databases from January 1, 2012, to December 31, 2019. Outcomes were measured descriptively.
Results: Of 41,391 patients with newly diagnosed schizophrenia, 1,836 (4%) received ≥ 1 LAI; 202 (< 1%) met eligibility criteria of successful LAI implementation following ≥ 1 second-generation OA. Median (range) time between diagnosis and first LAI was 289.5 (0–2,171) days, time between LAI initiation and successful implementation was 90.0 (90–1,061) days, and time to LAI discontinuation after successful implementation was 166.5 (91–799) days. Before LAI initiation, 58% received ≥ 2 OAs. For 86% with successful LAI implementation, the implementation was accomplished with the first LAI.
Conclusions: In this dataset of mainly commercially insured patients, LAI use in early-phase schizophrenia was very low (4%). For the majority for whom a LAI was successfully implemented per a priori definition, the implementation was accomplished with the first LAI and in a short period of time (90 days). However, even when LAIs were used in early-phase schizophrenia, they were generally not the first therapy, as most patients had several prior OA treatments.
J Clin Psychiatry 2023;84(3):22m14544
To cite: Kane JM, Mychaskiw MA, Lim S, et al. Treatment journey from diagnosis to the successful implementation of a long-acting injectable antipsychotic agent in young adults with schizophrenia. J Clin Psychiatry. 2023;84(3):22m14544.
To share: https://doi.org/10.4088/JCP.22m14544
© 2023 Physicians Postgraduate Press, Inc.
aThe Zucker Hillside Hospital, Northwell Health, Department of Psychiatry, Glen Oaks, New York
bDepartment of Psychiatry, Donald and Barbara Zucker School of Medicine at Hofstra/Northwell, Hempstead, New York
cFeinstein Institutes for Medical Research, Institute of Behavioral Science, Manhasset, New York
dTeva Branded Pharmaceutical Products R&D, Inc., Global Health Economics and Outcomes Research, West Chester, Pennsylvania
eTeva UK Limited, Global Medical Affairs, Harlow, United Kingdom
fTeva Branded Pharmaceutical Products R&D, Inc., Real World Evidence Statistics, West Chester, Pennsylvania
*Corresponding author: John M. Kane, MD, Senior Vice President, Behavioral Health Services, Zucker Hillside Hospital, Northwell Health, 75-59 263rd St, Glen Oaks, NY 11004 ([email protected]).
Schizophrenia is often a progressive and severely debilitating mental disorder that is characterized by positive (eg, hallucinations, delusions, disorganized speech or behavior), negative (eg, lack of motivation or emotional expressivity), and cognitive (eg, working memory, attention) symptoms.1 Although the lifetime global prevalence of schizophrenia is approximately 1%,2 the early age at typical disease onset, great degree of disability, and premature mortality rate associated with the illness underscore its substantial personal, social, and economic burden.3–5
Antipsychotic agents are an important part of treatment for many patients with schizophrenia.6 Since the initial development of these agents during the 1950s, the efficacy of antipsychotic agents, regardless of route of administration, has been thoroughly established7 and includes reductions in psychopathology, rates of relapse, rates of hospitalization, and mortality rates.8,9 Although early antipsychotic agents (ie, first-generation antipsychotic agents) demonstrated efficacy, they also were known to cause neurologic side effects, some of which could be very disabling. Newer antipsychotic agents (ie, second-generation antipsychotic agents) introduced in the 1990s generally have a higher degree of occupancy of the serotonergic 5-HT2A receptors.10 Given their pharmacodynamic properties, second-generation antipsychotic agents are generally tolerated better than first-generation drugs, particularly in early-phase psychosis.11 As a result, second-generation antipsychotic agents are the most commonly used type of antipsychotic agent.12
The benefits of antipsychotic agents may be more pronounced in patients receiving treatment with long-acting injectable antipsychotic agents (LAIs) compared with patients receiving oral antipsychotic agents (OAs).13–17 LAIs have demonstrated improvement in adherence rates and continuity of treatment among patients with schizophrenia compared with OAs.15,17,18 This may be particularly true for individuals in the early phase of the illness, when treatment interruption and nonadherence is most likely.18,19 Despite increasing data supporting the effectiveness of LAIs compared with OAs,14,20 LAIs are underutilized, with fewer than 14% of Medicaid beneficiaries with schizophrenia receiving LAIs.17 The degree to which LAIs are used in individuals with early-phase schizophrenia is not well understood, since previous epidemiologic studies typically captured individuals who were chronically ill and excluded patients with early-phase schizophrenia who are more likely to have private insurance.
Although continuous long-term antipsychotic therapy is most often required, poor treatment adherence represents a major obstacle in the management of schizophrenia.21,22 This is particularly true for individuals in the early phase, when treatment nonadherence/discontinuation is most common.
Treatment adherence among patients with schizophrenia hospitalized for first-episode psychosis is particularly poor, with 54.3% of these patients in one large cohort stopping their antipsychotic agent within 30 days of being discharged.19 Indeed, it is known that interruptions of antipsychotic treatment tend to be the greatest earlier in the illness, decreasing over time.18 Inadequate treatment, relapse, and prolonged periods of untreated psychosis, especially during the early stages of schizophrenia, are particularly concerning because of their association with the risk of developing treatment-resistant disease.23–27
Schizophrenia most often begins in late adolescence or early adulthood,28 and young adults with this illness are more likely to have commercial insurance (often through their parents until the age of 26 years) than those who have been ill for longer periods of time.29 Therefore, it is thought that many patients with first-episode psychosis and early-phase schizophrenia are not reflected in public insurance datasets in the US. However, most LAI utilization studies have been conducted using Medicaid/Medicare databases, which are more likely to include older patients29 with presumably more established disease.30 This study contributes to the advancement of knowledge by investigating trends in the use of LAIs in the initial phase of the illness, when the use of LAIs is particularly relevant yet data are most often unavailable in the existing literature.
