Objective: Differential predictors of response to alternative treatment options are needed to improve the outcomes in major depressive disorder. The symptom dimension comprising loss of interest and reduced activity has been reported as a predictor of poor outcome of treatment with antidepressants. We hypothesized that augmentation with partial dopamine agonist aripiprazole will be effective for individuals with pronounced interest-activity symptoms.
Methods: We tested the hypothesis in the 2-phase Canadian Biomarker Integration Network in Depression trial 1 (CAN-BIND-1). All participants had a primary diagnosis of major depressive disorder confirmed with the Mini-International Neuropsychiatric Interview. In phase 1, 188 individuals received escitalopram monotherapy 10-20 mg daily for 8 weeks. In phase 2, nonresponders received augmentation with aripiprazole 2-10 mg daily while responders continued escitalopram monotherapy for another 8 weeks. Outcomes were measured with the Montgomery-Åsberg Depression Rating Scale (MADRS) every 2 weeks. Effects of baseline interest-activity symptoms on outcomes were tested in repeated-measures mixed-effects models.
Results: Higher baseline interest-activity score (indicative of more severe loss of interest and reduction in activity) predicted worse outcome of escitalopram monotherapy in phase 1 (b = 1.75; 95% CI, 0.45 to 3.05; P = .009), but the association disappeared with the augmentation option in phase 2 (b = −0.19; 95% CI, −1.30 to 0.92; P = .739). A significant interaction between the baseline interest-activity score and aripiprazole reflected the opposite direction of the relationship between baseline interest-activity score and degree of improvement with escitalopram monotherapy versus aripiprazole augmentation (b = −1.60; 95% CI, −2.35 to −0.84; P < .001).
Conclusions: Individuals with prominent loss of interest and reduction in activity benefit less from escitalopram monotherapy and more from aripiprazole augmentation. Future trials may test the benefits of early prodopaminergic augmentation guided by interest-activity symptoms.
Trial Registration: ClinicalTrials.gov identifier: NCT01655706
See letter by Swami and Mishra and reply by Uher et al
ABSTRACT
Objective: Differential predictors of response to alternative treatment options are needed to improve the outcomes in major depressive disorder. The symptom dimension comprising loss of interest and reduced activity has been reported as a predictor of poor outcome of treatment with antidepressants. We hypothesized that augmentation with partial dopamine agonist aripiprazole will be effective for individuals with pronounced interest-activity symptoms.
Methods: We tested the hypothesis in the 2-phase Canadian Biomarker Integration Network in Depression trial 1 (CAN-BIND-1). All participants had a primary diagnosis of major depressive disorder confirmed with the Mini-International Neuropsychiatric Interview. In phase 1, 188 individuals received escitalopram monotherapy 10-20 mg daily for 8 weeks. In phase 2, nonresponders received augmentation with aripiprazole 2-10 mg daily while responders continued escitalopram monotherapy for another 8 weeks. Outcomes were measured with the Montgomery-Åsberg Depression Rating Scale (MADRS) every 2 weeks. Effects of baseline interest-activity symptoms on outcomes were tested in repeated-measures mixed-effects models.
Results: Higher baseline interest-activity score (indicative of more severe loss of interest and reduction in activity) predicted worse outcome of escitalopram monotherapy in phase 1 (b = 1.75; 95% CI, 0.45 to 3.05; P = .009), but the association disappeared with the augmentation option in phase 2 (b = −0.19; 95% CI, −1.30 to 0.92; P = .739). A significant interaction between the baseline interest-activity score and aripiprazole reflected the opposite direction of the relationship between baseline interest-activity score and degree of improvement with escitalopram monotherapy versus aripiprazole augmentation (b = −1.60; 95% CI, −2.35 to −0.84; P < .001).
Conclusions: Individuals with prominent loss of interest and reduction in activity benefit less from escitalopram monotherapy and more from aripiprazole augmentation. Future trials may test the benefits of early prodopaminergic augmentation guided by interest-activity symptoms.
Trial Registration: ClinicalTrials.gov identifier: NCT01655706
J Clin Psychiatry 2020;81(4):20m13229
To cite: Uher R, Frey BN, Quilty LC, et al. Symptom dimension of interest-activity indicates need for aripiprazole augmentation of escitalopram in major depressive disorder: a CAN-BIND-1 report. J Clin Psychiatry. 2020;81(4):20m13229.
