ABSTRACT
Objective: The study was undertaken to determine the prevalence of subclinical and overt thyroid dysfunction as well as thyroid autoimmunity in depressed adolescents in comparison to the general pediatric population. Additionally, the relationship between parameters of thyroid function and Beck Depression Inventory-II (BDI-II) scores was examined.
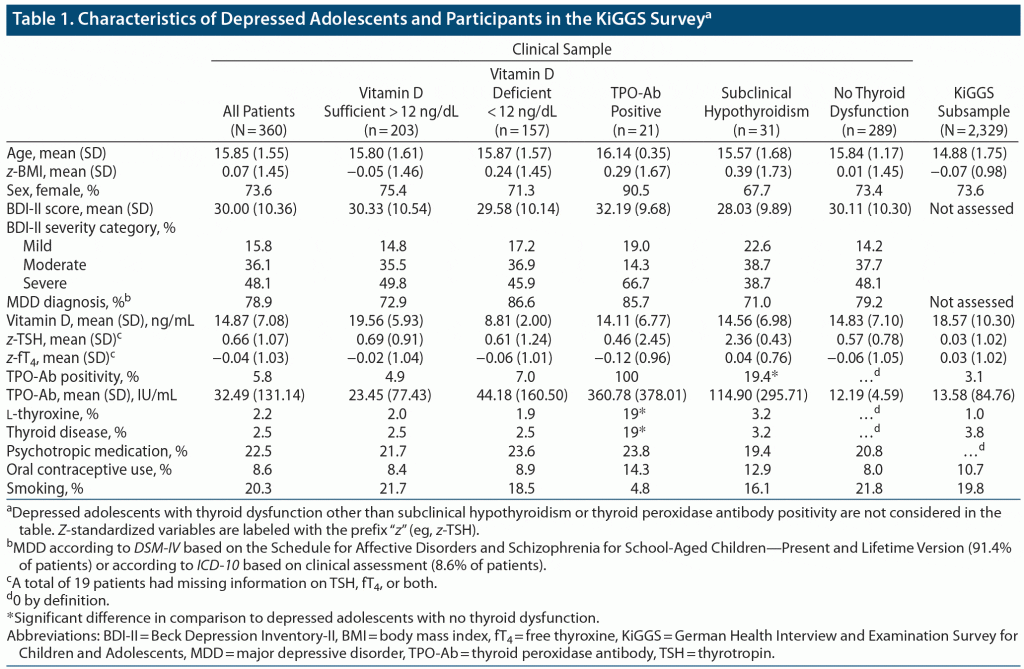
Methods: Parameters of thyroid function (thyrotropin, free thyroxine, thyroid peroxidase antibodies) and prevalence of thyroid dysfunction and autoimmunity were determined in 360 adolescents (11–19 years) with at least mild depression (BDI-II score > 13) between June 2016 and December 2019 and in a representative reference cohort without evidence of impaired mental health from a nationwide survey (German Health Interview and Examination Survey for Children and Adolescents [KiGGS], 2003–2006).
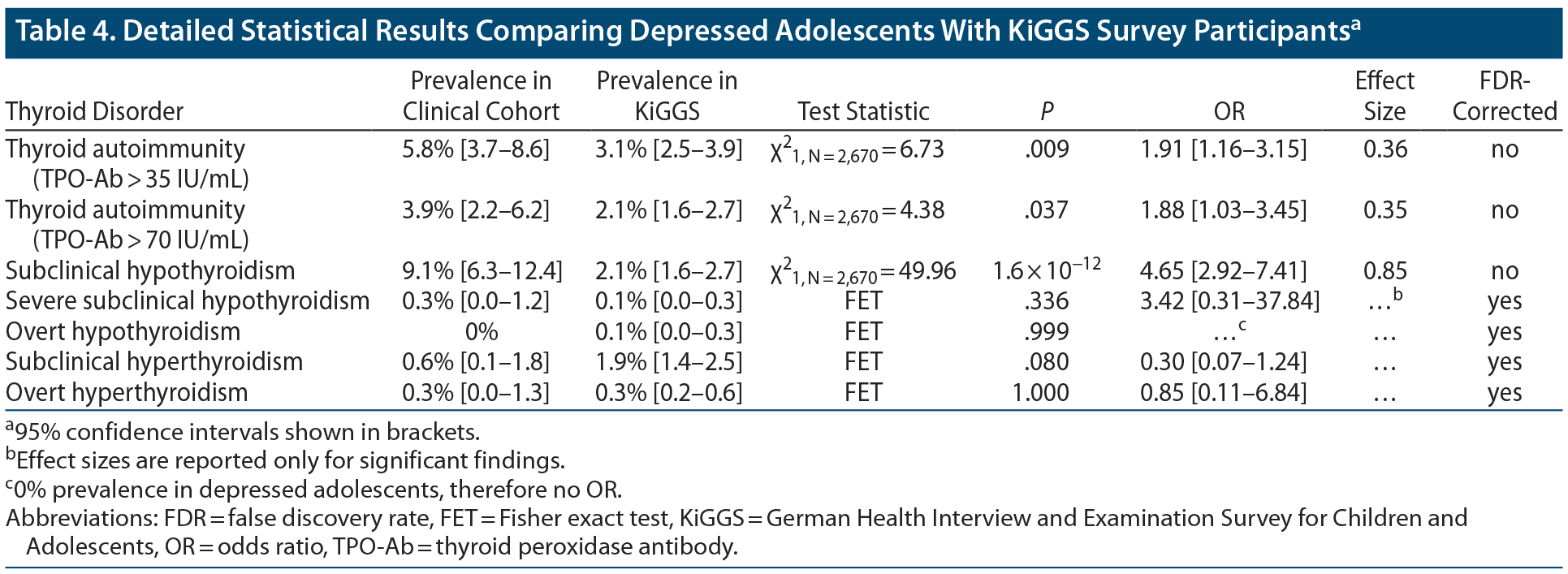
Results: There was a higher prevalence of thyroid peroxidase antibody positivity in depressed adolescents (mean ± SD BDI-II, 30.0 ± 10.4) compared to KiGGS participants (depressed adolescents: 5.8%, 95% CI [3.7–8.6]; odds ratio [OR] 1.9, P = .009, d = 0.36; KiGGS participants: 3.1%, 95% CI [2.5–3.9]). The prevalence of subclinical hypothyroidism was likewise higher in depressed adolescents (9.1%, 95% CI [6.3–12.4] vs KiGGS participants: 2.1%, 95% CI [1.6–2.7]; OR 4.7, P < .001, d = 0.85), but no other types of thyroid dysfunction had a higher prevalence. There was no significant relationship between parameters of thyroid function and BDI-II scores, as examined by multiple regression considering relevant covariates. The positive results were verified in a subsample of patients with a confirmed diagnosis of depression (N = 284).
