Objective: To analyze with a symptom-based approach the relationship between psychosis and diabetes mellitus in the general population.
Method: Nationally representative samples from the World Health Organization (WHO) World Health Survey, totaling 224,743 randomly selected adults 18 years and older from 52 countries worldwide, were interviewed to establish the presence of psychotic symptoms and diabetes mellitus. Presence of psychotic symptoms was established using questions pertaining to positive symptoms from the psychosis screening module of the Composite International Diagnostic Interview. Presence of diabetes was established with a response of “yes” to the question, “Have you ever been diagnosed with diabetes (high blood sugar)?” The World Health Survey was conducted between 2002 and 2004.
Results: An increasing number of psychotic symptoms was related to increasing likelihood of diabetes mellitus (OR = 1.27; 95% CI, 1.24-1.30). As compared to no symptoms, at least 1 psychotic symptom substantially elevated the risk (OR = 1.71; 95% CI, 1.61-1.81). In people with a lifetime diagnosis of schizophrenia or psychosis, the prevalence of diabetes was higher in those with current psychotic symptoms (7.3% vs 5.2%; OR = 1.65; 95% CI, 1.21-2.26), suggesting that the persistence of symptoms over time could play a central role. After controlling for different potential confounders, there was a clear increase in the probability of having diabetes as the number of psychotic symptoms increased. The relationship between psychotic symptoms and diabetes was tested with multiple mediation models and path analyses for categorical outcomes. Only body mass index appeared as a relevant mediator in a model with a good fit (ie, χ21 = 3.2, P = .0742; comparative fit index = 0.999).
Conclusions: Psychotic symptoms are related to increased rates of diabetes mellitus in nonclinical samples, independent of several potential confounders—including a clinical diagnosis of psychosis or schizophrenia, previous antipsychotic treatment, depression, lifestyle, and individual or country socioeconomic status. The findings highlight the worldwide relevance of the problem and the importance of identifying the specific paths of this association.
J Clin Psychiatry 2011;72(12):1592-1599
© Copyright 2011 Physicians Postgraduate Press, Inc.
Submitted: December 17, 2010; accepted March 18, 2011 (doi:10.4088/JCP.10m06801).
Corresponding author: Josש Luis Ayuso-Mateos, MD, Department of Psychiatry, Facultad de Medicina, Universidad Autónoma de Madrid, c/ Arzobispo Morcillo 4, 28029 Madrid (Spain) ([email protected]).
Increased Risk of Diabetes Mellitus
Among Persons With Psychotic Symptoms:
Results From the WHO World Health Survey
abstract
Objective: To analyze with a symptom-based approach the relationship between psychosis and diabetes mellitus in the general population.
Method: Nationally representative samples from the World Health Organization (WHO) World Health Survey, totaling 224,743 randomly selected adults 18 years and older from 52 countries worldwide, were interviewed to establish the presence of psychotic symptoms and diabetes mellitus. Presence of psychotic symptoms was established using questions pertaining to positive symptoms from the psychosis screening module of the Composite International Diagnostic Interview. Presence of diabetes was established with a response of “yes” to the question, “Have you ever been diagnosed with diabetes (high blood sugar)?” The World Health Survey was conducted between 2002 and 2004.
Results: An increasing number of psychotic symptoms was related to increasing likelihood of diabetes mellitus (OR = 1.27; 95% CI, 1.24–1.30). As compared to no symptoms, at least 1 psychotic symptom substantially elevated the risk (OR = 1.71; 95% CI, 1.61–1.81). In people with a lifetime diagnosis of schizophrenia or psychosis, the prevalence of diabetes was higher in those with current psychotic symptoms (7.3% vs 5.2%; OR = 1.65; 95% CI, 1.21–2.26), suggesting that the persistence of symptoms over time could play a central role. After controlling for different potential confounders, there was a clear increase in the probability of having diabetes as the number of psychotic symptoms increased. The relationship between psychotic symptoms and diabetes was tested with multiple mediation models and path analyses for categorical outcomes. Only body mass index appeared as a relevant mediator in a model with a good fit (ie, χ21 = 3.2, P = .0742; comparative fit index = 0.999).
Conclusions: Psychotic symptoms are related to
increased rates of diabetes mellitus in nonclinical samples, independent of several potential confounders—including a clinical diagnosis of psychosis or schizophrenia, previous antipsychotic treatment, depression, lifestyle, and individual or country socioeconomic status. The findings highlight the worldwide relevance of the problem and the importance of identifying the specific paths of this association.
J Clin Psychiatry 2011;72(12):1592–1599
© Copyright 2011 Physicians Postgraduate Press, Inc.
Submitted: December 17, 2010; accepted March 18, 2011 (doi:10.4088/JCP.10m06801).
Corresponding author: José Luis Ayuso-Mateos, MD, Department of Psychiatry, Facultad de Medicina, Universidad Autónoma de Madrid, c/ Arzobispo Morcillo 4, 28029 Madrid (Spain) ([email protected]).
Prevalence rates of diabetes mellitus in schizophrenia samples are about twice those in the general population.1–4 A review of data on diabetes prevalence in US schizophrenia samples found that the rate of lifetime diabetes was 14.9%,1 whereas in the general population (US national cross-sectional telephone survey with 184,450 adults) it was 7.3%.5 The excessive prevalence of diabetes mellitus in patients with psychosis compared with the general population remains even after controlling for other potential confounding variables.3
However, the basis of the relationship between psychosis and diabetes mellitus is still controversial. Three main paths for this connection may be proposed. First, diabetes mellitus and psychosis share several lifestyle and demographic risk conditions, including race and ethnicity, age, sedentary lifestyle, and obesity.6 Second, there is growing evidence that antipsychotic medications, especially some second-generation antipsychotics, are related to the onset or exacerbation of diabetes mellitus.4,7–9 Third, a still unknown and controversial physiopathologic link, which could presumably be based on genetics, inflammatory mechanisms, immunology, and/or
metabolism, may perhaps underlie the relationship between psychosis and diabetes mellitus.4,7,10
Studies in subjects with psychosis not exposed to antipsychotic medication have shown inconclusive results. Data from schizophrenia patients from the pre-antipsychotic era already showed that the prevalence of diabetes mellitus or glucose intolerance was higher in patients than in controls.11 On the other hand, recent studies assessing metabolic abnormalities (including glucose intolerance) in antipsychotic drug–naive samples have reported inconsistent results. Some studies found no differences between patients and controls,10,12,13 while other studies found that drug-naive patients were more likely to have diabetes mellitus or glucose intolerance.14,15 Most of these studies, however, used small samples.
