Objective: To evaluate the efficacy and safety of individualized dosing within the approved dose range for osmotic-release oral system (OROS) methylphenidate hydrochloride in adults with attention-deficit/hyperactivity disorder (ADHD).
Methods: A double-blind, 6-week trial was conducted between July 2009 and February 2010 at 35 US sites. Adults with ADHD (DSM-IV diagnostic criteria) and a screening ADHD Investigator Symptom Rating Scale (AISRS) score > 24 were randomly assigned to OROS methylphenidate 18 mg or matching placebo. Treatment dose could be increased at 18 mg increments, up to 72 mg/d, until an optimal dose was achieved. AISRS score changes from baseline to end point (primary outcome) were analyzed using analysis of covariance.
Results: At baseline, the intent-to-treat population of 169 OROS methylphenidate and 172 placebo subjects (mean age = 35.8 years) had mean (standard deviation [SD]) AISRS scores of 37.8 (6.94) and 37.0 (7.51), respectively. OROS methylphenidate-treated subjects exhibited a significantly greater mean (SD) AISRS score improvement than placebo subjects (-17.1 [12.44] vs -11.7 [13.30]; P < .001). In general, OROS methylphenidate-treated subjects experienced greater improvements than placebo subjects in secondary measures of symptom frequency, cognitive function, work productivity, and quality-of-life. Little effect of OROS methylphenidate was observed in exploratory sleep assessments. The adverse event pattern was similar to previous reports of stimulants in adults with ADHD.
Conclusions: OROS methylphenidate treatment with individualized doses titrated to achieve symptom remission demonstrated greater ADHD symptom reduction than placebo treatment. These data support the overall efficacy of OROS methylphenidate treatment in the management of adults with ADHD and provide new possibilities for additional intervention.
Trial Registration: ClinicalTrials.gov identifier: NCT00937040
This work may not be copied, distributed, displayed, published, reproduced, transmitted, modified, posted, sold, licensed, or used for commercial purposes. By downloading this file, you are agreeing to the publisher’s Terms & Conditions.
Randomized, 6-Week, Placebo-Controlled Study of Treatment for Adult Attention-Deficit/Hyperactivity Disorder:
Individualized Dosing of Osmotic-Release Oral System (OROS) Methylphenidate With a Goal of Symptom Remission

ABSTRACT
Objective: To evaluate the efficacy and safety of individualized dosing within the approved dose range for osmotic-release oral system (OROS) methylphenidate hydrochloride in adults with attention-deficit/hyperactivity disorder (ADHD).
Methods: A double-blind, 6-week trial was conducted between July 2009 and February 2010 at 35 US sites. Adults with ADHD (DSM-IV diagnostic criteria) and a screening ADHD Investigator Symptom Rating Scale (AISRS) score > 24 were randomly assigned to OROS methylphenidate 18 mg or matching placebo. Treatment dose could be increased at 18 mg increments, up to 72 mg/d, until an optimal dose was achieved. AISRS score changes from baseline to end point (primary outcome) were analyzed using analysis of covariance.
Results: At baseline, the intent-to-treat population of 169 OROS methylphenidate and 172 placebo subjects (mean age = 35.8 years) had mean (standard deviation [SD]) AISRS scores of 37.8 (6.94) and 37.0 (7.51), respectively. OROS methylphenidate-treated subjects exhibited a significantly greater mean (SD) AISRS score improvement than placebo subjects (-17.1 [12.44] vs -11.7 [13.30]; P < .001). In general, OROS methylphenidate-treated subjects experienced greater improvements than placebo subjects in secondary measures of symptom frequency, cognitive function, work productivity, and quality-of-life. Little effect of OROS methylphenidate was observed in exploratory sleep assessments. The adverse event pattern was similar to previous reports of stimulants in adults with ADHD.
Conclusions: OROS methylphenidate treatment with individualized doses titrated to achieve symptom remission demonstrated greater ADHD symptom reduction than placebo treatment. These data support the overall efficacy of OROS methylphenidate treatment in the management of adults with ADHD and provide new possibilities for additional intervention.
Trial Registration: ClinicalTrials.gov identifier: NCT00937040
J Clin Psychiatry 2017;78(1):105-114
dx.doi.org/10.4088/JCP.15m10348
© Copyright 2016 Physicians Postgraduate Press, Inc.
aSuburban Psychiatric Associates, LLC; Adult Attention Deficit Disorder Center of Maryland; and Department of Psychiatry and Behavioral Sciences, Johns Hopkins University School of Medicine, Baltimore, Maryland
bJanssen Scientific Affairs, LLC, Titusville, New Jersey
cJohnson & Johnson Consumer Inc, Horsham, Pennsylvania
dUniversity of Pennsylvania Adult ADHD Treatment and Research Program, Department of Psychiatry, University of Pennsylvania Health System, Philadelphia
eJanssen Pharmaceutical Development, LLC, Titusville, New Jersey
*Corresponding author: H. Lynn Starr, MD, Director, Medical Affairs, Janssen Scientific Affairs, LLC, 1125 Trenton-Harbourton Rd, Titusville, NJ 08560 ([email protected]).
Attention-deficit/hyperactivity disorder (ADHD) is a prevalent neurodevelopmental disorder that persists into adulthood in approximately two-thirds of individuals.1-3 In adults, the estimated prevalence of ADHD ranges from 1% to 6%.1,4-7 The symptoms defining ADHD impart a variety of life impairments including effects on work productivity, the rate of professional employment and job changes, cognitive skills, daily experiences, social interactions/relationships including divorce rates,8 and sleep difficulties.9,10 Quality-of-life is negatively correlated with symptom severity.8,11,12 Information on the impact of treatment on the broad range of life impairments, behaviors, and sleep or insomnia in adults with ADHD is limited.
Several studies have documented the efficacy of osmotic-release oral system (OROS) methylphenidate hydrochloride for ADHD symptom reduction in adults.13-15 These studies either randomized subjects to assigned OROS methylphenidate dosages or used a dose-titration strategy aimed at 30% reduction in ADHD symptoms, which, while decreasing ADHD symptom level, may still result in suboptimal clinical treatment benefit.16,17 By design, these studies did not explore the possibility that allowing additional OROS methylphenidate dose adjustment(s) might produce further improvement, remission of symptoms, or better tolerability.
Sleep may be a concern for individuals with ADHD; studies note sleep dysregulation in up to 40% of adults with ADHD.9,10,18-20 Clinical studies typically report insomnia as an adverse event, but there are limited data addressing relationships among sleep, ADHD, and treatment with methylphenidate.
This study in adults with ADHD compared the efficacy and safety of OROS methylphenidate treatment with placebo and had 3 main objectives: (1) evaluate a flexible-dose approach that balanced efficacy and tolerability with an ADHD symptom remission goal, (2) investigate the effects of treatment on daily functioning and well-being from a variety of perspectives, and (3) explore the potential relationship between sleep/insomnia and treatment using patient ratings supplemented by objective data obtained from actigraphy. The trial evaluations were performed from multiple perspectives: the investigator, the patient, and a patient-selected adult observer. Subjects also completed the ADHD Adult Self-Report Scale (ASRS) to assess the potential to use a scale as an alternative to a clinician-rated measure for adjusting dose during treatment.
METHODS
Study Design
This 6-week, double-blind, randomized, placebo-controlled trial was conducted at 35 clinical sites in the United States between July 2009 and February 2010. Institutional Review Boards at each site approved the study, and all subjects gave written informed consent to participate. The trial was registered with ClinicalTrials.gov (identifier: NCT00937040).