Conclusions: The prevalence of subclinical hypothyroidism and of thyroid autoimmunity in depressed adolescents is increased. The etiology of these observations is not well understood, and further studies to examine the underlying relationship are required. Moreover, thyroid autoimmunity may constitute an additional risk factor for depression on its own.
J Clin Psychiatry 2021;82(2):20m13511
To cite: Hirtz R, Föcker M, Libuda L, et al. Increased prevalence of subclinical hypothyroidism and thyroid autoimmunity in depressed adolescents: results from a clinical cross-sectional study in comparison to the general pediatric population. J Clin Psychiatry. 2021;82(2):20m13511.
To share: https://doi.org/10.4088/JCP.20m13511
© Copyright 2021 Physicians Postgraduate Press, Inc.
aDivision of Pediatric Endocrinology and Diabetology, Department of Pediatrics II, University Hospital Essen, University of Duisburg-Essen, Essen, Germany
bDepartment of Child and Adolescent Psychiatry and Psychotherapy, University Hospital Essen, University of Duisburg-Essen, Essen, Germany
cDepartment of Child and Adolescent Psychiatry, University Hospital Münster, Münster, Germany
dDepartment of Endocrinology, Diabetes and Metabolism, Division of Laboratory Research, University Hospital Essen, University of Duisburg-Essen, Essen, Germany
eDepartment of Epidemiology and Health Monitoring, Robert Koch Institute, Berlin, Germany
fDepartment of Pediatrics, Division of Rare Diseases, St Josef-Hospital, and CeSER, Ruhr-University Bochum, Bochum, Germany
*Corresponding author: Raphael Hirtz, MD, PhD, Division of Pediatric Endocrinology and Diabetology, Department of Pediatrics II, University Hospital Essen, Hufelandstr. 55, 45147 Essen, NRW, Germany ([email protected]).
Thyroid dysfunction and thyroid autoimmunity are known to affect mental health in adults. A recent large-scale meta-analysis confirmed a 3.3-fold increased risk for depression in hypothyroidism and autoimmune thyroiditis.1,2 In contrast, information on thyroid dysfunction in children and adolescents with depression is scarce, and studies are difficult to interpret due to conflicting findings, small sample size, and sample heterogeneity.3 However, recently, Luft et al4 published results on thyroid function in about 1,000 children and adolescents diagnosed with depression. In this cohort, 6% were affected by subclinical hypothyroidism (SCHYPO), defined by thyrotropin (TSH) levels above the age-specific reference range in lieu of normal free thyroxine (fT4) values. Less than 1% displayed a biochemical constellation of overt hypothyroidism with significantly elevated TSH and reduced fT4 levels, which led the authors to conclude that screening for thyroid dysfunction is indicated only in patients who display typical signs of overt hypothyroidism.4
Hashimoto thyroiditis, an autoimmune disorder of the thyroid gland, is the most common cause of thyroid dysfunction in children and adolescents. In adults, there is increasing evidence that thyroid autoimmunity itself may pose a risk factor for impaired mental health.1,5–7 Several mechanisms have been proposed to provide a theoretical framework for the relationship between thyroid autoimmunity and mental health. For example, Hashimoto thyroiditis is supposed to affect cerebral inflammation and myelinization5,6,8 as well as the release of neurotransmitters,9 thereby influencing neurocognition independent of thyroid functioning.1,5,6,10,11
The present study was intended to examine the prevalence of thyroid autoimmunity and to replicate findings by Luft et al4 in depressed adolescents. Additionally, the prevalence of thyroid dysfunction and autoimmunity was assessed in a representative reference cohort of German adolescents without impaired mental health from a nationwide survey (German Health Interview and Examination Survey for Children and Adolescents [KiGGS]), allowing our study to provide risk estimates.
Based on recent findings of Luft et al4 and Siegmann et al,1 an increased prevalence of SCHYPO (H1), as well as thyroid autoimmunity (H2), but not overt hypothyroidism (H3), was hypothesized in depressed adolescents. No hypotheses were derived regarding the prevalence of subclinical and overt hyperthyroidism in depressed adolescents due to conflicting previous results.4,12,13
METHODS
Study Design and Participants—Clinical Sample
Data for the depressed adolescents were derived from the baseline assessments of a 2-arm parallel-group, double-blind randomized controlled trial that investigated the effect of 25-hydroxyvitamin D deficiency (≤ 12 ng/mL [equivalent to ≤ 30 nmol/L]; German Clinical Trials Register identifier: DRKS00009758; N = 217) on depressive symptoms in psychiatric inpatients or day patients treated at the Department of Child and Adolescent Psychiatry, Psychosomatics and Psychotherapy (LVR-Klinikum Essen), Essen, Germany.14,15 Additionally, data from the follow-up study Nutrition and Mental Health, an ongoing cross-sectional study focusing on the relationship between nutrition and psychiatric disorders, were utilized. Until the end of 2019, data from 143 subjects were available. Both studies were conducted in accordance with the Declaration of Helsinki and approved by the local Ethics Committee (No. 15–6363-BO).
