Objective: Antipsychotic agents have serious metabolic adverse effects, among them dyslipidemia, which may necessitate secondary prophylaxis with cholesterol-lowering drugs. Second-generation antipsychotics (SGAs), particularly clozapine and olanzapine, are known to confer a higher risk of metabolic adverse effects than first-generation antipsychotics (FGAs). However, little is known regarding the real-life number of antipsychotic-treated patients receiving statins.
Method: By extracting data from the Norwegian prescription database, all patients 18-69 years old that started treatment with an antipsychotic during 2004-2012 formed the basis for analysis (n = 301,713). The primary outcome measure was the proportion of FGA and SGA users prescribed with cholesterol-lowering agent during the same period. We used Cox proportional hazards regression to evaluate the risk of redeeming a cholesterol-lowering drug for formerly antipsychotic drug-naive patients (n = 147,218).
Results: Statin prescription rates in patients receiving antipsychotic agents were lower (5.3%) than comparable rates in studies covering the general population (34%) and lower than would be expected based on the recognized negative impact of antipsychotics on serum lipids. Statin prescription rates were affected by patient age, antipsychotic dose, and the number of antipsychotic agents prescribed, but rates were only 5% elevated in patients receiving SGAs compared to patients receiving FGAs (P = .048).
Conclusions: Our results may support the notion that patients treated with antipsychotic agents receive suboptimal care with regard to metabolic adverse effects.
Incident Users of Antipsychotic Agents and Future Use of Cholesterol-Lowering Drugs: An Observational, Pharmacoepidemiologic Study

ABSTRACT
Objective: Antipsychotic agents have serious metabolic adverse effects, among them dyslipidemia, which may necessitate secondary prophylaxis with cholesterol-lowering drugs. Second-generation antipsychotics (SGAs), particularly clozapine and olanzapine, are known to confer a higher risk of metabolic adverse effects than first-generation antipsychotics (FGAs). However, little is known regarding the real-life number of antipsychotic-treated patients receiving statins.
Method: By extracting data from the Norwegian prescription database, all patients 18-69 years old that started treatment with an antipsychotic during 2004-2012 formed the basis for analysis (n = 301,713). The primary outcome measure was the proportion of FGA and SGA users prescribed with cholesterol-lowering agent during the same period. We used Cox proportional hazards regression to evaluate the risk of redeeming a cholesterol-lowering drug for formerly antipsychotic drug-naive patients (n = 147,218).
Results: Statin prescription rates in patients receiving antipsychotic agents were lower (5.3%) than comparable rates in studies covering the general population (34%) and lower than would be expected based on the recognized negative impact of antipsychotics on serum lipids. Statin prescription rates were affected by patient age, antipsychotic dose, and the number of antipsychotic agents prescribed, but rates were only 5% elevated in patients receiving SGAs compared to patients receiving FGAs (P = .048).
Conclusions: Our results may support the notion that patients treated with antipsychotic agents receive suboptimal care with regard to metabolic adverse effects.
J Clin Psychiatry 2015;76(1):e111-e116
© Copyright 2015 Physicians Postgraduate Press, Inc.
Submitted: January 13, 2014; accepted April 29, 2014 (doi:10.4088/JCP.14m08996).
Corresponding author: Silje Skrede, MD, PhD, Center for Medical Genetics and Molecular Medicine, Haukeland University Hospital, Bergen, 5021, Norway ([email protected]).
After their introduction in the 1950s, antipsychotic agents quickly became indispensable in the treatment of schizophrenia and other psychotic disorders, but they are also subject to extensive off-label use.1-3 In spite of the available treatment, mortality in patients suffering from serious mental disorders is 2-3 times higher than in the general population.4-6 Recent studies have demonstrated that the prevalence of somatic conditions such as cardiovascular disease, as well as mortality due to cardiovascular events, is increased 2- to 3-fold in these patients.4,7-9 This is partly due to lifestyle issues among patients, but the contribution by metabolic adverse effects of antipsychotic treatment is significant.4,8,10 The atypical antipsychotics, or second-generation antipsychotics (SGAs), particularly olanzapine and clozapine, are well known for their metabolic adverse effects, ie, weight gain, dyslipidemia, and diabetes.11-15 However, some SGAs, particularly clozapine, are also recognized to have superior symptom-relieving efficacy among the antipsychotics, and are more frequently prescribed than older, first-generation antipsychotics (FGAs).15
The magnitude of metabolic adverse effects during treatment with antipsychotic agents is significant. In clinical studies involving young or treatment-naive patients, an increase of ≥ 7% of baseline body mass index occurred in 80% of patients treated with olanzapine for a year, while an increase of 30%-50% in serum triglycerides during olanzapine treatment has consistently been reported.16-20 Furthermore, unfavorable alterations in cholesterol parameters, ie, increased serum total cholesterol and/or increased low-density lipoprotein cholesterol levels, have been reported during olanzapine treatment.18,20-22 Similarly, several studies on clozapine treatment have reported increased serum triglyceride and/or cholesterol levels.21,23,24
Antipsychotic-induced metabolic adverse effects are to some degree preventable through both lifestyle intervention and pharmacologic treatment.25-27 For instance, statins improved several lipid parameters that were negatively affected by antipsychotic treatment.27-30 Still, antipsychotic-induced dyslipidemia is underdiagnosed and undertreated.31-34 In particular, tangible data regarding coprescription of antipsychotics and lipid-lowering agents in patients treated with an antipsychotic agent are very limited. Clinical trials examining dysmetabolic effects of antipsychotic drugs often span a short period of time, and patients with somatic conditions are frequently excluded.35,36
AIMS OF THE STUDY
In this study, we aimed to assess the proportion of formerly treatment-naive patients being prescribed antipsychotic agents who subsequently received cholesterol-lowering agents and to examine whether the proportion of patients receiving cholesterol-lowering agents was higher in patient groups receiving SGAs, particularly clozapine or olanzapine, than in patients receiving FGAs.

- Antipsychotic agents, in particular second-generation antipsychotics, have dyslipidemic adverse effects.
- Several studies have confirmed the beneficial effects of lipid-lowering drugs in patients with antipsychotic-induced dyslipidemia.
- Many patients treated with antipsychotics most likely have undetected and untreated elevation in serum lipid levels.