The relationship between diabetes mellitus and schizophrenia still raises major questions that need to be addressed. It is unclear whether diabetes mellitus is correlated with the presence of psychotic symptoms, independently of any specific diagnosis. The boundaries of diagnostic categories of schizophrenia and other psychoses are now the subject of heated debate.16 Recent epidemiologic studies show that 3% of the general population have a psychotic disorder (including both affective and nonaffective psychoses).17 However, in population-based studies that focus on the prevalence of psychotic symptoms, estimates are significantly higher, ranging from 4%18 to 17.5%19 or, in a recent worldwide cross-national study,20 from 0.7% to 45.8% for the presence of at least 1 psychotic symptom. Therefore, the study of the relationship between diabetes mellitus and psychosis would benefit from an approach based on psychotic symptoms.
We analyzed data from the worldwide cross-national World Health Organization (WHO) World Health Survey,21 conducted between 2002 and 2004, to study the relationship between psychosis and diabetes mellitus using a symptom-based approach.
METHOD
Sample
Individuals from 52 countries from the World Health Survey who completed questions about diabetes and psychotic symptoms were included in the analysis. All samples were nationally representative and probabilistically selected and weighted. Countries were drawn from all regions of the world and different levels of epidemiologic and economic development, with 18 countries from the African region, 13 countries from the European region, 7 countries from the Americas region, 5 countries from the Western Pacific region, 5 countries from the South-East Asia region, and 4 countries from the Eastern Mediterranean region. Fifteen countries were classified in the high or upper-middle economic levels, according to the World Bank,22 with 37 in the lower-mid or low level. The individual global response rate was 98.5%. All samples were drawn from a current national frame using a multistage cluster design so as to allow each household and individual respondent to be assigned a known nonzero probability of selection. The sampling guidelines and summary descriptions of the sampling procedures for each site are available from the World Health Survey Web site.23 Post-stratification corrections were made to the weights to adjust for the population distribution obtained from the United Nations Statistics Division and for nonresponse.24
Informed consent was obtained from all respondents, and the study was approved by the ethical review committees at each site. The final sample comprised 224,743 subjects. All interviews were conducted by specifically trained interviewers. A standard procedure for the training and quality control was implemented at all sites and supervised periodically, as per the specified guidelines.21
Measures
All respondents were interviewed using the standardized World Health Survey instrument from the WHO.23 The interview collected data on health status; sociodemographic characteristics; weight and height; consumption of alcohol and tobacco; physical activity; fruit and vegetable intake; household economic status based on a list of permanent income indicators; functioning, health status, and quality of life; depressive symptoms; lifetime diagnosis of schizophrenia or psychosis; lifetime treatment for schizophrenia and psychotic symptoms and treatment during the last 12 months; and lifetime diagnosis of diabetes. A diagnosis of depression was established from questions of the World Health Survey version of the Composite International Diagnostic Interview (CIDI)25 using an algorithm in accordance with the ICD-10-DCR,26 as described elsewhere.27 Body mass
index (BMI) was coded using 3 dummy variables, with normal weight (18.5–25.0) as reference: underweight (< 18.5), overweight (25–30), and obesity (> 30). Alcohol consumption was coded using 3 dummy variables, with lifetime abstainers being the reference category, as classified in previous studies using the same assessment instrument28: occasional drinkers (those who consumed at least 1 unit during the last week, but less than a total of 15 units or not more than 4 units on 1 occasion); occasional heavy drinkers (those who consumed a total of 15 or more units in the previous week, but no more than 4 units on 1 occasion); and heavy drinkers (those who consumed 5 or more units on at least 1 occasion). Fruit and vegetables intake was dichotomized according to daily consumption: 0 = less than 5 times per day, 1 = 5 or more times. Smoking was dichotomized as not currently smoking any type of tobacco versus currently smoking tobacco daily. Participants were asked about the daily time spent in mild (walking), moderate, or vigorous physical activity. Subjects were then classified into 2 groups of physical activity: inadequate and adequate, based on a standard definition.29
Assessment of Psychotic Symptoms
Individual questions based on the World Health Survey version of the CIDI 3.025 were included to assess the presence of psychotic symptoms, including delusional mood, delusions of reference and persecution, delusions of control, and hallucinations, over the past 12 months. Thus, only questions from the psychosis screening module of the CIDI pertaining to positive psychotic symptoms, and not the full module, were included in the interview. The response format was dichotomous (ie, “yes” or “no”). The question on visual hallucinations specifically excluded symptoms related to substance use– or sleep-related states.
Dichotomous questions (yes/no) about lifetime diagnosis of schizophrenia or psychosis, and whether the person had ever been treated for it, were also included. The psychosis module of the CIDI has demonstrated high concordance with clinician ratings,30,31 although the goal of our study was not to detect clinical psychosis among respondents, but psychotic symptoms as present in the general population.
Assessment of Diabetes Mellitus
Responders were regarded as having diabetes mellitus if they responded “yes” to the question, “Have you ever been diagnosed with diabetes (high blood sugar)?” Type of diabetes was not assessed. Lifetime treatment and diabetes mellitus medications over the previous 2 weeks were also assessed.
Statistical Analysis
Weighted and age- and sex-standardized prevalence estimates were calculated for diabetes mellitus in persons with different numbers of psychotic symptoms and for persons with and without previous diagnoses of schizophrenia, with and without current symptoms. All of these estimates were calculated using post-stratified probability weights. Age and sex standardizations used the WHO’s World Standard Population for age32 and United Nations Statistics Division data for sex ratio.33 Differences in proportions across groups were compared with paired tests, adjusting the probability level for controlling for familywise type I error. A binary logistic regression analysis was then carried out, taking diabetes mellitus as the dependent variable and a number of psychotic symptoms as independent variables and controlling for different potential confounders coded as explained above: demographics, income level, work status, BMI, alcohol and tobacco consumption, physical activity, fruit and vegetables intake, diagnosis of depression, lifetime diagnosis of schizophrenia or psychosis, lifetime treatment for schizophrenia or psychosis, and countries as 51 dummy variables. All analyses were carried out with STATA, version 11.0.34 Data were missing for between 9.9% (previous diagnosis of schizophrenia) and 12.6% (delusions of reference and persecution) of respondents for the different variables included in the analyses, and 12.7% of the respondents had missing data for both diabetes mellitus diagnosis and at least 1 of the psychotic symptoms. STATA’s module for missing data based on the ICE program35 was used for performing multiple imputation procedures (10 additional samples) in order to estimate the values of these individuals in the logistic
regression analysis.