Patients were eligible for inclusion if they were (1) aged 11 to 18.9 years and (2) at least mildly depressed with a Beck Depression Inventory-II (BDI-II) sum score > 13. Exclusion criteria were a concurrent diagnosis of severe somatic disease and/or intellectual disability (IQ < 70).
Diagnostic Instruments
Patients with a total BDI-II score > 13 were classified as depressed.16 The BDI-II is a self-reported questionnaire, including 21 items covering DSM-IV diagnostic criteria for major depressive disorder (MDD).17,18
As the BDI-II is designed as a screening tool for depression, the diagnosis of MDD was confirmed either by the semistructured interview Schedule for Affective Disorders and Schizophrenia for School-Aged Children—Present and Lifetime Version (K-SADS-PL) according to DSM-IV19 (91.4% of patients) or by a clinical assessment according to ICD-10 (8.6%) if no K-SADS-PL was performed.
Study Design and Participants—KiGGS Study
The prevalence of thyroid dysfunction and of autoimmunity in the German general adolescent population were determined based on primary data of the first wave of the KiGGS survey. Details on the study design have been described elsewhere.20 Briefly, a representative cohort of 17,641 children and adolescents aged 0 and 18 years was studied between 2003 and 2006 by the Robert Koch Institute to determine the health status of German children and adolescents.20
KiGGS participants with available information on parameters of thyroid function (TSH, fT4, and thyroid peroxidase antibody [TPO-Ab] titers) and without evidence of a psychiatric disorder were included (N = 6,479; 3,127 girls; see Supplementary Methods for details).
TPO-Ab titers in children and adolescents are age- and sex-specific,21–23 a phenomenon that is not yet considered by reference ranges for TPO-Ab assays. To allow for a meaningful comparison of prevalence figures, the largest possible random subsample of eligible KiGGS participants was selected according to the distribution of age and sex in the depressed patients by employing the complex samples procedure implemented in SPSS 25.0 (IBM; Armonk, New York) and used for all subsequent analyses (N = 2,329). In all subsequent analyses, data from depressed adolescents were pooled and handled irrespective of vitamin D status, as vitamin D sufficient (≥ 12 ng/dL) and deficient patients (< 12 ng/dL) did not differ regarding thyroid functioning and autoimmunity (for details, see Supplementary Methods, Supplementary Results, and Table 1).
Laboratory Studies
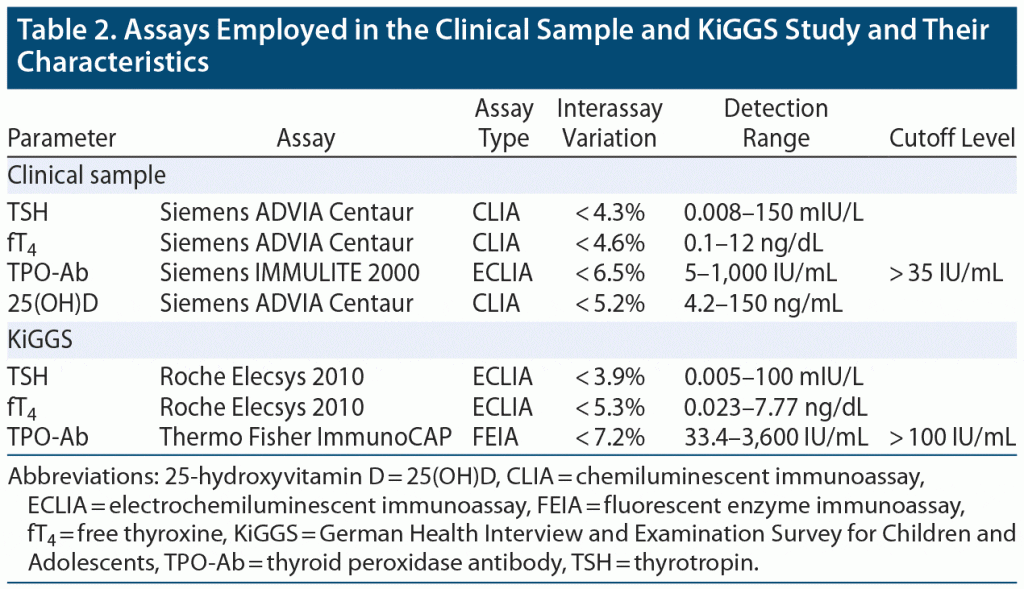
As the parameters of thyroid function were assessed using different laboratory analysis systems and assays for the 2 study groups (Table 2) and to correct for effects of age and sex, TSH and fT4 concentrations were z-transformed (see Supplementary Methods). The blood sampling procedure in depressed adolescents is described in the Supplementary Methods and elsewhere24 for the KiGGS participants.