METHOD
Data Extraction
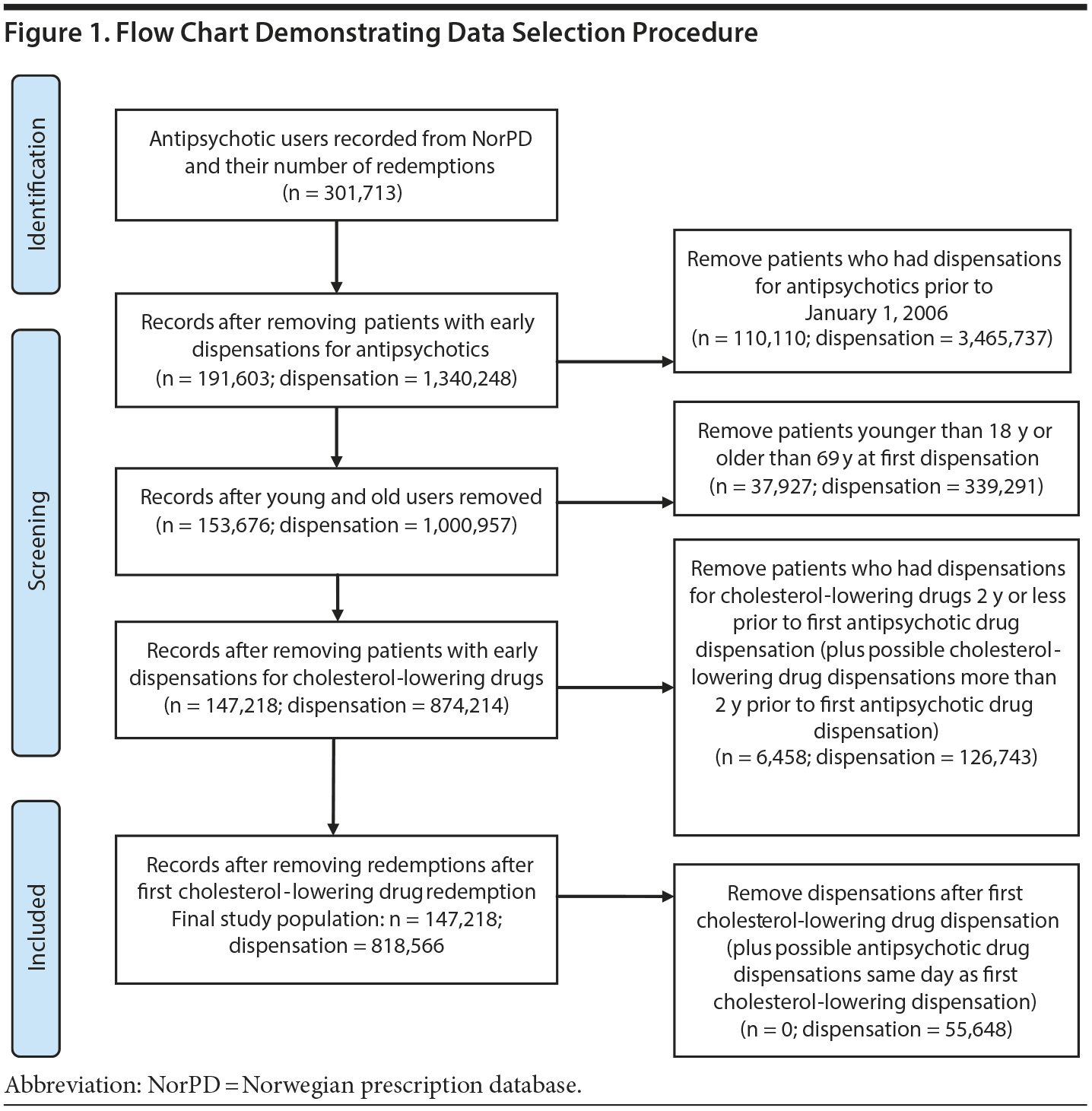
The data selection flow is presented in Figure 1. Data for the analysis were extracted from the Norwegian prescription database (NorPD) (http://www.norpd.no; accessed June 27, 2013; data can be obtained by applying to NorPD for data access) and initially comprised all adults who received at least 1 prescription for an antipsychotic drug during the years 2004 through 2012 (Figure 1). Adults encompass those from 18 to 69 years of age in the first redemption year. Information regarding the patients’ prescriptions for cholesterol-lowering drugs was also extracted. NorPD data are anonymized for researchers and thus approval by the Regional Committees for Medical and Health Research Ethics was not required (https://helseforskning.etikkom.no; accessed June 27, 2013).
Study Population
The initial group of antipsychotic users comprised 301,713 patients and included patients as described above, omitting those who died during the study period. We then defined drug-naive patients as patients with no prescription fulfilments prior to January 2006 for any of the antipsychotics included in the study (Figure 1), reducing the study population to 191,603 patients. By doing this, it is reasonable to assume that the remaining patients were new antipsychotic drug users. Patients with a previous history of redeeming prescriptions for cholesterol-lowering drugs (2 years or less prior to first antipsychotic drug redemption) were also omitted. This procedure reduced the dataset for analysis to 147,218 patients, encompassing a total of 818,566 prescriptions (Figure 1).