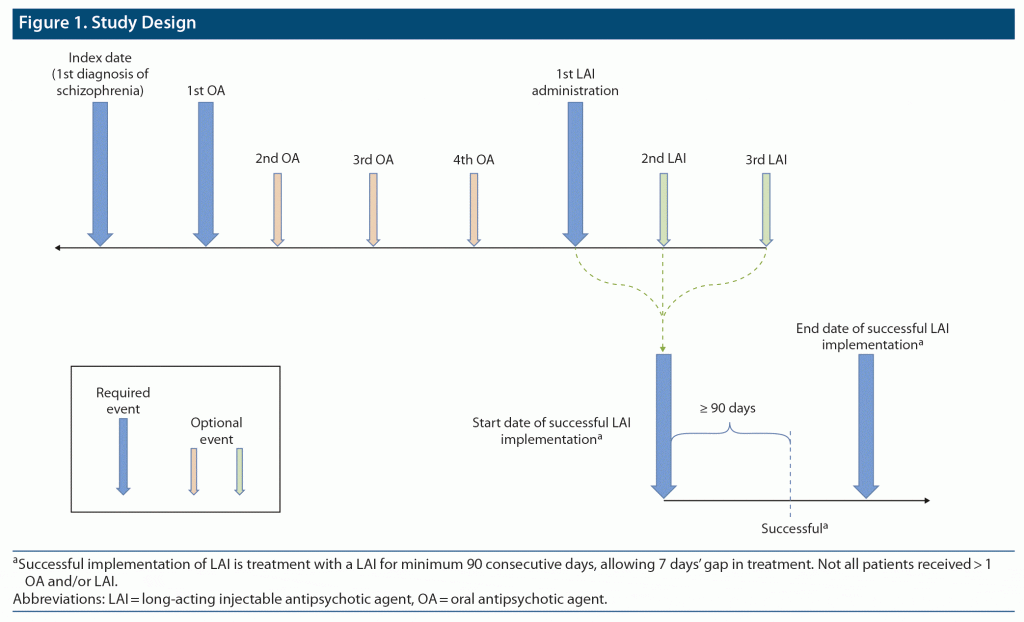
In this analysis, a US-based, real-world dataset of mainly commercially insured patients with schizophrenia was analyzed to descriptively evaluate treatment utilization patterns among young adults (ie, aged 18–40 years) with first-episode schizophrenia who were treated with second-generation LAIs. Patient data were analyzed from schizophrenia diagnosis to the first LAI administration and from first LAI administration to successful implementation (defined as continuous LAI use for ≥ 90 days, allowing 7-day gaps between each fill) to understand the early use of LAIs and barriers to successful implementation for patients and clinicians.
METHODS
Data Sources
The IBM MarketScan Commercial and Medicare Supplemental databases were used to analyze patient claims data from January 1, 2012, to December 31, 2019. The IBM MarketScan Commercial Database contains data regarding medical and pharmacy claims for individuals enrolled in fee-for-service, partially capitated, and fully capitated commercial health plans.31,32 The IBM MarketScan Medicare Supplemental Database captures medical and pharmacy claims data for individuals enrolled in Medicare Supplemental health plans.31,32 This study was exempt from review by an institutional review board because the database was compliant with the Health Insurance Portability and Accountability Act of 1996 and because the data do not include any identifiable patient information.
Patient Population and Cohorts
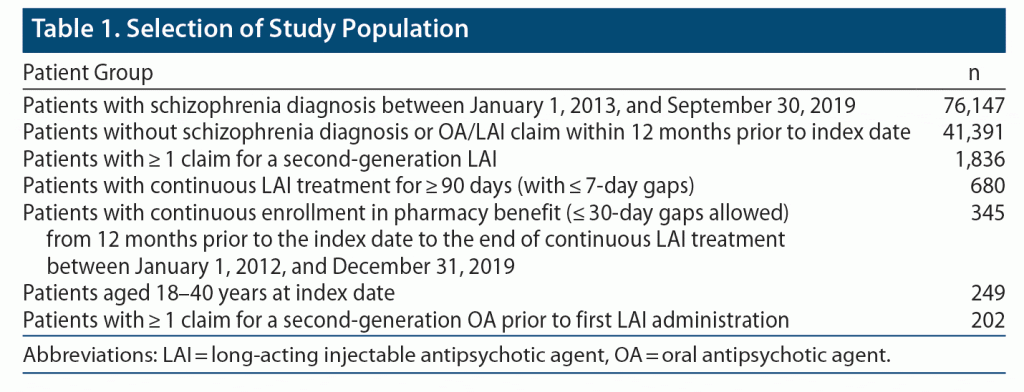
Patients with schizophrenia who were identified in the IBM MarketScan Commercial and Medicare Supplemental databases were eligible for inclusion in this analysis. Additional inclusion criteria (Table 1) were a diagnosis of schizophrenia (according to International Classification of Diseases, 9th Revision [ICD-9] or 10th Revision [ICD-10], codes for schizophrenia) between January 1, 2013, and September 30, 2019 (index date); no schizophrenia diagnosis or OA/LAI claims during 12-month pre-index period; pharmacy claim for a second-generation LAI (aripiprazole, olanzapine, paliperidone, or risperidone) and continuous use (≥ 90 consecutive days, allowing 7-day gaps between each fill); continuous enrollment in commercial or Medicare supplemental medical and pharmacy benefits from ≥ 12 months before the index date to the end of continuous LAI treatment; aged 18–40 years at index date; and ≥ 1 OA claim (aripiprazole, asenapine, brexpiprazole, cariprazine, clozapine, iloperidone, lurasidone, olanzapine, paliperidone, quetiapine, risperidone, or ziprasidone) prior to first LAI administration. The index date range of January 1, 2013, to September 30, 2019, was selected to allow a ≥ 12-month pre-index period during which no diagnosis of schizophrenia or administration of antipsychotic therapy was permitted and a ≥ 90-day post-index period to discern possible successful implementation of LAI therapy. These constraints were put in place to identify individuals with a recent onset of schizophrenia, as opposed to chronic illness.