Participants
Eligible participants were adults aged 18 to 65 years with a diagnosis of ADHD, as defined by the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition,21 and as evaluated at baseline with the adult ADHD Clinical Diagnostic Scale (ACDS), version 1.2,22,23 and the Mini-International Neuropsychiatric Interview.24 Prospective subjects had an adult ADHD Investigator Symptom Rating Scale25 (AISRS) score > 24 at the screening/baseline visit. Those with mild depression according to the Hamilton Depression Rating Scale26 (HDRS; HDRS score < 18) or mild anxiety according to the Hamilton Anxiety Rating Scale27 (HARS; HARS score < 21) were eligible for study participation. Subjects excluded were those with a history of diagnosis of substance or alcohol dependence or admission/hospitalization for rehabilitation for dependence, moderate or severe anxiety (HARS score ≥ 21), moderate or severe depression (HDRS score ≥ 18), and a history of stimulants or atomoxetine use within 5 years or other ADHD medications within 30 days and those for whom, in the investigator’s opinion, methylphenidate posed an unacceptable risk through a potential drug interaction or a concurrent medical, neurologic, or psychiatric illness.

- Response rates for reduction of symptoms of attention-deficit/hyperactivity disorder (ADHD) in adults using methylphenidate in randomized controlled trials are well-known, but few studies have focused on treatment to symptomatic remission, minimal or absent symptoms, and optimal functioning.
- Greater symptom improvements in ADHD have been associated with greater functional improvements.
- The study provided an opportunity to evaluate the effects of osmotic-release oral system methylphenidate on a wide range of functional abilities in adults with ADHD, including executive function, workplace productivity, collateral observations, sleep, and well-known ADHD efficacy measures.
Procedures
Subjects were randomly assigned by an interactive voice response system to 18 mg/d of OROS methylphenidate or matching placebo. Treatment dose could be increased at each of 3 subsequent weekly visits to 36 mg, 54 mg, and 72 mg (maximum) until the subject reached an AISRS score < 18 or a limit of tolerability.
Outcome Assessments and Descriptions
The primary efficacy assessment was the change from baseline to end point (week 6 or study discontinuation) in the investigator-rated AISRS, with remission defined as an AISRS score of < 18. This score was considered to be consistent with scores in adults without a diagnosis of ADHD. A broad range of secondary efficacy outcomes and exploratory analyses of sleep were assessed from the perspective of the investigator, the subject, and a subject-designated observer. If an enrolled subject did not designate an observer, the efficacy evaluations from that perspective were not performed.
Primary efficacy evaluation. The AISRS, an investigator-rated assessment of 18 core ADHD symptoms corresponding to the DSM-IV diagnostic symptoms for adults, served as the primary efficacy evaluation. Total scores range from 0 to 54; higher scores indicate greater symptom severity.25
Secondary efficacy evaluations-investigator rated. The Clinical Global Impressions-Severity (CGI-S) scale was used to assess the severity of the subject’s ADHD symptoms relative to investigators’ past experience with ADHD subjects.27
The Clinical Global Impressions-Improvement (CGI-I) scale, an investigator-rated scale, was used to assess how much the patient’s illness has improved or worsened relative to baseline.27
The Central Nervous System Vital Signs (CNSVS) Computerized Neurocognitive Battery rated subjects by using instrument-assisted measures on cognitive domains of (1) reaction time (in milliseconds) using a Stroop test domain, with lower scores indicating better functioning28; (2) vigilance using the Continuous Performance Test (CPT), with lower scores reflecting a decrease in the number of errors; (3) cognitive flexibility using the Shifting Attention Test (SAT), with higher scores indicating better accuracy; and (4) processing speed using the Symbol Digit Modalities Test, with higher scores indicating better functioning.29
Secondary efficacy evaluations-subject rated. The ASRS was used to assess subject-rated frequency of 18 ADHD symptoms. Total score ranges from 0 to 72; higher scores indicate greater symptom frequency.30,31
The Behavior Rating Inventory of Executive Function-Adult (BRIEF-A)32,33 was used by subjects and their designated observer to rate 75 items across 9 nonoverlapping clinical scales measuring various aspects of adult executive functioning and self-regulation in the subject’s everyday environment, with higher scoring indicating greater executive function impairment. The clinical scales form 2 broad indexes—the Behavioral Regulation Index and the Metacognition Index, with these scores combined for an overall Global Executive Composition (GEC) score.
The subject-rated Adult ADHD Impact Module (AIM-A)34 uses multi-item scales to assess global and ADHD-specific quality-of-life and functioning in 6 domains. Scores range from 0 to 100, with higher scores indicating less impact.
Overall productivity was assessed using the 25-item Endicott Work Productivity Scale (EWPS)35 that describes types of behaviors or subjective feelings likely to reduce work productivity or efficiency. Total score ranges from 0 to 10, with higher scores indicating worsening work productivity and efficiency.
The subject and their designated observer used the 10-item Dyadic Adjustment Scale (DAS)-Satisfaction subscale measure to assess relationship satisfaction.36 Total scores range from 0 to 50, with higher scores indicating better relationship satisfaction.
Subjects and their designated observer responded to the Satisfaction with Treatment Questionnaire, which comprised 4 treatment-related questions regarding the extent of ADHD symptom change since medication initiation, the benefit received from the medication, the extent of advantages, if any, outweighing the disadvantages, and their overall satisfaction with the medication.
Secondary efficacy evaluations-designated observer rated. The subject’s designated observer rated the frequency of 18 ADHD symptoms using the Adult ADHD Significant-Other Rating Scale. Total score ranges from 0 to 54; higher scores indicate greater symptom frequency.37
As described in the subject-rated section, subject’s designated observer also completed the BRIEF-A.
As described in the subject-rated section, subject’s designated observer also completed the DAS-Satisfaction.
Exploratory analyses of sleep measures-subject rated. Subjects rated their sleep quality and quantity during the prior 2 weeks using the 7-domain Pittsburgh Sleep Quality Index (PSQI).38 Lower scores indicate better sleep quality.
Using the Epworth Sleepiness Scale (ESS), subjects rated how likely they would be to “doze off” on a scale of “0 = never” to “3 = high chance”39; higher scores indicate a greater probability of dozing.
Continuous monitoring of movement during sleep (actigraphy) was employed. Subjects wore a wristwatch-type device (Actiwatch-Spectrum, Mini-Mitter Company, Inc [a Respironics Inc Company], Bend, Oregon) to measure movement during the entire 14-day assessment period, with these data used to infer sleep and wake periods and quality of other sleep aspects.
Safety and Tolerability
Adverse event reports were collected throughout the study. Original terms used by investigators to identify adverse events were coded using the Medical Dictionary for Regulatory Activities (MedDRA).40 An assessment of suicidality was performed at each visit using the modified InterSePT Scale for Suicidal Thinking (ISST-Plus).41
Data Analyses
Based on previous AISRS work and an expected mean between-treatment group AISRS change from baseline to end point difference of 4.1 (standard deviation [SD] 11.114), it was estimated that 312 subjects would provide 90% power to determine statistical significance at an α level of .05.
Outcomes were assessed for the intent-to-treat population (ie, all subjects randomized who received at least 1 dose of study drug and had any [excluding ASRS] postbaseline efficacy outcome data). The last observation carried forward was used to impute missing data at the final visit for change from baseline to end point in AISRS score. AISRS score changes were tested using an analysis of covariance (ANCOVA) model with treatment and pooled study center as factors and baseline AISRS score as a covariate. Two-sided α level was .05.