Statistical Analysis
Overview. Data handling and statistical analyses were performed with SPSS. Results were considered significant at P < .05 when specific hypotheses were tested (H1–3). Analyses without hypotheses were tested 2-tailed and corrected for multiple comparisons controlling the false discovery rate at q < 0.05.25
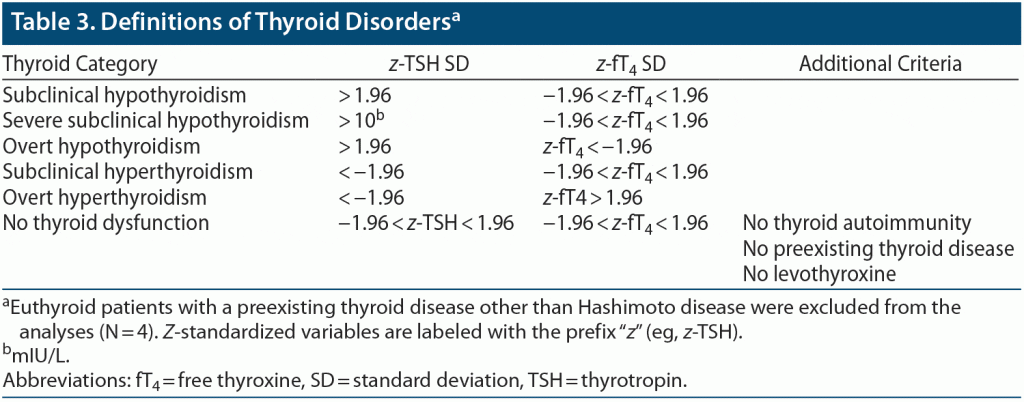
Prevalence figures. The prevalence of TPO-Ab positivity (TPO+), its prevalence in SCHYPO as well as the prevalence of SCHYPO in TPO+ were estimated in a random subsample of the KiGGS participants as outlined above and compared to depressed patients by χ2 tests of independence and Fisher’s exact test in case of cell counts < 5. Effect size was determined by the odds ratio (OR) and converted to Cohen d according to Borenstein et al.26 The same procedure of analysis was also applied to compare the prevalence of all types of thyroid dysfunction (see Table 3 for definitions) between depressed patients and KiGGS participants.
A sensitivity analysis regarding the prevalence of TPO+ was conducted applying a cutoff level twice the recommended threshold specified by the manufacturer to avoid conclusions based on spurious thyroid autoimmunity (for details, see Supplementary Methods). Results regarding the prevalence of thyroid dysfunction and autoimmunity were verified in a subsample of patients with a BDI-II score above 13 and a confirmed diagnosis of depression. Sensitivity analyses were considered exploratory and, therefore, not corrected for multiple comparisons.
In depressed adolescents, the frequency of TPO+ and presence of SCHYPO was compared between adolescents with mild (BDI-II score 14–19), moderate (20–28), and severe (29–63) depression.18 Considering the observation of an increased prevalence of SCHYPO in obesity,27 we also examined the relationship between categorical body mass index (BMI) (underweight: < 10th percentile, normal: 10th–90th percentile, overweight: 90th–97th percentile, obese: > 97th percentile) and the frequency of SCHYPO in depressed adolescents. All analyses relied on χ2 tests of independence.
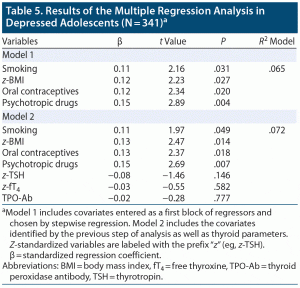
Multiple regression. In depressed adolescents and in the subsample of patients with SCHYPO and with TPO+, the relationship between BDI-II scores and z-standardized hormone levels (TSH and ft4) and TPO-Ab levels was assessed by multiple regression combining a standard hierarchical approach with a stepwise regression. Covariates with potential influence on thyroid function (vitamin D, intake of antidepressant28–30 and psychotropic drugs,31 thyroid hormone medication, use of oral contraceptives,32,33 smoking,34,35 and z-standardized BMI36) were entered as first block of regressors and assessed via stepwise regression. Then, z-standardized TSH and fT4 as well as TPO-Ab titers were entered as second block of regressors. The combined variance, which was accounted for in BDI-II scores by the independent variables (thyroid parameters), was assessed by testing the change in R2 against zero.
RESULTS
Prevalence of TPO-Ab Positivity
There was a significantly higher prevalence of TPO+ in depressed patients (5.8%) than in KiGGS participants (3.1%) considering the age and sex distribution of the clinical sample (P = .009, Table 4).
Despite a statistical trend, there was no difference regarding the prevalence of SCHYPO between depressed patients (28.6%, 95% CI [12.5–49.6]) and KiGGS participants (8.2%, 95% CI [3.4–16.0]) affected by TPO+ (χ21, N = 94 = 6.07, P = .024; OR = 4.47, 95% CI [1.26–15.79]) when correcting for multiple comparisons. However, a significantly higher percentage of depressed adolescents than KiGGS participants was affected by TPO+ as well as SCHYPO (depressed: 1.7%, 95% CI [0.7–3.3]; KiGGS: 0.3%, 95% CI [0.1–0.5]; χ21, N = 2,689 = 13.93, P = .003; OR = 6.56, 95% CI [2.11–20.52]).
The severity of depression was not associated with TPO+, as an equal fraction of patients with TPO+ was observed in the groups with mild (7.0%), moderate (2.3%), and severe (8.1%) depressive symptoms (χ22, N = 360 = 4.70, P = .096).
Prevalence of Thyroid Dysfunction
Data from 23 patients were not considered for analysis: in 19 patients, TSH, fT4, or both were not assessed, and 4 patients with a preexisting thyroid disease other than Hashimoto disease were excluded.
There was a significantly increased prevalence of SCHYPO (9.1%) in depressed patients in comparison to KiGGS participants (2.1%, P = 1.6 × 10−12). However, neither the risks for severe subclinical (depressed: 0.3%, KiGGS: 0.1%; PFisher’s exact test (FET) = .336) and overt hypothyroidism (depressed: 0.0%, KiGGS: 0.1%; PFET = .999) nor subclinical (depressed: 0.6%; KiGGS: 1.9%; PFET = .080) and overt hyperthyroidism (depressed: 0.3%; KiGGS: 0.3%; PFET = 1.000) were increased in depressed adolescents compared to KiGGS participants.