Patients included in this analysis were grouped according to the LAI used for the successful LAI implementation (defined a priori as continuous LAI use for ≥ 90 days, allowing 7-day gaps between each fill). Because the recommended dose frequencies of LAIs available at the time of this research ranged up to once every 3 months, the threshold set for successful LAI implementation was 90 consecutive days to increase the likelihood of capturing at least 2 administrations of a LAI. Although comparison between LAIs was not in the scope of this analysis, grouping patients according to the LAI used in successful implementation allowed for descriptive examination.
Outcome Measures
Figure 1 describes the sequence of events used for this analysis. Analyzed outcomes included the time between diagnosis of schizophrenia (index date) and first LAI administration; time between first administration and successful implementation of a LAI, per the aforementioned definition; time to LAI discontinuation (ie, > 7-day gaps between injections); number of different OAs received between the index date and first administration of a LAI; number of and overall treatment patterns with different LAIs received between first administration and successful implementation of a LAI; and proportion of patients for whom the initial LAI was successfully implemented.
Statistical Analysis
The distribution of patient demographic characteristics, use of second-generation antipsychotic agents, and time to LAI initiation and successful implementation were descriptively evaluated for each LAI group. Numbers and proportions were calculated for categorical variables, whereas means, standard deviations (SDs), medians, and ranges were calculated for continuous variables.
RESULTS
Analysis Set
Claims data for 98,416,202 individuals were present in the IBM MarketScan commercial and Medicare supplemental databases from January 1, 2012, to December 31, 2019 (index date between January 1, 2013, and September 30, 2019); among these individuals, 76,147 patients with any schizophrenia diagnosis (according to ICD-9 or ICD-10 codes for schizophrenia) within the index date range were identified. Of these patients, 41,391 had no schizophrenia diagnosis and no LAI/OA claim during 12 months prior to the index date (ie, newly diagnosed), of whom 1,836 (4%) had ≥ 1 LAI claim after the index date. Among these patients with newly diagnosed schizophrenia, 202 patients with successful LAI implementation met eligibility criteria and composed the analysis set for this study (Table 1).
Patient Demographics and Baseline Characteristics
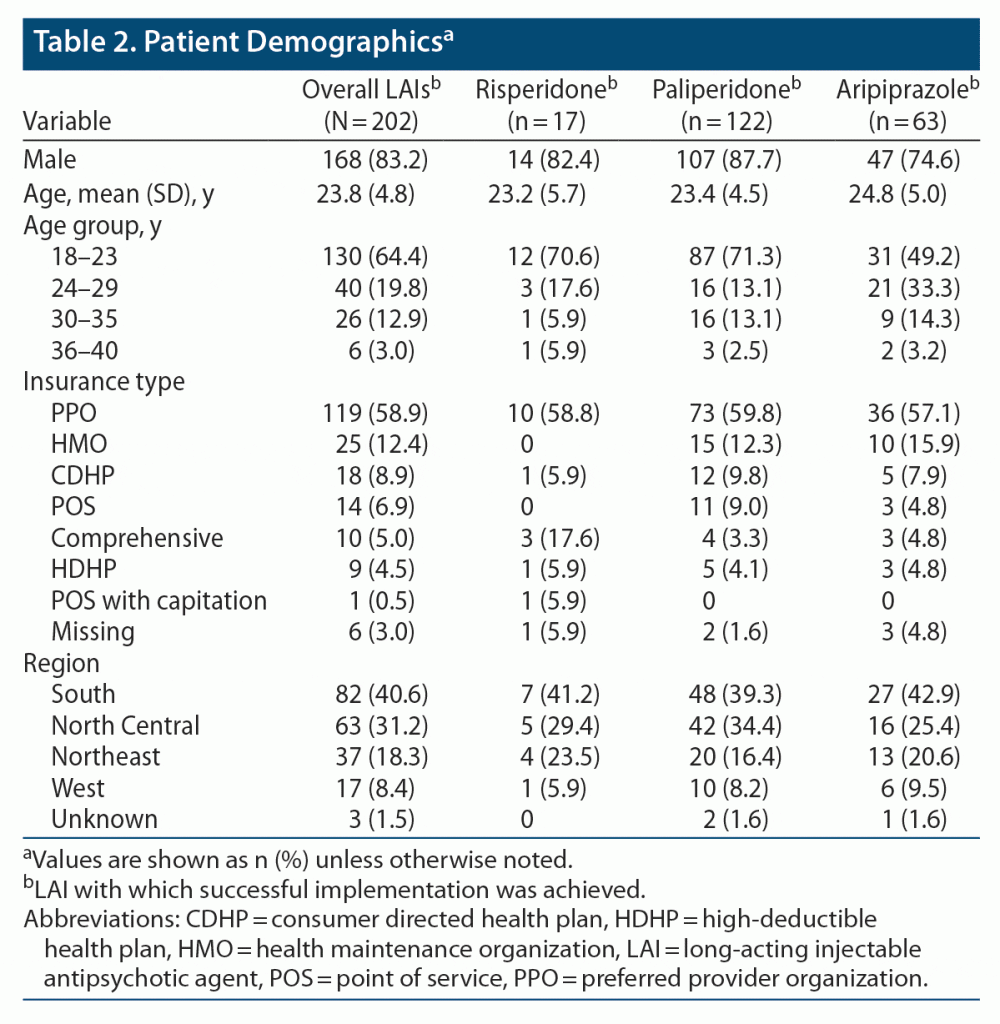
At the time of first diagnosis (ie, index date), patient demographics and baseline disease characteristics were similar among successful LAI implementation groups (Table 2). Mean (SD) age of included patients was 23.8 (4.8) years with 64.4% of the population aged 18–23 years and 19.8% aged 24–29 years. Most (83.2%) of the patients in this study were male.