Next, a mediation model was tested to determine whether different variables related to lifestyle (BMI, activity, tobacco and alcohol consumption, or diet) accounted for the relationship between psychotic symptoms and diabetes. If complete mediation was not found, estimation of the strength of the indirect effects through the Sobel test36 was performed. Psychotic symptoms were dichotomized (0 vs at least 1 symptom) for this analysis in order to simplify the pattern of relationships and given that the presence of at least 1 symptom had proved to be a good indicator of severity.20
Finally, to examine the pattern of associations among the variables, path analysis using the weighted least squares means and variance adjusted estimator was employed to test the fit of different models. From the simple model of a
direct relationship between psychotic symptoms and diabetes, potential mediators were added one by one according to the size of their mediation effects (BMI dummies were added in 1 step), and goodness-of-fit indices were assessed
according to the usual recommendations.37 Mplus version 5.2138 was used for these analyses.
RESULTS
Relationship Between
Psychotic Symptoms and Diabetes
Prevalence estimates of diabetes mellitus (adjusting for sampling weights and standardizing for age and sex) across persons with different numbers of psychotic symptoms and persons with at least 1 symptom are shown in Table 1. The pooled age- and sex-standardized prevalence of diabetes was 3.63% (95% CI, 3.41%–3.85%), whereas the crude rate of diabetes was 3.25%. The prevalence rates widely varied among countries, from 10.90% (95% CI, 8.92%–12.88%) in South Africa to 0.21% (95% CI, 0.07%–0.36%) in Malawi (specific figures available from the corresponding author upon request).
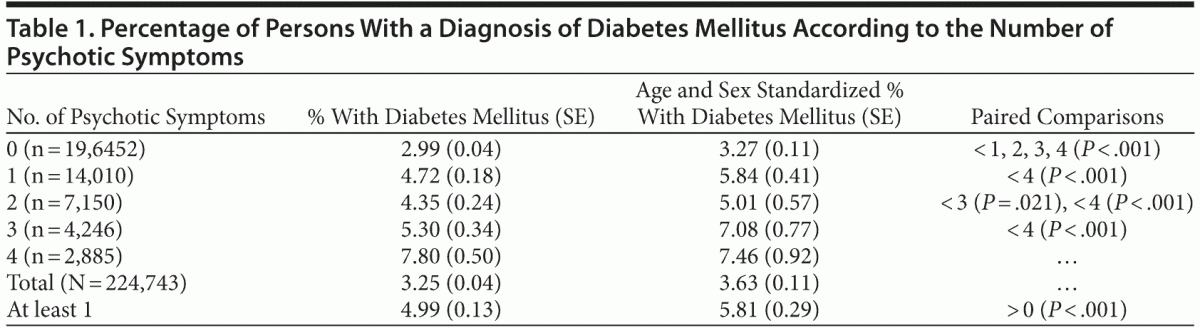
There was a statistically significant association of the overall number of symptoms with the diagnosis of diabetes (OR = 1.27, SE = 0.02; P < .001; 95% CI, 1.24–1.30). The odds ratio for at least 1 symptom clearly indicated a higher probability of diabetes in that group compared with the absence of symptoms (OR = 1.71, SE = 0.05; P < .001; 95% CI, 1.61–1.81). The linear relationship of an increased probability of diabetes mellitus with a higher number of psychotic symptoms is graphically presented in Figure 1. Paired comparisons indicated that the prevalence of diabetes was lower in persons without psychotic symptoms compared with each group with different numbers of symptoms (P < .001 in all cases). Likewise, the prevalence of diabetes was higher in persons with 4 symptoms compared with those who had fewer symptoms. Persons with 3 symptoms had a higher prevalence of diabetes mellitus than persons with 1 symptom and an only marginally higher prevalence than persons with 2 symptoms (P = .021), but the finding lacked significance after adjusting the probability level for multiple comparisons (P = .05/10 = .005).
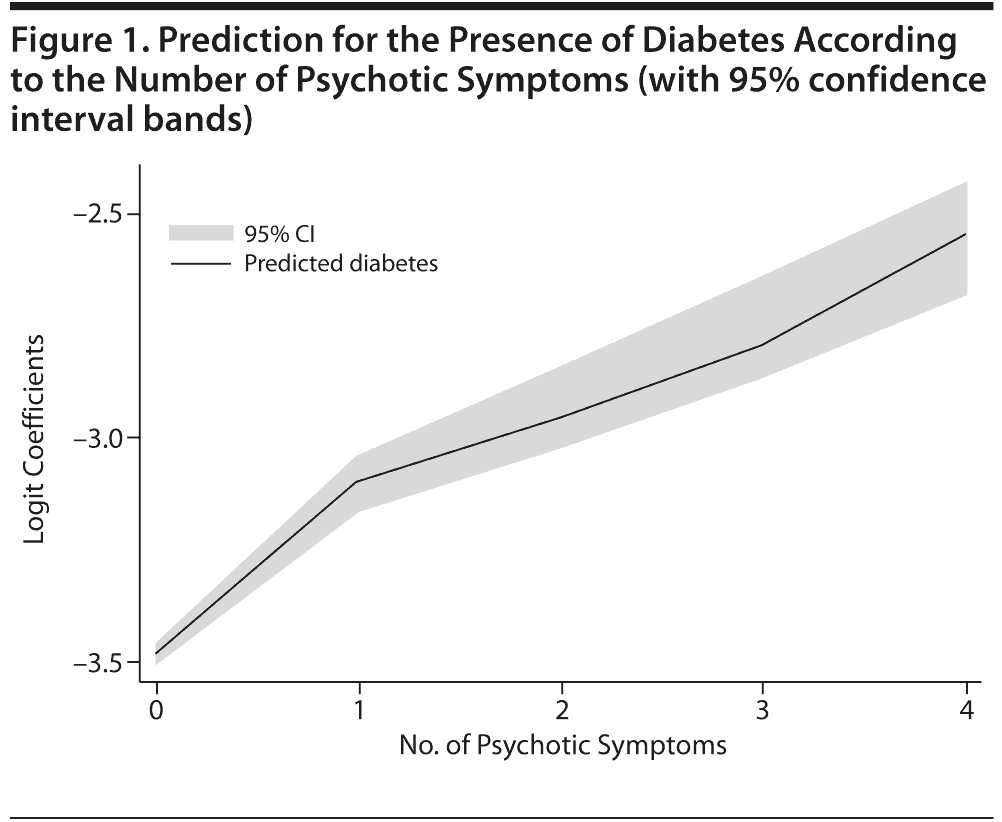
The binary logistic regression analysis with diabetes mellitus as the dependent variable, and controlling for different potential confounders, showed that each of the different individual numbers of symptoms was statistically significant in comparison to the absence of symptoms (Table 2). In paired post hoc comparisons (with P level adjusted for multiple comparisons to .05/6 = .0083), the ORs for the group with 4 symptoms were higher than for the groups with 2 symptoms (χ21 = 26.9, P < .001) and 1 symptom (χ21 = 18.2, P < .001), without differences for the group with 3 symptoms (χ21 = 4.8, P = .028). The group with 3 symptoms also had a significantly higher OR than the group with 2 symptoms (χ21 = 8.1, P = .005), with no differences between the rest of the groups.
