Objective: INP105 is a drug-device combination of olanzapine and technology that delivers a powder formulation of olanzapine to the vascular-rich upper nasal space. This study evaluated the pharmacokinetics, pharmacodynamics, safety, and tolerability of single ascending doses of INP105, olanzapine intramuscular (OLZ IM), and olanzapine oral disintegrating tablet (OLZ ODT).
Methods: This was a phase 1, active and double-blind placebo comparator-controlled, ascending-dose, 2-period, incomplete-block, 1-way crossover study in 40 healthy subjects, randomized to single doses of OLZ IM (5 or 10 mg) or OLZ ODT (10 mg) in Period 1 and then 1 of 3 doses (5 mg, 10 mg, or 15 mg) of INP105 or placebo in Period 2 between July and October 2018. Sedation and attention were evaluated by visual analog scale (VAS), the Agitation/Calmness Evaluation Scale (ACES), and the Digit Symbol Substitution Test (DSST).
Results: At equivalent doses, INP105 provided similar area under the drug concentration-time curve (AUC) from time 0 to the last measurable concentration, AUC from time 0 to infinity, and maximum observed concentration (Cmax) as OLZ IM and greater Cmax than but similar AUCs to OLZ ODT. Median time to maximum concentration was less for INP105 (15, 10, and 9.5 min for 5 mg, 10 mg, and 15 mg, respectively) than for OLZ IM (20 and 15 min for 5 mg and 10 mg, respectively) or OLZ ODT (120 min). Effects as measured with the VAS, ACES, and DSST with INP105 5 mg were comparable to those with OLZ IM 5 mg, with earlier onset for INP105 10 mg and 15 mg and greater effects than placebo and OLZ ODT. The incidence of treatment-emergent adverse events with INP105 5 mg, 10 mg, and 15 mg was 80%, 66.7%, and 75%, respectively, compared to 90% and 100% for OLZ IM 5 mg and 10 mg, respectively, and 83.3% for OLZ ODT; most common were dizziness, hypotension, and orthostatic symptoms.
Conclusions: INP105 has rapid absorption and pharmacodynamic effects and may represent an effective, convenient, noninvasive, and well-tolerated alternative for treating acutely agitated patients by self- or caregiver administration in the home, community, or hospital environments.
Trial Registration: ClinicalTrials.gov identifier: NCT03624322

ABSTRACT
Objective: INP105 is a drug-device combination of olanzapine and technology that delivers a powder formulation of olanzapine to the vascular-rich upper nasal space. This study evaluated the pharmacokinetics, pharmacodynamics, safety, and tolerability of single ascending doses of INP105, olanzapine intramuscular (OLZ IM), and olanzapine oral disintegrating tablet (OLZ ODT).
Methods: This was a phase 1, active and double-blind placebo comparator-controlled, ascending-dose, 2-period, incomplete-block, 1-way crossover study in 40 healthy subjects, randomized to single doses of OLZ IM (5 or 10 mg) or OLZ ODT (10 mg) in Period 1 and then 1 of 3 doses (5 mg, 10 mg, or 15 mg) of INP105 or placebo in Period 2 between July and October 2018. Sedation and attention were evaluated by visual analog scale (VAS), the Agitation/Calmness Evaluation Scale (ACES), and the Digit Symbol Substitution Test (DSST).
Results: At equivalent doses, INP105 provided similar area under the drug concentration-time curve (AUC) from time 0 to the last measurable concentration, AUC from time 0 to infinity, and maximum observed concentration (Cmax) as OLZ IM and greater Cmax than but similar AUCs to OLZ ODT. Median time to maximum concentration was less for INP105 (15, 10, and 9.5 min for 5 mg, 10 mg, and 15 mg, respectively) than for OLZ IM (20 and 15 min for 5 mg and 10 mg, respectively) or OLZ ODT (120 min). Effects as measured with the VAS, ACES, and DSST with INP105 5 mg were comparable to those with OLZ IM 5 mg, with earlier onset for INP105 10 mg and 15 mg and greater effects than placebo and OLZ ODT. The incidence of treatment-emergent adverse events with INP105 5 mg, 10 mg, and 15 mg was 80%, 66.7%, and 75%, respectively, compared to 90% and 100% for OLZ IM 5 mg and 10 mg, respectively, and 83.3% for OLZ ODT; most common were dizziness, hypotension, and orthostatic symptoms.
Conclusions: INP105 has rapid absorption and pharmacodynamic effects and may represent an effective, convenient, noninvasive, and well-tolerated alternative for treating acutely agitated patients by self- or caregiver administration in the home, community, or hospital environments.
Trial Registration: ClinicalTrials.gov identifier: NCT03624322
J Clin Psychiatry 2020;81(4):19m13086
To cite: Shrewsbury SB, Hocevar-Trnka J, Satterly KH, et al. The SNAP 101 double-blind, placebo/active-controlled, safety, pharmacokinetic, and pharmacodynamic study of INP105 (nasal olanzapine) in healthy adults. J Clin Psychiatry. 2020;81(4):19m13086.
To share: https://doi.org/10.4088/JCP.19m13086
© Copyright 2020 Physicians Postgraduate Press, Inc.
aImpel NeuroPharma, Inc, Seattle, Washington
bNucleus Network, Melbourne, Australia
*Corresponding author: Stephen B. Shrewsbury, Impel NeuroPharma, Inc, 201 Elliott Ave West, Ste 260, Seattle, WA 98119 ([email protected]).
Agitation is a behavior comprising aggressive and nonaggressive verbal and physical components marked by motor restlessness, heightened responsivity to internal and external stimuli, irritability, inappropriate or purposeless verbal or motor activity, and an unstable course1 and is recognized as a component of many psychiatric disorders.2 In a recent US survey,3 81% of caregivers of community-based treatment-resistant schizophrenia patients reported having observed agitation, hostility, and/or irritability in their care recipients. Schizophrenia underlies over 80% of treated emergencies for acute psychosis-related agitation or aggression.4
Agitation is usually seen in bipolar patients during acute manic states, which are associated with increased psychomotor activity and reduced sleep need, but also occurs during mixed and depressive states.5 Most of the 30 million Americans diagnosed with schizophrenia or bipolar disorder experience at least 1 episode of acute agitation annually, up to a mean of 12 episodes.6 There are over 10 million annual hospital admissions in the United States, many (though not all) related to unmanageable agitation from any cause, each requiring 6-10 days for disease stabilization.7 Many patients attending emergency departments with agitation may be sedated,8 although this may no longer be the preferred outcome and can delay definitive treatment for the underlying cause.9 A European survey of patients with schizophrenia or bipolar disorder living in the community2 found they had an average of 22.4 mild, 15.4 moderate, 6.8 moderate-intense, and 2.9 severe episodes of agitation per year, of which 2.7 required hospital attendance.