Antipsychotic Agent Use and Utilization Outcomes
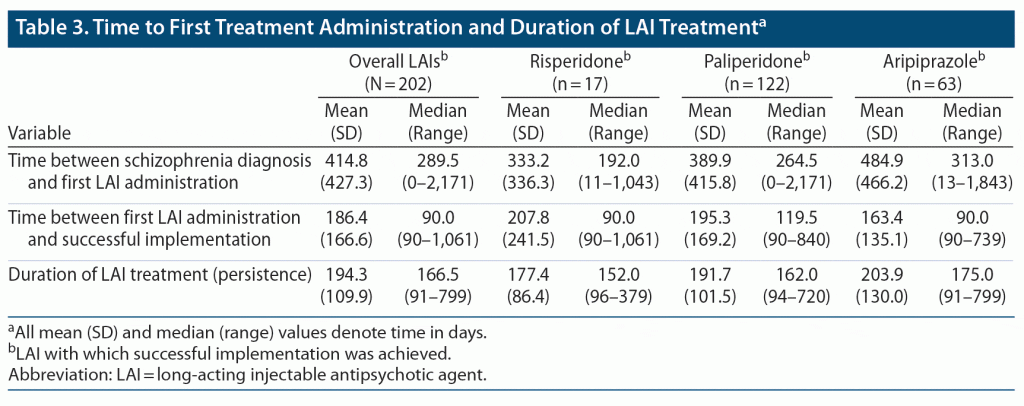
The median (range) time between the index date and initial treatment with a LAI among all patients in this analysis was 289.5 (0–2,171) days; for patients initially treated with LAI risperidone, LAI paliperidone, or LAI aripiprazole, the time between diagnosis and first LAI use were 192.0 (11–1,043) days, 264.5 (0–2,171) days, and 313.0 (13–1,843) days, respectively (Table 3). The median (range) time between initial treatment with a LAI and successful implementation was 90.0 (90–1,061) days for patients overall, 90.0 (90–1,061) days for those who achieved successful implementation with risperidone, 119.5 (90–840) days with paliperidone, and 90.0 (90–739) days with aripiprazole (Table 3). Overall, the median (range) persistence with a successfully implemented LAI was 166.5 (91–799) days, and 152.0 (96–379) days, 162.0 (94–720) days, and 175.0 (91–799) days for patients who achieved successful implementation with risperidone, paliperidone, and aripiprazole, respectively (Table 3).
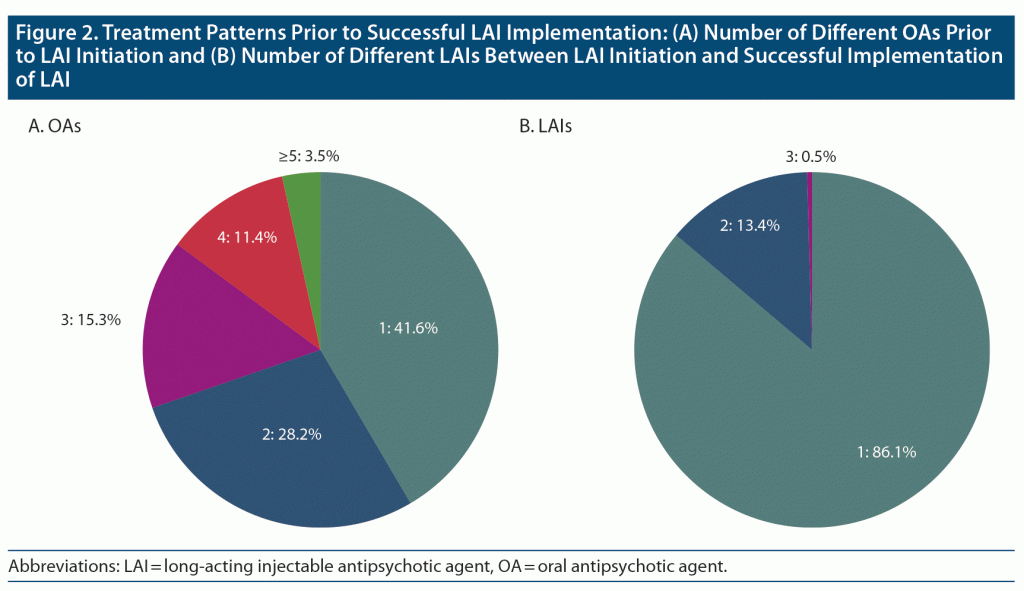
Prior to LAI initiation, risperidone (34.7%, n = 70) was the most frequently used first-line OA among patients who would later have a successful LAI implementation, followed by aripiprazole (16.3%, n = 33), olanzapine (14.9%, n = 30), paliperidone (13.4%, n = 27), and quetiapine (10.9%, n = 22). As second-line therapy, aripiprazole (22.9%, n = 27/118) and paliperidone (22.0%, n = 26/118) were the most utilized OA agents. Before initiating a LAI, more than half (58.4%) of patients received ≥ 2 distinct OAs; most patients received treatment with either 1 (41.6%), 2 (28.2%), or 3 (15.3%) distinct OAs before initiating treatment with a LAI (Figure 2).