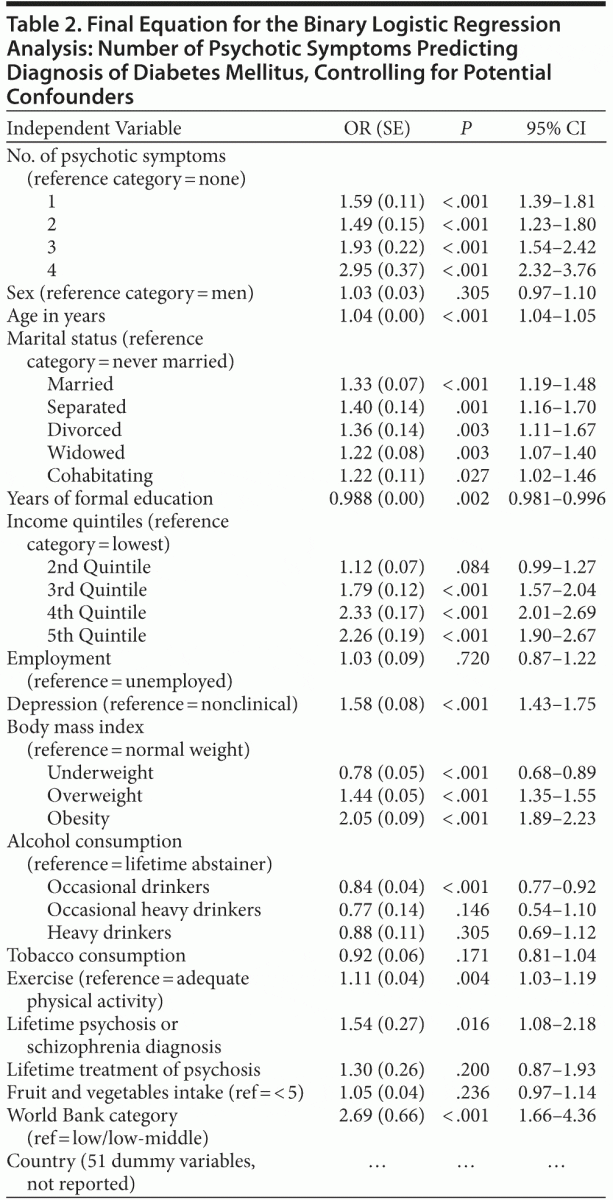
Known risk and protective factors for diabetes mellitus were related to a significantly augmented or reduced probability of having the disease, respectively. Consumption of alcohol was negatively related to the probability of diabetes mellitus, but only for an occasional drinking pattern compared with lifetime abstainers, without statistically significant effects for an occasional heavy or heavy pattern of consumption. On the other hand, a diagnosis of depression, low levels of physical activity, and older age were positively related to the probability of diabetes mellitus; every marital status compared with never married was positively related to diabetes mellitus; income level was also positively related to diabetes mellitus with a progressive and linear increase in the effect with increases in income; and the World Bank category classification of the country indicated a significantly increased probability of diabetes mellitus in high-middle or high income level countries. BMI had also a significant relation with diabetes mellitus: taking normal weight as reference, being underweight was negatively related to the probability of diabetes mellitus, whereas overweight and obesity were positively related. Finally, previous diagnosis of psychosis, but not previous treatment of schizophrenia or psychosis (although information about specific drugs used was not available), was positively related to diabetes mellitus. There
was no significant effect for fruit and vegetables intake, tobacco consumption, sex, education level, or work status. Low rates of comorbidity between psychotic symptoms and diabetes do not allow making specific comparisons in specific countries, and the country is thus considered as a potential confounder in the analyses. These results are summarized in Table 2. This pattern of results was also checked under more restrictive conditions for establishing the presence of diabetes mellitus: additional logistic regression analyses were performed including only persons with lifetime self-reported diabetes mellitus who had been treated for that condition or who had taken medication for diabetes in the last 2 weeks. Results were nearly identical to those reported here (specific results available upon request from the corresponding author).
Diagnosis of Psychosis and Diabetes
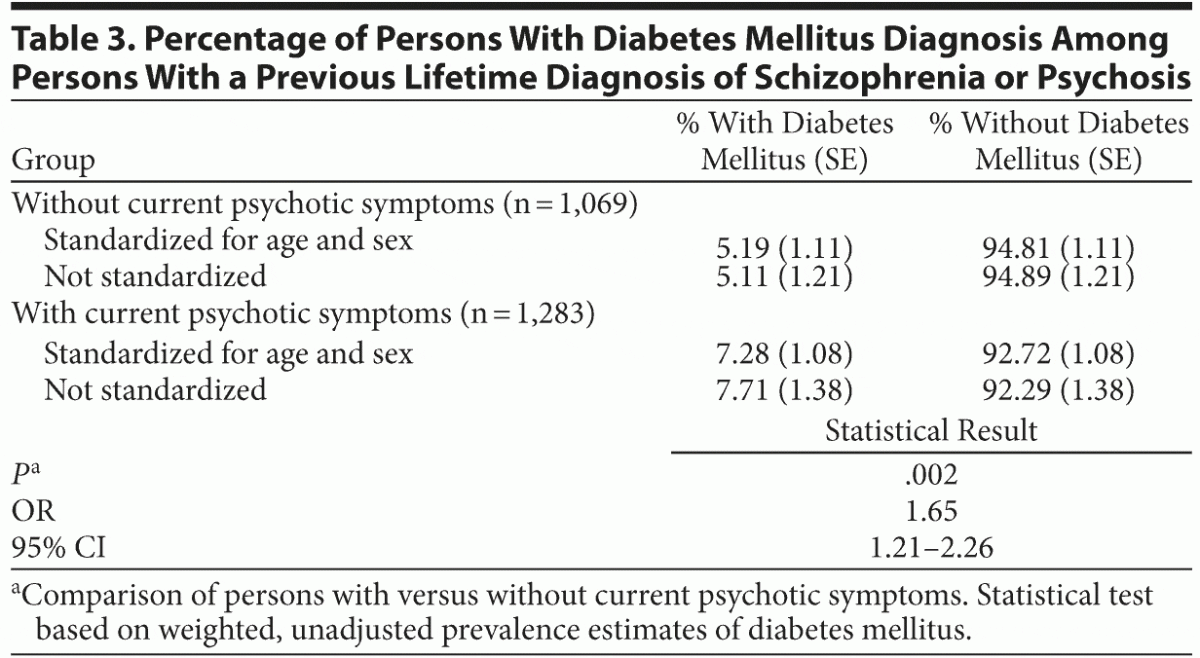
In separate analyses of persons with a lifetime diagnosis of schizophrenia or psychosis, a significantly higher percentage of people had diabetes mellitus in the subsample with current psychotic symptoms compared with the subsample without current symptoms. These results are presented in Table 3.