In depressed adolescents (19.4%, 95% CI [8.2–35.4]) and KiGGS participants (12.2%, 95% CI [5.1–23.3]) affected by SCHYPO, there was no significant difference in the prevalence of TPO+ (χ21, N = 80 = 0.75, P = .386).
Likewise, in thyroid autoimmunity, the severity of depression was not associated with SCHYPO, as an equal fraction of patients with SCHYPO was observed in patients with mild (13.5%), moderate (9.7%), and severe (7.3%) depression (χ22, N = 341 = 1.91, P = .384). Despite a descriptive tendency, the prevalence of SCHYPO was independent of categorical z-standardized BMI of depressed patients (underweight: 5.6%, normal weight: 8.4%, overweight: 9.8%, obese: 19.4%; χ21, N = 341 = 4.93, P = .177).
Sensitivity Analyses
Except for a slightly lower prevalence of SCHYPO (8.2%) in patients with a confirmed diagnosis of depression, all of the above-reported prevalence figures regarding thyroid dysfunction and autoimmunity were confirmed by sensitivity analyses (for details, see Supplementary Results and Supplementary Table 2).
Regression Analysis
In depressed adolescents, BDI-II scores were associated with thyroid hormone levels neither by bivariate correlation analysis (z-TSH: r353 = –0.08, P = .126; z-fT4: r339 = –0.04, P = .482) nor by multiple regression considering the covariates smoking, psychotropic drugs, oral contraceptives, and BMI (see Table 5). This also applied to TPO-Ab titers (TPO-Ab: r358 = –0.00, P = .974). Moreover, the combined parameters (z-TSH, z-fT4, TPO-Ab titers) did not account for a significant proportion of variance in BDI-II scores (R2change|thyroid F3,333 = 0.81, P = .488), which also applied to the subsample of depressed adolescents affected by SCHYPO and TPO+ (for details, see Supplementary Results and Supplementary Table 3).
DISCUSSION
In the present study of 360 depressed adolescents, an increased prevalence of thyroid autoimmunity and SCHYPO in comparison to the general pediatric population was found and confirmed by sensitivity analyses. Subsequently, these prevalence figures will be discussed in greater detail with a focus on the pathophysiology of thyroid autoimmunity in depression and their clinical implications, especially regarding the utility of screening for thyroid dysfunction in depressed adolescents.
Prevalence of TPO-Ab Positivity
The finding of an increased prevalence of TPO+ is well in line with hypothesis H2 based on results from a recent large-scale meta-analysis in adults in whom a 3.3-fold increased risk of depression in thyroid dysfunction and autoimmunity1,2 was found. Importantly, 71.4% of depressed adolescents with TPO+ of the present study were biochemically euthyroid, which may indicate that TPO+ constitutes a risk for depression, independent of thyroid functioning. Moreover, the increased prevalence of TPO+ in adolescents as well as in adults argues for a pathophysiologic relevance of thyroid autoimmunity in the etiology of depression in at least a subsample of patients regardless of age.
In addition to this epidemiologic evidence, there is accumulating evidence for a pathophysiologic framework indicating how thyroid and systemic autoimmunity may affect mental health in a significant manner.5,7,8,37 It has been shown that TPO-Abs bind to cerebellar astrocytes,8 potentially mediating a direct effect on the brain. Moreover, the presence of TPO-Abs may indicate an autoimmune involvement of the central nervous system (CNS), as CNS-Abs were detected in a significant proportion of adult patients with Hashimoto thyroiditis.6,10,38 These CNS-Abs disturb myelogenesis, induce inflammation, and potentially impair neurotransmission,5,38 which may causally contribute to the clinical phenotype of Hashimoto thyroiditis, including an increased risk of depression,1,39 suicidal tendencies,40 and reports of encephalopathy.38,41
Considering these findings in adults, further studies are needed to determine the prevalence of CNS-Abs in (depressed) children and adolescents affected by Hashimoto thyroiditis and to explore the relationship between the presence of CNS-Abs and the mental health phenotype in thyroid autoimmunity.
Thyroid Dysfunction
While neither the prevalence of overt hypothyroidism nor the prevalence of subclinical and overt hyperthyroidism was increased in comparison to the general pediatric population (for details, see Supplementary Discussion), SCHYPO was observed in 9.1% of at least mildly depressed adolescents, which is in line with hypothesis H1. These numbers are slightly higher than in the study by Luft et al,4 possibly due to differences in the study designs regarding the time of day when blood was sampled and different criteria for the diagnosis of depression (for details, see Supplementary Discussion). Thus, further studies are needed to provide a reliable estimate of the prevalence of SCHYPO in adolescent depression considering established confounders of thyroid functioning and a diagnosis of SCHYPO based on 2 independent TSH measurements. However, despite a short half-life of TSH of about 30 to 60 minutes, variations in serum TSH levels are intraindividually small42 and even smaller in SCHYPO than in euthyroidism.43 Thus, the diagnosis of SCHYPO has quite likely been assigned correctly in the present study.44
Despite slightly diverging estimates of the prevalence of SCHYPO in depressed adolescents, its prevalence is significantly increased compared to the general pediatric population. However, whether SCHYPO is causally related to depression remains unclear. A recent meta-analysis of epidemiologic studies found a significant relationship between SCHYPO and depression only in elderly adults,45 and in a meta-analysis of intervention studies comprising 2,192 adults of the general population with SCHYPO, no beneficial effects of levothyroxine treatment on health-related quality of life, including depressive symptoms, were described.46 In addition to these findings, so far, no plausible pathophysiologic mechanism by which SCHYPO would affect mental health in biochemically euthyroid individuals has been presented. In accordance with the meta-analyses, no relationship between thyroid parameters and depression in the depressed adolescents was detected in the present study, not even in those patients affected by SCHYPO. Thus, SCHYPO may be the consequence rather than the cause of an unknown mechanism either favoring depression or resulting from depression. However, the mechanisms discussed to explain the etiology of SCHYPO in depression, including increased cortisol levels47,48 as well as altered serotonin49,50 and catecholamine signaling,49,50 have not conclusively been confirmed.49–51 To summarize, the exact nature of the relationship between SCHYPO and depression remains to be determined by future studies, as discussed below.