Among patients who had a successful LAI implementation, paliperidone was most frequently used as first-line and second-line LAI treatment (57.9% [n = 117] and 50.0% [n = 14/28], respectively), followed by aripiprazole (27.7% [n = 56], 28.6% [n = 8/28], respectively) and risperidone (12.9% [n = 26], 21.4% [n = 6/28], respectively). The LAIs with which patients achieved successful implementation were paliperidone (60.4%, n = 122), aripiprazole (31.2%, n = 63), and risperidone (8.4%, n = 17); none of the individuals achieved successful LAI implementation with olanzapine. Most patients (86.1%, n = 174) who achieved a successful LAI implementation achieved it with their first LAI (Figure 2).
DISCUSSION
Although several clinical studies have previously described LAI treatment patterns in individuals with first-episode schizophrenia,33–35 this study marks the first time, to the best of our knowledge, that the utilization patterns of LAIs in a national cohort of individuals were analyzed. Our main finding was that LAI utilization among patients early in the course of illness is very low. For the majority of those for whom a LAI was utilized, a LAI was initiated relatively soon after diagnosis (median = 290 days), although the sustained use of the LAI after first administration was limited (median = 167 days).
The rates of LAI acceptability among those prescribed LAIs aligns with a previous report from a clinical trial focused on the patients with early-phase illness.36 In that study, the vast majority of those to whom a LAI was offered agreed to use it at least once,36 indicating that younger patients may be accepting of an alternative to taking oral medication daily. In this study, the majority (84%) of the population were aged 18–29 years, making the onset congruent with the early onset of disease described previously.28,37 In addition, it seems that patients with early-phase schizophrenia would be among those who would benefit the most from LAI formulations, as they are the most likely to interrupt treatment.18,19 This population also may not respond to reintroduced treatments as well as they responded to the initial implementation.26 There is a growing body of evidence suggesting that LAIs are acceptable and advantageous for a variety of outcomes in early-phase schizophrenia.38 Early treatment with LAIs also aligns with recently revised treatment guidelines, which are increasingly supportive of the use of LAIs among younger individuals with early-phase disease.39
However, one of the other main findings was that only 4% of patients newly diagnosed with schizophrenia were treated with a LAI medication. This finding emphasizes the relevance of studying specific patterns of LAI utilization among patients with first-episode psychosis, as rates of antipsychotic agent use may be substantially different from those in public insurance databases reflective of individuals further along in the course of illness. For instance, a recent evaluation of Medicaid data reported that the LAI (both first- and second-generation) utilization rate in the US ranges between 4% and 22% depending on the state,40 which, although lower than recommended, is a higher rate than observed in our study. This finding may indicate that there is still a prioritization of LAIs for older individuals, who may have already experienced several relapses and who may not benefit as much from relapse prevention as younger individuals would. The disconnect between the data on acceptability and utilization of LAIs among patients with early-phase schizophrenia is striking and suggests that LAIs are not yet offered often enough to younger individuals by clinicians and that their use is mostly reactive (ie, following several relapses after treatment discontinuation) rather than proactive (ie, earlier on before relapses have occurred). Although data describing patients declining a LAI were not captured, utilization by clinicians along with data on the acceptance of LAIs by patients suggest the need to provide training and treatment systems to health care providers so younger individuals are offered LAI formulations as an alternative to OA medication. In addition to misperceptions of LAIs by clinicians, barriers to the utilization of LAIs may also include payers’ requiring prior authorization or a preference over a first-generation LAI (ie, haloperidol decanoate). Also, these data suggest that, even when LAIs were used proactively in patients with early-phase schizophrenia, they were generally not the first therapy used, as most individuals in this study had several prior treatment episodes with OAs. This finding indicates that LAIs are often not administered as a standard-of-care by clinicians, even among patients who will eventually accept them. Thus, there is more work needed to update treatment practices so that the utilization of LAIs in individuals in the early phase of the illness is more proactive. In addition, LAIs were in general used for only about 1 year following initiation. This finding aligns with the literature on treatment discontinuation in patients with early-phase schizophrenia. We did not compare time to treatment discontinuation between LAIs and OAs, since it was out of the scope of this analysis; however, it is well documented that LAIs are used for longer periods of time than their oral counterparts.17,18 Nonetheless, further work is necessary to reduce the risk of unjustified LAI discontinuation.
