Abstract
Objective: Aripiprazole lauroxil (AL), a long-acting injectable antipsychotic, has 2 initiation options: 1-day (AL NanoCrystal Dispersion [ALNCD] injection plus 30 mg oral aripiprazole on day 1 only) and 21-day (15 mg oral aripiprazole for 21 days). This post hoc analysis assessed the safety and tolerability of both initiation approaches.
Methods: We analyzed data from the first 4 weeks of 2 AL studies, one using the 1-day initiation regimen (conducted between November 2017 and March 2019) and the other using the 21-day initiation regimen (conducted between December 2011 and March 2014). Outcomes of interest during the matched 4-week period included the likelihood of adverse events (AEs), including those associated with discontinuation, rated as serious, or of special interest (injection site reactions [ISRs] and akathisia).
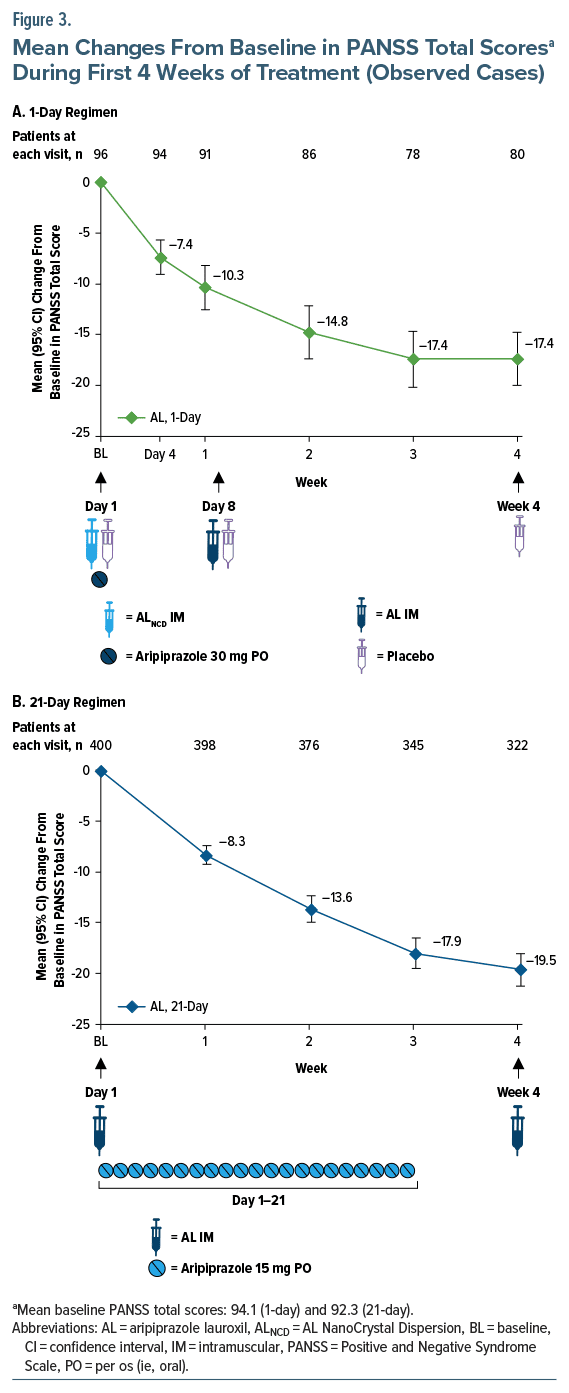
Results: The 1-day (n = 99) and 21-day (n = 415) initiation regimens had comparable rates of AEs (57.6% and 52.0%, respectively; most were mild), serious AEs (2.0% and 1.4%), and AEs leading to discontinuation (4.0% and 3.1%). The incidence of ISRs was 11.1% after the ALNCD injection (day 1) in the 1-day initiation regimen. ISR rates for the AL starting doses were 9.2% for the 1-day regimen (AL 1064 mg on day 8) and 3.9% for the 21-day regimen (AL 441 mg/882 mg on day 1). Rates of akathisia were 9.1% and 11.1% for the 1-day and 21-day regimens, respectively. One patient discontinued because of an ISR in the 21-day study, and 2 patients in the 21-day study discontinued because of akathisia. Mean changes from baseline in week 4 Positive and Negative Syndrome Scale total scores were −17.4 (1-day) and −19.5 (21-day).
Conclusions: Four-week safety and tolerability were similar following the initiation of AL with either the 1-day or 21-day regimen, supporting the utility of both initiation regimens. Engaging patients in discussions regarding options for initiating AL may help facilitate shared decision-making and personalization of treatment for patients with schizophrenia.
Trial Registration: ClinicalTrials.gov identifiers: NCT03345979 and NCT01469039
J Clin Psychiatry 2024;85(3):23m15132
Author affiliations are listed at the end of this article.
Patients with schizophrenia can start treatment with the long-acting injectable medication aripiprazole lauroxil (or AL) using either a 1-day or 21-day initiation regimen with their first dose of AL.
The 1-day approach was designed to provide the same drug concentrations as the 21-day regimen, but clinicians often ask whether the two approaches are equally safe.
We looked at the safety of the two initiation methods in separate studies and found that the numbers of people who had side effects were similar and most side effects were mild in both studies.
These results tell us that patients with schizophrenia and their doctors can choose between two ways of safely starting AL, either a 1-day initiation regimen or a 21-day initiation regimen.