Secondary efficacy end points were tested at a 2-sided .05 α level, using a fixed-sequence gatekeeper approach, given that the primary efficacy variable was statistically significant. A sequence of multiple hypotheses (that OROS methylphenidate would be superior to placebo) was tested individually, one after the other, in order to maintain the overall Type I error at .05. Following a prespecified fixed sequence of testing for the secondary efficacy end points, the first end point was tested at the .05 level of significance. If that null hypothesis was rejected, then the second end point was tested. This analysis was stopped when the P value for a test was greater than .05. P values of subsequent end point comparisons in the sequence were calculated, but no unqualified statements about the statistical significance may be made. The first 4 end points (in sequence) after the primary were from the CNSVS Computerized Neurocognitive Battery: reaction time, vigilance, cognitive flexibility, and processing speed. Continuous secondary end points and exploratory end points (ie, PSQI and ESS) were analyzed using ANCOVA models (or ranked ANCOVA if normality assumption did not hold). Cochran-Mantel-Haenszel tests were used to evaluate treatment effects on remission rate and treatment satisfaction. All variables were analyzed as the change from baseline to the end point. Final visit actigraphy scores were analyzed using analysis of variance. Descriptive statistics were used for analysis of baseline demographic and disease variables and adverse events.
RESULTS
Subjects
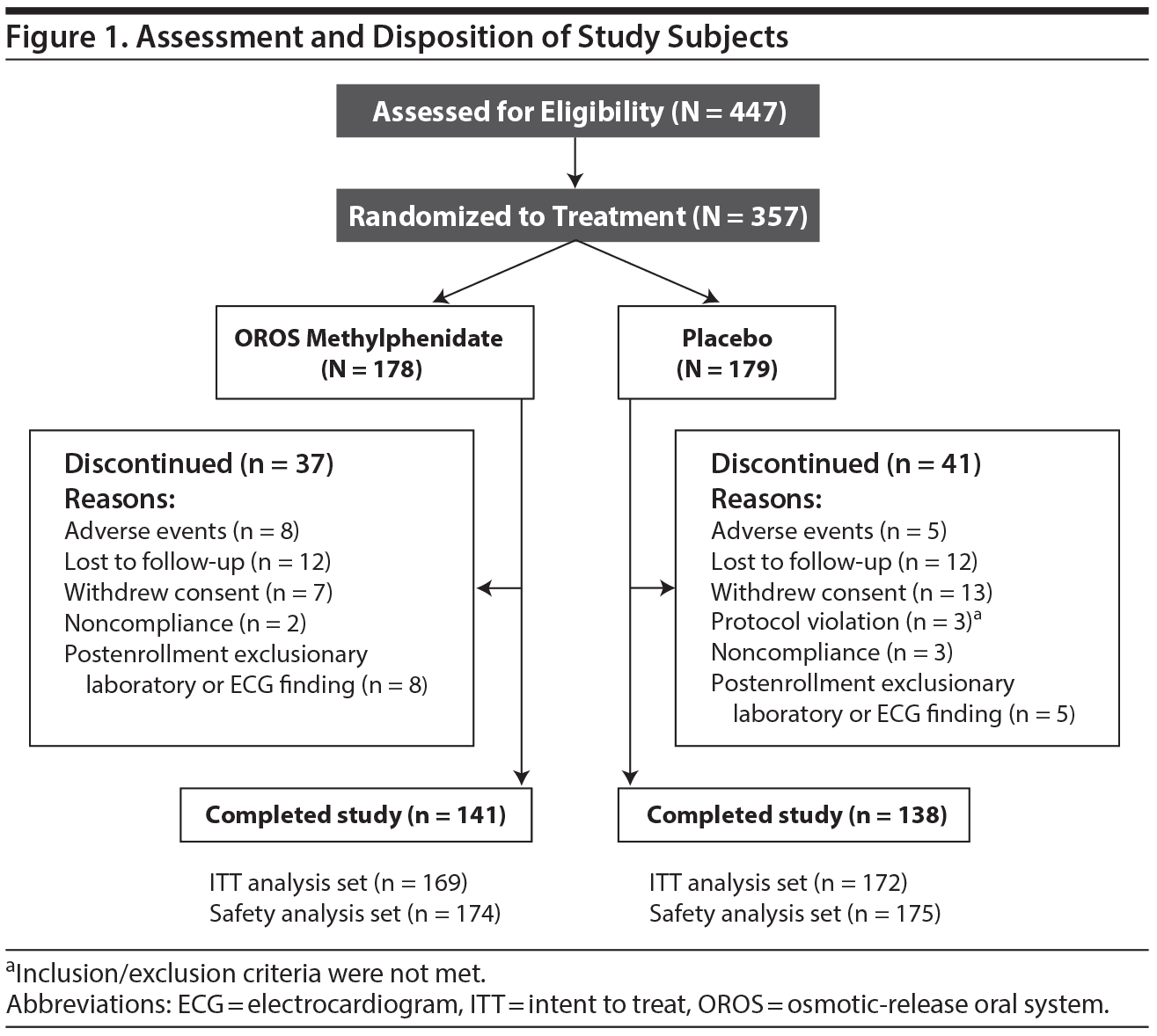
Subject assessment and disposition are illustrated in Figure 1. Five subjects in the placebo arm discontinued due to adverse events; the reported events were increased blood pressure (1 subject), headache (2 subjects), suicidal ideation (considered serious; 1 subject), and tinnitus and rash (1 subject). Eight subjects in the OROS methylphenidate arm discontinued due to adverse events; the reported events were increased blood pressure (2 subjects), headache and gastroenteritis (1 subject), tremor (1 subject), anxiety (1 subject), anxiety and insomnia (1 subject), oral pain (1 subject), and hypertension (1 subject).
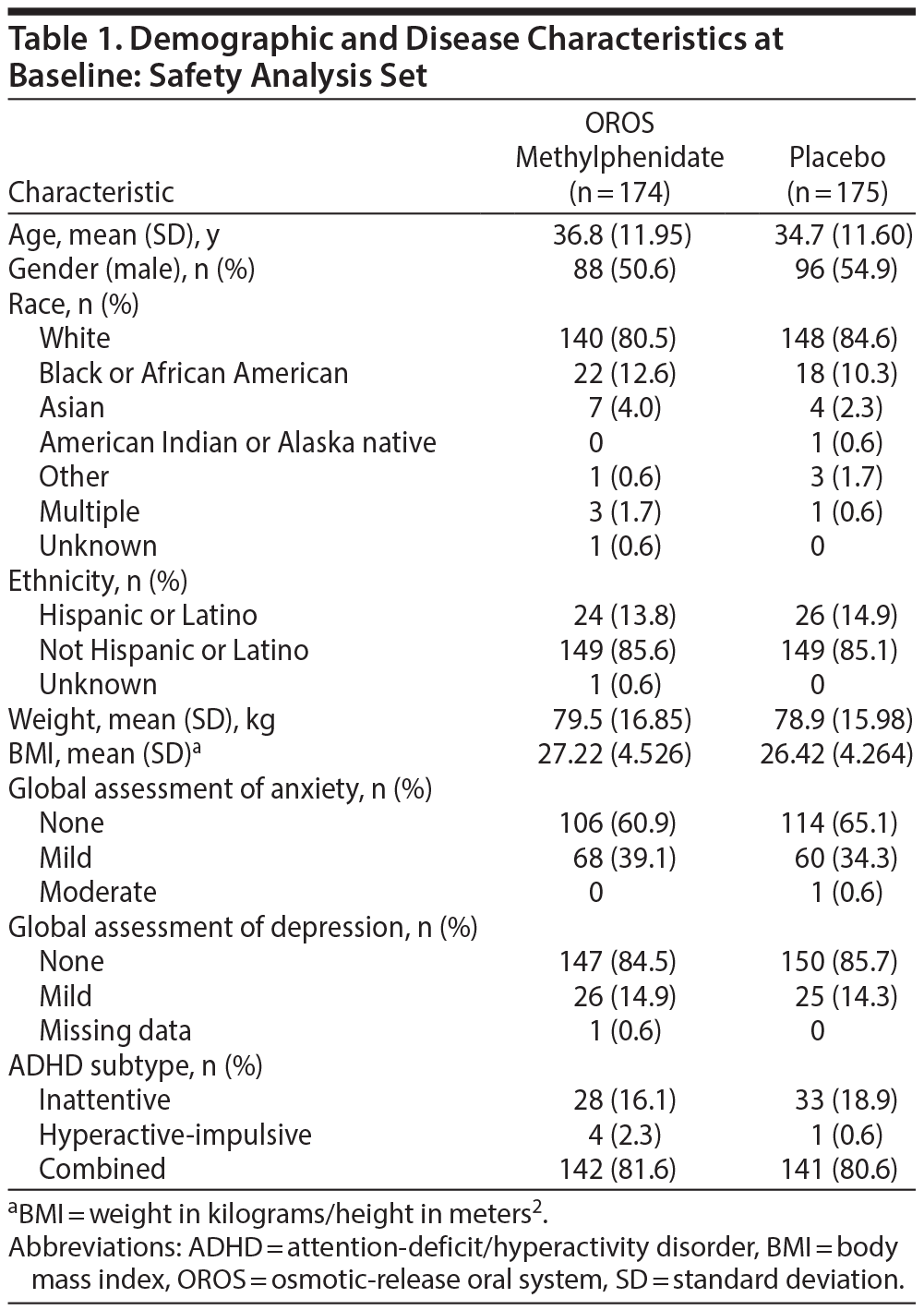
Baseline characteristics including weight appeared similar between treatment groups (Table 1). Approximately 50% of subjects reported prior use of other medications (other than ADHD medications), the most common of which were anti-inflammatory and anti-rheumatoid products. At the final study visit, the respective percentage of subjects receiving 18 mg of OROS methylphenidate was 13.6% and placebo, 11.0%; 36 mg of OROS methylphenidate was 23.1% and placebo, 20.3%; 54 mg of OROS methylphenidate was 24.3% and placebo, 9.3%; and 72 mg of OROS methylphenidate was 39.1% and placebo, 59.3%. The mean (SD) daily dose was 54.89 mg (19.809 mg) in the OROS methylphenidate group and 61.27 mg (15.746 mg) in the placebo comparison group. Approximately 95% of subjects in each group took > 80% and < 120% of their intended doses and were considered adherent with treatment.