Mediational and Path Analyses
The direct effect of psychotic symptoms on diabetes was positive and significant (ie,
greater number of symptoms was related to higher probability of diabetes mellitus; B = 0.235, SE = 0.014, P < .001). All of the potential mediators had a statistically significant relationship both with psychotic symptoms and with the presence of diabetes. When they were added one by one in models of simple mediation, the mediation effects were low; ie, the total mediation effect for smoking according to the Sobel test accounted for 1.56% (z = 7.5, P < .001), and the maximum effect was for BMI: total effect mediated was 5.49%. When all potential mediators were included together in a multiple mediation model, only 6.79% of the effect of psychotic symptoms on diabetes was mediated by these factors.
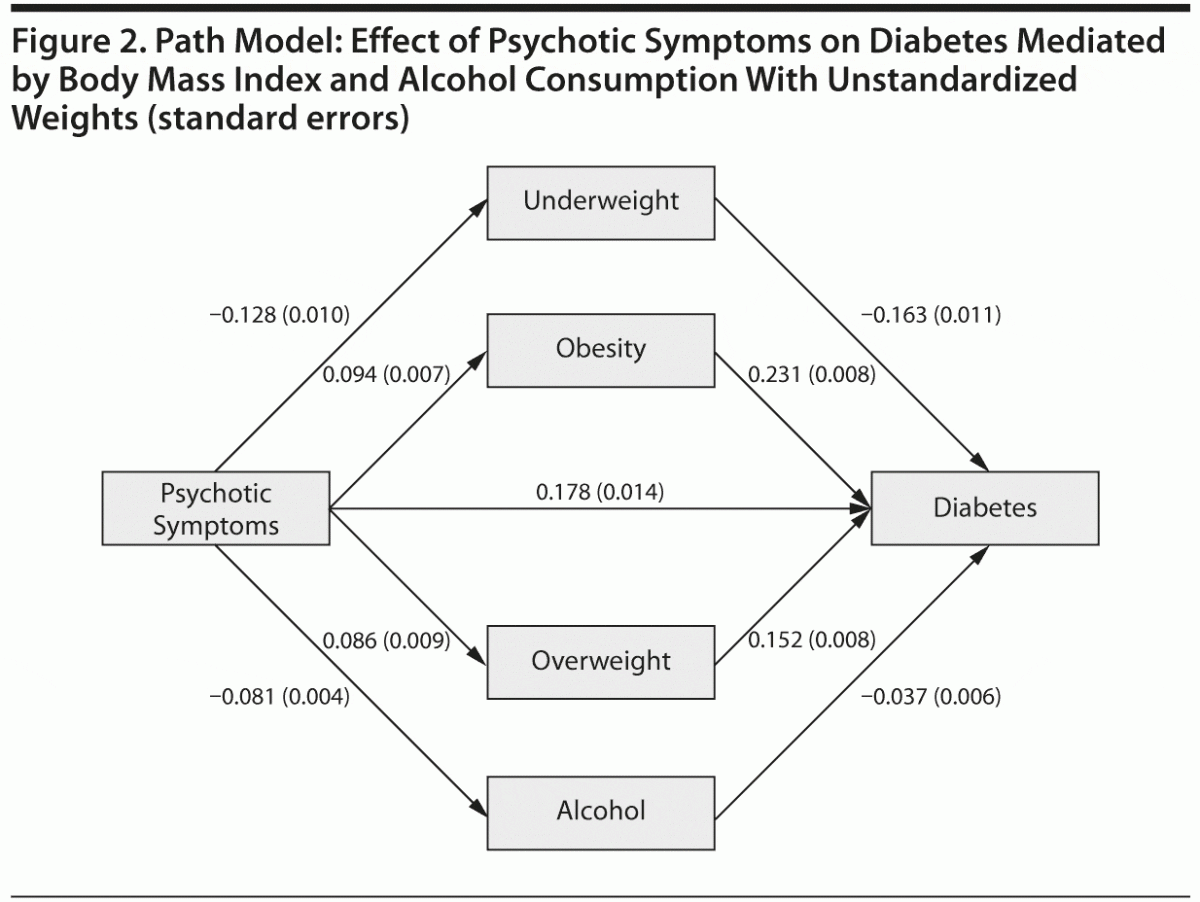
The relationship between psychotic symptoms and diabetes was modeled through path analysis. Mediators were included one by one according to the sizes of their effects in the previous multiple mediation model. Thus, the 3 BMI dummy variables (normal weight as reference) were first included as mediators between psychotic symptoms and diabetes mellitus. This model demonstrated good fit indices (ie, χ21 = 3.2, P = .0742; comparative fit index [CFI] = 0.999; Tucker-Lewis index [TLI] = 0.990; root mean square error of approximation [RMSEA] = 0.003, weighted root mean square residual [WRMR] = 0.826), with a negative regression coefficient for underweight (–0.163, P < .001) and positive coefficients for overweight (0.152, P < .001) and obesity (0.231, P < .001). Modification indices did not suggest relevant sources of misspecification. When additional variables were added to the model in a second step, the goodness-of-fit indices were clearly not satisfactory; only the inclusion of alcohol consumption offered indices that can be considered as acceptable (χ23 = 63.1, P < .001; CFI = 0.970; TLI = 0.889; RMSEA = 0.008, WRMR = 2.452). This model with the standardized weights is graphically represented in Figure 2.
DISCUSSION
The results show that prevalence of diabetes mellitus was related to the presence of psychotic symptoms, independent of a self-reported lifetime diagnosis of schizophrenia or psychosis. This finding supports the relevance of the symptom-based approach. Among the strengths of the present study are that it was conducted across a range of countries spread globally, including those with a wide range of differences in their levels of economic development, lifestyle, and the availability of treatment for psychotic symptoms and diabetes. Moreover, most previous studies on the relationship between schizophrenia and diabetes mellitus have focused on clinical samples of persons diagnosed with schizophrenia seeking treatment or in contact with clinical settings, and most of these studies have been carried out in Western countries where treatment coverage for schizophrenia is high. The relationship between psychosis and diabetes mellitus in the general population has been understudied; the present work is, to our knowledge, the first epidemiologic study of this subject in a representative sample of the general population on such a scale.