Long-acting injectable (LAI) antipsychotics provide patients with continuous drug exposure over dosing intervals of up to 1 month or longer.1–3 Because LAI formulations are associated with a slow rate of dissolution, a challenge in starting any LAI versus an oral formulation is the delay in achieving plasma drug concentrations that are relevant for efficacy.1,4,5 This delay when starting LAIs can be mitigated in several ways, including by covering with oral antipsychotic doses for a period of time after the first administration of the LAI dose or by using a “loading dose” (starting with a higher initial dose of the LAI than would be used for maintenance treatment).1,6 The potential downsides of oral supplementation include complexity of the regimen, risk of dosing errors, and undetected nonadherence to the oral antipsychotic, which could reduce efficacy during initiation.4,7,8 The use of loading doses to initiate LAI antipsychotics has the potential to result in supratherapeutic plasma drug concentrations in individual patients due to genetic, drug interaction–, or disease-related changes in drug metabolism, and any consequent effects on safety and tolerability cannot be addressed rapidly with dose changes.6,9
An alternative approach to initiating LAI treatment is coadministration of a long-acting formulation optimized for faster dissolution. Such an approach achieves relevant plasma concentrations without multiple-day oral supplementation and avoids the risk of supratherapeutic antipsychotic plasma concentrations caused by a loading dose. The atypical LAI antipsychotic aripiprazole lauroxil (AL; Aristada; Alkermes, Inc, Waltham, MA), which is indicated for treatment of schizophrenia in adults, can be initiated using one of 2 regimens approved by the US Food and Drug Administration (FDA). AL can either be initiated as an intramuscular AL injection followed by 21 days of oral aripiprazole supplementation (21-day regimen) or, alternatively, initiated using a single intramuscular dose of the NanoCrystal Dispersion formulation of AL (ALNCD; Aristada Initio; Alkermes, Inc) and one 30-mg dose of oral aripiprazole administered together on day 1 (1-day regimen).4 ALNCD, developed for initiating AL treatment, contains the same prodrug as AL but is formulated for faster dissolution in the bloodstream.4 The use of the ALNCD injection to initiate AL treatment is not the same as a loading-dose strategy. A “loading dose” uses the exact same agent given at higher doses for the purpose of achieving relevant concentrations faster than simply giving a maintenance dose at the beginning of treatment.4 Oral overlap strategies for initiating LAI antipsychotics make up for the delay that occurs in reaching relevant antipsychotic blood concentrations due to slow drug release from LAI formulations (longer time to peak concentration) with the first injection. ALNCD was developed specifically to release drug at a rate closely matching the 21-day oral overlap initiation regimen.10 When using the 1-day regimen, the first injection of the chosen AL maintenance dose can be administered on the same day as the ALNCD injection or up to 10 days later.11 FDA approval of the 1-day regimen was based on a phase 1 pharmacokinetic study comparing the 1-day and 21-day regimens (Supplementary Figure 1A).4,10
The objective of this post hoc analysis was to assess the safety and tolerability of the 2 FDA-approved initiation regimens for AL. These 2 initiation options have been assessed for efficacy in separate clinical studies in acutely ill patients with schizophrenia.12,13 The AL pivotal study, which provided the basis for the initial FDA approval for AL 441 or 882 mg monthly, used the 21-day initiation regimen (15 mg of oral aripiprazole supplementation for 21 days, started on the day of the first AL dose). The second study evaluated the efficacy of a longer dosing interval of AL (1064 mg AL every 2 months) using the 1-day initiation regimen (a single ALNCD injection along with a single 30-mg dose of oral aripiprazole). These 2 studies had similar key enrollment criteria and study design features12,13 (Supplementary Table 1), thus allowing a cross-study, indirect comparison of the safety and tolerability of the 1-day and 21-day regimens. Using data from the first 4 weeks of treatment in these studies, the current post hoc analysis was conducted to assess the incidence and severity of common adverse events (AEs) associated with LAI antipsychotic treatment (particularly akathisia and injection site reactions [ISRs])14,15 and to evaluate the effectiveness of the 2 regimens based on schizophrenia symptom improvement after initiation.
METHODS
Data for this post hoc analysis were derived from 2 studies: the phase 3b ALPINE (Aripiprazole Lauroxil and Paliperidone palmitate: INitiation Effectiveness) study and the phase 3 pivotal study that supported the FDA’s approval of AL. ALPINE (1-day regimen study; ClinicalTrials.gov identifier NCT03345979) was conducted between November 2017 and March 201912; the AL pivotal study (21-day regimen study; ClinicalTrials.gov identifier NCT01469039) was conducted between December 2011 and March 2014.13 Both studies were designed and carried out in accordance with the principles of Good Clinical Practice that have their origin in the Declaration of Helsinki and its amendments16 and in accordance with local regulations and International Council for Harmonization guidelines.17 Study protocols were approved by the independent ethics committee/institutional review board for each study site. All patients provided written informed consent before participating.
Study Design and Patient Populations
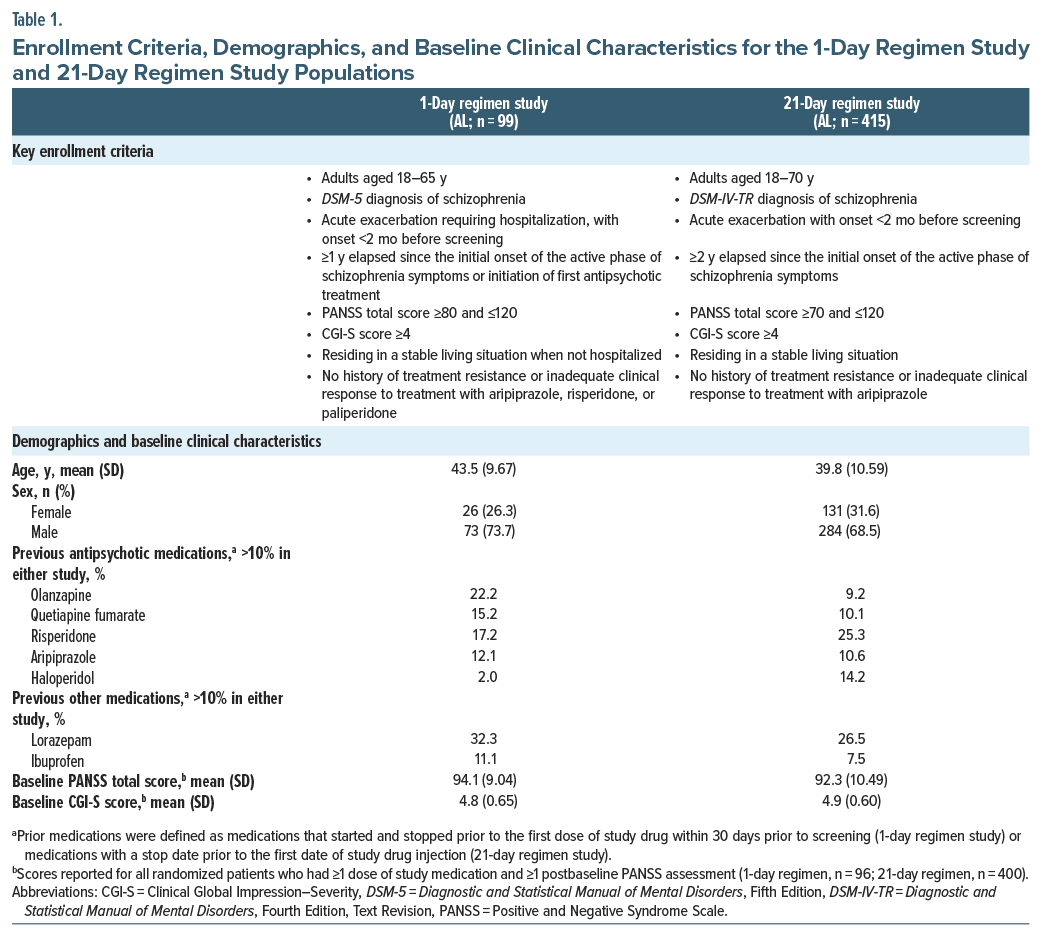
Study designs, treatment schedules, and assessments for the 2 studies were described in detail in their respective primary publications12,13 and are summarized in Supplementary Table 1. Key enrollment criteria for the 2 studies are listed in Table 1.