Primary Efficacy Assessment
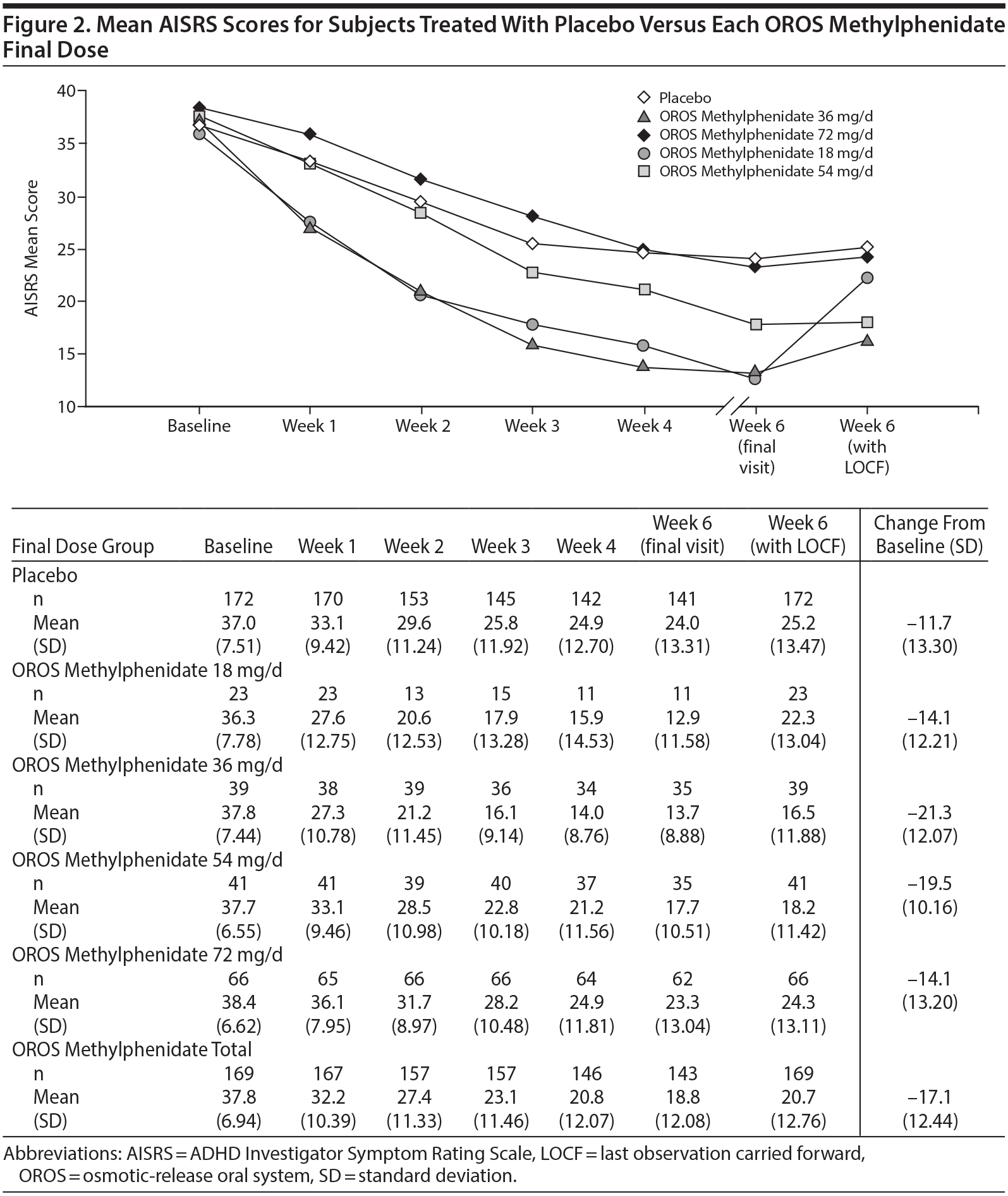
The mean (SD) AISRS score at baseline was 37.8 (6.94) for the OROS methylphenidate group and 37.0 (7.51) for the placebo group. At end point, subjects receiving OROS methylphenidate had a greater change from baseline (−17.1 [12.44]) than placebo subjects (−11.7 [13.30]) (Figure 2). Treatment difference was significantly better for the OROS methylphenidate-treated group with a least squares mean (LS mean) difference of −5.0 (−16.9 and −12.0, respectively; P < .001]. Remission (ie, AISRS score of < 18) was attained by a significantly greater percentage of OROS methylphenidate-treated than placebo-treated subjects (45.0% [76/169] vs 30.8% [53/172]; P = .0008).