Olanzapine intramuscular (OLZ IM) is often used to treat acute agitation because of a shorter time to peak concentration, leading to more rapid onset of efficacy than oral or orally disintegrating tablet (ODT) formulations, and a lower risk of extrapyramidal adverse effects than first-generation antipsychotics. However, IM administration requires either restraint or some level of patient cooperation, is invasive, and can be painful.4,10 In less cooperative patients, it poses a risk for needle stick injury to health care workers11 or the patients themselves,12 making non-injectable drugs preferred when possible.13 However, currently approved oral products have slower onset of effect, often requiring observation of the medicated patient for a sustained period of time until the agitation resolves.14,15
Acute agitation may be managed by the misapplication (eg, covert administration16) or use of involuntary psychotropic medications,17 involuntary detention,18 physical restraints, and seclusion.4,19 This practice has become a focus of attention for mental health professionals, policy makers, and the lay public, the media, and patient advocacy organizations. In a survey for improving psychiatric emergency care,20 patients repeatedly stressed the importance of having staff treat them with respect, talk to them, listen to them, and involve them in treatment decisions.
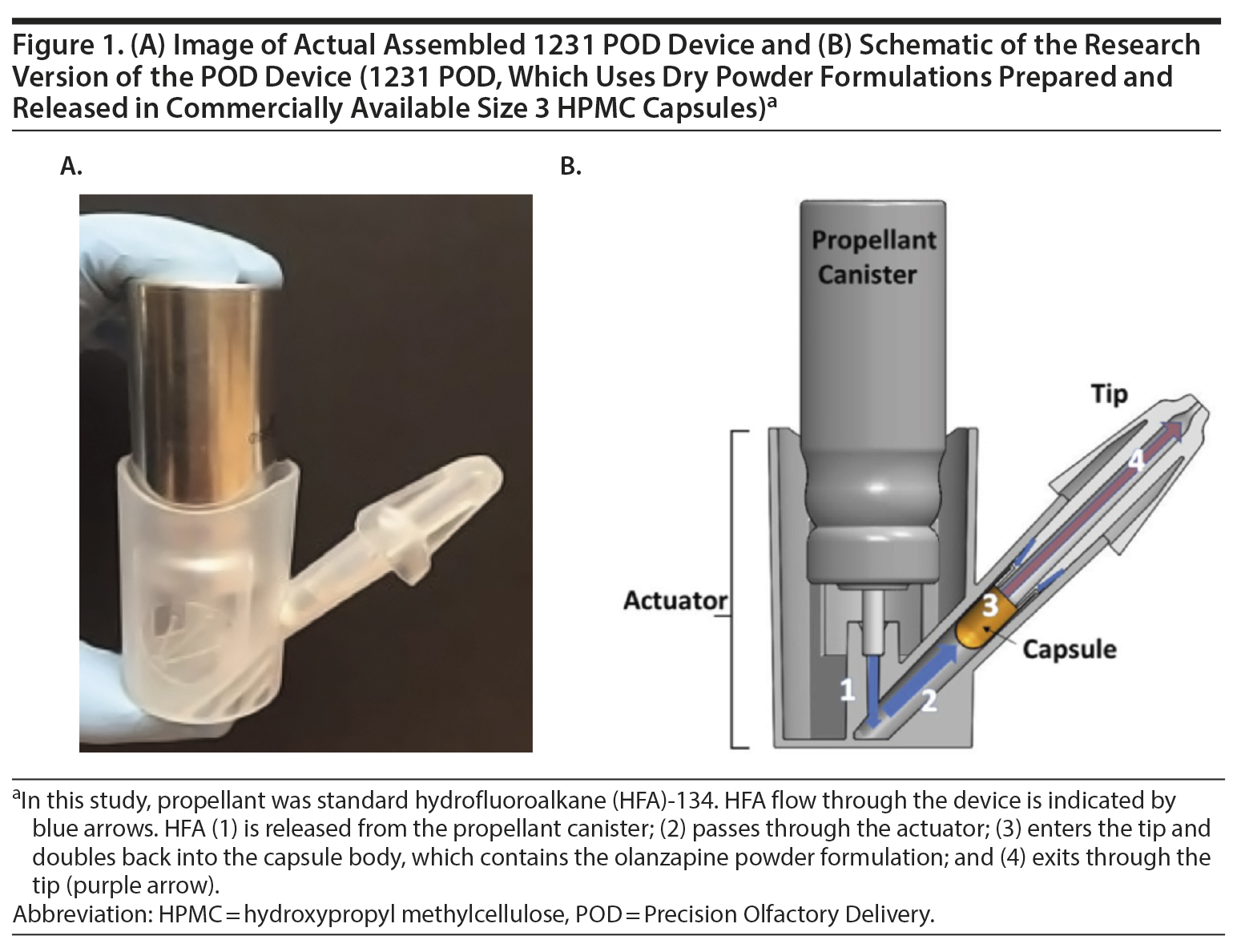
INP105 is a drug-device combination product consisting of a powder formulation of olanzapine and Precision Olfactory Delivery (POD) technology that delivers olanzapine to the vascular-rich upper nasal space.21 By consistently and predictably delivering therapeutics to the upper nasal cavity, the POD technology is designed to improve overall bioavailability of drugs without intravenous or IM injection. The I231 POD device is a handheld, manually actuated, gas-propelled, investigational administration device intended to deliver a powder drug formulation specifically to the upper nasal cavity (ie, olanzapine or placebo) from a commercially available size 3 capsule (Figure 1A and 1B). Most nasally administered products deliver to the anterior, lower nasal space where absorption and hence effective plasma drug levels may vary.22,23 The INP105 is the first powder formulation combination product utilizing POD technology. INP105 is designed to propel a nasal formulation of olanzapine with a total target dose of 5 mg active pharmaceutical ingredient (API) with each actuation into the upper nasal space.
The objectives of this study were to evaluate the safety and tolerability of 3 single ascending doses of INP105; to compare the pharmacokinetic and pharmacodynamic effects of those 3 doses with OLZ IM (5 and 10 mg), OLZ ODT 10 mg, and placebo; and to explore pharmacokinetic/pharmacodynamic and dose-response relationships for INP105.
METHODS
The study was conducted at Nucleus Network in Melbourne, Australia, in accordance with all appropriate regulations, after protocol approval and written informed consent had been obtained (ClinicalTrials.gov identifier: NCT03624322).
Study Design
This was a phase 1, randomized, active and double-blind, placebo comparator-controlled, ascending-dose study in healthy subjects to evaluate the safety, tolerability, pharmacokinetics, and pharmacodynamics of INP105, OLZ IM, and OLZ ODT conducted between July and October 2018. A randomization list containing the randomization number, treatment kit number, and treatment information was generated by an unblinded statistician and transferred to the good manufacturing practices facility that measured olanzapine and placebo into capsules and packaged and labeled kits for the study.

- Acute agitation in patients with psychiatric illness is common, and current treatment options are suboptimal. Few, if any, rapidly acting pharmacotherapeutic options are available for acute agitation in the community or even in hospital without recourse to intramuscular injection.
- A novel, nasally administered, fast-acting, well-tolerated olanzapine product showed rapid absorption and pharmacodynamic effects and may be a useful addition to the psychiatrist’s pharmacopoeia.