The 1-day regimen study was a 25-week, double blind efficacy and safety trial in patients with schizophrenia randomized to AL 1064 mg every 2 months, initiated using the 1-day regimen, or an active control (paliperidone palmitate).12 The 21-day regimen study was a 12-week, double-blind, randomized, controlled trial of 2 AL dose levels (441 or 882 mg monthly), started with the 21-day regimen, versus placebo in patients with schizophrenia.13 This post hoc analysis examined safety and tolerability, as well as effectiveness, associated with the 2 AL initiation regimens, and only data from the first 4 weeks of each study were considered (Figure 1). Both studies began with an inpatient period that included screening and washout, followed by randomization and treatment initiation on study day 1. Patients were discharged at least 2 weeks after treatment initiation and followed as outpatients for the remainder of their respective study.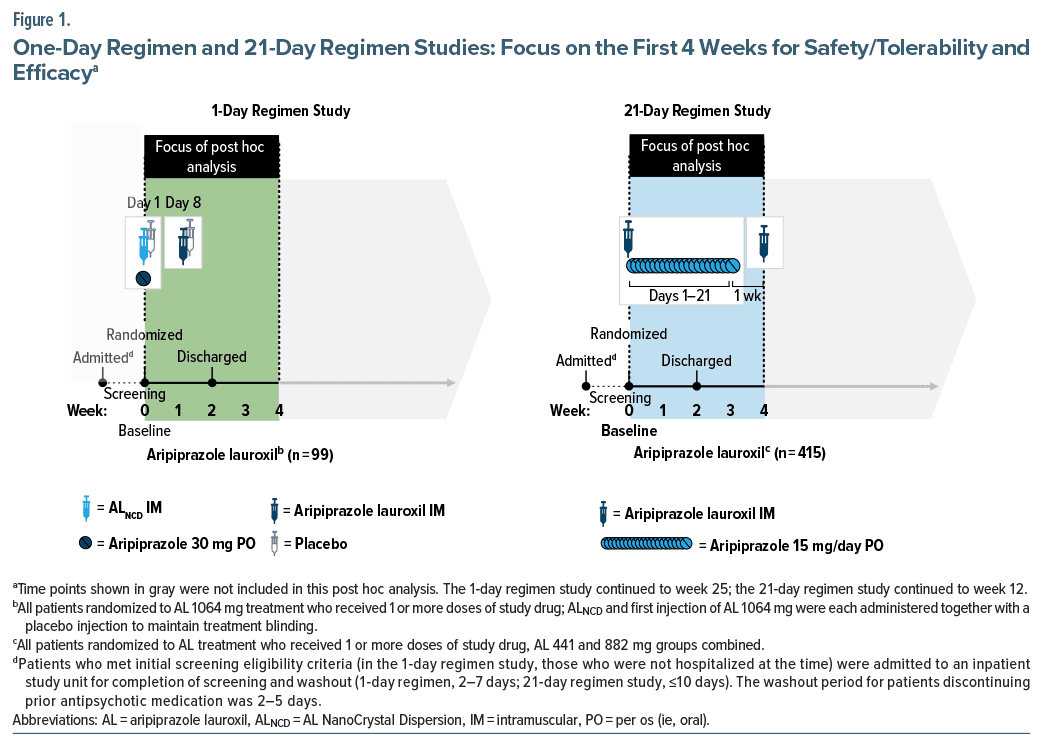
Both studies enrolled adults with a diagnosis of schizophrenia based on criteria from the Diagnostic and Statistical Manual of Mental Disorders (Fifth Edition18 [1-day] or Fourth Edition, Text Revision19 [21-day]) who had experienced an onset of acute exacerbation <2 months before screening. Patients enrolled in the 1-day regimen study additionally required hospitalization for their symptoms. The post hoc analysis included all patients randomized to AL who received ≥1 dose of study drug (Supplementary Figure 2); other randomized groups (active control and placebo in the 1-day and 21-day regimen studies, respectively) were excluded. The fixed dose AL groups in the 21-day regimen study were pooled for the 21-day regimen outcomes reported here, given the lack of any differential findings associated with efficacy or safety with the 2 AL doses administered in that study.13
Study Treatment Through Week 4
In patients who had no prior exposure to aripiprazole, tolerability was assessed in both studies using a test dose of oral aripiprazole (5 mg) administered daily for 2 days before randomization.12,13
1-Day regimen. Patients randomly assigned to AL treatment in the 1-day regimen study received an initiation regimen consisting of intramuscular ALNCD plus a single 30- mg oral aripiprazole tablet on day 1 followed by their initial AL 1064-mg injection administered on day 8. The rationale for administering the first AL dose on day 8 (rather than on day 1 with ALNCD) was to maintain blinding against the active comparator. Patients received an additional dose of AL 1064 mg every 8 weeks thereafter (last dose, week 17).