Antipsychotics and Cholesterol-Lowering Drugs
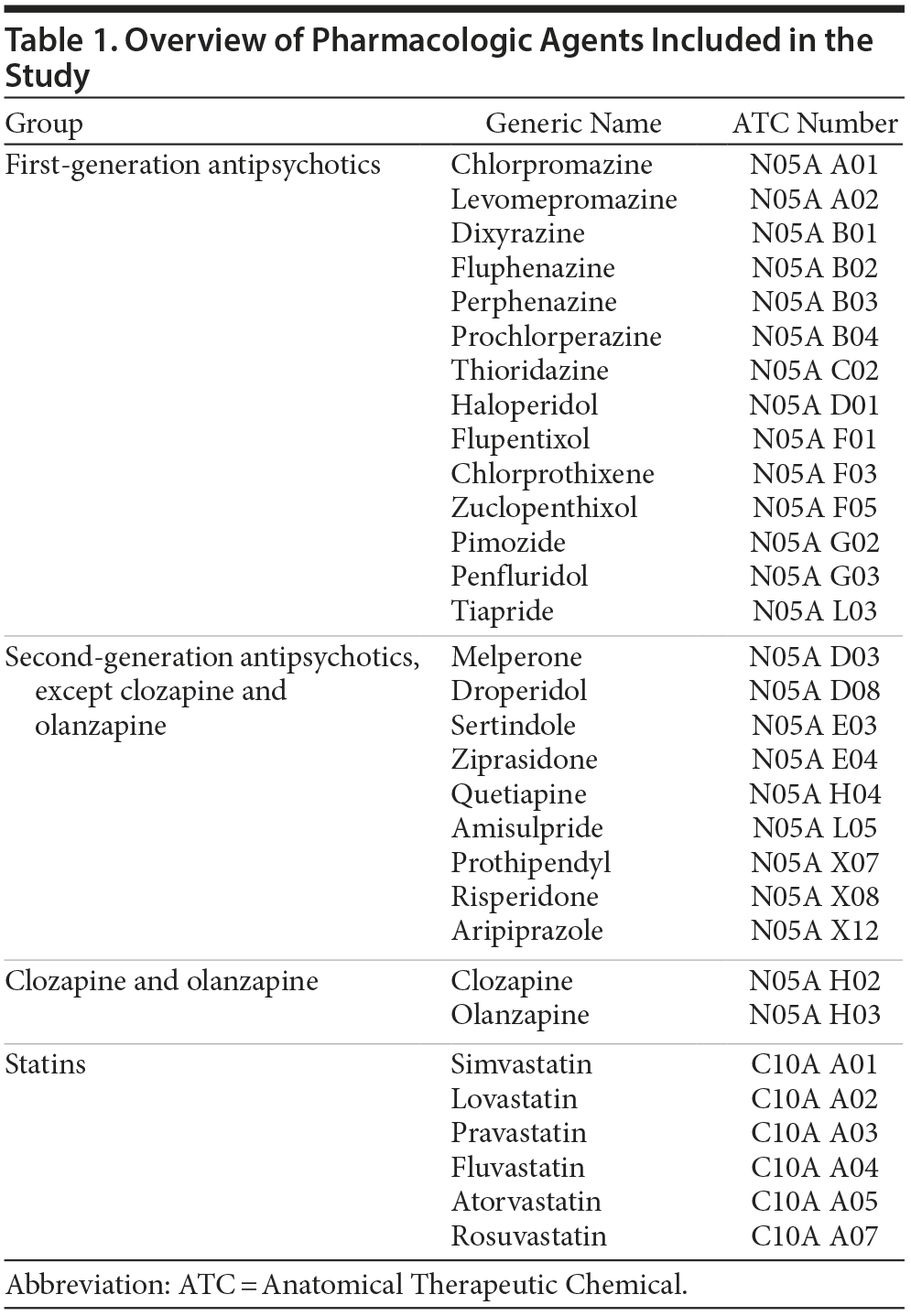
We divided the antipsychotics into 3 groups (Table 1): a first group of FGAs/typical antipsychotics (chlorpromazine, levomepromazine, dixyrazine, fluphenazine, perphenazine, prochlorperazine, thioridazine, haloperidol, flupentixol, chlorprothixene, zuclopenthixol, pimozide, penfluridol, and tiapride), a second group of SGAs/atypical antipsychotics (melperone, droperidol, sertindole, ziprasidone, quetiapine, amisulpride, prothipendyl, risperidone, and aripiprazole), and a third group consisting of the 2 SGAs olanzapine and clozapine (the olanzapine/clozapine group). The cholesterol-lowering drugs considered were all statins (Table 1): simvastatin, lovastatin, pravastatin, fluvastatin, atorvastatin, and rosuvastatin.
Patient Characteristics
Patients were characterized in terms of gender; age at first redemption; antipsychotic drug group from which they first redeemed; amount of antipsychotics redeemed, measured as numbers of defined daily doses37; number of different antipsychotic drug groups redeemed; and time from redemption of the first antipsychotic to the first redemption of a cholesterol-lowering drug (or throughout December 2012 if no redemption of a cholesterol-lowering drug).
Survival Analysis
The Kaplan-Meier method was used to initially generate survival curves using the date of the first redeemed antipsychotic drug as zero time. We conducted a survival analysis by considering a Cox proportional hazards regression model with both time-independent and time-dependent explanatory variables to examine the effect of these on the time to a first redemption for a cholesterol-lowering drug after starting antipsychotic treatment. Possible explanatory time-independent variables included gender, age, and antipsychotic drug group at first redemption, and time-dependent variables included the amount of drug redeemed and the number of different antipsychotic drug groups redeemed. The outcome (survival time) was time from the first redemption of an antipsychotic drug until the time of a first redemption for a cholesterol-lowering drug. If a patient redeemed a prescription for a cholesterol-lowering drug, this was recorded as an event, while, if a patient had no such redemption, no event was recorded and the patient was censored with the censoring time December 31, 2012. We chose a step-wise model selection approach for considering the explanatory variables’ relevance, and conducted partial likelihood ratio tests for the alternative models, applying a significance level of P < .05. Data were analyzed using R (survival package).38