Secondary Efficacy and Exploratory Assessments
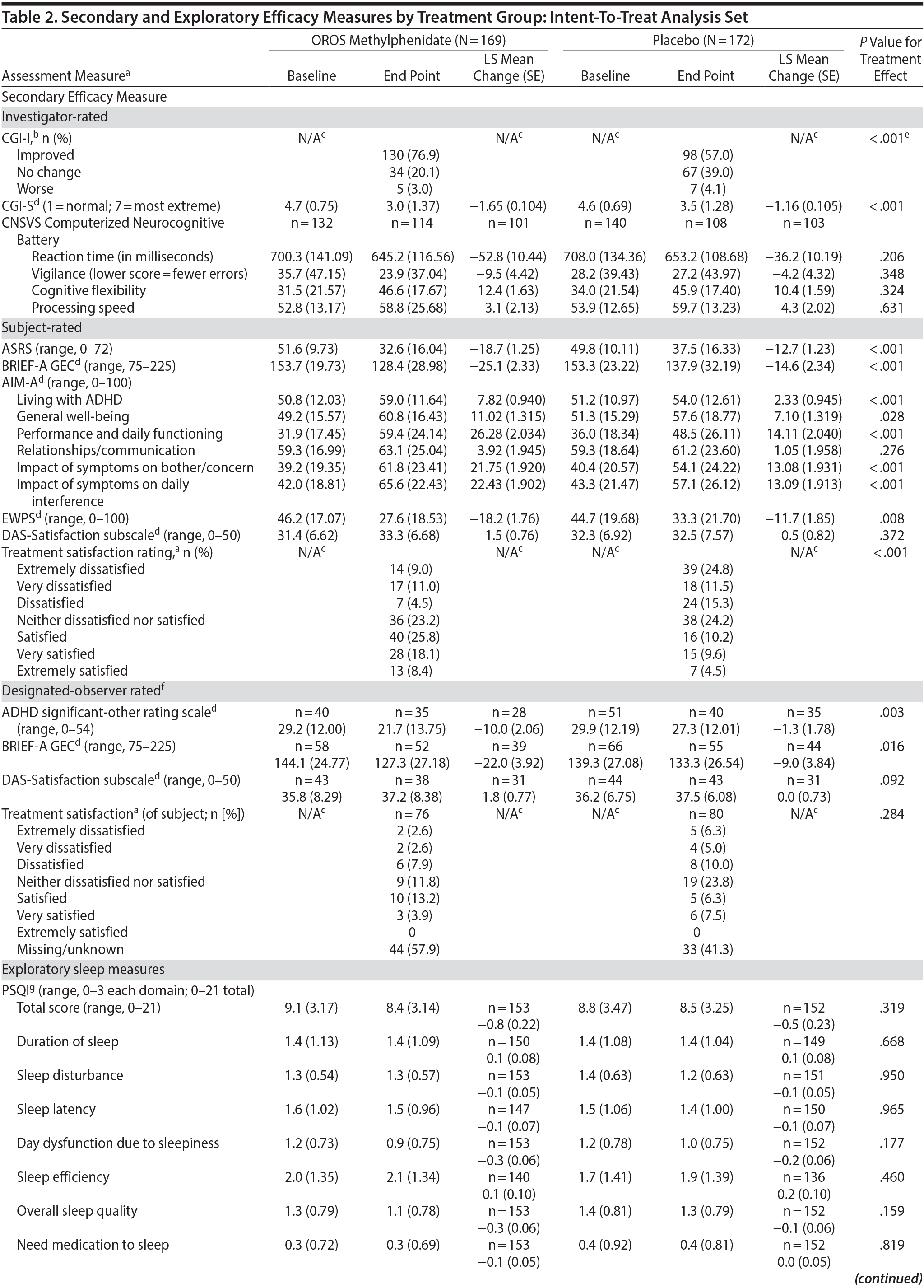
Secondary and exploratory assessment data are summarized in Table 2. In the investigator-rated assessments, OROS methylphenidate-treated subjects exhibited greater illness improvement (CGI-I; P < .001) and a greater decrease in illness severity (CGI-S; P < .001) compared to placebo treated-subjects.
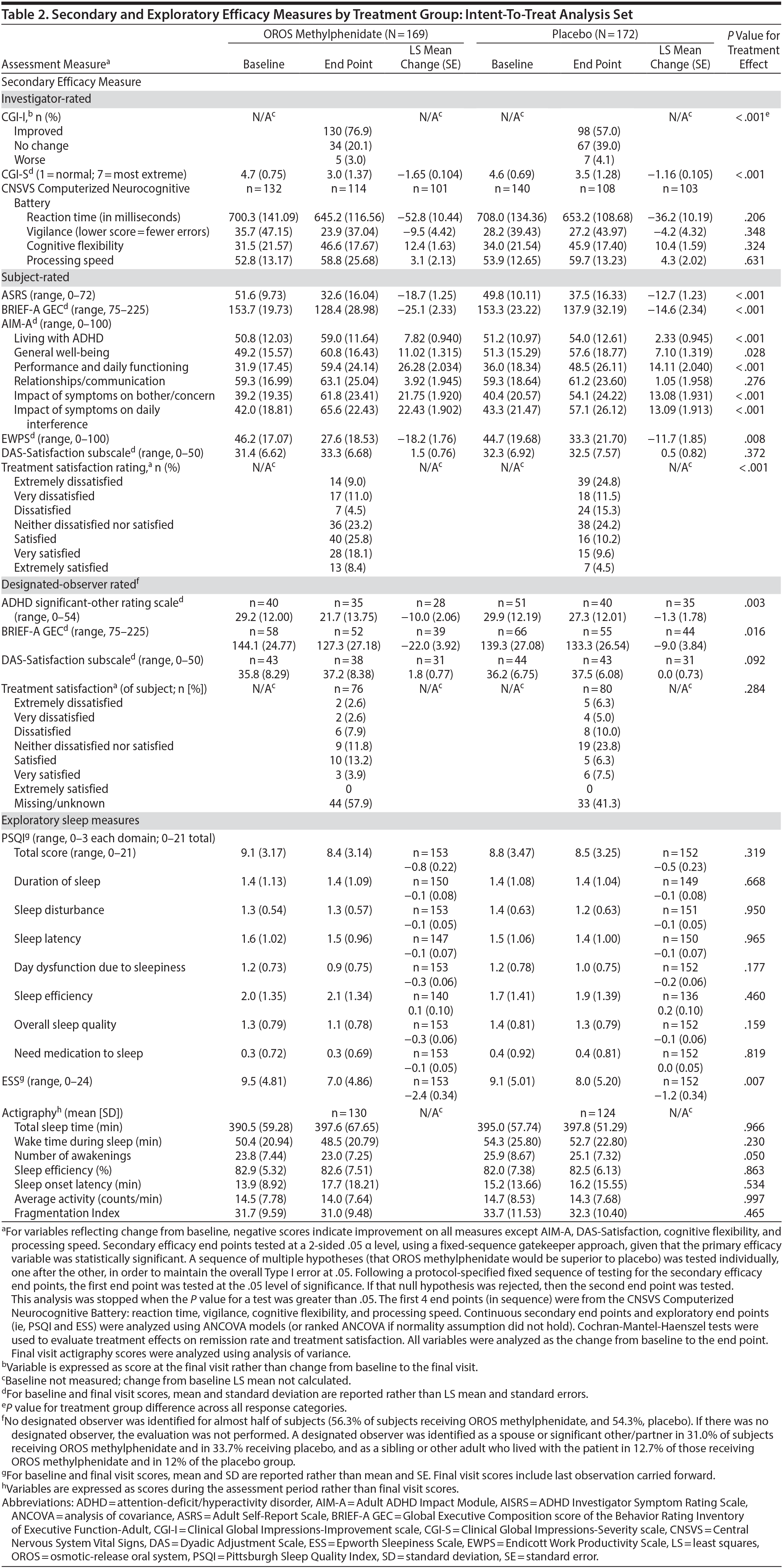
In subject-rated assessments, the OROS methylphenidate group reported greater improvements in symptom frequency (ASRS; P < .001), executive function and self-regulation (BRIEF-A GEC; P < .001), quality-of-life and everyday functioning (AIM-A domains [except relationship/communication]; P ≤ .028), and improved work productivity or efficacy (EWPS; P = .008). There were no significant treatment-effect differences in the AIM-A domain of relationship/communication (P = .276) or the DAS assessment of relationship satisfaction (P = .372). Most (52.3%) OROS methylphenidate-treated subjects and 24.2% of placebo-treated subjects were satisfied, very satisfied, or extremely satisfied with their treatment.