In the 1-day regimen study, placebo injections were used to maintain blinding of AL or active control treatment assignment; there was no placebo treatment group. Because initiation of the 2 treatments required injections in different sites (gluteal vs deltoid), patients were administered a placebo injection at the alternate site with the day 1 and day 8 injections of AL or active control.
21-Day regimen. Patients randomly assigned to AL in the 21-day regimen study received their first AL injection (441 or 882 mg) on day 1, with oral aripiprazole supplementation beginning on day 1 and continuing for 21 days, at a dose of 15 mg/day. Adherence to the last week of the 21-day oral aripiprazole regimen prescribed after discharge was estimated using pill counts. The second AL injection was administered on day 29, and the last dose was administered on day 57.
Assessments Included in Post Hoc Analyses
Safety and tolerability during the first 4 weeks of each study were assessed based on rates of AEs, AEs leading to discontinuation, and serious AEs (SAEs). (AEs reported during the first 4 weeks of the 1-day regimen study were also summarized in the primary publication; in the 21-day regimen study primary publication, AEs were summarized for the full 12-week study period.) AEs of special interest in the current analysis were ISRs, akathisia, and AEs in the Medical Dictionary for Regulatory Activities System Organ Class category of psychiatric disorders. For the 1-day regimen study, rates of ISRs associated with the ALNCD injection and with the initial AL injection were assessed and reported separately, excluding ISRs associated with placebo injections. For the 21-day regimen study, ISRs associated with the initial AL injection only were assessed. Assessments of akathisia included time to onset relative to the first dose of study drug, severity, and numbers of patients who received treatment for AEs of akathisia.
Efficacy over the first 4 weeks of treatment was assessed in each study using the Positive and Negative Syndrome Scale (PANSS)20 and the Clinical Global Impression–Severity (CGI-S)21 scale.
Statistical Analysis
Safety and efficacy end points during the first 4 weeks of treatment were summarized descriptively for the 1-day and 21-day regimens. Because of the between-study nature of this analysis, no inferential statistics were calculated. Analyses were based on observed data; missing data were not imputed.
RESULTS
Patients
A total of 514 patients initiating AL treatment were included in the analysis, including 99 patients from the 1-day regimen study and 415 from the 21-day regimen study (Supplementary Figure 2). All patients treated with AL in the 21-day regimen study were adherent to oral aripiprazole dosing based on returned pill counts. Demographics and baseline clinical characteristics for the study populations are summarized in Table 1. Mean (SD) ages at baseline were 43.5 (9.67) and 39.8 (10.59) years for patients administered the 1-day and 21-day regimens, respectively; 73.7% and 68.4% of patients, respectively, were male. Baseline clinical characteristics were comparable between patients receiving the 2 initiation regimens; mean (SD) baseline PANSS total scores were 94.1 (9.04) and 92.3 (10.49) for the 1-day and 21-day regimens, respectively.
Safety Outcomes
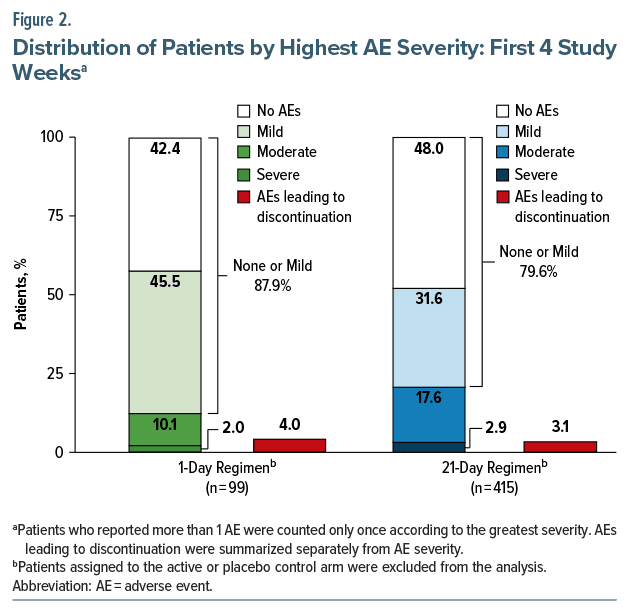
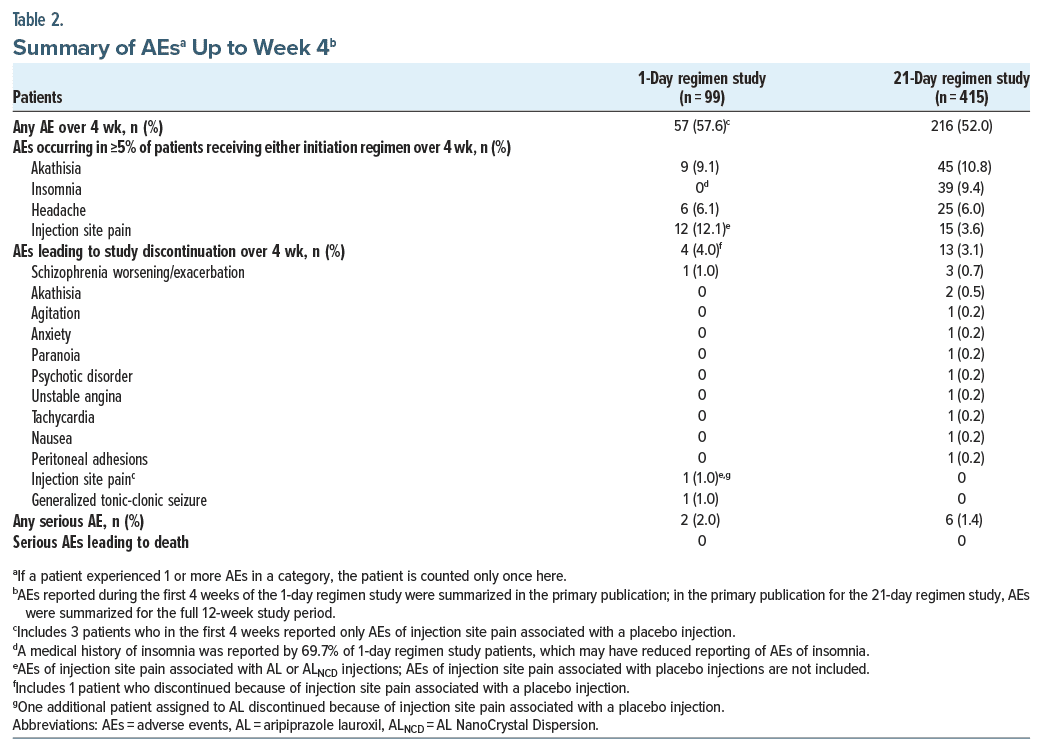
Rates of AEs over the first 4 weeks of AL treatment were 57.6% (57/99) for patients administered the 1-day regimen and 52.0% (216/415) for those administered the 21-day regimen (Table 2). For both regimens, AEs reported during the first 4 weeks of AL treatment were predominantly mild or moderate in severity (Figure 2). Through week 4, 2.0% (2/99) of patients who received the 1-day regimen and 2.9% (12/415) who received the 21-day regimen reported severe AEs.