Period 1 was an open-label evaluation of 2 doses (5 mg and 10 mg) of OLZ IM and 1 dose (10 mg) of OLZ ODT (Supplementary Figure 1). Period 1 was to include 3 treatment arms of 12 subjects, each receiving either 5 mg OLZ IM, 10 mg OLZ IM, or 10 mg OLZ ODT. After the first 2 subjects from Cohort 1 in Period 1 received OLZ IM 10 mg and suffered severe hypotension that prevented standing blood pressure recording and pharmacodynamic assessments, this treatment arm was dropped and Period 1 was subsequently modified, for safety reasons, to 2 treatment arms of either 5 mg OLZ IM or 10 mg OLZ ODT. Period 2 was a double-blind phase to evaluate 3 single ascending doses of INP105 (5 mg, 10 mg, and 15 mg) or matched placebo (9:3 active:placebo per cohort) and to compare effects of INP105 with OLZ in Period 1. Dose escalation was staggered in Period 2 to allow a monitoring committee to assess the safety and tolerability of the prior INP105 dose. Some subjects were dosed in Period 2 first and then returned to dose in Period 1 a minimum of 14 days later. The Safety Monitoring Committee comprised the investigator, Sponsor Medical Expert, and Local Medical Monitor and convened, at least 72 hours after the last subject was dosed in a Period 2 cohort, to review blinded safety data (specifically, adverse events and vital signs) to determine if any safety signal was present that would prevent planned escalation to the next higher Period 2 dosing cohort. During Period 2, INP105 or placebo was delivered nasally using the POD device. Doses were delivered as 1, 2 or 3 actuations of 5 mg each. All subjects were observed as inpatients for at least 72 hours post-dosing, with follow-up at 4, 5, and 14 days after dosing in each study period.
Subject Selection
Adult male or female subjects aged 18 to 55 years inclusive, with a body mass index of 18 to 32 kg/m2 were eligible if they were in good general health with no significant medical history or clinically significant physical or laboratory abnormalities at screening and a negative urine drug screen and alcohol breath test at screening and prior to randomization. Adequate contraception was required during the study.
Subjects were excluded for hypersensitivity to, or use of, olanzapine within the past 3 months; for use of cytochrome P450 (CYP) 1A2 inducers or inhibitors, prescription medications, or over-the-counter medications or supplements that could interfere with the conduct of the study; if they had a history of hypo- or hypertension or were current or recent smokers; if they had significant surgery in the previous 3 months or recent significant acute illness; or if they had nasal congestion or physical blockage of either nostril or sero-positivity for human immunodeficiency virus (HIV) or hepatitis B or C.
Study Assessments
Safety was assessed from adverse event reporting, physical examination including nasal passages, vital signs (supine and standing blood pressure [BP], heart rate, respiratory rate and body temperature), 12-lead electrocardiogram (ECG), and clinical laboratory tests (hematology, serum chemistry, urinalysis). A nasal examination using an otoscope was conducted coincident with directed and full physical examination at scheduled time points in both Period 1 and Period 2. Nasal examination was performed before and after dosing in both periods, and findings were rated based on the following: mucosal irritation (scored 0-5), epistaxis (0-3), mucosal edema (0-3), nasal discharge (0-3), and mucosal crusting (0-3).
Sedation and attention pre- and post-dose were assessed using the Agitation/Calmness Evaluation Scale (ACES) (Supplementary Appendix 1) ,24,25 a visual analog scale (VAS) (Supplementary Appendix 2),26 and the Digit Symbol Substitution Test (DSST).27 For the ACES, the investigator assessed the level of agitation or calmness on a 9-point scale. For the VAS, subjects were asked to mark their level of sedation on each of three 100-mm scales using the descriptive terms Alertâ„Drowsy, Clear/Foggy-Headed, and Energeticâ„Lethargic as the boundaries, yielding a maximum score of 300. For the DSST, subjects were assessed for response speed, sustained attention, visual-spatial skills, and set shifting when decoding a series of symbols outlined on a test paper. The number of symbols correctly substituted for the corresponding digits within 90 seconds was scored.
Blood samples for olanzapine levels were collected predose; at 5, 10, 15, 20, 30, and 45 minutes; at 1, 2, 4, 6, 8, 12, 18, 24, 36, 48, 60, and 72 hours (domiciled); and at 96 and 120 hours.
Plasma olanzapine concentrations were determined using a validated liquid chromatography-tandem mass spectrometry (LC-MS/MS) method. The following pharmacokinetic parameters for individual subjects were calculated using noncompartmental analysis (Phoenix WinNonlin v8.1; June 2018; Certara USA, Inc; Princeton, New Jersey): maximum observed concentration (Cmax), time to maximum observed concentration (Tmax), apparent elimination half-life (t1/2), area under the drug concentration-time curve from time 0 to the last measurable concentration (AUC0-last), AUC from time 0 to infinity (AUC0-inf), terminal elimination rate constant (kel), apparent clearance (CL/F), and volume of distribution (Vz/F).
Statistical Analysis
All randomized subjects who received any study drug (safety population) in this exploratory study were included in the analyses. Data from all placebo recipients in Period 2 were pooled. Pharmacokinetic and pharmacodynamic findings for each treatment were summarized using descriptive statistics. Pharmacodynamic effects were compared between olanzapine and placebo using paired t test and between INP105 doses and placebo using analysis of covariance (ANCOVA) with treatment group as a factor and baseline pharmacodynamic result as a covariate. Pharmacokinetic parameters were computed from the individual plasma concentration-time profiles using non-compartmental analysis.
RESULTS
Subject Disposition
The severity of hypotension in the first 2 volunteers receiving OLZ IM 10 mg caused discontinuation of this dose. Their lowest supine systolic/diastolic BP recorded was 71/34 mm Hg and 78/34 mm Hg; however, both recovered and went on to receive medication in Period 2. It was not unexpected that OLZ IM 10 mg would cause some hypotension in a population of healthy volunteers, but, after an urgent meeting of the Safety Monitoring Committee, a decision was made that, due to the severity of the observed hypotension, this treatment arm was dropped for safety concerns. Subsequent subjects in Period 1 were randomized to OLZ IM 5 mg or OLZ ODT 10 mg. Forty subjects were randomized and treated in Period 1. Three subjects were withdrawn from Period 2 prior to dosing: 1 subject each in the INP105 5 mg and 10 mg groups for noncompliance/protocol violation and withdrawal of consent, respectively, and 1 subject in the INP105 15 mg group for an unrelated adverse event of gastroenteritis (Supplementary Figure 2). All 40 subjects were included in the safety, pharmacokinetics, and pharmacodynamics populations.
Baseline Characteristics
Treatment groups appeared comparable for baseline characteristics (Supplementary Table 1), although subjects in the OLZ ODT 10 mg group were approximately 5 years older.