Nearly one-half of subjects in each treatment group did not designate an observer. Of those providing data, the LS mean (SE) change in ADHD Significant-Other Rating Scale was −10.0 (2.06) in the OROS methylphenidate group compared to −1.3 (1.78) in the placebo group (P = .003); LS mean change (SE) in executive function and self-regulation (BRIEF-A GEC) was −22.0 (3.92) in the OROS methylphenidate group and −9.0 (3.84) in the placebo group (P = .016).
With the exception of a greater reduction in daytime sleepiness (ESS) in the OROS methylphenidate-treatment group, no significant between-treatment differences were observed in other investigator-, subject-, or designated-observer-rated measures.
Safety Analyses
Table 3 summarizes the percentages of subjects in each group with a treatment-emergent adverse event and the adverse events reported in ≥ 5% of subjects in either treatment group. Severe events were reported in 6 subjects treated with OROS methylphenidate (3.4%; anxiety, restlessness, tension headache, fatigue, nervousness and feeling jittery, and gastroenteritis) and in 3 placebo-treated subjects (1.7%; headache and fatigue, insomnia, and increased blood pressure). One placebo-treated subject experienced a serious adverse event of suicidal ideation. No deaths occurred, and there were no clinically important results in relation to anxiety, depression, or suicidality measures.
DISCUSSION
OROS methylphenidate treatment for ADHD in adults, dosed to balance clinical remission and tolerability, produced a significantly (P < .001) greater decrease in ADHD symptoms, with significantly more subjects achieving remission (P = .0008) than placebo treatment. This is consistent with findings of a prior meta-analysis of randomized controlled trials enrolling children and adolescents that found higher remission rates with OROS methylphenidate in comparison to stimulant medications available at that time.17
In the current study, AISRS score reductions occurred with each OROS methylphenidate dose suggesting that although some adults may require US Food and Drug Administration maximum dosing (72 mg/d), others show a good response with doses as low as 18 mg/d. This broad range of dose effect suggests that clinicians should closely examine individual responses when adjusting the dose of OROS methylphenidate treatment. The numbers of subjects in the 18-mg and 36-mg dosage groups were smaller than the number in the 72-mg group. It is possible that the changes in group size due to dropouts and dose changes disproportionately impacted the time response patterns in the lower dose groups. ADHD symptom reduction, however, has been observed clinically during stabilized dose treatment and has been attributed to variability of ADHD symptoms over time. This same pattern, to a lesser extent, also occurred in the placebo arm.
OROS methylphenidate dosages used in clinical practice and studied have ranged from 18 to 108 mg/d.13-15,42,43 As seen in Figure 2, in the current study, there was a progressive decline in mean AISRS scores over the 6 weeks of treatment, with approximately one-half (62/143) of OROS methylphenidate-treated subjects receiving 72 mg/d and 20% each receiving 36 mg/d or 54 mg/d at the end point—with these later dosage groups (ie, 36 and 54 mg/d) having the greatest decrease from baseline to end point in their AISRS scores. This improvement over time as well as the waxing and waning of symptoms and responses suggests that clinicians may consider allowing a period of time, weeks or longer, between OROS methylphenidate dosage adjustments with continuous monitoring such that the benefits of a particular dose have sufficient time to emerge. The AISRS score improvement findings observed in this study with OROS methylphenidate doses ranging from 18 to 72 mg/d are similar to reductions in rating scales in previously published OROS methylphenidate studies performed in adults with ADHD.13,15,44
Subjects treated with OROS methylphenidate also reported greater improvement in the BRIEF-A GEC score and in AIM-A quality-of-life scores of “living with ADHD,” “general well-being,” “performance and daily functioning,” “impact of symptoms on bother/concern,” and “impact of symptoms on daily interference” compared with placebo-treated subjects. Interestingly, the only AIM-A quality-of-life scale in which the OROS methylphenidate and placebo groups did not differ was that of “relationships/communication.” They also did not differ in the DAS-Satisfaction subscale scores. Symptom reduction during treatment may lead to improved productivity (as evidenced by improvement in the EWPS) and, in turn, enhanced satisfaction with daily functioning; however, the skills for relationships and communication may require more time and/or specific therapy to demonstrate meaningful change. Improvement in relationships also requires that others accept that persons with ADHD are functioning more consistently. During treatment for ADHD, a progression may occur from symptom reduction, to performance improvement, to enhanced personal satisfaction, and finally, to relationship amendments, as seems to be the trajectory of improvement anecdotally observed in clinical practice.
Overall, the efficacy measures that were rated by investigators, subjects, and designated observers all reflected similar patterns of improvement in both treatment groups with greater improvement in the OROS methylphenidate group. However, little improvement occurred in the computerized neuropsychological battery, and there was no between-treatment difference. The lack of a between-treatment difference in the neuropsychological battery suggests that neuropsychological assessments may not accurately measure ADHD symptom improvement; however, to our knowledge, there are no data that rigorously prove why these evaluations and symptom responses do not correlate. In other studies as well as a meta-analysis of neuropsychological functioning, measures directly related to attention seem to exhibit the greatest consistency in improvement.14,45,46 The neuropsychological test measures in the current study were performed only at baseline and end point and, therefore, were of limited use for guiding dosing or gauging clinical response in this study. Other more frequently used symptom measures such as the AISRS and observer ratings were used to inform dose decisions. The choice to have neuropsychological test measures as the first end points in the gatekeeper sequence limits the conclusions that can be drawn from the secondary efficacy measures in this study.
Across the measures of sleep quality and quantity administered (PSQI, ESS, and actigraphy), OROS methylphenidate did not appear to affect sleep negatively in comparison to placebo. On the measure of daytime sleepiness (ESS score), the OROS methylphenidate group reported greater improvement than the placebo group (P = .007). No significant between-treatment difference in the exploratory PSQI scores for overall quality of sleep, in actigraphy results for sleep interval variables (ie, sleep onset latency, total and average activity, and sleep efficacy percentage), or for the active interval or daily interval variables was seen. However, higher percentages of OROS methylphenidate than placebo subjects reported insomnia (6.9% and 2.3%, respectively) and initial insomnia (9.8% and 1.7%, respectively). The discrepant results of a lack of difference in sleep efficiency, with higher reports of insomnia in the treated condition, suggest the need for additional study.
Limitations of the study include a sample size potentially inadequate for a robust assessment of other instruments, as it was based on expected effects on the AISRS. Many subjects had no designated observer. Some measures, particularly those requiring changes in social skills and interactions, may not be adequately assessed during a 6-week study. Several placebo-controlled trials or pooled analyses of trials involving adults with ADHD have reported substantial response rates among those treated with placebo, with several of these performing analyses to identify possible predictors of a greater or lower placebo response.47-51 Potential predictors of a greater placebo response have included a history of comorbid anxiety disorders,47 higher severity of ADHD symptoms, younger age, shorter time since diagnosis, and lower educational level.48 Study design characteristics may also play a role in the comparisons between active and placebo treatment.49 The placebo response observed in the present study may reflect a sample population not receiving ADHD-related medications at study entry, demonstrating some benefit from study-related monitoring and visits. Strengths of this study include the broad range of efficacy measures evaluated from a variety of perspectives and the use of a dose titration strategy that targeted symptom remission, subject to tolerability constraints. In addition, the population enrolled in this study represents the broad demographic range of adult subjects with ADHD seen by clinicians and internists, without excluding subjects with substance abuse, anxiety, or depression, as has been done in most ADHD treatment studies.
CONCLUSIONS
Treatment with OROS methylphenidate in individualized doses with the goal of symptom remission resulted in greater ADHD symptom reduction and a 50% greater remission rate in OROS methylphenidate-treated subjects compared to placebo. These data support the known efficacy of OROS methylphenidate treatment in the management of adults with ADHD and the importance of individualized dosing to achieve the goal of symptom remission.
Submitted: August 24, 2015; accepted January 25, 2016.
Online first: August 2, 2016.