Our results provide evidence for a direct relationship between diabetes mellitus and psychotic symptoms. It is clear that the prevalence of diabetes mellitus increases as the number of reported psychotic symptoms rises; for
instance, diabetes mellitus prevalence for people reporting
4 symptoms is 2.6 times higher than for people not reporting any symptoms. Even a single psychotic symptom is clearly related to higher rates of diabetes mellitus. This is compatible with the idea of a continuum of psychotic symptoms19 and with the recent finding that even minimal presentations of psychotic symptoms in nonclinical general populations have a potential impact on health and functioning in daily life.20
The relationship between diabetes mellitus and psychotic symptoms depends on the current presence of symptoms and not on a previous diagnosis of psychosis or schizophrenia: in the subsample of people with a previous diagnosis, for those who reported current symptoms compared with those who did not, the prevalence of diabetes mellitus was clearly higher. This finding suggests that in the complex relationship between diabetes mellitus and psychosis, the persistence of symptoms over time could play a central role, although this possibility cannot be addressed with the cross-sectional data of this study.
Results of the binary logistic regression show that, even after controlling for the many potential confounders (including several known risk factors for diabetes regarding lifestyle, as well as individual and country socioeconomic level and the country of residence), the probability of having diabetes mellitus rises as the number of positive symptoms increases. This effect was present even after controlling for the presence of a previous diagnosis or treatment for schizophrenia or other psychosis. Thus, it seems that psychotic symptoms in nonclinical populations are related to diabetes mellitus,
independent of diagnoses and treatments, which could support indirect pathways for the relationship between psychosis and diabetes mellitus; for example, they share genetic and/or environmental factors.7 Although the relationship between depression and diabetes mellitus39 and general stress40 has recently been highlighted, clinical depression did not directly affect the relationship between psychotic symptoms and diabetes mellitus and was not a relevant mediator in our analysis.
Finally, the mediational analyses show that the relationship between diabetes mellitus and psychosis is not clearly mediated by other variables because the indirect effect of the different potential mediators was clearly low in a multiple mediation model. Furthermore, when the relationship between psychotic symptoms and diabetes mellitus was modeled in a path analysis, only BMI figured in the model, and more complex models clearly did not fit the data. Thus, the relationship between diabetes and psychotic symptoms was not reducible to known metabolic risk factors and was only partially accounted for by differences in BMI, in agreement with recent findings that compared persons with schizoaffective disorder or schizophrenia with persons with bipolar disorder.41
Psychotic symptoms in the general population significantly affect health and functioning even in the absence of clinical diagnosis.20 Likewise, it is well known that individuals with psychosis have poorer access to health services, weaker support networks, and unhealthier lifestyles,42,43 and it has been suggested that diabetes could contribute to mortality in persons with severe mental illness.44 In this context, the results of the present study suggest that people reporting psychotic symptoms should be screened for diabetes mellitus. Moreover, due to the relationship between BMI and diabetes among subjects with psychotic symptoms, interventions to produce lifestyle changes should be promoted among this population. As recommended in recent guidelines, screening and monitoring of diabetes among those with a diagnosis of schizophrenia and during the use of antipsychotic medication should be considered given that both of these factors can increase the risk for diabetes.45,46
Limitations
The diagnosis of diabetes was established through a self-reported item of previous diagnosis, which may imply an underestimation of real diabetes mellitus prevalence as many patients could be unaware that they have diabetes mellitus.47 This is also reflected in the wide variance in the reported diagnosis of diabetes mellitus across countries in our study, which is perhaps related to available health care services in each location. In any case, personal and country socioeconomic levels, as well as country of residence and differences in lifestyle (all potential reasons for the differences between countries in adult-onset diabetes), were statistically controlled for in the analysis, possibly reducing the bias these factors may introduce. Nonetheless, the same methodology has been used in previous studies.24 In a telephone survey in the United States using the same item (self-report of diabetes), Mokdad et al5 calculated that they underestimated diabetes mellitus prevalence by 2.7%. High agreement has been found for self-reported diabetes and clinical records.48 Furthermore, although it would appear that this procedure increases the percentage of false-negatives, it probably minimizes the percentage of false-positives, as well. Therefore, even though the strength of the relationship between psychosis and diabetes mellitus might be underestimated in our study, the pattern of associations remains valid and interpretable.
An additional limitation was that the type of diabetes was not assessed. Therefore, we did not present data on the relationship between psychosis and type of diabetes. Most of the studies on the association between diabetes and psychosis emphasize the relationship between psychosis and type 2 diabetes.1 However, there are few data about the association of type 1 diabetes and psychosis,49 and they point to lower rates of psychosis in persons with type 1 diabetes,50 which suggests that the relationship between diabetes and psychotic symptoms reported in the present study could have been underestimated. Further studies should analyze this issue in more detail. Missing data in the main variables (diabetes and psychotic symptoms) are also a potential problem, although multiple imputation methods partially address this problem, and our specific analyses of patterns of missingness suggest that this should not detract from the main results of our study.
Finally, we did not assess all psychotic symptoms and hence are unable to comment, on the basis of our nonexhaustive list, on whether other psychotic symptoms would have the same relationship with diabetes mellitus as observed in our study. Likewise, the type of medication for psychotic symptoms or the temporal relationship between medication and symptoms could not be analyzed due to a lack of available data in the World Health Survey, and we only considered past or current treatment as a general nonspecific potential confounder in our analyses.
CONCLUSIONS
Our results provide support for the association between psychotic symptoms and diabetes mellitus. This relationship is independent of several potential confounders—including a clinical diagnosis of psychosis or schizophrenia, previous antipsychotic treatment, depression, lifestyle, and individual or country socioeconomic status—and it occurs in nonclinical samples. This highlights the global relevance of the problem and the importance of identifying the specific paths through which the association is mediated. Interventions to address lifestyle changes in this population to reduce the risks of diabetes are required.
Disclosure of off-label usage: The authors have determined that, to the best of their knowledge, no investigational information about pharmaceutical agents that is outside US Food and Drug Administration–approved labeling has been presented in this article.
Author affiliations: Instituto de Salud Carlos III, Centro de Investigación Biomédica en Red de Salud Mental, CIBERSAM, Madrid, Spain
(Drs Nuevo, Fraguas, Arango, and Ayuso-Mateos); Department of Psychiatry, Universidad Autonoma de Madrid, Hospital Universitario de la Princesa, Madrid, Spain (Drs Nuevo and Ayuso-Mateos); Department of Health Statistics and Informatics, World Health Organization, Geneva, Switzerland (Drs Chatterji and Verdes and Ms Naidoo); Complejo Hospitalario Universitario de Albacete, Albacete, Spain (Dr Fraguas); and Adolescent Unit, Department of Psychiatry, Hospital General Universitario Gregorio Marañón, Madrid, Spain (Dr Arango).