Pharmacokinetics
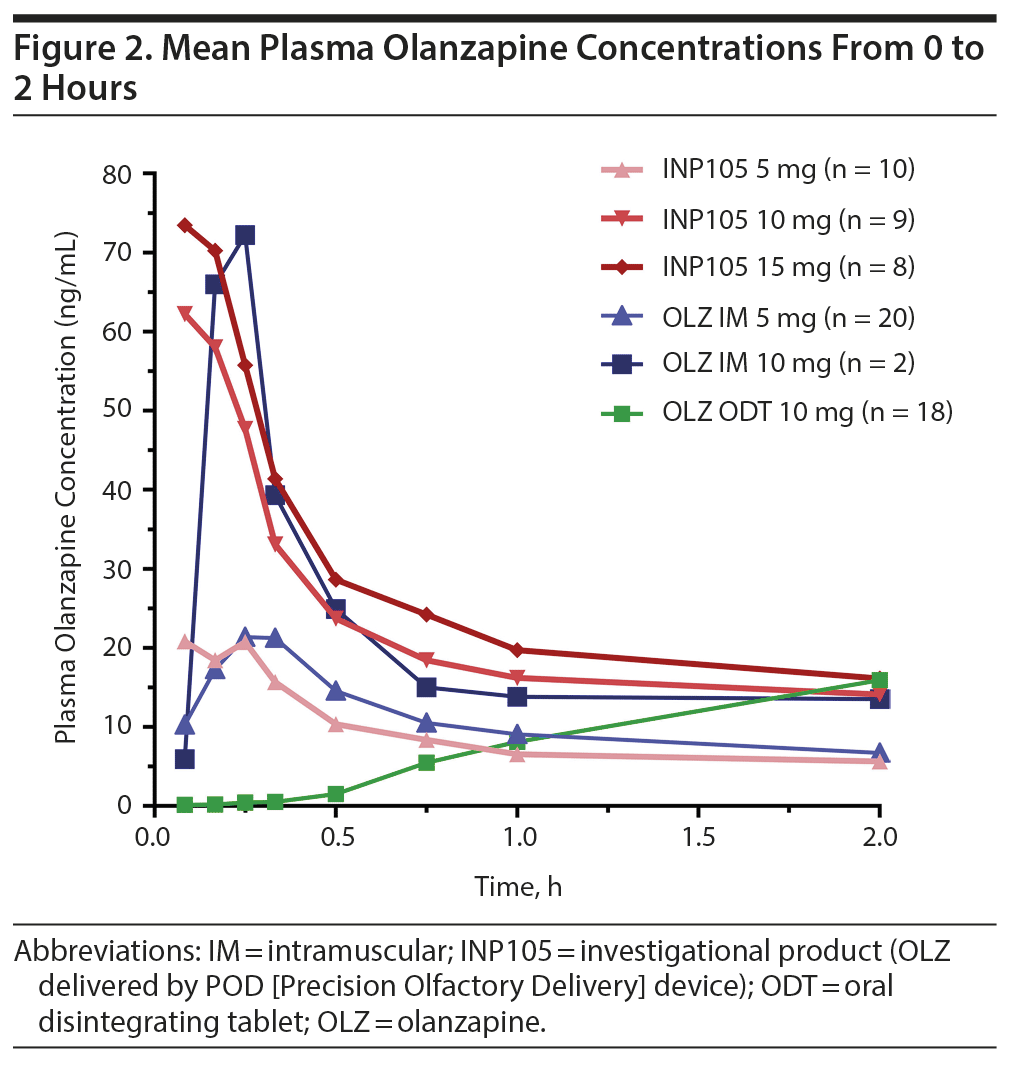
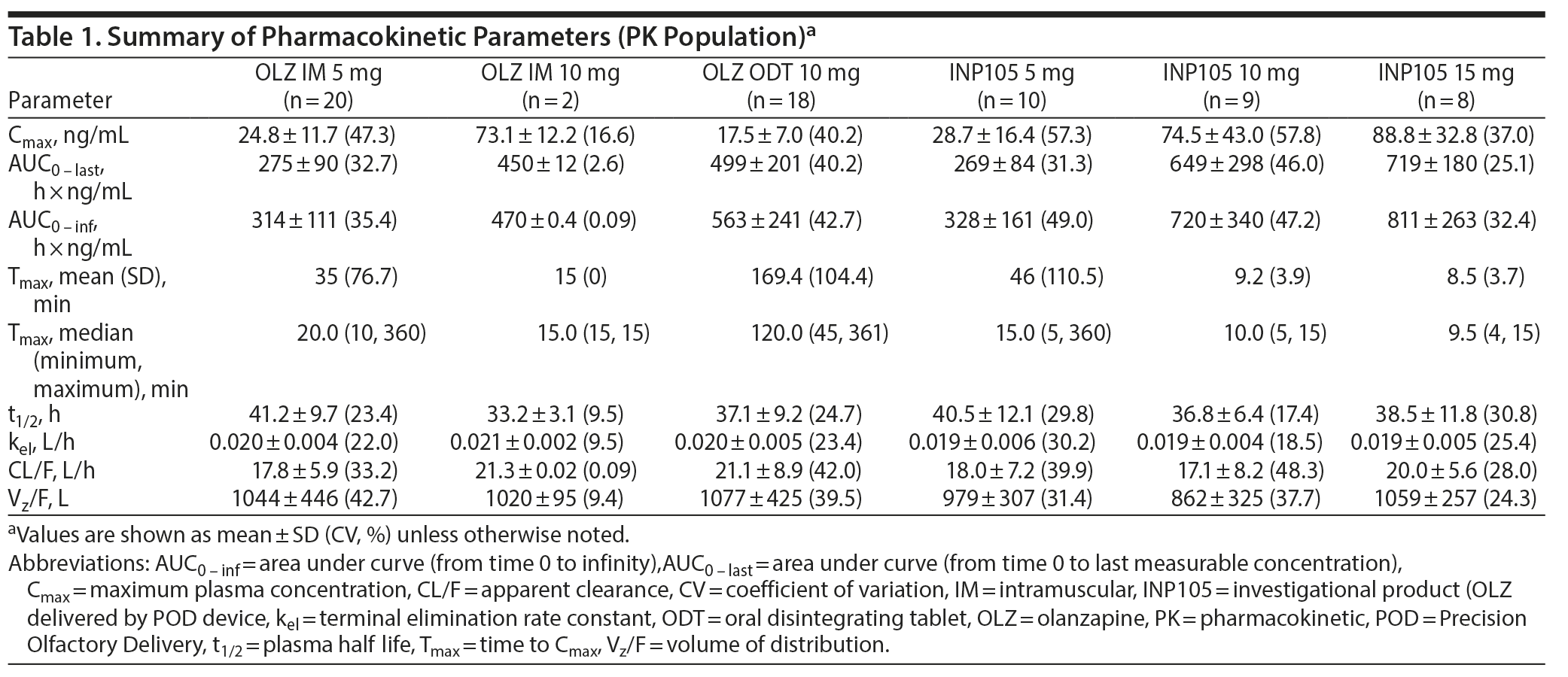
Absorption of olanzapine was fastest after administration of INP105, followed by IM administration. Peak plasma olanzapine concentrations were achieved within 30 minutes for all treatment groups (except OLZ ODT) and thereafter declined in a monoexponential manner by 60 minutes (Figure 2). Further, at least 40% of the individuals that received INP105 5 mg (4 of 10), 10 mg (4 of 9), or 15 mg (4 of 8) had the highest measured plasma concentration at the first blood draw time point (5 min), indicating that Tmax/Cmax may occur prior to 5 minutes for at least some subjects. Median Tmax was between 9.5 to 15 minutes for INP105 (depending on dose) versus 20 minutes for OLZ IM 5 mg and 120 minutes with OLZ ODT (Table 1). Mean Cmax with INP105 5 mg was similar to that for OLZ IM 5 mg and approximately 1.6-fold greater than that for OLZ ODT 10 mg. The Cmax for 10 mg INP105 was 2.6-fold higher than that for 5 mg INP105, and Cmax for 15 mg INP105 was 3-fold higher. Exposure (AUC0-last and AUC0-inf) was similar for INP105 5 mg and OLZ IM 5 mg (Table 1). Therefore, at equivalent doses, INP105 provided exposure similar to that of OLZ IM and OLZ ODT. An increase in exposure (AUC) for INP105 10 mg compared to INP105 5 mg was observed (2.4- and 2.2-fold for AUC0-last and AUC0-inf, respectively) and for INP105 15 mg compared to INP105 5 mg (2.7- and 2.5-fold for AUC0-last and AUC0-inf, respectively) (Table 1).