Drug names: atomoxetine (Strattera and others), osmotic-release oral system methylphenidate (Concerta).
Potential conflicts of interest: Dr Goodman discloses the following financial considerations: consultant and/or reports associated with WebMD, Medscape, Temple University, American Professional Society of ADHD and Related Disorders, Neuroscience Education Institute, Children and Adults with ADHD Association, Shire, McNeil, Cephalon, Teva, Lundbeck, Janssen US and Canada, OptumInsight (Ingenix Pharmaceutical Services), Sunovion, Thomson Reuters, GuidePoint Global, Otsuka, Med-IQ, Novartis, Ironshore Pharmaceuticals, Neos Therapeutics, Rhodes Pharmaceuticals, Avacat, Major League Baseball, National Football League, American Physician Institute for Advanced Professional Studies, LLC, Healthequity Corporation, Prescriber’s Letter, Consumer Reports, and Pontifax. Dr Rostain is an employee of the University of Pennsylvania; has been on the scientific advisory boards of Pearson and Alcobra; has been a consultant to the State University of New York (SUNY) at Albany; has received grant/research support from the Agency for Healthcare Research and Quality and the National Institute of Mental Health; has made educational presentations/received honoraria from WebMD, MedScape, and the National Association for Continuing Education; and has received royalties from Routledge/Taylor Francis Group. Dr Armstrong discloses the following financial considerations relating to Johnson & Johnson: stockholder, stock options holder, and pension. Dr Ma is an employee of Johnson & Johnson Consumer, Inc, and is a Johnson & Johnson stockholder. Dr Ascher is an employee of Janssen Pharmaceutical Development, LLC, and is a stockholder of Johnson & Johnson as well as a holder of stock options. Dr Starr is an employee of Janssen Scientific Affairs, LLC, and is a Johnson & Johnson stockholder.
Funding/support: This study was funded by Ortho-McNeil Janssen Scientific Affairs, LLC.
Role of the sponsor: The funding agency developed and approved the study design and received regular updates on the progress of the study. The funding agency supported the data collection and analyzed the data. All authors, including those within the funding agency, collaborated on the interpretation of the data, writing of the manuscript, and decision to submit the paper for publication.
Previous presentation: Poster presented at the US Psychiatric & Mental Health Congress in Orlando, Florida, November 20, 2010.
Acknowledgments: The authors thank Ellen Stoltzfus, PhD, JK Associates, Inc, and Susan Ruffalo, PharmD, MedWrite, Inc, for providing medical writing and editorial assistance in preparation of this manuscript and Tony Mirra, BA, JK Associates, Inc, for project management support. This assistance was funded by Ortho-McNeil Janssen.
REFERENCES
1. Ebejer JL, Medland SE, van der Werf J, et al. Attention deficit hyperactivity disorder in Australian adults: prevalence, persistence, conduct problems and disadvantage. PLoS ONE. 2012;7(10):e47404. PubMed doi:10.1371/journal.pone.0047404
2. Faraone SV, Biederman J, Mick E. The age-dependent decline of attention deficit hyperactivity disorder: a meta-analysis of follow-up studies. Psychol Med. 2006;36(2):159-165. PubMed doi:10.1017/S003329170500471X
3. Gray S, Woltering S, Mawjee K, et al. The Adult ADHD Self-Report Scale (ASRS): utility in college students with attention-deficit/hyperactivity disorder. PeerJ. 2014;2:e324. PubMed doi:10.7717/peerj.324
4. Fayyad J, De Graaf R, Kessler R, et al. Cross-national prevalence and correlates of adult attention-deficit hyperactivity disorder. Br J Psychiatry. 2007;190(5):402-409. PubMed doi:10.1192/bjp.bp.106.034389
5. Polanczyk G, Rohde LA. Epidemiology of attention-deficit/hyperactivity disorder across the lifespan. Curr Opin Psychiatry. 2007;20(4):386-392. PubMed doi:10.1097/YCO.0b013e3281568d7a
6. Kessler RC, Adler L, Barkley R, et al. The prevalence and correlates of adult ADHD in the United States: results from the National Comorbidity Survey Replication. Am J Psychiatry. 2006;163(4):716-723. PubMed doi:10.1176/ajp.2006.163.4.716
7. Simon V, Czobor P, Bálint S, et al. Prevalence and correlates of adult attention-deficit hyperactivity disorder: meta-analysis. Br J Psychiatry. 2009;194(3):204-211. PubMed doi:10.1192/bjp.bp.107.048827
8. Safren SA, Sprich SE, Cooper-Vince C, et al. Life impairments in adults with medication-treated ADHD. J Atten Disord. 2010;13(5):524-531. PubMed doi:10.1177/1087054709332460
9. Kooij JJS, Bijlenga D. The circadian rhythm in adult attention-deficit/hyperactivity disorder: current state of affairs. Expert Rev Neurother. 2013;13(10):1107-1116. PubMed doi:10.1586/14737175.2013.836301
10. Pitts M, Mangle L, Asherson P. Impairments, diagnosis and treatments associated with attention-deficit/hyperactivity disorder (ADHD) in UK adults: results from the lifetime impairment survey. Arch Psychiatr Nurs. 2015;29(1):56-63. PubMed doi:10.1016/j.apnu.2014.10.001
11. Mick E, Faraone SV, Spencer T, et al. Assessing the validity of the Quality of Life Enjoyment and Satisfaction Questionnaire Short Form in adults with ADHD. J Atten Disord. 2008;11(4):504-509. PubMed doi:10.1177/1087054707308468
12. Wilens TE, Faraone SV, Biederman J. Attention-deficit/hyperactivity disorder in adults. JAMA. 2004;292(5):619-623. PubMed doi:10.1001/jama.292.5.619
13. Adler LA, Zimmerman B, Starr HL, et al. Efficacy and safety of OROS methylphenidate in adults with attention-deficit/hyperactivity disorder: a randomized, placebo-controlled, double-blind, parallel group, dose-escalation study. J Clin Psychopharmacol. 2009;29(3):239-247. PubMed doi:10.1097/JCP.0b013e3181a390ce
14. Biederman J, Mick E, Surman C, et al. A randomized, placebo-controlled trial of OROS methylphenidate in adults with attention-deficit/hyperactivity disorder. Biol Psychiatry. 2006;59(9):829-835. PubMed doi:10.1016/j.biopsych.2005.09.011
15. Medori R, Ramos-Quiroga JA, Casas M, et al. A randomized, placebo-controlled trial of three fixed dosages of prolonged-release OROS methylphenidate in adults with attention-deficit/hyperactivity disorder. Biol Psychiatry. 2008;63(10):981-989. PubMed doi:10.1016/j.biopsych.2007.11.008
16. Goodman D, Faraone SV, Adler LA, et al. Interpreting ADHD rating scale scores: linking ADHD rating scale scores and CGI levels in two randomized controlled trials of lisdexamfetamine dimesylate in ADHD. Prim Psychiatry. 2010;17(3):44-52.
17. Steele M, Jensen PS, Quinn DM. Remission versus response as the goal of therapy in ADHD: a new standard for the field? Clin Ther. 2006;28(11):1892-1908. PubMed doi:10.1016/j.clinthera.2006.11.006
18. Surman CBH, Adamson JJ, Petty C, et al. Association between attention-deficit/hyperactivity disorder and sleep impairment in adulthood: evidence from a large controlled study. J Clin Psychiatry. 2009;70(11):1523-1529. PubMed doi:10.4088/JCP.08m04514
19. Stein MA, Weiss M, Hlavaty L. ADHD treatments, sleep, and sleep problems: complex associations. Neurotherapeutics. 2012;9(3):509-517. PubMed doi:10.1007/s13311-012-0130-0
20. Kooij JJ, Middelkoop HA, van Gils K, et al. The effect of stimulants on nocturnal motor activity and sleep quality in adults with ADHD: an open-label case-control study. J Clin Psychiatry. 2001;62(12):952-956. PubMed doi:10.4088/JCP.v62n1206
21. American Psychiatric Association. Diagnostic and Statistical Manual for Mental Disorders. Fourth Edition, Text Revision. Washington, DC: American Psychiatric Association; 2000.