RESULTS
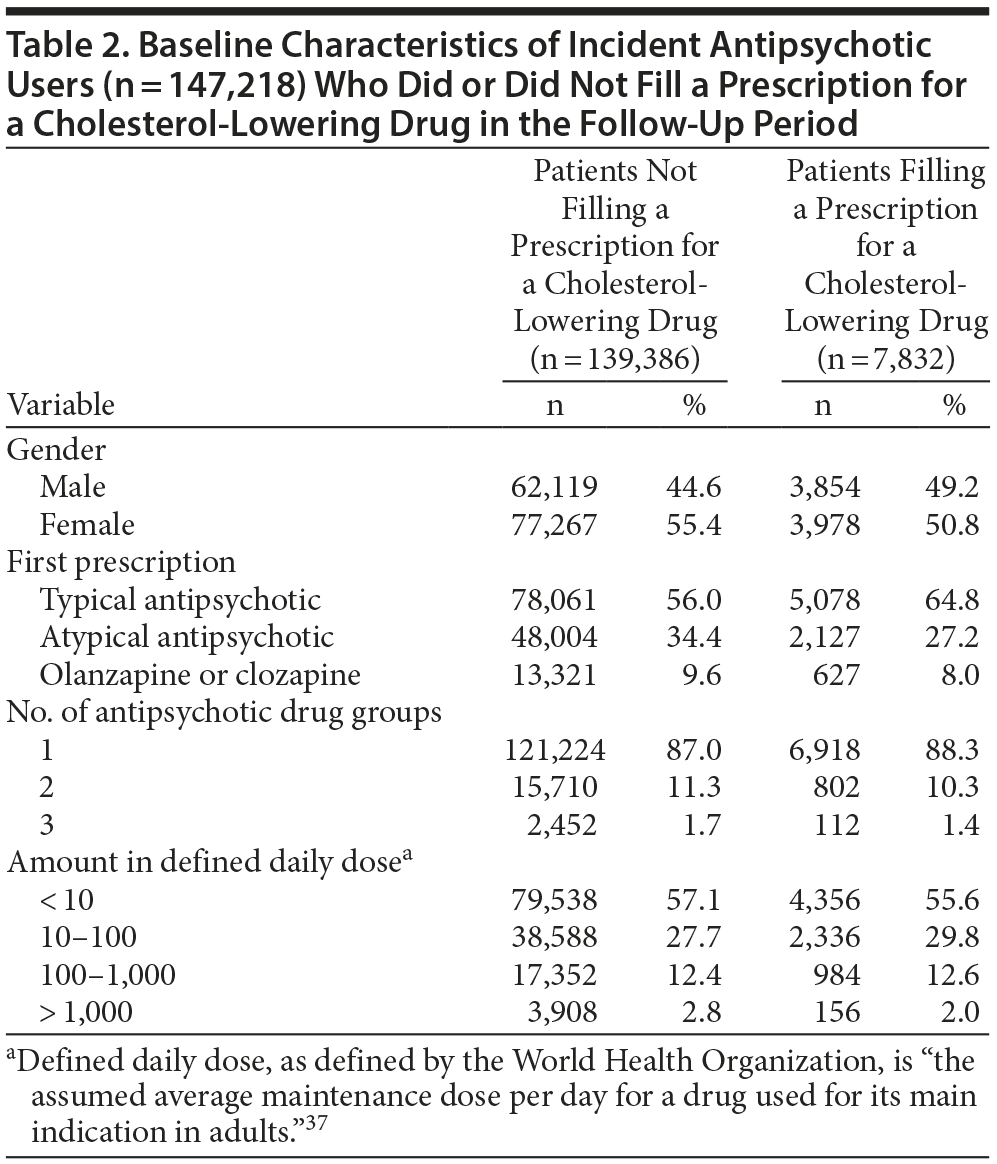
Baseline characteristics of the 147,218 patients are summarized in Table 2. There were 65,973 (44.8%) male and 81,245 (55.2%) female patients, with an mean age of 42.6 years. Of these, 83,139 (56.5%) filled a prescription for an FGA, 50,131 (34.1%) for an SGA (not including olanzapine or clozapine), while 13,948 (9.5%) filled their first prescription for clozapine or olanzapine. Most patients filled prescriptions for only 1 antipsychotic (128,142; 87.0%), while 19,076 (13.0%) filled prescriptions for at least 2 different antipsychotics.
During the follow-up period, 7,832 patients (5.3%) filled a prescription for a cholesterol-lowering drug. The mean age of patients in this group was higher than that of the general study population (54.4 years). In absolute numbers, a higher share of patients that were prescribed cholesterol-lowering agents filled a prescription for an FGA (n = 5,078; 64.8%) than for SGAs (n = 2,127; 27.2%) or clozapine/olanzapine (n = 627; 8.0%). The percentage of patients receiving more than 1 antipsychotic was similar in patients prescribed a cholesterol-lowering drug (11.7%) and in patients not prescribed cholesterol-lowering medication (13.0%) (Table 2).
Kaplan-Meier Curves
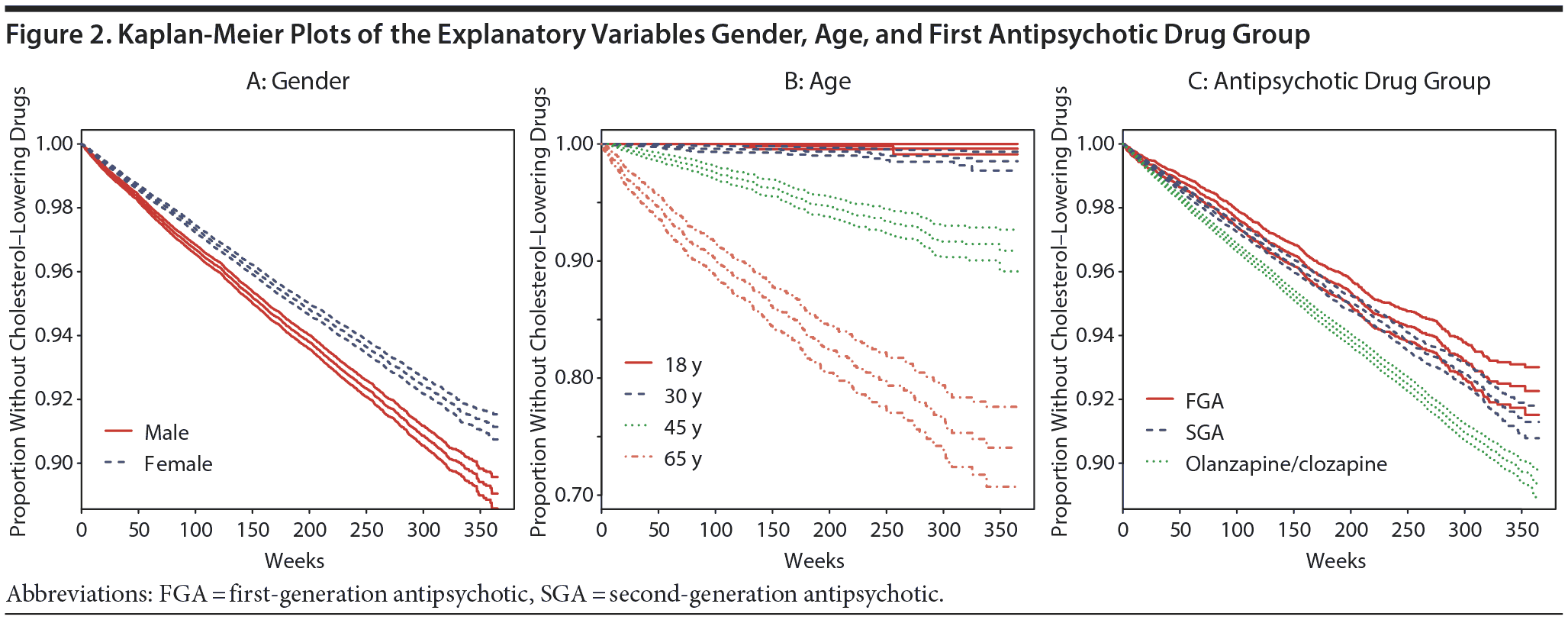
The 147,218 patients represented a total of 27,036,548 weeks (mean = 184) after filling of the first prescription for an antipsychotic drug until (1) redeeming a prescription for a cholesterol-lowering drug or (2) the end of the observation period, 938,846 weeks (mean = 120) for the 7,832 who filled a prescription for a cholesterol-lowering drug, and 26,097,703 weeks (mean = 187) for the 139,386 patients who were not prescribed a cholesterol-lowering drug. Mean time until the first redemption of a cholesterol-lowering drug was 118 weeks (median = 101) for men and 121 weeks (median = 107) for women. The Kaplan-Meier curves in Figure 2 show the differences in risk for redeeming a cholesterol-lowering drug depending upon gender, age, and the antipsychotic drug first redeemed. Male patients were at a higher risk for redeeming a cholesterol-lowering drug than female patients.