Financial disclosure: Dr Fraguas has received consultancy fees from Otsuka, payment for preparing manuscripts and educational presentations from Bristol-Myers Squibb, and travel/accommodation fees from Otsuka and Janssen-Cilag. Dr Arango has received fees for his institution from CIBERSAM and the Alicia Koplowitz Foundation. Dr Ayuso-Mateos has received consultancy fees from Lundbeck and Risk Management Resources, LLC and provided expert testimony for Sanofi-Aventis.
Drs Nuevo, Chatterji, and Verdes and Ms Naidoo have no personal
affiliations or financial relationships with any commercial interest to
disclose relative to the article.
Funding/support: Supported by the Instituto de Salud Carlos III, Centro de Investigación Biomédica en Red de Salud Mental, CIBERSAM Spain, and the World Health Organization.
Role of sponsor: The sponsors played no role in study design or conduct of the study; the collection, management, analysis, or interpretation of data; the preparation, review, and approval of the manuscript; or the
decision to submit the manuscript for publication.
Disclaimer: The views expressed in this paper are those of the author(s) and do not necessarily represent the views or policies of the World Health Organization.
REFERENCES
1. Dixon L, Weiden P, Delahanty J, et al. Prevalence and correlates of diabetes in national schizophrenia samples. Schizophr Bull. 2000;26(4):903–912. PubMed
2. Lamberti JS, Crilly JF, Maharaj K, et al. Prevalence of diabetes mellitus among outpatients with severe mental disorders receiving atypical antipsychotic drugs. J Clin Psychiatry. 2004;65(5):702–706. doi:10.4088/JCP.v65n0517 PubMed
3. Mukherjee S, Decina P, Bocola V, et al. Diabetes mellitus in schizophrenic patients. Compr Psychiatry. 1996;37(1):68–73. doi:10.1016/S0010-440X(96)90054-1 PubMed
4. Newcomer JW. Medical risk in patients with bipolar disorder and schizophrenia. J Clin Psychiatry. 2006;67(suppl 9):25–30, discussion 36–42. doi:10.4088/JCP.1106e16 PubMed
5. Mokdad AH, Bowman BA, Ford ES, et al. The continuing epidemics
of obesity and diabetes in the United States. JAMA. 2001;286(10):
1195–1200. doi:10.1001/jama.286.10.1195 PubMed
6. Fusar-Poli P, De Marco L, Cavallin F, et al. Lifestyles and cardiovascular risk in individuals with functional psychoses. Perspect Psychiatr Care. 2009;45(2):87–99. doi:10.1111/j.1744-6163.2009.00202.x PubMed
7. Henderson DC, Ettinger ER. Schizophrenia and diabetes. Int Rev Neurobiol. 2002;51:481–501. doi:10.1016/S0074-7742(02)51014-X PubMed
8. Sernyak MJ, Leslie DL, Alarcon RD, et al. Association of diabetes mellitus with use of atypical neuroleptics in the treatment of schizophrenia. Am J Psychiatry. 2002;159(4):561–566. doi:10.1176/appi.ajp.159.4.561 PubMed
9. Simon V, van Winkel R, De Hert M. Are weight gain and metabolic side effects of atypical antipsychotics dose dependent? a literature review.
J Clin Psychiatry. 2009;70(7):1041–1050. doi:10.4088/JCP.08r04392 PubMed
10. Fernandez-Egea E, Bernardo M, Donner T, et al. Metabolic profile
of antipsychotic-naive individuals with non-affective psychosis.
Br J Psychiatry. 2009;194(5):434–438. doi:10.1192/bjp.bp.108.052605 PubMed
11. Henneman DH, Altschule MD, Goncz RM. Carbohydrate metabolism in brain disease, 2: glucose metabolism in schizophrenic, manic-depressive, and involutional psychoses. AMA Arch Intern Med. 1954;94(3):402–416. PubMed
12. Sengupta S, Parrilla-Escobar MA, Klink R, et al; Ying Kin Ng. Are metabolic indices different between drug-naïve first-episode psychosis patients and healthy controls? Schizophr Res. 2008;102(1–3):329–336. doi:10.1016/j.schres.2008.02.013 PubMed
13. Zhang ZJ, Yao ZJ, Liu W, et al. Effects of antipsychotics on fat deposition and changes in leptin and insulin levels: magnetic resonance imaging study of previously untreated people with schizophrenia. Br J Psychiatry. 2004;184(1):58–62. doi:10.1192/bjp.184.1.58 PubMed
14. Spelman LM, Walsh PI, Sharifi N, et al. Impaired glucose tolerance in first-episode drug-naïve patients with schizophrenia. Diabet Med. 2007;
24(5):481–485. doi:10.1111/j.1464-5491.2007.02092.x PubMed
15. Verma SK, Subramaniam M, Liew A, et al. Metabolic risk factors in drug-naive patients with first-episode psychosis. J Clin Psychiatry. 2009;70(7):997–1000. doi:10.4088/JCP.08m04508 PubMed
16. van Os J, Kapur S. Schizophrenia. Lancet. 2009;374(9690):635–645. doi:10.1016/S0140-6736(09)60995-8 PubMed
17. Perälä J, Suvisaari J, Saarni SI, et al. Lifetime prevalence of psychotic and bipolar I disorders in a general population. Arch Gen Psychiatry. 2007;64(1):19–28. doi:10.1001/archpsyc.64.1.19 PubMed
18. Wiles NJ, Zammit S, Bebbington P, et al. Self-reported psychotic symptoms in the general population: results from the longitudinal study of
the British National Psychiatric Morbidity Survey. Br J Psychiatry. 2006;
188(6):519–526. doi:10.1192/bjp.bp.105.012179 PubMed
19. van Os J, Hanssen M, Bijl RV, et al. Strauss (1969) revisited: a psychosis continuum in the general population? Schizophr Res. 2000;45(1-2):
11–20. doi:10.1016/S0920-9964(99)00224-8 PubMed
20. Nuevo R, Chatterji S, Verdes E, et al. The continuum of psychotic symptoms in the general population: a cross-national study.[published online ahead of print September 13, 2010].Schizophr Bull. 2010; doi:10.1093/schbul/sbq099 PubMed
21. Ustun TB, Chatterji S, Mechbal A, et al. WHS Collaborating Groups. Quality assurance in surveys: standards, guidelines and procedures.
In: United Nations Statistics Division, Department for Economic and Social Affairs, ed. Household Sample Surveys in Developing and Transition Countries. New York, NY: United Nations; 2005;199–230.