The most common AEs, reported by ≥5% of patients receiving the 1-day regimen, were injection site pain (12.1%, associated with AL or ALNCD injections but excluding injection site pain after placebo injections), akathisia (9.1%), and headache (6.1%) (Table 2). For patients receiving the 21-day regimen, the most common AEs were akathisia (10.8%), insomnia (9.4%), and headache (6.0%). Few patients reported SAEs during the first 4 weeks of treatment (1-day, 2.0% [2/99]; 21-day, 1.4% [6/415]). For patients receiving the 1-day regimen, 1 SAE each of schizophrenia worsening or exacerbation and generalized tonic-clonic seizure were reported, both assessed as possibly related to study treatment. For those receiving the 21-day regimen, one SAE each of unstable angina, peritoneal adhesions, appendicitis, hypoglycemia, drug abuse, and akathisia were reported over the first 4 weeks. Only the SAE of akathisia was assessed as related to study treatment. AEs resulted in discontinuation of 4.0% (4/99) of patients administered the 1-day regimen and 3.1% (13/415) of those receiving the 21-day regimen (Table 2). No deaths were reported in AL-treated patients in either study.
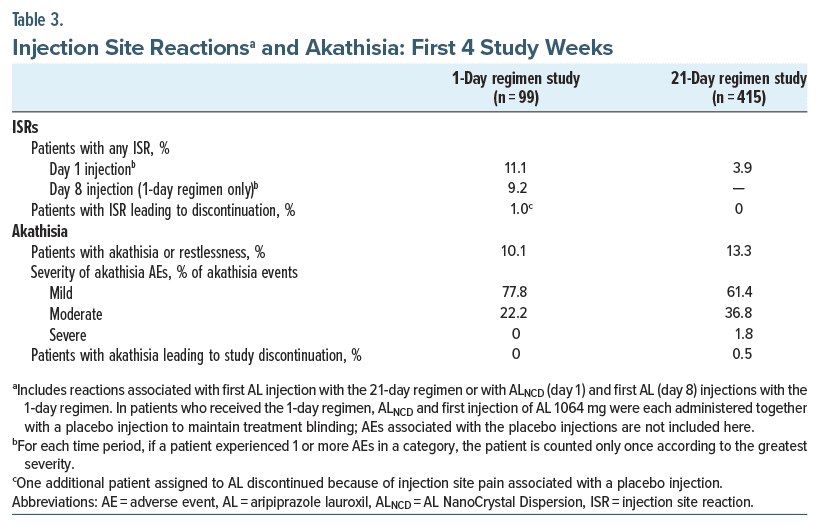
ISRs were associated most frequently with the first AL injection administered for each regimen. ISRs were reported by 11.1% (11/99) and 3.9% (16/415) of patients after day 1 injections of the 1-day initiation regimen (ALNCD) and the 21-day initiation regimen (AL 441 or 882 mg), respectively (Table 3; Supplementary Table 2). The day 8 injection in the 1-day regimen study (AL 1064 mg) was associated with ISRs in 9.2% (8/87) of patients. ISRs experienced after the 1-day regimen injection were mild (22 ISRs) or moderate (3 ISRs) in severity; 1 patient discontinued because of an AE of injection site pain of moderate severity after receiving the ALNCD injection. All but one of the ISRs in patients administered the 21-day regimen were mild (one was moderate in severity); no patient receiving the 21-day regimen was discontinued because of an ISR.
Akathisia occurred in 9.1% (9/99) of patients receiving the 1-day regimen and 10.8% (45/415) of patients receiving the 21-day regimen; restlessness was reported in 1.0% (1/99) of patients receiving the 1-day regimen and 2.4% (10/415) of patients receiving the 21-day regimen (Table 3; Supplementary Table 2). Median (95% CI) times to onset of AEs of akathisia or restlessness were 2.5 (1.0–8.0) days (1-day regimen) and 7.0 (5.0–9.0) days (21-day regimen). All AEs of akathisia or restlessness following the 1-day regimen were mild or moderate in severity; one severe AE of akathisia was reported for a patient receiving the 21-day regimen. No patient using the 1-day regimen and 2/415 (0.5%) patients on the 21-day regimen discontinued treatment because of akathisia in the first 4 weeks.
Psychiatric disorder AEs were reported by 5.1% (5/99) of patients receiving the 1-day regimen and 18.3% (76/415) of those receiving the 21-day regimen in the first 4 weeks of AL treatment. Psychiatric disorder AEs reported by ≥1% of patients up to week 4 with the 1-day regimen were restlessness (1.0% [1/99]), schizophrenia worsening or exacerbation (1.0% [1/99]), agitation (3.0% [3/99]), and anhedonia (1.0% [1/99]) and with the 21-day regimen were insomnia (9.4% [39/415]), anxiety (3.4% [14/415]), restlessness (2.4% [10/415]), schizophrenia worsening or exacerbation (1.4% [6/415]), agitation (1.2% [5/415]), and suicidal ideation (1.0% [4/415]).
Efficacy Outcomes
Improvements in PANSS total scores from baseline to week 4 were observed in both the 1-day regimen and 21-day regimen studies. Mean (95% CI) changes from baseline to week 4 in PANSS total score based on observed cases were −17.4 (−20.0 to −14.8) for patients who received the 1-day regimen (Figure 3A) and −19.5 (−21.1 to −17.9) for patients initiating AL with the 21- day regimen (Figure 3B). Mean (95% CI) changes from baseline in CGI-S at week 4 were −1.1 (−1.3 to −0.9) for AL using the 1-day regimen and −1.2 (−1.3 to −1.0) for AL using the 21-day regimen (Supplementary Figure 3A and 3B).