Implications, Limitations, and Future Directions
Neither the European nor the American Thyroid Association44,52 recommends treatment for subclinical hypothyroidism in children and adolescents with a TSH < 10 mIU/L. Despite a prevalence of SCHYPO of 9.1%, a TSH level > 10 mIU/L was found in only a single patient in the present study, which corresponds to a prevalence of 0.3% of subclinical hypothyroidism in depressed adolescents in need of treatment. In other studies, in at least 70% of patients with a TSH between 5.5 and 10, subclinical hypothyroidism rarely progresses to overt hypothyroidism but resolves spontaneously within 5 years.44 However, whether this observation also applies to depressed adolescents is unknown. The risk for thyroid autoimmunity in depressed adolescents is increased almost 2-fold, and the risk for SCHYPO in TPO+ is increased more than 4-fold. This results in a significant, 6.6-fold increased risk of thyroid dysfunction in depressed adolescents with thyroid autoimmunity. These patients are at least in need of follow-up, since children and adolescents with Hashimoto thyroiditis and SCHYPO are prone to experience deterioration of thyroid functioning in comparison to children and adolescents with SCHYPO but without Hashimoto thyroiditis.44
Based on these results, clinicians should biochemically assess thyroid function in depressed adolescents and test for thyroid autoimmunity in patients with SCHYPO to identify those who are at risk for progression to overt hypothyroidism. These suggestions apply irrespective of the severity of depression at the time of diagnosis.
However, the present study does not allow causal implication of thyroid dysfunction or autoimmunity in the etiology of adolescent depression due to its cross-sectional design. Thus, caution is warranted to avoid reverse causation, especially when considering the inconclusive and partially contradictory findings in adults regarding the relationship between (subclinical) thyroid dysfunction and mental health45,46 and its hypothesized underlying mechanisms.49–51 Moreover, the results of the present study should independently be confirmed, and the diagnosis of autoimmune thyroiditis should be verified by ultrasonography of the thyroid gland even though it is likely that patients of the present study who evidenced TPO+ and SCHYPO were affected by Hashimoto thyroiditis.
Considering these limitations as well as the limitations from previous studies, longitudinal studies are needed to understand the role and natural history of thyroid dysfunction and autoimmunity in adolescent depression.50 To further explore the meaning of systemic autoimmunity in depressed adolescents with thyroid autoimmunity, future research should also address the prevalence of antibodies targeting the CNS as well as the effect of immunosuppressive therapy on (treatment-resistant) depression in depressed adolescents with CNS autoimmunity, as this approach has proven successful in patients affected by Hashimoto encephalopathy53 and may provide causal evidence of the meaning of CNS-Abs in depression.
Submitted: June 3, 2020; accepted September 21, 2020.
Published online: February 23, 2021.
Potential conflicts of interest: The authors report no financial or other relationship relevant to the subject of this article.
Funding/support: Dr Hirtz was supported by the UMEA Clinical Scientist Program by the Faculty of Medicine of the University of Duisburg-Essen and the German Research Foundation (DFG).
Role of the sponsor: The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Supplementary material: Available at Psychiatrist.com.
Clinical Points
- Preliminary evidence suggests an increased prevalence of thyroid dysfunction in adolescent depression, but its relation to thyroid autoimmunity remains to be determined.
- An increased risk of subclinical hypothyroidism but also autoimmunity in adolescent depression was found.
- Based on the risk of progression to overt hypothyroidism if thyroid autoimmunity is present, assessment of thyroid function is suggested in depressed adolescent patients.
Editor’s Note: We encourage authors to submit papers for consideration as a part of our Focus on Childhood and Adolescent Mental Health section. Please contact Karen D. Wagner, MD, PhD, at [email protected].