Pharmacodynamics
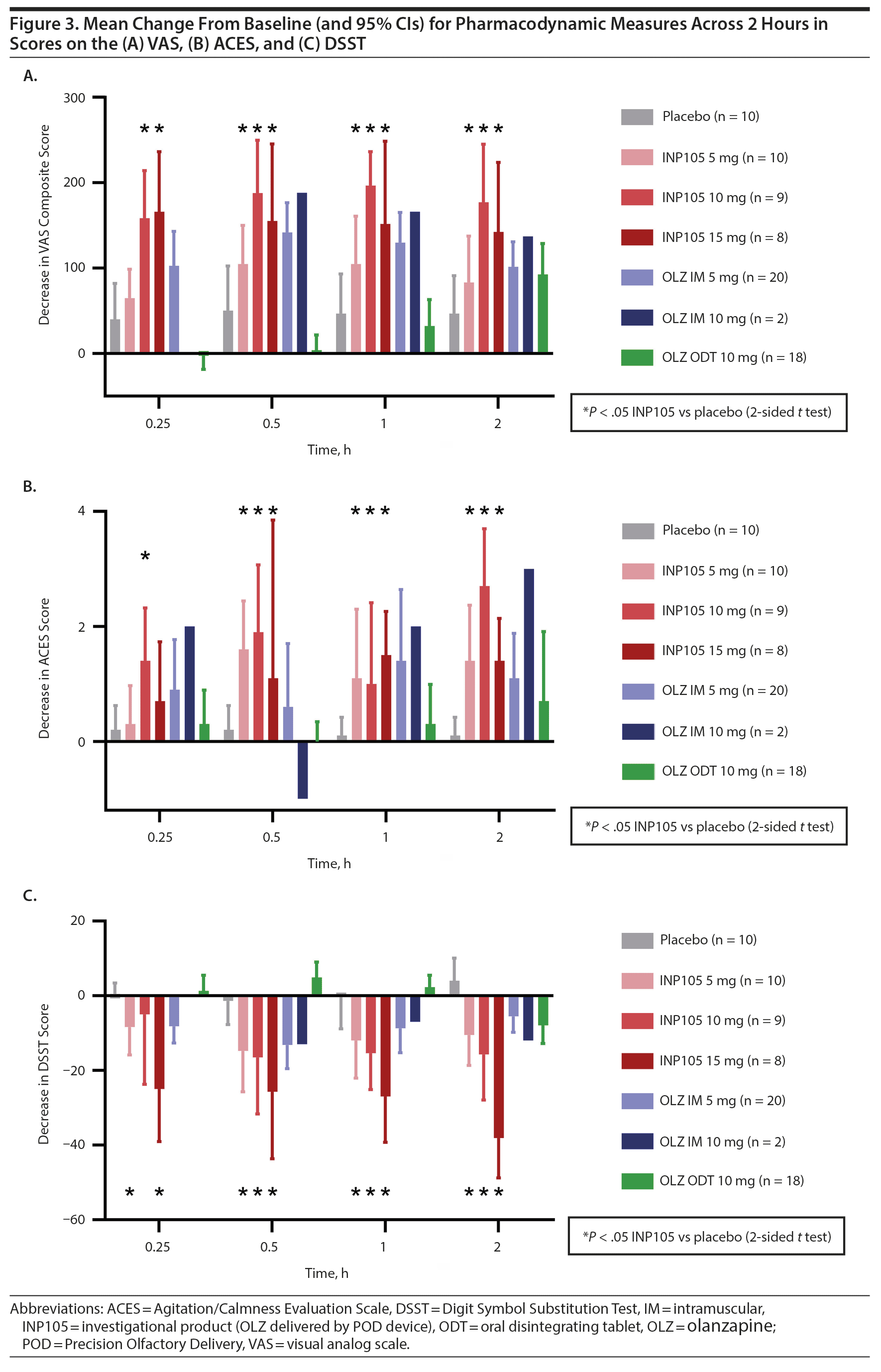
A decrease (desired effect) was observed for mean change from baseline to 15 minutes for the VAS score with all 3 doses of INP105 and OLZ IM 5 mg (Figure 3A). No significant change occurred with OLZ ODT or placebo. The mean maximum change in VAS scores showed greater subjective central nervous system effects with all active groups than with placebo. For the ACES, INP105 10 mg showed calming by 15 minutes, and mean change from baseline score during 60 minutes increased by 1 to 2 points with all doses of INP105 and OLZ IM 5 mg but was unchanged with OLZ ODT 10 mg and placebo (Figure 3B). Similarly, greater dose-related mean changes from baseline during 2 hours for the DSST were noted with INP105 and OLZ IM, but no change was observed with placebo and changes were observed with OLZ ODT 10 mg only at 2 hours (Figure 3C).
Safety and Tolerability
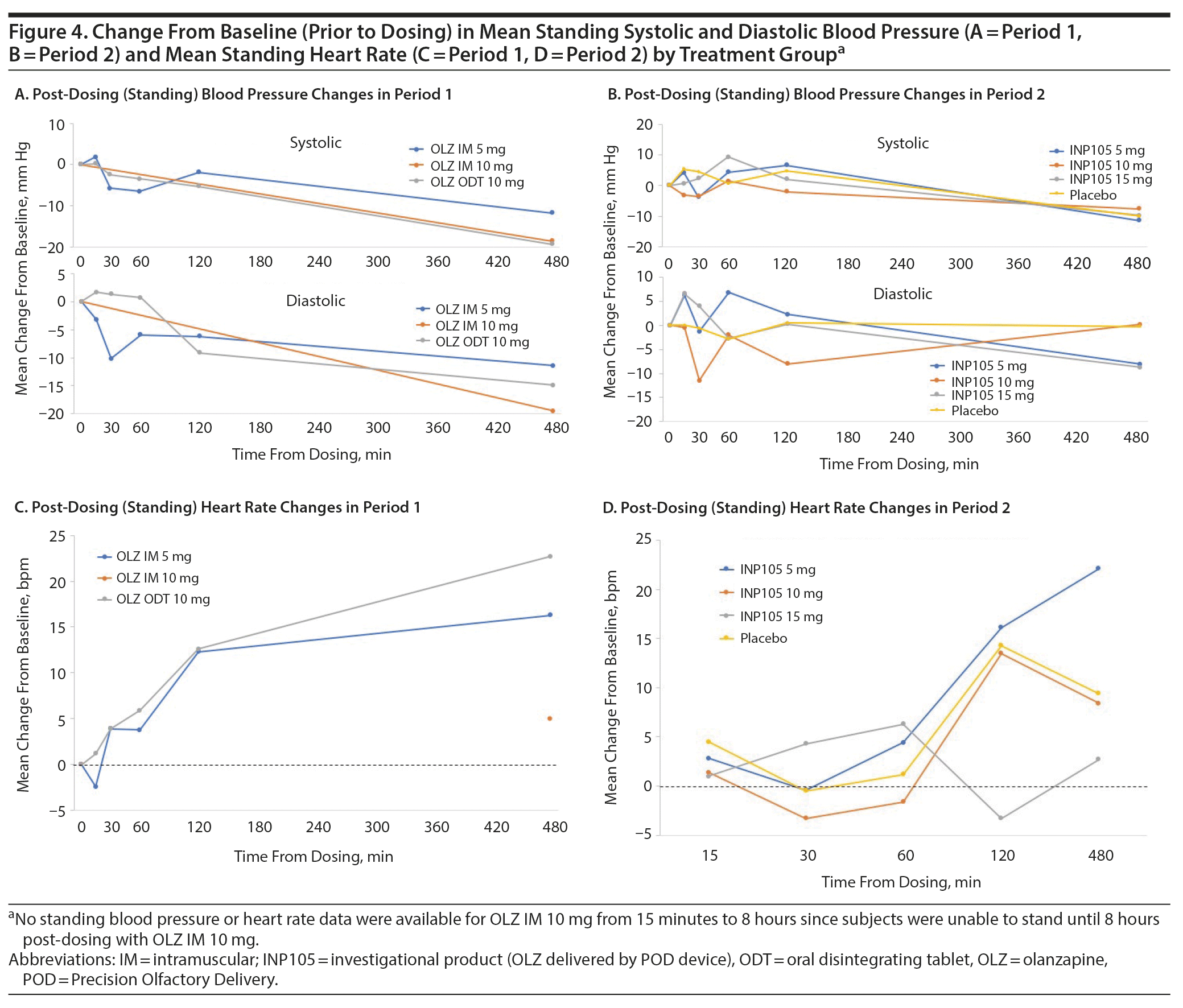
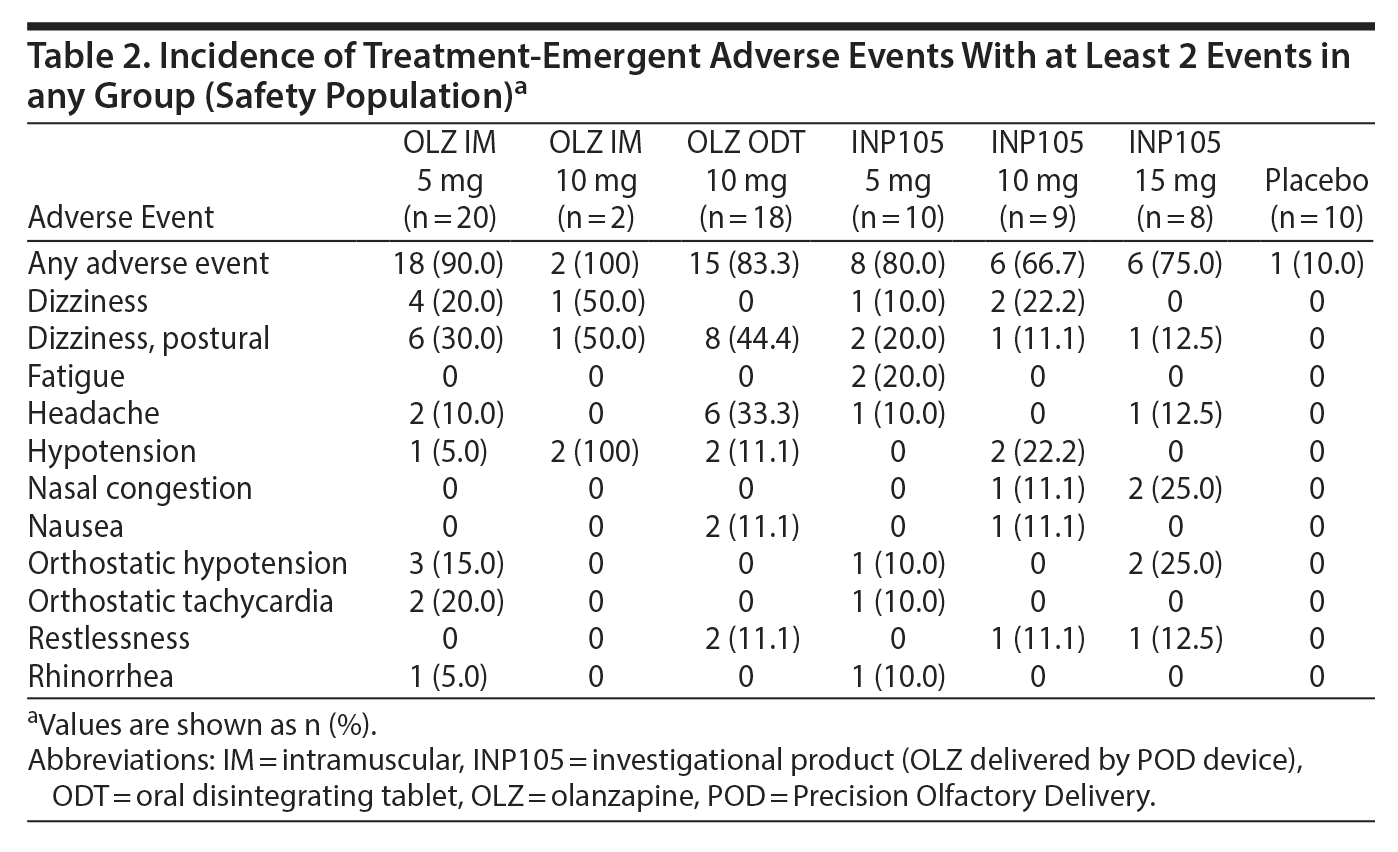
The incidence of treatment-emergent adverse events (TEAEs) was 100% for OLZ IM 10 mg, 90.0% for OLZ IM 5 mg, and 83.3% for OLZ ODT (Table 2) compared to 80% for INP105 5 mg, 66.7% for INP105 10 mg, 75.0% for INP105 15 mg, and 10% for placebo. The most commonly reported TEAEs were postural dizziness/dizziness, headache, and orthostatic hypotension/hypotension, all occurring at higher frequency after reference treatment in Period 1 compared to after INP105 dosing in Period 2 (Table 2). TEAEs were mostly mild in intensity, although 16 (40%) reported moderate-intensity TEAEs in Period 