22. World Bank. World Bank List of Economies, 2007. http://data.worldbank.org/indicator/all. Accessed March 9, 2010.
23. World Health Survey. World Health Organization Web site. http://www.who.int/healthinfo/survey/en/index.html. Accessibility verified September 13, 2011.
24. Moussavi S, Chatterji S, Verdes E, et al. Depression, chronic diseases,
and decrements in health: results from the World Health Surveys. Lancet. 2007;370(9590):851–858. doi:10.1016/S0140-6736(07)61415-9 PubMed
25. Kessler RC, Ustün TB. The World Mental Health (WMH) Survey Initiative Version of the World Health Organization (WHO) Composite International Diagnostic Interview (CIDI). Int J Methods Psychiatr Res. 2004;13(2):93–121. doi:10.1002/mpr.168 PubMed
26. World Health Organization. The ICD-10 Classification of Mental and Behavioural Disorders: Diagnostic Criteria for Research (ICD-10-DCR). Geneva, Switzerland: WHO; 1993.
27. Ayuso-Mateos JL, Nuevo R, Verdes E, et al. From depressive symptoms to depressive disorders: the relevance of thresholds. Br J Psychiatry. 2010;
196(5):365–371. doi:10.1192/bjp.bp.109.071191 PubMed
28. Clausen T, Rossow I, Naidoo N, et al. Diverse alcohol drinking patterns in 20 African countries. Addiction. 2009;104(7):1147–1154. doi:10.1111/j.1360-0443.2009.02559.x PubMed
29. Guthold R, Ono T, Strong KL, et al. Worldwide variability in physical inactivity a 51-country survey. Am J Prev Med. 2008;34(6):486–494. doi:10.1016/j.amepre.2008.02.013 PubMed
30. Cooper L, Peters L, Andrews G. Validity of the Composite International Diagnostic Interview (CIDI) psychosis module in a psychiatric setting.
J Psychiatr Res. 1998;32(6):361–368. doi:10.1016/S0022-3956(98)00021-1 PubMed
31. Hanssen MS, Bijl RV, Vollebergh W, et al. Self-reported psychotic experiences in the general population: a valid screening tool for DSM-III-R psychotic disorders? Acta Psychiatr Scand. 2003;107(5):369–377. doi:10.1034/j.1600-0447.2003.00058.x PubMed
32. Ahmad OB, Boschi-Pint C, López AD, et al. Age Standardization of Rates: A New WHO Standard. Geneva, Switzerland: World Health Organization; 2001.
33. United Nations Statistics Division Web site. http://unstats.un.org/unsd/default.htm. Accessibility verified September 13, 2011.
34. StataCorp. Stata Statistical Software: Release 11. College Station, TX: StataCorp LP; 2009.
35. van Buuren S, Oudshoorn CGM. Multivariate Imputation by Chained Equations. MICE V 1.0 User’s Manual. Leiden, the Netherlands: TNO Preventie en Gezondheid; 2009.
36. Sobel ME. Asymptotic confidence intervals for indirect effects in structural equation models. In: Leinhardt S, ed. Sociological Methodology. Washington, DC: American Sociological Association; 1982:290–312.
37. Schreiber JB, Stage FK, King J, et al. Reporting structural equation modelling and confirmatory factor analysis results: a review. J Educ Res. 2006;99(6):323–338. doi:10.3200/JOER.99.6.323-338
38. Muthén LK, Muthén BO. Mplus User’s Guide. 6th ed. Los Angeles, CA: Muthén & Muthén; 2010.
39. Campayo A, de Jonge P, Roy JF, et al; ZARADEMP Project. Depressive disorder and incident diabetes mellitus: the effect of characteristics of depression. Am J Psychiatry. 2010;167(5):580–588. doi:10.1176/appi.ajp.2009.09010038 PubMed
40. Fisher L, Skaff MM, Mullan JT, et al. Clinical depression versus distress among patients with type 2 diabetes: not just a question of semantics. Diabetes Care. 2007;30(3):542–548. doi:10.2337/dc06-1614 PubMed
41. van Winkel R, van Os J, Celic I, et al. Psychiatric diagnosis as an independent risk factor for metabolic disturbances: results from a comprehensive, naturalistic screening program. J Clin Psychiatry. 2008;69(8):1319–1327. doi:10.4088/JCP.v69n0817 PubMed
42. Brown S, Inskip H, Barraclough B. Causes of the excess mortality of schizophrenia. Br J Psychiatry. 2000;177(3):212–217. doi:10.1192/bjp.177.3.212 PubMed
43. Dickerson FB, Brown CH, Kreyenbuhl JA, et al. Obesity among
individuals with serious mental illness. Acta Psychiatr Scand. 2006;
113(4):306–313. doi:10.1111/j.1600-0447.2005.00637.x PubMed
44. Brown C, Leith J, Dickerson F, et al. Predictors of mortality in patients with serious mental illness and co-occurring type 2 diabetes. Psychiatry Res. 2010;177(1-2):250–254. doi:10.1016/j.psychres.2010.01.004 PubMed
45. De Hert M, Dekker JM, Wood D, et al. Cardiovascular disease and diabetes in people with severe mental illness position statement from the European Psychiatric Association (EPA), supported by the European Association for the Study of Diabetes (EASD) and the European Society of Cardiology (ESC). Eur Psychiatry. 2009;24(6):412–424. doi:10.1016/j.eurpsy.2009.01.005 PubMed
46. Fleischhacker WW, Cetkovich-Bakmas M, De Hert M, et al. Comorbid somatic illnesses in patients with severe mental disorders: clinical, policy, and research challenges. J Clin Psychiatry. 2008;69(4):514–519. doi:10.4088/JCP.v69n0401 PubMed
47. Cowie CC, Rust KF, Ford ES, et al. Full accounting of diabetes and pre-diabetes in the US population in 1988–1994 and 2005–2006. Diabetes Care. 2009;32(2):287–294. doi:10.2337/dc08-1296 PubMed
48. Okura Y, Urban LH, Mahoney DW, et al. Agreement between self-report questionnaires and medical record data was substantial for diabetes, hypertension, myocardial infarction and stroke but not for heart failure.
J Clin Epidemiol. 2004;57(10):1096–1103. doi:10.1016/j.jclinepi.2004.04.005 PubMed
49. Muthukrishnan J, Kiranmayi L, Verma A, et al. Type 1 diabetes mellitus: correlation between etiological factors and associated conditions.
Int J Diab Dev Ctries. 2007;27(2):46–49.
50. Juvonen H, Reunanen A, Haukka J, et al. Incidence of schizophrenia in a nationwide cohort of patients with type 1 diabetes mellitus. Arch Gen Psychiatry. 2007;64(8):894–899. doi:10.1001/archpsyc.64.8.894 PubMed
This PDF is free for all visitors!
Save
Cite