References (53)

- Siegmann EM, Müller HHO, Luecke C, et al. Association of depression and anxiety disorders with autoimmune thyroiditis: a systematic review and meta-analysis. JAMA Psychiatry. 2018;75(6):577–584. PubMed CrossRef NLM
- Siegmann E-M, Grömer TW. Additional data from omitted study in a meta-analysis of the association of depression and anxiety with autoimmune thyroiditis. JAMA Psychiatry. 2019;76(8):871. PubMed CrossRef NLM
- Kaufman J, Martin A, King RA, et al. Are child-, adolescent-, and adult-onset depression one and the same disorder? Biol Psychiatry. 2001;49(12):980–1001. PubMed CrossRef NLM
- Luft MJ, Aldrich SL, Poweleit E, et al. Thyroid function screening in children and adolescents with mood and anxiety disorders. J Clin Psychiatry. 2019;80(5):18m12626. PubMed CrossRef NLM
- Leyhe T, Müssig K. Cognitive and affective dysfunctions in autoimmune thyroiditis. Brain Behav Immun. 2014;41:261–266. PubMed CrossRef NLM
- Müssig K, Leyhe T, Holzmüller S, et al. Increased prevalence of antibodies to central nervous system tissue and gangliosides in Hashimoto’s thyroiditis compared to other thyroid illnesses. Psychoneuroendocrinology. 2009;34(8):1252–1256. PubMed CrossRef NLM
- Iseme RA, McEvoy M, Kelly B, et al. Autoantibodies and depression: evidence for a causal link? Neurosci Biobehav Rev. 2014;40:62–79. PubMed CrossRef NLM
- Blanchin S, Coffin C, Viader F, et al. Anti-thyroperoxidase antibodies from patients with Hashimoto’s encephalopathy bind to cerebellar astrocytes. J Neuroimmunol. 2007;192(1-2):13–20. PubMed CrossRef NLM
- Bauer M, Heinz A, Whybrow PC. Thyroid hormones, serotonin and mood: of synergy and significance in the adult brain. Mol Psychiatry. 2002;7(2):140–156. PubMed CrossRef NLM
- Matsunaga A, Yoneda M. Anti-NAE autoantibodies and clinical spectrum in Hashimoto’s encephalopathy [in Japanese]. Rinsho Byori. 2009;57(3):271–278. PubMed NLM
- Zettinig G, Asenbaum S, Fueger BJ, et al. Increased prevalence of sublinical brain perfusion abnormalities in patients with autoimmune thyroiditis: evidence of Hashimoto’s encephalitis? Clin Endocrinol (Oxf). 2003;59(5):637–643. PubMed CrossRef NLM
- Leo RJ, Batterman-Faunce JM, Pickhardt D, et al. Utility of thyroid function screening in adolescent psychiatric inpatients. J Am Acad Child Adolesc Psychiatry. 1997;36(1):103–111. PubMed CrossRef NLM
- Zader SJ, Williams E, Buryk MA. Mental health conditions and hyperthyroidism. Pediatrics. 2019;144(5):e20182874. PubMed CrossRef NLM
- Föcker M, Antel J, Grasemann C, et al. Effect of an vitamin D deficiency on depressive symptoms in child and adolescent psychiatric patients: a randomized controlled trial: study protocol. BMC Psychiatry. 2018;18(1):57. PubMed CrossRef NLM
- Libuda L, Timmesfeld N, Antel J, et al. Effect of vitamin D deficiency on depressive symptoms in child and adolescent psychiatric patients: results of a randomized controlled trial. Eur J Nutr. 2020;59(8):3415–3424. PubMed CrossRef NLM
- Beck A, Steer R, Brown G. Manual for Beck Depression Inventory–II. San Antonio, TX: Psychological Corporation; 1996.
- Straub J, Plener PL, Koelch M, et al. Agreement between self-report and clinician’s assessment in depressed adolescents, using the example of BDI-II and CDRS-R [in German]. Z Kinder Jugendpsychiatr Psychother. 2014;42(4):243–252. PubMed CrossRef NLM
- Kumar G, Steer RA, Teitelman KB, et al. Effectiveness of Beck Depression Inventory-II subscales in screening for major depressive disorders in adolescent psychiatric inpatients. Assessment. 2002;9(2):164–170. PubMed CrossRef NLM
- Kaufman J, Birmaher B, Brent D, et al. Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Version (K-SADS-PL): initial reliability and validity data. J Am Acad Child Adolesc Psychiatry. 1997;36(7):980–988. PubMed CrossRef NLM
- Kurth BM, Kamtsiuris P, Hölling H, et al. The challenge of comprehensively mapping children’s health in a nation-wide health survey: design of the German KiGGS-Study. BMC Public Health. 2008;8(1):196. PubMed CrossRef NLM
- Kabelitz M, Liesenkötter KP, Stach B, et al. The prevalence of anti-thyroid peroxidase antibodies and autoimmune thyroiditis in children and adolescents in an iodine replete area. Eur J Endocrinol. 2003;148(3):301–307. PubMed CrossRef NLM
- Kaloumenou I, Mastorakos G, Alevizaki M, et al. Thyroid autoimmunity in schoolchildren in an area with long-standing iodine sufficiency: correlation with gender, pubertal stage, and maternal thyroid autoimmunity. Thyroid. 2008;18(7):747–754. PubMed CrossRef NLM
- Taubner K, Schubert G, Pulzer F, et al. Serum concentrations of anti-thyroid peroxidase and anti-thyroglobulin antibodies in children and adolescents without apparent thyroid disorders. Clin Biochem. 2014;47(1–2):3–7. PubMed CrossRef NLM
- Dortschy R, Rosario AS, Scheidt-Nave C, et al. Bevölkerungsbezogene Verteilungswerte ausgewählter Laborparameter aus der Studie zur Gesundheit von Kindern und Jugendlichen in Deutschland (KiGGS). Berlin, Germany: Mercedes-Druck; 2009.
- Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. Journal of the Royal Statistical Society Series B. 1995;57(1):289–300.
- Converting among effect sizes. In: Borenstein M, Hedges LV, Higgins JP, et al. Introduction to Meta-Analysis. John Wiley & Sons: 2009:45–49.