DISCUSSION
The results of this post hoc analysis support the use of both the 1-day and 21-day initiation regimens as safe, tolerable, and effective options for initiating AL. These options allow for flexibility in choosing the initiation regimen to account for factors such as patient preference, care setting, and access to support systems. An overlapping oral supplementation regimen for starting LAI treatment may be preferred in certain circumstances, such as if the clinician plans to use a different oral dose during AL initiation than the 30-mg aripiprazole dose administered in the 1-day initiation regimen22 or if the patient would prefer 1 less injection and there are no adherence concerns.4 Alternatively, clinicians may elect to fully initiate AL treatment in 1 day (eg, in a single office visit or during an inpatient stay) using ALNCD and AL injections together with the single 30-mg dose of oral aripiprazole. Importantly, the 1-day initiation regimen (ALNCD + 30-mg oral aripiprazole) is not a loading-dose strategy; it is a titration strategy specifically developed to mimic the pharmacokinetics that are operant when using oral antipsychotic supplementation with the 21-day initiation regimen. ALNCD has a faster dissolution rate than AL; plasma aripiprazole concentrations peak within approximately 27 days of ALNCD administration5 compared with 6–7 weeks with AL.23 Therefore, the 1-day regimen obviates the need for continued oral antipsychotic dosing after discharge (avoiding undetected nonadherence),12 provides continuous exposure from day 1,10 and forestalls concerns about potential supratherapeutic plasma drug concentrations associated with a loading dose.6
The 1-day and 21-day initiation regimens for AL had comparable safety and tolerability profiles based on AE rates and severity, incidence of SAEs, and rates of AEs leading to discontinuation in the first 4 weeks after initiation. In addition, the 2 initiation regimens resulted in similar improvements in PANSS total and CGI-S scores. These results are consistent with pharmacokinetic modeling and phase 1 study data in which the ranges of plasma aripiprazole concentrations from the 1-day and 21-day regimens were overlapping.10
ISRs were of particular interest in this analysis, owing to a common apprehension among healthcare professionals and patients receiving LAI treatment that the 1-day regimen’s increased number of injections could result in a greater number of ISRs.24,25 The 1-day regimen was associated with a numerically higher rate of ISRs. Most were mild in severity, and only 1 patient treated with the 1-day regimen discontinued because of an ISR after the ALNCD injection. It is important to note that although the use of the 1-day regimen requires an additional injection when initiating AL treatment in clinical practice, the 1-day regimen study design required 3 additional injections: the ALNCD injection on day 1 and placebo injections on days 1 and 8 to maintain blinding. The fact that the 1-day regimen group received an additional injection in the regimen and placebo injections at each visit presented a challenge for reporting ISRs. This methodologic difference suggests caution in interpreting the incidence of ISRs with each regimen. Differences between the 2 studies in the dosage strengths used also may have contributed to the number of observed ISRs. The AL 1064-mg dosage strength used in the 1-day regimen study required a greater injection volume (3.9 mL) compared with the dosage strengths used in the 21-day regimen study (441 mg, 1.6 mL; 882 mg, 3.2 mL).26 However, incidences of ISRs were comparable for the 441-mg q4wk, 882-mg q6wk, and 1064-mg q8wk AL regimens when assessed head-to head within a single-phase 1 study.27 The ALNCD injection also differed from the AL 441-mg and 882-mg injections with respect to injection volume (2.4 mL28) and potentially other characteristics such as viscosity. Additionally, some patients in the 21-day regimen study may have received AL injections using a smaller-diameter needle compared with those in the 1-day regimen study (23 vs 20 gauge22,28). In both studies, injection providers were fully trained in administration to ensure consistency in their technique.
Akathisia is commonly associated with LAI and oral antipsychotics14,15,29,30 and was of interest in this analysis because there is a perception that the total amount of drug administered within 1–10 days (as in the 1-day regimen) may increase the incidence of akathisia. Akathisia is generally observed early in treatment14,31 and is more likely to be reported at higher antipsychotic doses and with rapid initiation of treatment.14,15 Little difference in the occurrence or severity of akathisia was observed in this analysis between the 2 regimens, again consistent with the observed overlap in plasma aripiprazole concentrations in the pharmacokinetic study that compared the 2 regimens.10 For each regimen, akathisia was generally mild or moderate in severity and reported early in treatment. The median time to the first episode of akathisia or restlessness was shorter with the 1-day regimen than with the 21-day regimen but was within the first week in both studies.
Interpretation of the results of this post hoc analysis is limited because the regimens were assessed in 2 separate studies that, although similar in design, differed in some study procedures and enrollment criteria. Eligibility criteria for the 1-day regimen study included a shorter duration since the initial onset of active-phase schizophrenia symptoms compared with the 21-day regimen study (≥1 vs ≥2 years, respectively). Participants in the 1-day regimen study were required to be hospitalized for their current exacerbation of symptoms, whereas in the 21-day study, participants did not have this requirement. However, differences in study entry criteria were unlikely to have affected results, given that the mean severity of symptom scores at baseline was comparable for patients from the 2 studies. In the 1-day regimen study, the first AL injection was administered on day 8 to maintain study blinding and was not combined with the ALNCD injection on day 112; thus, these results may not reflect the real-world incidence of AEs when both AL and ALNCD are administered on the same day. Based on pharmacokinetic modeling analysis11 and consistent with AL prescribing information,22 the first AL injection can be administered up to 10 days after the ALNCD injection and still maintain clinically relevant plasma aripiprazole exposures associated with efficacy (Supplementary Figure 1B). The AL doses initiated using the 1-day and 21-day regimens differed (AL 1064 mg every 2 months and AL 441 or 882 mg monthly, respectively); however, pharmacokinetic modeling indicates that plasma aripiprazole concentrations resulting from the 1-day regimen are similar through 2 weeks for all AL doses and remain similar for AL 1064 mg every 2 months and AL 882 mg monthly through at least week 4.11 Additional potential confounders include the smaller study population who used the 1-day regimen and inclusion of an active control versus a placebo control. Raters’ observations were conducted in the context of the 100% likelihood of patients receiving an active drug in the 1-day regimen study vs 67% likelihood in the 21-day regimen study, which may have affected efficacy findings and AE reporting. The inclusion of a placebo vs an active control group also could affect perception of the blinding.