1 and 9 INP105-dosed subjects (33.3%) reported moderate TEAEs in Period 2. TEAEs were considered treatment related by 70% overall in Period 1 and 70.4% of INP105-dosed subjects in Period 2. With INP105, no subject discontinued for a treatment-related TEAE, and no severe or serious TEAEs were observed. Additional safety monitoring using ECG recording only at baseline and end of study found no clinically significant abnormal changes; vital signs (temperature, respiratory rate) also showed no clinically significant difference in changes up to 14 days post-dosing. Changes in standing systolic and diastolic BP were similar across treatment groups in Period 1 and Period 2 (with the exception of OLZ IM 10 mg, for which changes in standing BP could not be adequately assessed until 8 hours post-dosing as subjects were severely hypotensive and stayed in the recumbent position) (Figures 4A and 4B). There was little change in heart rate (HR) from baseline in the first hour (with the exception of OLZ IM 10 mg, for which standing HR was not obtained until 8 hours post dosing) (Figures 4C and 4D). Two of the 4 subjects who dosed in Period 2 before dosing in Period 1 (as was allowed by the protocol) developed postural dizziness and 1 developed orthostatic tachycardia—both with INP105 5 mg. One of the 2 subjects dosed first with INP105 10 mg developed hypotension and bradycardia. Laboratory tests (chemistry, hematology, and urinalysis) showed small numbers of fluctuations but none that were judged to be clinically significant or with a discernible consistent pattern.