- Salerno M, Capalbo D, Cerbone M, et al. Subclinical hypothyroidism in childhood: current knowledge and open issues. Nat Rev Endocrinol. 2016;12(12):734–746. PubMed CrossRef NLM
- Eker SS, Akkaya C, Sarandol A, et al. Effects of various antidepressants on serum thyroid hormone levels in patients with major depressive disorder. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32(4):955–961. PubMed CrossRef NLM
- Gambi F, De Berardis D, Sepede G, et al. Effect of mirtazapine on thyroid hormones in adult patients with major depression. Int J Immunopathol Pharmacol. 2005;18(4):737–744. PubMed CrossRef NLM
- Gitlin M, Altshuler LL, Frye MA, et al. Peripheral thyroid hormones and response to selective serotonin reuptake inhibitors. J Psychiatry Neurosci. 2004;29(5):383–386. PubMed NLM
- Bou Khalil R, Richa S. Thyroid adverse effects of psychotropic drugs: a review. Clin Neuropharmacol. 2011;34(6):248–255. PubMed CrossRef NLM
- Sänger N, Stahlberg S, Manthey T, et al. Effects of an oral contraceptive containing 30 mcg ethinyl estradiol and 2 mg dienogest on thyroid hormones and androgen parameters: conventional vs extended-cycle use. Contraception. 2008;77(6):420–425. PubMed CrossRef NLM
- Wiegratz I, Kutschera E, Lee JH, et al. Effect of four oral contraceptives on thyroid hormones, adrenal and blood pressure parameters. Contraception. 2003;67(5):361–366. PubMed CrossRef NLM
- Park S, Kim WG, Jeon MJ, et al. Serum thyroid-stimulating hormone levels and smoking status: data from the Korean National Health and Nutrition Examination Survey VI. Clin Endocrinol (Oxf). 2018;88(6):969–976. PubMed CrossRef NLM
- Wiersinga WM. Smoking and thyroid. Clin Endocrinol (Oxf). 2013;79(2):145–151. PubMed CrossRef NLM
- Thamm M, Ellert U, Thierfelder W, et al. Iodine intake in Germany: results of iodine monitoring in the German Health Interview and Examination Survey for Children and Adolescents (KiGGS) [in German]. Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz. 2007;50(5-6):744–749. PubMed CrossRef NLM
- Siriwardhane T, Krishna K, Ranganathan V, et al. Exploring systemic autoimmunity in thyroid disease subjects. J Immunol Res. 2018;2018:6895146. PubMed CrossRef NLM
- Hilberath JM, Schmidt H, Wolf GK. Steroid-responsive encephalopathy associated with autoimmune thyroiditis (SREAT): case report of reversible coma and status epilepticus in an adolescent patient and review of the literature. Eur J Pediatr. 2014;173(10):1263–1273. PubMed CrossRef NLM
- Endres D, Perlov E, Stich O, et al. Steroid responsive encephalopathy associated with autoimmune thyroiditis (SREAT) presenting as major depression. BMC Psychiatry. 2016;16(1):184. PubMed CrossRef NLM
- Shen Y, Wu F, Zhou Y, et al. Association of thyroid dysfunction with suicide attempts in first-episode and drug naïve patients with major depressive disorder. J Affect Disord. 2019;259:180–185. PubMed CrossRef NLM
- Lee J, Yu HJ, Lee J. Hashimoto encephalopathy in pediatric patients: homogeneity in clinical presentation and heterogeneity in antibody titers. Brain Dev. 2018;40(1):42–48. PubMed CrossRef NLM
- Ehrenkranz J, Bach PR, Snow GL, et al. Circadian and circannual rhythms in thyroid hormones: determining the TSH and free T4 reference intervals based upon time of day, age, and sex. Thyroid. 2015;25(8):954–961. PubMed CrossRef NLM
- Karmisholt J, Andersen S, Laurberg P. Variation in thyroid function tests in patients with stable untreated subclinical hypothyroidism. Thyroid. 2008;18(3):303–308. PubMed CrossRef NLM
- Lazarus J, Brown RS, Daumerie C, et al. 2014 European Thyroid Association guidelines for the management of subclinical hypothyroidism in pregnancy and in children. Eur Thyroid J. 2014;3(2):76–94. PubMed CrossRef NLM
- Tang R, Wang J, Yang L, et al. Subclinical hypothyroidism and depression: a systematic review and meta-analysis. Front Endocrinol (Lausanne). 2019;10:340. PubMed CrossRef NLM
- Feller M, Snel M, Moutzouri E, et al. Association of thyroid hormone therapy with quality of life and thyroid-related symptoms in patients with subclinical hypothyroidism: a systematic review and meta-analysis. JAMA. 2018;320(13):1349–1359. PubMed CrossRef NLM
- Tamada D, Onodera T, Kitamura T, et al. Hyperthyroidism due to thyroid-stimulating hormone secretion after surgery for Cushing’s syndrome: a novel cause of the syndrome of inappropriate secretion of thyroid-stimulating hormone. J Clin Endocrinol Metab. 2013;98(7):2656–2662. PubMed CrossRef NLM
- Walter KN, Corwin EJ, Ulbrecht J, et al. Elevated thyroid stimulating hormone is associated with elevated cortisol in healthy young men and women. Thyroid Res. 2012;5(1):13. PubMed CrossRef NLM
- Bahls S-C, de Carvalho GA. The relation between thyroid function and depression: a review [in Portuguese]. Br J Psychiatry. 2004;26(1):41–49. PubMed CrossRef NLM
- Hage MP, Azar ST. The link between thyroid function and depression. J Thyroid Res. 2012;2012:590648. PubMed CrossRef NLM
- Bunevicius R, Prange AJ Jr. Thyroid disease and mental disorders: cause and effect or only comorbidity? Curr Opin Psychiatry. 2010;23(4):363–368. PubMed CrossRef NLM
- Jonklaas J, Bianco AC, Bauer AJ, et al; American Thyroid Association Task Force on Thyroid Hormone Replacement. Guidelines for the treatment of hypothyroidism: prepared by the American Thyroid Association Task Force on Thyroid Hormone Replacement. Thyroid. 2014;24(12):1670–1751. PubMed CrossRef NLM
- Laurent C, Capron J, Quillerou B, et al. Steroid-responsive encephalopathy associated with autoimmune thyroiditis (SREAT): characteristics, treatment and outcome in 251 cases from the literature. Autoimmun Rev. 2016;15(12):1129–1133. PubMed CrossRef NLM
Enjoy free PDF downloads as part of your membership!
Save
Cite
Advertisement
GAM ID: sidebar-top