Cox Proportional Hazards Regression
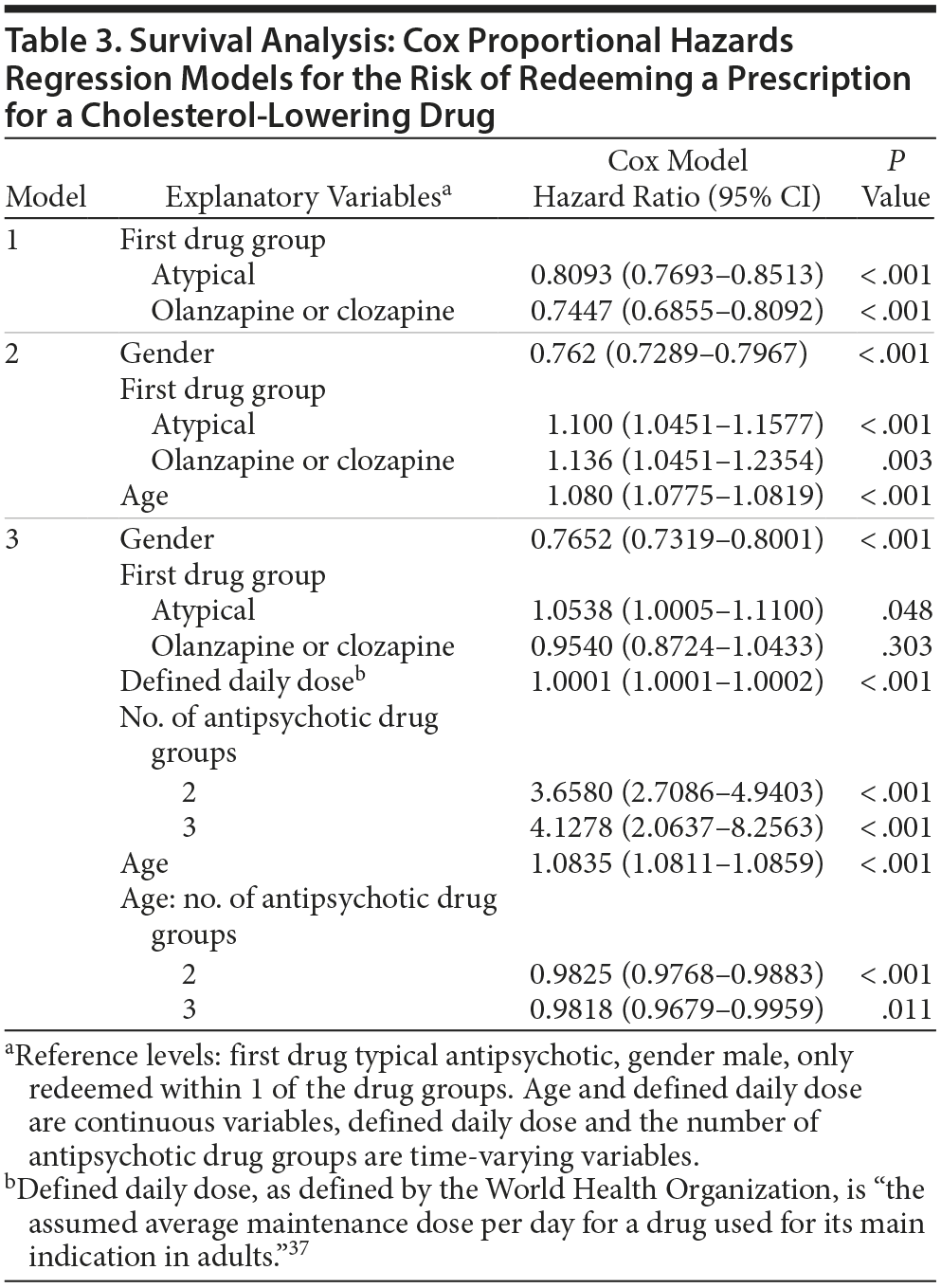
In a univariate analysis (Table 3, model 1), we found that patients initially receiving FGAs had a significantly higher risk of redeeming a prescription for a cholesterol-lowering drug compared to those who initially received SGAs or olanzapine/clozapine, 23.5% and 34.3%, respectively. Patients receiving FGAs, however, were older, and, when adjusting for gender and age (Table 3, model 2), we found that patients who initially received SGAs or olanzapine/clozapine had a slight but statistically significant increase in the risk of being prescribed a cholesterol-lowering drug compared to those initially receiving FGAs (hazard ratios [HRs] [95% CIs] = 1.100 [1.045-1.158], P < .001, and 1.136 [1.045-1.235], P = .003, for the 2 groups, respectively) (Table 3).
Using model 3, ie, adjusting for age, gender, initial antipsychotic drug group, number of different antipsychotic drug groups, amount of drug prescribed, and the interaction of age with number of different antipsychotic drug groups (model obtained after a stepwise model selection approach), a slight increase (HR = 1.0538; ie, 5.4%; P = .048) in the risk for statin redemption was maintained in patients initially redeeming SGAs other than olanzapine/clozapine compared to those initially redeeming FGAs (Table 3). There was no longer any significant difference between risk in patients receiving FGAs and clozapine/olanzapine. Notably, patients with redemptions within all 3 antipsychotic drug groups were 4.1 times more likely to have a redemption for a cholesterol-lowering drug than a patient with redemption(s) within just 1 antipsychotic drug group.