One subject (10%) experienced moderate nasal discharge on day 1 following INP105 5 mg administration (possibly related, but fully resolved by day 2); other observations of nasal discharge were mild in nature and occurred at a lower incidence than observed at baseline. One subject (11.1%) who received INP105 10 mg was noted to have mild mucosal crusting, but only on day 4, that fully resolved. Mucosal edema following INP105 occurred at a lower incidence than observed at baseline. Lastly, epistaxis was observed in 6 (42.9%) of the 14 subjects who were examined on day 2 after they received OLZ ODT, an observation not noted at baseline or end of study follow-up. No concerning trends in the nasal examination results were noted for any treatment group.
DISCUSSION
INP105 offers the potential for noninvasive, needle-free delivery of olanzapine in acutely agitated patients with satisfactory tolerability, potentially a more rapid onset of effect than IM or ODT, and a durable response that may allow self- or caregiver administration in at-home, residential, or inpatient psychiatric locations. In addition, use of INP105 in lieu of IM injections may create an environment that enables patient engagement and verbal de-escalation for the acutely agitated patient either in the community, in a psychiatric bed, or in an emergency department. INP105 may obviate the need for heavily sedating IM medications.
Results from this phase 1 study demonstrated that INP105 absorption was rapid, reaching median peak plasma levels 5 minutes earlier than the corresponding OLZ IM dose and 110 minutes sooner than OLZ ODT. Olanzapine exposure (Cmax and AUC) following INP105 closely matched the exposure following the same dose delivered IM and exceeded that of OLZ ODT at the same dose.
All pharmacodynamic measures of sedation and attention obtained from the ACES, VAS, and DSST in these healthy subjects suggested clinically meaningful and statistically significant early changes, often starting as soon as 15 minutes after dosing with INP105 compared to placebo.
In a randomized, controlled study of OLZ IM 5 mg in acutely agitated patients,25 agitation was reduced but without excessive sedation as measured on the ACES. The mean change from a baseline score of 4.05 on the ACES was 1.88 at 2 hours post-injection. Earlier timepoints were not reported. This increase was similar to the increase from baseline in ACES score reported in the present study, albeit in nonagitated healthy volunteers, of 1.4, 2.7, and 1.4 with INP105 at doses of 5, 10, and 15 mg, respectively, at 2 hours and compared to increases of 1.1, 3.0, and 0.7 with OLZ IM 5 mg, OLZ IM 10 mg, and OLZ ODT 10 mg, respectively, in the present study.
There is a recognized need for an alternative to IM injections for acute agitation. Oral disintegrating tablets are bioequivalent to standard oral tablets, and any reports of faster onset of action compared to standard oral tablets are anecdotal and marginal.28 Inhaled loxapine is rapid and effective for treating acute agitation, but its use may be limited by a risk of bronchospasm that necessitates screening all patients for a history of pulmonary disease and an examination including chest auscultation for respiratory abnormalities prior to administration,4 which is restricted to “an enrolled healthcare facility that has immediate access, on site, to supplies and personnel trained to manage acute bronchospasm.”29
Using the nasal route to treat acute agitation may be preferred by patients as well as health care practitioners because it avoids injections, avoids “cheeking,” is easy to use, and can be self- or caregiver-administered in a variety of settings.13,30 In addition, delivering olanzapine with INP105 may achieve a faster onset of effect compared to oral or IM administration. Nasal delivery of the full dose of olanzapine in one-tenth of a second is possible, while oral therapy has a slow onset of action of approximately 1 hour or more. Intravenous (IV) therapy requires establishing IV access and may be associated with unacceptable adverse events,4,31,32 and IM and IV delivery have the risk of needle stick injuries to patients or health care workers.
Limitations include that this was a single-dose study and does not provide any data on what would be seen—in terms of either pharmacokinetics or pharmacodynamics—with a second dose, neither does it investigate how often that need may arise. This factor may be investigated in future studies. Descriptive statistics (not formal statistical methods) were used to compare data on participant characteristics, pharmacokinetics, and adverse effects. This study was conducted in healthy adult volunteers and may not predict the pharmacokinetic or pharmacodynamic effects that would be encountered in patients who might be taking chronic oral olanzapine treatment. Nevertheless, it does provide a preliminary intrasubject comparison of at least 2 forms of olanzapine—either commercially available IM or ODT and INP105 at 3 doses. There could be a period effect, and reduced TEAEs might be encountered in Period 2 accounting for the favorable tolerability profile of INP105. Only 4 subjects received Period 2 dosing first (2 got 5 mg and 2 got 10 mg INP105) and then returned for Period 1 dosing 14 days later. The 2 subjects who received 5 mg both reported postural dizziness and 1 of them also reported orthostatic tachycardia, and 1 of the 2 who received 10 mg first reported postural hypotension and bradycardia, but the numbers were too small for any hypothesis about tachyphylaxis to be developed. This possible period effect may be examined further in a future 2-way cross-over pharmacokinetics study.
Summary
In summary, INP105 offers noninvasive delivery of olanzapine into the upper nasal cavity enabling rapid systemic absorption without an injection. In this study, INP105 was well tolerated in healthy subjects after a single dose. Olanzapine exposure with INP105 5 mg closely matched exposure with OLZ IM 5 mg but with peak plasma levels occurring within 10-15 minutes with INP105 versus 20 minutes with OLZ IM 5 mg. Clinically meaningful calming effects were observed with INP105 versus placebo within 15 minutes. Thus, INP105 may represent an alternative for treating acutely agitated patients and could become a valuable needle-free option for self- or caregiver administration in the psychiatric ward, board and care homes or other residential accommodations, or in the home environment.
Submitted: September 16, 2019; accepted March 31, 2020.
Published online: June 30, 2020.
Author contributions: All authors had full access to all the data in the study and had full responsibility for the content of the manuscript for publication. The corresponding author was responsible for the final review and had final responsibility for the decision to submit for publication.
Potential conflicts of interest: Drs Shrewsbury, Hocevar-Trnka, Satterly, Craig, and Hoekman are full-time employees of Impel NeuroPharma, Inc, and own stock. Dr Lickliter is a full-time employee of Nucleus Network, where this study was conducted, but has no financial interest in Impel NeuroPharma, Inc.
Funding/support: Funding for this study was provided by Impel NeuroPharma, Inc, Seattle, Washington.
Role of the sponsor: The funder of the study was involved in study design, data analysis, data interpretation, and writing of the report.
Previous presentation: Previously presented as a poster at the 2019 American Psychiatric Association Annual Meeting; May 18-22, 2019; San Francisco, California ▪ 2019 American Society of Clinical Psychopharmacology Annual Meeting; May 28-31, 2019; Scottsdale, Arizona.
Acknowledgments: The authors would like to acknowledge the editorial assistance of Richard S. Perry, PharmD (Scientific Communications Consulting), in the development of this manuscript, which was supported by Impel NeuroPharma, Inc, Seattle, Washington.
Supplementary material: See accompanying pages.