Among patients with newly diagnosed schizophrenia who received at least 1 LAI, approximately 11% (202 of 1,836 patients) achieved successful LAI implementation per the a priori definition of continuous use for ≥ 90 days, allowing 7-day gaps. The low proportion of patients who used LAIs longer than 90 days could be attributed to suboptimal efficacy or tolerability; however, it is also possible that the study failed to capture patients who continued to receive LAI treatment after successful implementation but had a gap longer than 7 days between dosing periods. In addition, the low rate of successful LAI implementation lasting longer than 90 days supports recent findings on treatment discontinuation18; although time to treatment discontinuation may be short, it is still a long period for maintenance treatment for schizophrenia in general. It is also possible that the low rate could have been affected by patients who either switched insurance quickly after successful LAI implementation, missed follow-up office visits, or encountered financial and/or accessibility issues related to subsequent administrations. Regardless of the reason, patients with newly diagnosed schizophrenia could benefit from initiatives that encourage continued treatment, including patient assistance programs, reminders of scheduled office visits, and other mitigation strategies that can lessen the impact of limited accessibility.
Some additional limitations need to be considered when interpreting these data. First, our data are not reflective of the use of first-generation LAIs. However, the market share of first-generation LAIs is very small and consists largely of patients who initiated LAI treatment before second-generation LAIs came to market and have continued such treatment. Thus, we do not believe that exclusion of first-generation LAIs had a major impact on these results, since our focus was patients with early-phase schizophrenia. Second, these data were generated from insurance claims and are subject to the common limitations of these datasets, such as the exclusion of patients for whom there was interrupted coverage. Third, our follow-up period did not cover the entire initial 5 years of treatment for each individual, which is considered by many specialty programs as the period of early-phase schizophrenia. Because the objective of this analysis was to examine patients from diagnosis (ie, index date) to successful implementation, some patients may have had less than 5 years between their index date and the end of the study period. Therefore, it is possible that there is a more comprehensive pattern of LAI utilization in the early phases of illness that was not captured by these data. Lastly, although the MarketScan databases capture comprehensive commercial medical and pharmacy claims data, the databases consist of convenience samples that use nonrandom methods to select patients and might not be representative of the national population of patients with early-phase schizophrenia. However, because many patients are still on their parent’s or guardian’s insurance (ie, commercially available) at the time of diagnosis, the MarketScan databases are more likely to capture younger patients with early-phase schizophrenia compared with traditional analyses that use public insurance (ie, Medicaid/Medicare) databases.
In conclusion, these data indicate that despite the overwhelming evidence in favor of LAIs over OAs for relapse prevention41 and the evidence that indicated LAI treatment during the early phase of schizophrenia is most effective,18,19,39 LAIs are markedly underutilized among patients with early-phase schizophrenia. Our data support previous findings about the acceptability of LAIs in younger individuals with schizophrenia, highlighting a disconnect between treatment utilization and acceptability that should be addressed in future research and clinical practice.
Submitted: May 25, 2022; accepted November 17, 2022.
Published online: April 19, 2023.
Relevant financial relationships: Dr Kane has been a consultant for or received honoraria from Alkermes, EnVivo Pharmaceuticals (Forum), Forest (Allergan), Genentech, Intra-Cellular Therapies, Janssen, Johnson & Johnson, Karuna Therapeutics, LB Pharmaceuticals, Lilly, Lundbeck, Lyndra Therapeutics, Merck, Neurocrine Biosciences, Otsuka, Pierre Fabre, Reviva Pharmaceuticals, Roche, Saladax Biomedical, Sunovion, Takeda, and Teva Pharmaceuticals; has received grant support from Janssen, Lundbeck, and Otsuka; and is a shareholder of LB Pharmaceuticals and Vanguard Research Group. Drs Mychaskiw, Suett, and Tian are employees and stockholders of Teva Pharmaceuticals. Mr Lim is an employee of Teva Pharmaceuticals. Dr Rubio has been a consultant for and has received support for attending meetings/travel from Teva Pharmaceuticals; has received honoraria from Lundbeck; has received grants from Alkermes and the National Institute of Mental Health (NIMH); has received royalties/licensing fees from UpToDate; and owns stock/stock options in Doximity.
Funding/support: This study was supported by Teva Branded Pharmaceutical Products R&D, Inc.
Role of the sponsor: The sponsor participated in the design and conduct of the study and in the collection, management, analysis, and interpretation of the data and funded the preparation of the manuscript. All authors, including those affiliated with the sponsor, fulfilled all authorship criteria and participated in the review and approval of the manuscript and in the decision to submit the manuscript for publication.
Previous presentations: Presented at the European College of Neuropsychopharmacology Congress; Lisbon, Portugal; October 2–5 2021 • Annual Psych Congress; San Antonio, Texas; October 29–November 1, 2021 • American College of Neuropsychopharmacology Annual Meeting; San Juan, Puerto Rico; December 5–8, 2021.
Acknowledgments: The authors would like to thank Seojin Park, PharmD, of Teva Branded Pharmaceutical Products R&D, Inc., at the time of this research, for contributions to the conduct of the study. Medical writing support was provided by Mark Skopin, PhD, CMPP, and Jennifer C. Jaworski, MS, BCMAS, and editorial support by Kelsey Hogan, MS, of Ashfield MedComms, an Inizio company, and was funded by Teva Branded Pharmaceutical Products R&D, Inc. The individuals acknowledged received indirect compensation from Teva Branded Pharmaceutical Products R&D, Inc., for their roles in the conduct of the study and development of this manuscript.