DISCUSSION
It is well known that SGAs, particularly clozapine and olanzapine, are associated with a higher risk of metabolic adverse effects than FGAs.15,33 In this observational study, we determined the proportion and characteristics of antipsychotic-naive patients starting treatment with antipsychotics who also received prescriptions for a statin during a median follow-up period of 3.5 years. During the follow-up period, 5.3% of the patients filled a prescription for a statin. In comparison, a prospective study on a Norwegian cohort showed that among individuals with low risk of cardiovascular disorder, 8% of individuals were prescribed statins during a 2-year follow-up period.39 In individuals with a high risk of cardiovascular disorder, likely comparable to that of patients treated with antipsychotics, the statin prescription rate was 34%.39 It is, however, challenging to answer the question of how many patients receiving antipsychotics “should” be treated with lipid-lowering agents. The prevalence of dyslipidemia in our study population is unknown, but it has been reported at 20%-30% in patients receiving antipsychotics in general, and within the 30%-40% range in olanzapine-/clozapine-treated patients.40,41 In light of these reports, it seems fair to claim that more patients than 5.3% in a cohort receiving antipsychotic agents could benefit from treatment with statins.
We used 3 different approaches to estimate the effect of the antipsychotic group first prescribed on the risk of being prescribed a statin, revealing that the impact of antipsychotic class was low. In order to examine whether the slight increase in incidence of cholesterol-lowering prescriptions in the SGA and clozapine/olanzapine groups was dose-related, we adjusted for the amount of prescribed drug (defined daily dose) in addition to gender and age. Indeed, high doses of antipsychotic seemed to increase the likelihood of statin prescription. Furthermore, we found that the likelihood of being prescribed a statin was increased 4-fold in patients redeeming antipsychotic agents from all 3 antipsychotic groups included. A former, relatively small study42 examining prescription redemptions and biochemical diagnosis also failed to demonstrate differential influence of antipsychotic class on redemption of lipid-lowering drugs, but we are not aware of former studies on the impact of the number or dose of different antipsychotics on statin prescription rates.
In light of the recognized propensity for SGAs, in particular clozapine and olanzapine, to induce dyslipidemia, the small differences between antipsychotic groups may seem surprising. One important reason may be the low overall prescription rate of clozapine and olanzapine in the study population (9.5%). In addition, antipsychotics with intermediate risk for metabolic adverse effects (such as risperidone and quetiapine) included in the SGA group may have masked clozapine/olanzapine effects in our multivariate model. Possibly, overadjustment for antipsychotic dose could also contribute to masked differences in statin prescription among the different antipsychotic groups.
Several limitations to the study deserve mentioning. First, the a priori grouping of atypical agents into FGAs, SGAs, and clozapine/olanzapine most likely influenced our results. Several authors have argued against the conventional FGA/SGA grouping.15,43 However, as well as facilitating comparison with other studies, our grouping largely follows risk profile, with the FGA group consisting mostly of low-risk agents, the SGA group containing mostly intermediate-risk agents, and the clozapine and olanzapine group containing the most metabolically potent agents.33 Second, selecting statin prescription as an outcome will fail to identify patients receiving other lipid-lowering agents, such as fibrates and drug combinations, ie, ezetimib + simvastatin (Anatomical Therapeutic Chemical classification code C10B A02). According to the NorPD database, however, general prescription rates of such drugs were very low in Norway during the study period. A third weakness of the study is the lack of access to data regarding serum lipid levels and lifestyle issues of patients. The actual prevalence of dyslipidemia in the patients would provide a more refined indicator of statin requirement than the prevalence data mentioned above.
In summary, our findings indicate that differences in prescription rates for adjuvant cholesterol-lowering agents in patients treated with SGAs, including clozapine and olanzapine, and FGAs, are not as large as would be expected based on the high prevalence of dyslipidemia described in patients treated with SGAs. Importantly, our data support previous reports that, in spite of published recommendations for systematic monitoring for dyslipidemic and diabetogenic effects of antipsychotic agents, such adverse effects are frequently undertreated.31,32,44-46 Issues such as patient compliance and prejudice and lack of awareness among health care providers, in addition to separation of somatic and mental health care systems, most likely contribute to neglect of somatic health in patients diagnosed with serious mental disorders.7,9,32,47,48 Future studies should combine data regarding prescription rates and the prevalence of dyslipidemia in naturalistic settings. Such data could contribute to improved general care for a vulnerable patient group.
Drug names: aripiprazole (Abilify), atorvastatin (Lipitor and others), clozapine (Clozaril, FazaClo, and others), droperidol (Inapsine and others), fluvastatin (Lescol and others), ezetimib (Liptruzet, Zetia), haloperidol (Haldol and others), lovastatin (Altoprev and others), olanzapine (Zyprexa and others), pimozide (Orap), pravastatin (Pravachol and others), prochlorperazine (Compro and others), quetiapine (Seroquel and others), risperidone (Risperdal and others), rosuvastatin (Crestor), simvastatin (Simcor, and others), ziprasidone (Geodon and others).
Author affiliations: Dr Einar Martens Research Group for Biological Psychiatry, Center for Medical Genetics and Molecular Medicine, Haukeland University Hospital, Bergen; The Norwegian Centre for Mental Disorders Research (NORMENT) and the K.G. Jebsen Centre for Psychosis Research, Department of Clinical Science, University of Bergen (Drs Skrede and Steen); The Norwegian Computing Center (Dr Tvete); Norwegian Center for Addiction Research, University of Oslo (Drs Tanum and Bramness); Diakonhjemmet Hospital, Centre for Psychopharmacology (Dr Bramness), Oslo; and Akershus University Hospital, L׸renskog (Dr Tanum), Norway.
Potential conflicts of interest: None reported.
Funding/support: This study was funded in conjunction with the Norwegian Centre for Addiction Research (SERAF) Oslo, Norway; the Research Council of Norway (NORMENT CoE grant), University of Oslo, Norway; Akershus University Hospital, L׸renskog, Norway; the K.G. Jebsen Foundation, Bergen, Norway; and Dr Einar Martens Foundation for Research on Serious Mental Disorders, Bergen, Norway.
Role of the sponsors: The sponsors did not influence collection, interpretation, or reporting of the results.
Additional information: Access to the Norwegian prescription database (NorPD) can be obtained by applying to the NorPD at http://www.norpd.no.