CONCLUSIONS
Both 1-day and 21-day initiation regimens were generally well tolerated, with safety profiles through 4 weeks consistent with the known profiles for aripiprazole and AL; no unexpected safety findings were observed. Rates of reported akathisia (including SAEs and discontinuations due to akathisia) were comparable for the 1-day and 21-day regimens. Both initiation regimens were efficacious, with no meaningful differences observed through 4 weeks. Results from this post hoc analysis support the safety and utility of both the 1-day and 21- day regimens. Engaging patients in discussion regarding options for initiating AL may help facilitate shared decision-making and personalization of treatment for patients with schizophrenia.
Article Information
Published Online: August 12, 2024. https://doi.org/10.4088/JCP.23m15132
© 2024 Physicians Postgraduate Press, Inc.
Submitted: October 2, 2023; accepted June 6, 2024.
To Cite: Sommi RW, Saklad SR, Weiden PJ, et al. Initiating aripiprazole lauroxil: post hoc analysis of safety and tolerability of 1-day and 21-day regimens. J Clin Psychiatry. 2024;85(3):23m15132.
Author Affiliations: School of Pharmacy, University of Missouri-Kansas City, Kansas City, Missouri (Sommi); Division of Pharmacotherapy, College of Pharmacy, The University of Texas at Austin, San Antonio, Texas (Saklad); Renaissance School of Medicine at Stony Brook University, Stony Brook, New York (Weiden); Alkermes, Inc, Waltham, Massachusetts (Still, Wang, Yagoda).
Corresponding Author: Sergey Yagoda, MD, PhD, Alkermes, Inc, 900 Winter St, Waltham, MA 02451 ([email protected]).
Author Contributions: Study design: (Weiden); principal investigator: (Weiden) (ALPINE); data analysis: (Wang, Weiden); data interpretation, manuscript preparation, manuscript review and revisions, and final approval of manuscript: all authors.
Relevant Financial Relationships: Dr Sommi is Emeritus Professor of Pharmacy and Psychiatry at the University of Missouri-Kansas City Schools of Pharmacy and Medicine and Pharmacy Consultant to the Missouri Department of Mental Health; has received grant support from or is a consultant or speaker for Alkermes, AstraZeneca, Auspex, Avanir, Boots, Boehringer Ingelheim, Bristol Myers Squibb, Eli Lilly, Forest, Hoechst Marion Roussel, Intracellular Therapies, Janssen, Karuna, Merck, Neurocrine, NIMH, Novartis, Ortho-McNeil Janssen, Otsuka, Pfizer, Sanofi-Aventis, Shire, SmithKline Beecham, Solvay, Sunovion, Teva, Upjohn, and Wyeth-Ayerst; serves on the Business Development Council for the American Association of Psychiatric Pharmacists; has served as a defendant and plaintiff expert witness; and has no direct stock ownership in any pharmaceutical corporation. Dr Saklad is an employee of The University of Texas at Austin College of Pharmacy; is appointed to the Texas Health and Human Services Commission, San Antonio State Hospital, and UT Health San Antonio Long School of Medicine; has consulted for Alkermes, BioXcel, Genomind, Janssen, Karuna, and Otsuka; has participated on speakers bureaus for BioXcel, Neurocrine, Otsuka PsychU, Teva, Texas Society of Health-System Pharmacists, and several professional organizations; serves on the Business Development Council for the College of Psychiatric and Neurologic Pharmacists; has served as a defendant and plaintiff expert witness; and has no direct stock ownership in any pharmaceutical corporation. Dr Weiden has been a consultant for Alkermes, Lyndra, MapLight, and Teva and was employed by Alkermes at the time of the study and may be a shareholder. Drs Still, Wang, and Yagoda are or were employees of Alkermes and may be shareholders.
Funding/Support: This study was sponsored by Alkermes, Inc (Waltham, Massachusetts).
Role of the Sponsor: Alkermes, Inc, is a pharmaceutical company that developed and markets aripiprazole lauroxil, used to improve mood, thoughts, and behaviors in adults who have schizophrenia, in the United States, and funded this study. Alkermes, Inc, was involved in the design, collection, and analysis of the data. Interpretation of the results was performed by the authors, and the decision to submit the manuscript for publication was made by the authors.
Previous Presentation: Poster presented at the College of Psychiatric and Neurologic Pharmacists (CPNP) 2020 Annual Meeting (Virtual); April 27, 2020.
Acknowledgments: Medical writing and editorial support were provided by Kathleen M. Dorries, PhD, and John H. Simmons, MD, of Peloton Advantage, LLC, an OPEN Health company, and funded by Alkermes, Inc. The authors also thank Mark S. Todtenkopf, PhD, of Alkermes, Inc, who assisted in the preparation and proofreading of the manuscript. Mark S. Todtenkopf, PhD, is an employee of Alkermes, Inc, and may also own stock.
Supplementary Material: Available at Psychiatrist.com.
Clinical Points
- Safety and tolerability profiles were similar for the 1-day and 21-day strategies for initiating AL.
- Choosing a 1-day regimen allows clinicians to initiate AL quickly and safely.
- Complexities of oral overlap are potentially reduced with a 1-day initiation regimen.