REFERENCES
1.Lindenmayer JP. Treatment refractory schizophrenia. Psychiatr Q. 2000;71(4):373-384. PubMed CrossRef
2.Roberts J, Gracia Canales A, Blanthorn-Hazell S, et al. Characterizing the experience of agitation in patients with bipolar disorder and schizophrenia. BMC Psychiatry. 2018;18(1):104. PubMed CrossRef
3.Brain C, Kymes S, DiBenedetti DB, et al. Experiences, attitudes, and perceptions of caregivers of individuals with treatment-resistant schizophrenia: a qualitative study. BMC Psychiatry. 2018;18(1):253. PubMed CrossRef
4.Zeller SL, Citrome L. Managing agitation associated with schizophrenia and bipolar disorder in the emergency setting. West J Emerg Med. 2016;17(2):165-172. PubMed CrossRef
5.Alderfer BS, Allen MH. Treatment of agitation in bipolar disorder across the life cycle. J Clin Psychiatry. 2003;64(suppl 4):3-9. PubMed
6.Dundar Y, Greenhalgh J, Richardson M, et al. Pharmacological treatment of acute agitation associated with psychotic and bipolar disorder: a systematic review and meta-analysis. Hum Psychopharmacol. 2016;31(4):268-285. PubMed CrossRef
7.Trends in Psychiatric Inpatient Capacity, United States and Each State, 1970 to 2014. National Association of State Mental Health Program Directors website. https://www.nasmhpd.org/sites/default/files/TACPaper.2.Psychiatric-Inpatient-Capacity_508C.pdf. Accessed September 15, 2019.
8.Klein LR, Driver BE, Miner JR, et al. Intramuscular midazolam, olanzapine, ziprasidone, or haloperidol for treating acute agitation in the emergency department. Ann Emerg Med. 2018;72(4):374-385. PubMed CrossRef
9.Zeller S, Wilson M, Nordstrom K. Intramuscular midazolam, olanzapine, ziprasidone, or haloperidol for treating acute agitation. Ann Emerg Med. 2019;73(4):422-423. PubMed CrossRef
10.Sacchetti E, Amore M, Di Sciascio G, et al. Psychomotor agitation in psychiatry: an Italian Expert Consensus. Evididence-Based Psychiatric Care. 2017;1:1-24.
11.Garus-Pakowska A, Górajski M. Epidemiology of needlestick and sharp injuries among health care workers based on records from 252 hospitals for the period 2010-2014, Poland. BMC Public Health. 2019;19(1):634. PubMed CrossRef
12.Bailey AM, Baum RA, Horn K, et al. Review of intranasally administered medications for use in the emergency department. J Emerg Med. 2017;53(1):38-48. PubMed CrossRef
13.J׸rgensen TR, Emborg C, Dahlen K, et al. Patient preferences for medicine administration for acute agitation: results from an internet-based survey of patients diagnosed with bipolar disorder or schizophrenia in two Nordic countries. Psychol Health Med. 2018;23(1):30-38. PubMed CrossRef
14.Garriga M, Pacchiarotti I, Kasper S, et al. Assessment and management of agitation in psychiatry: expert consensus. World J Biol Psychiatry. 2016;17(2):86-128. PubMed CrossRef
15.Wilson MP, Pepper D, Currier GW, et al. The psychopharmacology of agitation: consensus statement of the american association for emergency psychiatry project BETA psychopharmacology workgroup. West J Emerg Med. 2012;13(1):26-34. PubMed CrossRef
16.Latha KS. The noncompliant patient in psychiatry: the case for and against covert/surreptitious medication. Mens Sana Monogr. 2010;8(1):96-121. PubMed CrossRef
17.Bennion E. A right to remain psychotic? a new standard for involuntary treatment in light of current science. Loyola of Losa Angeles Law Review. 2013;47(1):251-317.
18.Steinert T. Ethics of coercive treatment and misuse of psychiatry. Psychiatr Serv. 2017;68(3):291-294. PubMed CrossRef
19.Zun LS. Use of restraint and seclusion in the emergency department. Psychiatric Times website. https://www.psychiatrictimes.com/view/use-restraint-and-seclusion-emergency-department. 2005. Accessed September 15, 2019.
20.Allen MH, Carpenter D, Sheets JL, et al. What do consumers say they want and need during a psychiatric emergency? J Psychiatr Pract. 2003;9(1):39-58. PubMed CrossRef
21.Hoekman JD, Ho RJY. Enhanced analgesic responses after preferential delivery of morphine and fentanyl to the olfactory epithelium in rats. Anesth Analg. 2011;113(3):641-651. PubMed
22.Silberstein SD, Shrewsbury SB, Hoekman J. Dihydroergotamine (DHE)—then and now: a narrative review. Headache. 2020;60(1):40-57. PubMed CrossRef
23.Shrewsbury SB, Jeleva M, Satterly KH, et al. STOP 101: a phase 1, randomized, open-label, comparative bioavailability study of ONP104, dihydroergotamine mesylate (DHE) administered intranasally by a I123 Precision Olfactory Delivery (POD) device, in healthy adult subjects. Headache. 2019;59(3):394-409. PubMed CrossRef
24.Battaglia J, Lindborg SR, Alaka K, et al. Calming versus sedative effects of intramuscular olanzapine in agitated patients. Am J Emerg Med. 2003;21(3):192-198. PubMed CrossRef
25.Meehan KM, Wang H, David SR, et al. Comparison of rapidly acting intramuscular olanzapine, lorazepam, and placebo: a double-blind, randomized study in acutely agitated patients with dementia. Neuropsychopharmacology. 2002;26(4):494-504. PubMed CrossRef
26.Bond A, Lader M. The use of analogue scales in rating subjective feelings. Br J Med Psychol. 1974;47(3):211-218. CrossRef
27.Jaeger J. Digit Symbol Substitution Test: the case for sensitivity over specificity in neuropsychological testing. J Clin Psychopharmacol. 2018;38(5):513-519. PubMed CrossRef
28.Markowitz JS, DeVane CL, Malcolm RJ, et al. Pharmacokinetics of olanzapine after single-dose oral administration of standard tablet versus normal and sublingual administration of an orally disintegrating tablet in normal volunteers. J Clin Pharmacol. 2006;46(2):164-171. PubMed CrossRef
29.Adasuve (loxapine) inhalation powder [package insert]. Souderton, PA: Galen, US, Inc; 2017.
30.J׸rgensen TR, Emborg C, Dahlen K, et al. The effect of the medicine administration route on health-related quality of life: results from a time trade-off survey in patients with bipolar disorder or schizophrenia in 2 Nordic countries. BMC Psychiatry. 2016;16(1):244. PubMed CrossRef
31.Hankin CS, Bronstone A, Koran LM. Agitation in the inpatient psychiatric setting: a review of clinical presentation, burden, and treatment. J Psychiatr Pract. 2011;17(3):170-185. PubMed CrossRef
32.Ng AT, Zeller SL, Rhoades RW. Clinical challenges in the pharmacologic management of agitation. Prim Psychiatry. 2010;17(8):46-52.
Editor’s Note: We encourage authors to submit papers for consideration as a part of our Focus on Psychosis section. Please contact Ann K. Shinn, MD, MPH, at [email protected].
Save
Cite
Advertisement
GAM ID: sidebar-top