Clinical Points
- A real-world dataset of mainly commercially insured patients with schizophrenia was analyzed to evaluate treatment utilization patterns among young adults with first-episode schizophrenia who were treated with second-generation long-acting injectable antipsychotic agents (LAIs).
- These data indicate that despite the overwhelming evidence in favor of LAIs over oral antipsychotic agents for relapse prevention and the evidence that indicated LAI treatment during the early phase of schizophrenia is most effective, LAIs are markedly underutilized among patients with early-phase schizophrenia.
- These data support previous findings about the acceptability of LAIs in younger individuals with schizophrenia, highlighting a disconnect between treatment utilization and acceptability that should be addressed in future research and clinical practice.
References (41)

- Patel KR, Cherian J, Gohil K, et al. Schizophrenia: overview and treatment options. P&T. 2014;39(9):638–645. PubMed
- Biagi E, Capuzzi E, Colmegna F, et al. Long-acting injectable antipsychotics in schizophrenia: literature review and practical perspective, with a focus on aripiprazole once-monthly. Adv Ther. 2017;34(5):1036–1048. PubMed CrossRef
- Charlson FJ, Ferrari AJ, Santomauro DF, et al. Global epidemiology and burden of schizophrenia: findings from the Global Burden of Disease Study 2016. Schizophr Bull. 2018;44(6):1195–1203. PubMed CrossRef
- Cloutier M, Aigbogun MS, Guerin A, et al. The economic burden of schizophrenia in the United States in 2013. J Clin Psychiatry. 2016;77(6):764–771. PubMed CrossRef
- Hjorthøj C, Stürup AE, McGrath JJ, et al. Years of potential life lost and life expectancy in schizophrenia: a systematic review and meta-analysis. Lancet Psychiatry. 2017;4(4):295–301. PubMed CrossRef
- The American Psychiatric Association Practice Guideline for the Treatment of Patients With Schizophrenia. 3rd ed. American Psychiatric Association; 2021. Accessed March 8, 2022. https://psychiatryonline.org/doi/book/10.1176/appi.books.9780890424841
- Haddad PM, Correll CU. The acute efficacy of antipsychotics in schizophrenia: a review of recent meta-analyses. Ther Adv Psychopharmacol. 2018;8(11):303–318. PubMed CrossRef
- Leucht S, Tardy M, Komossa K, et al. Antipsychotic drugs versus placebo for relapse prevention in schizophrenia: a systematic review and meta-analysis. Lancet. 2012;379(9831):2063–2071. PubMed CrossRef
- Tiihonen J, Mittendorfer-Rutz E, Torniainen M, et al. Mortality and cumulative exposure to antipsychotics, antidepressants, and benzodiazepines in patients with schizophrenia: an observational follow-up study. Am J Psychiatry. 2016;173(6):600–606. PubMed CrossRef
- Dazzan P, Morgan KD, Orr K, et al. Different effects of typical and atypical antipsychotics on grey matter in first episode psychosis: the AESOP study. Neuropsychopharmacology. 2005;30(4):765–774. PubMed CrossRef
- Fusar-Poli P, McGorry PD, Kane JM. Improving outcomes of first-episode psychosis: an overview. World Psychiatry. 2017;16(3):251–265. PubMed CrossRef
- Hálfdánarson Ó, Zoëga H, Aagaard L, et al. International trends in antipsychotic use: a study in 16 countries, 2005–2014. Eur Neuropsychopharmacol. 2017;27(10):1064–1076. PubMed CrossRef
- Nielsen RE, Hessellund KB, Valentin JB, et al. Second-generation LAI are associated to favorable outcome in a cohort of incident patients diagnosed with schizophrenia. Schizophr Res. 2018;202:234–240. PubMed CrossRef
- Tiihonen J, Mittendorfer-Rutz E, Majak M, et al. Real-world effectiveness of antipsychotic treatments in a nationwide cohort of 29,823 patients with schizophrenia. JAMA Psychiatry. 2017;74(7):686–693. PubMed CrossRef
- Greene M, Yan T, Chang E, et al. Medication adherence and discontinuation of long-acting injectable versus oral antipsychotics in patients with schizophrenia or bipolar disorder. J Med Econ. 2018;21(2):127–134. PubMed CrossRef
- Kishimoto T, Nitta M, Borenstein M, et al. Long-acting injectable versus oral antipsychotics in schizophrenia: a systematic review and meta-analysis of mirror-image studies. J Clin Psychiatry. 2013;74(10):957–965. PubMed CrossRef
- Pilon D, Joshi K, Tandon N, et al. Treatment patterns in Medicaid patients with schizophrenia initiated on a first- or second-generation long-acting injectable versus oral antipsychotic. Patient Prefer Adherence. 2017;11:619–629. PubMed CrossRef
- Rubio JM, Taipale H, Tanskanen A, et al. Long-term continuity of antipsychotic treatment for schizophrenia: a nationwide study. Schizophr Bull. 2021;47(6):1611–1620. PubMed CrossRef
- Tiihonen J, Haukka J, Taylor M, et al. A nationwide cohort study of oral and depot antipsychotics after first hospitalization for schizophrenia. Am J Psychiatry. 2011;168(6):603–609. PubMed CrossRef
- Kane JM, Schooler NR, Marcy P, et al. Effect of long-acting injectable antipsychotics vs usual care on time to first hospitalization in early-phase schizophrenia: a randomized clinical trial. JAMA Psychiatry. 2020;77(12):1217–1224. PubMed CrossRef