References (31)

- Brissos S, Veguilla MR, Taylor D, et al. The role of long-acting injectable antipsychotics in schizophrenia: a critical appraisal. Ther Adv Psychopharmacol. 2014;4(5):198–219. PubMed CrossRef
- Hard ML, Mills RJ, Sadler BM, et al. Pharmacokinetic profile of a 2-month dose regimen of aripiprazole lauroxil: a phase I study and a population pharmacokinetic model. CNS Drugs. 2017;31(7):617–624. PubMed
- Ravenstijn P, Remmerie B, Savitz A, et al. Pharmacokinetics, safety, and tolerability of paliperidone palmitate 3-month formulation in patients with schizophrenia: a phase-1, single-dose, randomized, open-label study. J Clin Pharmacol. 2016;56(3):330–339. PubMed CrossRef
- Jain R, Meyer J, Wehr A, et al. Size matters: the importance of particle size in a newly developed injectable formulation for the treatment of schizophrenia. CNS Spectr. 2020;25(3):323–330. PubMed CrossRef
- Ehret MJ, Davis E, Luttrell SE, et al. Aripiprazole lauroxil NanoCrystal® dispersion technology (Aristada Initio®). Clin Schizophr Relat Psychoses. 2018;12(2):92–96. PubMed CrossRef
- Preskorn SH. How loading dose strategies for depot paliperidone can go wrong. J Psychiatr Pract. 2022;28(2):130–137. PubMed CrossRef
- Masand PS, Roca M, Turner MS, et al. Partial adherence to antipsychotic medication impacts the course of illness in patients with schizophrenia: a review. Prim Care Companion J Clin Psychiatry. 2009;11(4):147–154. PubMed CrossRef
- Robinson D, Woerner MG, Alvir JM, et al. Predictors of relapse following response from a first episode of schizophrenia or schizoaffective disorder. Arch Gen Psychiatry. 1999;56(3):241–247. PubMed CrossRef
- Morabito BD, Paulison B. Drug-induced parkinsonism: a case report. Ment Health Clin. 2017;7(2):65–68. PubMed
- Hard ML, Wehr AY, Du Y, et al. Pharmacokinetic evaluation of a 1-day treatment initiation option for starting long-acting aripiprazole lauroxil for schizophrenia. J Clin Psychopharmacol. 2018;38(5):435–441. PubMed
- Hard ML, Wehr AY, Sadler BM, et al. Population pharmacokinetic analysis and model-based simulations of aripiprazole for a 1-day initiation regimen for the long acting antipsychotic aripiprazole lauroxil. Eur J Drug Metab Pharmacokinet. 2018;43(4):461–469. PubMed CrossRef
- Weiden PJ, Claxton A, Kunovac J, et al. Efficacy and safety of a 2-month formulation of aripiprazole lauroxil with 1-day initiation in patients hospitalized for acute schizophrenia transitioned to outpatient care: phase 3, randomized, double-blind, active control ALPINE study. J Clin Psychiatry. 2020;81(3):19m13207. PubMed CrossRef
- Meltzer HY, Risinger R, Nasrallah HA, et al. A randomized, double-blind, placebo controlled trial of aripiprazole lauroxil in acute exacerbation of schizophrenia. J Clin Psychiatry. 2015;76(8):1085–1090. PubMed CrossRef
- Yoshimura B, Sato K, Sakamoto S, et al. Incidence and predictors of acute akathisia in severely ill patients with first-episode schizophrenia treated with aripiprazole or risperidone: secondary analysis of an observational study. Psychopharmacology (Berl). 2019;236(2):723–730. PubMed CrossRef
- Jouini L, Ouali U, Ouanes S, et al. Akathisia among patients undergoing antipsychotic therapy: prevalence, associated factors, and psychiatric impact. Clin Neuropharmacol. 2022;45(4):89–94. PubMed
- World Medical Association. Declaration of Helsinki—ethical principles for medical research involving human subjects. World Medical Association; 2022. Accessed February 13, 2023. https://www.wma.net/policies-post/wmadeclaration-of-helsinki-ethical-principles-for-medical-research-involving-humansubjects
- European Medicines Agency. International conference on harmonisation of technical requirements for registration of pharmaceuticals for human use (ICH). E6 R2. Guideline for good clinical practice. European Medicines Agency; 2017. Accessed February 13, 2023. https://www.ema.europa.eu/en/ich-e6-r2-goodclinical-practice-scientific-guideline
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. American Psychiatric Publishing; 2013.
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, Text Revision: DSM-IV-TR. 4th ed. American Psychiatric Association; 2000.
- Kay SR, Fiszbein A, Opler LA. The positive and negative Syndrome scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13(2):261–276. PubMed CrossRef
- Guy W. CGI Clinical Global Impressions. ECDEU Assessment Manual for Psychopharmacology. US Department of Health, Education, and Welfare, National Institute of Mental Health; 1976:217–222.
- Aristada [package insert]. Waltham, MA: Alkermes, Inc; 2023.
- Hard ML, Mills RJ, Sadler BM, et al. Aripiprazole lauroxil: pharmacokinetic profile of this long-acting injectable antipsychotic in persons with schizophrenia. J Clin Psychopharmacol. 2017;37(3):289–295. PubMed CrossRef
- Jaeger M, Rossler W. Attitudes towards long-acting depot antipsychotics: a survey of patients, relatives and psychiatrists. Psychiatry Res. 2010;175(1–2):58–62. PubMed CrossRef
- Geerts P, Martinez G, Schreiner A. Attitudes towards the administration of long acting antipsychotics: a survey of physicians and nurses. BMC Psychiatry. 2013;13:58. PubMed CrossRef
- Farwick S, Hickey MB, Jacobs G, et al. Best practices for aripiprazole lauroxil administration: from formulation development to injection technique. J Psychiatr Pract. 2019;25(2):82–90. PubMed
- Weiden PJ, Du Y, von Moltke L, et al. Pharmacokinetics, safety, and tolerability of a 2-month dose interval regimen of the long-acting injectable antipsychotic aripiprazole lauroxil: results from a 44-week phase i study. CNS Drugs. 2020;34(9):961–972. PubMed CrossRef
- Aristada Initio [package insert]. Waltham, MA: Alkermes, Inc; 2023.
- Thomas JE, Caballero J, Harrington CA. The incidence of akathisia in the treatment of schizophrenia with aripiprazole, asenapine and lurasidone: a meta analysis. Curr Neuropharmacol. 2015;13(5):681–691. PubMed CrossRef
- Chow CL, Kadouh NK, Bostwick JR, et al. Akathisia and newer second-generation antipsychotic drugs: a review of current evidence. Pharmacotherapy. 2020;40(6):565–574. PubMed CrossRef
- Demyttenaere K, Detraux J, Racagni G, et al. Medication-induced akathisia with newly approved antipsychotics in patients with a severe mental illness: a systematic review and meta-analysis. CNS Drugs. 2019;33(6):549–566. PubMed
This PDF is free for all visitors!
Save
Cite