REFERENCES
1. Procyshyn RM, Honer WG, Wu TK, et al. Persistent antipsychotic polypharmacy and excessive dosing in the community psychiatric treatment setting: a review of medication profiles in 435 Canadian outpatients. J Clin Psychiatry. 2010;71(5):566-573. PubMed doi:10.4088/JCP.08m04912gre
2. Maglione M, Maher AR, Hu J, et al. Off-Label Use of Atypical Antipsychotics: An Update. AHRQ Comparative Effectiveness Reviews. Report No 11-EHC087-EF. http://www.effectivehealthcare.ahrq.gov/ehc/products/150/778/CER43_Off-LabelAntipsychotics_20110928.pdf. Updated September 2011. Accessed November 4, 2014.
3. Alexander GC, Gallagher SA, Mascola A, et al. Increasing off-label use of antipsychotic medications in the United States, 1995-2008. Pharmacoepidemiol Drug Saf. 2011;20(2):177-184. PubMed doi:10.1002/pds.2082
4. Brown S, Kim M, Mitchell C, et al. Twenty-five year mortality of a community cohort with schizophrenia. Br J Psychiatry. 2010;196(2):116-121. PubMed doi:10.1192/bjp.bp.109.067512
5. Laursen TM, Munk-Olsen T, Vestergaard M. Life expectancy and cardiovascular mortality in persons with schizophrenia. Curr Opin Psychiatry. 2012;25(2):83-88. PubMed doi:10.1097/YCO.0b013e32835035ca
6. McGrath J, Saha S, Chant D, et al. Schizophrenia: a concise overview of incidence, prevalence, and mortality. Epidemiol Rev. 2008;30(1):67-76. PubMed doi:10.1093/epirev/mxn001
7. Laursen TM, Munk-Olsen T, Agerbo E, et al. Somatic hospital contacts, invasive cardiac procedures, and mortality from heart disease in patients with severe mental disorder. Arch Gen Psychiatry. 2009;66(7):713-720. PubMed doi:10.1001/archgenpsychiatry.2009.61
8. Osborn DP, Levy G, Nazareth I, et al. Relative risk of cardiovascular and cancer mortality in people with severe mental illness from the United Kingdom’s General Practice Research Database. Arch Gen Psychiatry. 2007;64(2):242-249. PubMed doi:10.1001/archpsyc.64.2.242
9. Nordentoft M, Wahlbeck K, Hפllgren J, et al. Excess mortality, causes of death and life expectancy in 270,770 patients with recent onset of mental disorders in Denmark, Finland and Sweden. PLoS ONE. 2013;8(1):e55176. PubMed doi:10.1371/journal.pone.0055176
10. Foley DL, Morley KI. Systematic review of early cardiometabolic outcomes of the first treated episode of psychosis. Arch Gen Psychiatry. 2011;68(6):609-616. PubMed doi:10.1001/archgenpsychiatry.2011.2
11. Meyer JM, Koro CE. The effects of antipsychotic therapy on serum lipids: a comprehensive review. Schizophr Res. 2004;70(1):1-17. PubMed doi:10.1016/j.schres.2004.01.014
12. Newcomer JW, Meyer JM, Baker RA, et al. Changes in non-high-density lipoprotein cholesterol levels and triglyceride/high-density lipoprotein cholesterol ratios among patients randomized to aripiprazole versus olanzapine. Schizophr Res. 2008;106(2-3):300-307. PubMed doi:10.1016/j.schres.2008.09.002
13. Rummel-Kluge C, Komossa K, Schwarz S, et al. Head-to-head comparisons of metabolic side effects of second generation antipsychotics in the treatment of schizophrenia: a systematic review and meta-analysis. Schizophr Res. 2010;123(2-3):225-233. PubMed doi:10.1016/j.schres.2010.07.012
14. Daumit GL, Goff DC, Meyer JM, et al. Antipsychotic effects on estimated 10-year coronary heart disease risk in the CATIE schizophrenia study. Schizophr Res. 2008;105(1-3):175-187. PubMed doi:10.1016/j.schres.2008.07.006
15. Leucht S, Cipriani A, Spineli L, et al. Comparative efficacy and tolerability of 15 antipsychotic drugs in schizophrenia: a multiple-treatments meta-analysis. Lancet. 2013;382(9896):951-962. PubMed doi:10.1016/S0140-6736(13)60733-3
16. Sheitman BB, Bird PM, Binz W, et al. Olanzapine-induced elevation of plasma triglyceride levels. Am J Psychiatry. 1999;156(9):1471-1472. PubMed
17. Osser DN, Najarian DM, Dufresne RL. Olanzapine increases weight and serum triglyceride levels. J Clin Psychiatry. 1999;60(11):767-770. PubMed doi:10.4088/JCP.v60n1109
18. Meyer JM. A retrospective comparison of weight, lipid, and glucose changes between risperidone- and olanzapine-treated inpatients: metabolic outcomes after 1 year. J Clin Psychiatry. 2002;63(5):425-433. PubMed doi:10.4088/JCP.v63n0509
19. Patel JK, Buckley PF, Woolson S, et al; CAFE Investigators. Metabolic profiles of second-generation antipsychotics in early psychosis: findings from the CAFE study. Schizophr Res. 2009;111(1-3):9-16. PubMed doi:10.1016/j.schres.2009.03.025
20. Perez-Iglesias R, Mata I, Pelayo-Teran JM, et al. Glucose and lipid disturbances after 1 year of antipsychotic treatment in a drug-naׯve population. Schizophr Res. 2009;107(2-3):115-121. PubMed doi:10.1016/j.schres.2008.09.028
21. Lindenmayer JP, Czobor P, Volavka J, et al. Changes in glucose and cholesterol levels in patients with schizophrenia treated with typical or atypical antipsychotics. Am J Psychiatry. 2003;160(2):290-296. PubMed doi:10.1176/appi.ajp.160.2.290
22. Gaulin BD, Markowitz JS, Caley CF, et al. Clozapine-associated elevation in serum triglycerides. Am J Psychiatry. 1999;156(8):1270-1272. PubMed
23. Henderson DC, Cagliero E, Gray C, et al. Clozapine, diabetes mellitus, weight gain, and lipid abnormalities: a five-year naturalistic study. Am J Psychiatry. 2000;157(6):975-981. PubMed doi:10.1176/appi.ajp.157.6.975
24. Wu RR, Zhao JP, Liu ZN, et al. Effects of typical and atypical antipsychotics on glucose-insulin homeostasis and lipid metabolism in first-episode schizophrenia. Psychopharmacology (Berl). 2006;186(4):572-578. PubMed doi:10.1007/s00213-006-0384-5