- Haddad PM, Brain C, Scott J. Nonadherence with antipsychotic medication in schizophrenia: challenges and management strategies. Patient Relat Outcome Meas. 2014;5:43–62. PubMed CrossRef
- Penttilä M, Jääskeläinen E, Hirvonen N, et al. Duration of untreated psychosis as predictor of long-term outcome in schizophrenia: systematic review and meta-analysis. Br J Psychiatry. 2014;205(2):88–94. PubMed CrossRef
- Andreasen NC, Liu D, Ziebell S, et al. Relapse duration, treatment intensity, and brain tissue loss in schizophrenia: a prospective longitudinal MRI study. Am J Psychiatry. 2013;170(6):609–615. PubMed CrossRef
- Drake RJ, Husain N, Marshall M, et al. Effect of delaying treatment of first-episode psychosis on symptoms and social outcomes: a longitudinal analysis and modelling study. Lancet Psychiatry. 2020;7(7):602–610. PubMed CrossRef
- Rubio J, Taipale H, Correll CU, et al. Psychotic relapse during maintenance antipsychotic treatment: a nationwide study. Schizophr Bull. 2019;45(suppl 2):S174–S175. CrossRef
- Takeuchi H, Siu C, Remington G, et al. Does relapse contribute to treatment resistance? antipsychotic response in first- vs second-episode schizophrenia. Neuropsychopharmacology. 2019;44(6):1036–1042. PubMed CrossRef
- Taipale H, Tanskanen A, Correll CU, et al. Real-world effectiveness of antipsychotic doses for relapse prevention in patients with first-episode schizophrenia in Finland: a nationwide, register-based cohort study. Lancet Psychiatry. 2022;9(4):271–279. PubMed CrossRef
- Häfner H. From onset and prodromal stage to a life-long course of schizophrenia and its symptom dimensions: how sex, age, and other risk factors influence incidence and course of illness. Psychiatry J. 2019;2019:9804836. PubMed CrossRef
- Cohen RA, Cha AE. National Center for Health Statistics. Health insurance coverage: early release of quarterly estimates from the national health interview survey, January–December 2019. CDC website. https://www.cdc.gov/nchs/data/nhis/earlyrelease/Quarterly_estimates_2019_Q14-508.pdf. 2020. Accessed May 9, 2022.
- Lin D, Thompson-Leduc P, Ghelerter I, et al. Real-world evidence of the clinical and economic impact of long-acting injectable versus oral antipsychotics among patients with schizophrenia in the United States: a systematic review and meta-analysis. CNS Drugs. 2021;35(5):469–481. PubMed CrossRef
- IBM Watson Health. IBM MarketScan Research Databases for life sciences researchers. IBM Watson Health website. https://www.ibm.com/downloads/cas/OWZWJ0QO. Accessed March 8, 2022
- Roy B, Shah A, Bloomgren G, et al. Disease prevalence, comorbid conditions, and medication utilization among patients with schizophrenia in the United States. CNS Spectr. 2021;26(2):157. CrossRef
- Lian L, Kim DD, Procyshyn RM, et al. Efficacy of long-acting injectable versus oral antipsychotic drugs in early psychosis: a systematic review and meta-analysis. Early Interv Psychiatry. 2022;16(6):589–599. PubMed CrossRef
- Giordano G, Tomassini L, Cuomo I, et al. Aripiprazole long-acting injection during first episode schizophrenia—an exploratory analysis. Front Psychiatry. 2020;10:935. PubMed CrossRef
- Abdel-Baki A, Medrano S, Maranda C, et al. Impact of early use of long-acting injectable antipsychotics on psychotic relapses and hospitalizations in first-episode psychosis. Int Clin Psychopharmacol. 2020;35(4):221–228. PubMed CrossRef
- Kane JM, Schooler NR, Marcy P, et al. Patients with early-phase schizophrenia will accept treatment with sustained-release medication (long-acting injectable antipsychotics): results from the recruitment phase of the PRELAPSE trial. J Clin Psychiatry. 2019;80(3):18m12546. PubMed CrossRef
- Reist C, Valdes E, Ren Y, et al. Using claims data to assess treatment quality of first-episode psychosis. Psychiatr Serv. 2021;72(3):247–253. PubMed CrossRef
- Subotnik KL, Casaus LR, Ventura J, et al. Long-acting injectable risperidone for relapse prevention and control of breakthrough symptoms after a recent first episode of schizophrenia. A randomized clinical trial. JAMA Psychiatry. 2015;72(8):822–829. PubMed CrossRef
- 2019–2020 Florida Best Practice Psychotherapeutic Medication Guidelines for Adults (Florida Agency for Health Care Administration. The University of South Florida website. https://floridabhcenter.org/adult-guidelines/2019-2020-florida-best-practice-psychotherapeutic-medication-guidelines-for-adults/. 2020. Accessed March 8, 2022.
- Bareis N, Olfson M, Wall M, et al. Variation in psychotropic medication prescription for adults with schizophrenia in the United States. Psychiatr Serv. 2022;73(5):492–500. PubMed CrossRef
- Kishimoto T, Hagi K, Kurokawa S, et al. Long-acting injectable versus oral antipsychotics for the maintenance treatment of schizophrenia: a systematic review and comparative meta-analysis of randomised, cohort, and pre-post studies. Lancet Psychiatry. 2021;8(5):387–404. PubMed CrossRef
Save
Cite
Advertisement
GAM ID: sidebar-top