22. Adler L, Cohen J. Diagnosis and evaluation of adults with attention-deficit/hyperactivity disorder. Psychiatr Clin North Am. 2004;27(2):187-201. PubMed doi:10.1016/j.psc.2003.12.003
23. Kessler RC, Green JG, Adler LA, et al. Structure and diagnosis of adult attention-deficit/hyperactivity disorder: analysis of expanded symptom criteria from the Adult ADHD Clinical Diagnostic Scale. Arch Gen Psychiatry. 2010;67(11):1168-1178. PubMed doi:10.1001/archgenpsychiatry.2010.146
24. Sheehan DV, Lecrubier Y, Sheehan KH, et al. The Mini-International Neuropsychiatric Interview (MINI): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. 1998;59(suppl 20):22-33, quiz 34-57. PubMed
25. Spencer TJ, Adler LA, Meihua Qiao, et al. Validation of the Adult ADHD Investigator Symptom Rating Scale (AISRS). J Atten Disord. 2010;14(1):57-68. PubMed doi:10.1177/1087054709347435
26. Lipman RS. Differentiating anxiety and depression in anxiety disorders: use of rating scales. Psychopharmacol Bull. 1982;18(4):69-77. PubMed
27. Rush AJ, First MB, Blacker D. Handbook of Psychiatric Measures. 2nd ed. Arlington, VA: American Psychiatric Publishing, Inc; 2008.
28. Howieson DB, Lezak MD, Loring DW. Orientation and Attention. Neuropsychological Assessment. Oxford, UK: Oxford University Press; 2004:3365-3367.
29. Gualtieri CT, Johnson LG. Reliability and validity of a computerized neurocognitive test battery, CNS Vital Signs. Arch Clin Neuropsychol. 2006;21(7):623-643. PubMed doi:10.1016/j.acn.2006.05.007
30. Kessler RC, Adler L, Ames M, et al. The World Health Organization Adult ADHD Self-Report Scale (ASRS): a short screening scale for use in the general population. Psychol Med. 2005;35(2):245-256. PubMed doi:10.1017/S0033291704002892
31. Kessler RC, Adler LA, Gruber MJ, et al. Validity of the World Health Organization Adult ADHD Self-Report Scale (ASRS) Screener in a representative sample of health plan members. Int J Methods Psychiatr Res. 2007;16(2):52-65. PubMed doi:10.1002/mpr.208
32. McCandless S, O’ Laughlin L. The Clinical Utility of the Behavior Rating Inventory of Executive Function (BRIEF) and the diagnosis of ADHD. J Atten Disord. 2007;10(4):381-389. PubMed doi:10.1177/1087054706292115
33. Roth RM, Lance CE, Isquith PK, et al. Confirmatory factor analysis of the Behavior Rating Inventory of Executive Function-Adult version in healthy adults and application to attention-deficit/hyperactivity disorder. Arch Clin Neuropsychol. 2013;28(5):425-434. PubMed doi:10.1093/arclin/act031
34. Landgraf JM. Monitoring quality of life in adults with ADHD: reliability and validity of a new measure. J Atten Disord. 2007;11(3):351-362. PubMed doi:10.1177/1087054707299400
35. Endicott J, Nee J. Endicott Work Productivity Scale (EWPS): a new measure to assess treatment effects. Psychopharmacol Bull. 1997;33(1):13-16. PubMed
36. Spanier GB. Measuring dyadic adjustment: new scales for assessing the quality of marriage and similar dyads. J Marriage Fam. 1976;38(1):15-28. doi:10.2307/350547
37. DuPaul GJ, Power TJ, Anastopoulos AD, et al. ADHD Rating Scale-IV: Checklists, Norms and Clinical Interpretation. New York, NY: The Guilford Press; 1998.
38. Buysse DJ, Reynolds CF, Monk TH, et al. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric research and practice. Psychiatry Res. 1989;28(2):193-213. PubMed doi:10.1016/0165-1781(89)90047-4
39. Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. 1991;14(6):540-545. PubMed
40. MedDRA, International Federation of Pharmaceutical Manufacturers & Associations [IFPMA] on behalf of International Conference on Harmonization of Technical Requirements for Registration of Pharmaceuticals for Human Use [ICH], Geneva, Switzerland. http://www.meddra.org/. Accessed February 9, 2016.
41. Lindenmayer JP, Czobor P, Alphs L, et al; InterSePT Study Group. The InterSePT Scale for Suicidal Thinking reliability and validity. Schizophr Res. 2003;63(1-2):161-170. PubMed doi:10.1016/S0920-9964(02)00335-3
42. Rostain AL. Attention-deficit/hyperactivity disorder in adults: evidence-based recommendations for management. Postgrad Med. 2008;120(3):27-38. PubMed doi:10.3810/pgm.2008.09.1905
43. Reimherr FW, Williams ED, Strong RE, et al. A double-blind, placebo-controlled, crossover study of osmotic release oral system methylphenidate in adults with ADHD with assessment of oppositional and emotional dimensions of the disorder. J Clin Psychiatry. 2007;68(1):93-101. PubMed doi:10.4088/JCP.v68n0113
44. Buitelaar JK, Trott GE, Hofecker M, et al. Long-term efficacy and safety outcomes with OROS-MPH in adults with ADHD. Int J Neuropsychopharmacol. 2012;15(1):1-13. PubMed doi:10.1017/S1461145711001131
45. Bouffard R, Hechtman L, Minde K, et al. The efficacy of 2 different dosages of methylphenidate in treating adults with attention-deficit hyperactivity disorder. Can J Psychiatry. 2003;48(8):546-554. PubMed
46. Boonstra AM, Oosterlaan J, Sergeant JA, et al. Executive functioning in adult ADHD: a meta-analytic review. Psychol Med. 2005;35(8):1097-1108. PubMed doi:10.1017/S003329170500499X
47. Biederman J, Mick E, Spencer T, et al. Is response to OROS-methylphenidate treatment moderated by treatment with antidepressants or psychiatric comorbidity? a secondary analysis from a large randomized double blind study of adults with ADHD. CNS Neurosci Ther. 2012;18(2):126-132. PubMed doi:10.1111/j.1755-5949.2010.00233.x
48. Buitelaar JK, Sobanski E, Stieglitz RD, et al. Predictors of placebo response in adults with attention-deficit/hyperactivity disorder: data from 2 randomized trials of osmotic-release oral system methylphenidate. J Clin Psychiatry. 2012;73(8):1097-1102. PubMed doi:10.4088/JCP.11m07528
49. Faraone SV, Glatt SJ. A comparison of the efficacy of medications for adult attention-deficit/hyperactivity disorder using meta-analysis of effect sizes. J Clin Psychiatry. 2010;71(6):754-763. PubMed doi:10.4088/JCP.08m04902pur
50. Spencer T, Wilens T, Biederman J, et al. A double-blind, crossover comparison of methylphenidate and placebo in adults with childhood-onset attention-deficit hyperactivity disorder. Arch Gen Psychiatry. 1995;52(6):434-443. PubMed doi:10.1001/archpsyc.1995.03950180020004
51. Waxmonsky JG, Waschbusch DA, Glatt SJ, et al. Prediction of placebo response in 2 clinical trials of lisdexamfetamine dimesylate for the treatment of ADHD. J Clin Psychiatry. 2011;72(10):1366-1375. doi:10.4088/JCP.10m05979pur
This PDF is free for all visitors!
Save
Cite