25. Bushe CJ, McNamara D, Haley C, et al. Weight management in a cohort of Irish inpatients with serious mental illness (SMI) using a modular behavioural programme: a preliminary service evaluation. BMC Psychiatry. 2008;8(1):76. PubMed doi:10.1186/1471-244X-8-76
26. Pendlebury J, Bushe CJ, Wildgust HJ, et al. Long-term maintenance of weight loss in patients with severe mental illness through a behavioural treatment programme in the UK. Acta Psychiatr Scand. 2007;115(4):286-294. PubMed doi:10.1111/j.1600-0447.2006.00906.x
27. Maayan L, Correll CU. Management of antipsychotic-related weight gain. Expert Rev Neurother. 2010;10(7):1175-1200. PubMed doi:10.1586/ern.10.85
28. Carrizo E, Fernández V, Connell L, et al. Extended release metformin for metabolic control assistance during prolonged clozapine administration: a 14 week, double-blind, parallel group, placebo-controlled study. Schizophr Res. 2009;113(1):19-26. PubMed doi:10.1016/j.schres.2009.05.007
29. Ojala K, Repo-Tiihonen E, Tiihonen J, et al. Statins are effective in treating dyslipidemia among psychiatric patients using second-generation antipsychotic agents. J Psychopharmacol. 2008;22(1):33-38. PubMed doi:10.1177/0269881107077815
30. De Hert M, Kalnicka D, van Winkel R, et al. Treatment with rosuvastatin for severe dyslipidemia in patients with schizophrenia and schizoaffective disorder. J Clin Psychiatry. 2006;67(12):1889-1896. PubMed doi:10.4088/JCP.v67n1208
31. Larsen JT, Fagerquist M, Holdrup M, et al. Metabolic syndrome and psychiatrists’ choice of follow-up interventions in patients treated with atypical antipsychotics in Denmark and Sweden. Nord J Psychiatry. 2011;65(1):40-46. PubMed doi:10.3109/08039488.2010.486443
32. Johnsen E, Gjestad R, Kroken RA, et al. Cardiovascular risk in patients admitted for psychosis compared with findings from a population-based study. Nord J Psychiatry. 2011;65(3):192-202. PubMed doi:10.3109/08039488.2010.522729
33. De Hert M, Detraux J, van Winkel R, et al. Metabolic and cardiovascular adverse effects associated with antipsychotic drugs. Nat Rev Endocrinol. 2011;8(2):114-126. PubMed doi:10.1038/nrendo.2011.156
34. De Hert M, Vancampfort D, Correll CU, et al. Guidelines for screening and monitoring of cardiometabolic risk in schizophrenia: systematic evaluation. Br J Psychiatry. 2011;199(2):99-105. PubMed doi:10.1192/bjp.bp.110.084665
35. Thornley B, Adams C. Content and quality of 2000 controlled trials in schizophrenia over 50 years. BMJ. 1998;317(7167):1181-1184. PubMed doi:10.1136/bmj.317.7167.1181
36. Leucht S, Heres S, Hamann J, et al. Methodological issues in current antipsychotic drug trials. Schizophr Bull. 2008;34(2):275-285. PubMed doi:10.1093/schbul/sbm159
37. Definition and general considerations. WHO Collaborating Centre for Drug Statistics Methodology Web site. http://www.whocc.no/ddd/definition_and_general_considera/. Accessed November 21, 2014.
38. R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing; 2010.
39. Selmer R, Sakshaug S, Skurtveit S, et al. Statin treatment in a cohort of 20 212 men and women in Norway according to cardiovascular risk factors and level of education. Br J Clin Pharmacol. 2009;67(3):355-362. PubMed doi:10.1111/j.1365-2125.2008.03360.x
40. Birkenaes AB, Birkeland KI, Engh JA, et al. Dyslipidemia independent of body mass in antipsychotic-treated patients under real-life conditions. J Clin Psychopharmacol. 2008;28(2):132-137. PubMed doi:10.1097/JCP.0b013e318166c4f7
41. Saari K, Koponen H, Laitinen J, et al. Hyperlipidemia in persons using antipsychotic medication: a general population-based birth cohort study. J Clin Psychiatry. 2004;65(4):547-550. PubMed doi:10.4088/JCP.v65n0415
42. Lund BC, Perry PJ, Brooks JM, et al. Clozapine use in patients with schizophrenia and the risk of diabetes, hyperlipidemia, and hypertension: a claims-based approach. Arch Gen Psychiatry. 2001;58(12):1172-1176. PubMed doi:10.1001/archpsyc.58.12.1172
43. Fischer-Barnicol D, Lanquillon S, Haen E, et al; Working Group ‘ Drugs in Psychiatry’ . Typical and atypical antipsychotics—the misleading dichotomy: results from the Working Group ‘ Drugs in Psychiatry’ (AGATE). Neuropsychobiology. 2008;57(1-2):80-87. PubMed doi:10.1159/000135641
44. American Diabetes Association; American Psychiatric Association; American Association of Clinical Endocrinologists, et al. Consensus development conference on antipsychotic drugs and obesity and diabetes. J Clin Psychiatry. 2004;65(2):267-272. PubMed doi:10.4088/JCP.v65n0219
45. Lamberti JS, Olson D, Crilly JF, et al. Prevalence of the metabolic syndrome among patients receiving clozapine. Am J Psychiatry. 2006;163(7):1273-1276. PubMed doi:10.1176/appi.ajp.163.7.1273
46. Nasrallah HA, Meyer JM, Goff DC, et al. Low rates of treatment for hypertension, dyslipidemia and diabetes in schizophrenia: data from the CATIE schizophrenia trial sample at baseline. Schizophr Res. 2006;86(1-3):15-22. PubMed doi:10.1016/j.schres.2006.06.026
47. Björkenstam E, Ljung R, Burström B, et al. Quality of medical care and excess mortality in psychiatric patients—a nationwide register-based study in Sweden. BMJ Open. 2012;2(1):e000778. PubMed doi:10.1136/bmjopen-2011-000778
48. Morrato EH, Newcomer JW, Kamat S, et al. Metabolic screening after the American Diabetes Association’s consensus statement on antipsychotic drugs and diabetes. Diabetes Care. 2009;32(6):1037-1042. PubMed doi:10.2337/dc08-1720
Enjoy free PDF downloads as part of your membership!
Save
Cite
Advertisement
GAM ID: sidebar-top