To share: https://doi.org/10.4088/JCP.20m13229
© Copyright 2020 Physicians Postgraduate Press, Inc.
aDepartment of Psychiatry, Dalhousie University, Halifax, Nova Scotia, Canada
bNova Scotia Health Authority, Halifax, Nova Scotia, Canada
cDepartment of Psychiatry and Behavioural Neurosciences, McMaster University, Hamilton, Ontario, Canada
dSt Joseph’s Healthcare, Hamilton, Ontario, Canada
eCentre for Addiction and Mental Health, Toronto, Ontario, Canada
fDepartment of Psychiatry, University of Toronto, Toronto, Ontario, Canada
gDepartment of Psychiatry, St Michael’s Hospital, University of Toronto, Toronto, Ontario, Canada
hRoyal’s Institute of Mental Health Research, University of Ottawa, Ottawa, Ontario, Canada
iDepartment of Psychiatry & Behavioural Neurosciences, St Joseph’s Healthcare, Hamilton, Ontario, Canada
jDepartments of Psychiatry and Psychology, Queen’s University, Providence Care Hospital, Kingston, Ontario, Canada
kDouglas Institute, Department of Psychiatry, McGill University, Montreal, Quebec, Canada
lDepartment of Psychiatry, University of Michigan, Ann Arbor, Michigan
mUniversity of Calgary, Hotchkiss Brain Institute, Calgary, Alberta, Canada
nDepartment of Psychiatry, University of British Columbia, Vancouver, British Columbia, Canada
oDepartment of Psychiatry, University Health Network, Toronto, Ontario, Canada
pKrembil Research Centre, University Health Network, University of Toronto, Canada
qMembers of the CAN-BIND Investigator Team are listed at www.canbind.ca/about-can-bind/our-team/.
*Corresponding author: Rudolf Uher, MD, PhD, Dalhousie University, 5909 Veterans Memorial Lane, Halifax, NS, B3H 2E2, NS, Canada ([email protected]).
Major depressive disorder (MDD) continues to be a leading cause of disability partly because of limited efficacy of available treatments.1 While dozens of antidepressants and augmentation strategies are available, each one has variable success, and fewer than half of individuals with depression experience remission with their first prescribed treatments.2 Most individuals with depression eventually find an effective treatment after several trials,3 but there is a significant personal and societal cost attached to the delay in reaching remission. Many individuals with depression give up on trying other treatments if the first treatment attempt does not provide benefits.4,5 In combination, the availability of multiple treatment options and individually variable outcomes suggest that there is potential to reduce depression burden with personalized indications for existing treatments.6
Tapping the potential of existing treatments requires reliable predictors of treatment-specific outcomes.7,8 Several predictors of depression treatment outcomes have been identified, but most predictors are not robust enough to be clinically meaningful or are not specific to a particular treatment.8-11 Some biological differential predictors of alternative treatment outcomes have been reported and are awaiting replication,12,13 but symptom-based predictors may be more feasible for clinical practice. A symptom dimension of loss of interest and reduced activity was previously reported to be a strong and replicable predictor of poor outcome of treatment with commonly used antidepressants.14 The symptom score is easy and inexpensive to obtain, and it predicted outcome with a clinically meaningful effect size. However, higher scores on the symptom dimension predicted worse outcome with all antidepressants that were examined.14 If it is not known what alternative treatment is needed for those with predicted poor outcome with standard treatment, the clinical application of the predictor remains limited.
Interest and activity are part of the positive mood domain that may be distinct from the distress/negative mood domain of depressive symptoms. It has been suggested that positive and negative affect domains may be modulated through different monoamine neurotransmitter systems. While the distress dimension may be primarily modulated by serotonin, the positive mood dimension may be modulated through dopaminergic signaling.15,16 Individuals with MDD who report profound loss of interest and severely reduced activity responded less well to both serotonergic (citalopram, escitalopram) and noradrenergic (nortriptyline) antidepressants.14 In a separate study,17,18 the prodopaminergic antidepressant bupropion led to greater improvement in positive mood symptoms, including increases in interest and activity. The potential of other, more potent dopaminergic treatments, such as partial dopamine agonists aripiprazole and brexpiprazole, to improve interest and activity remains to be established. Animal data suggest that antidepressant effects of an aripiprazole-escitalopram combination are mediated through specific effects on activity levels.19 Dopamine agonists have also been shown to be effective in MDD when they are used in combination with other antidepressants and are often used as augmentation of selective serotonin reuptake inhibitors (SSRIs).20,21 We hypothesized that individuals with MDD who score high on the loss of interest and activity symptom dimension will respond poorly to monotherapy with an SSRI but will benefit from augmentation with a partial dopamine agonist. We tested this hypothesis in a clinical trial with initial escitalopram monotherapy followed by aripiprazole augmentation (see Kennedy et al22 for the initial report of results from this trial).
METHODS
Participants
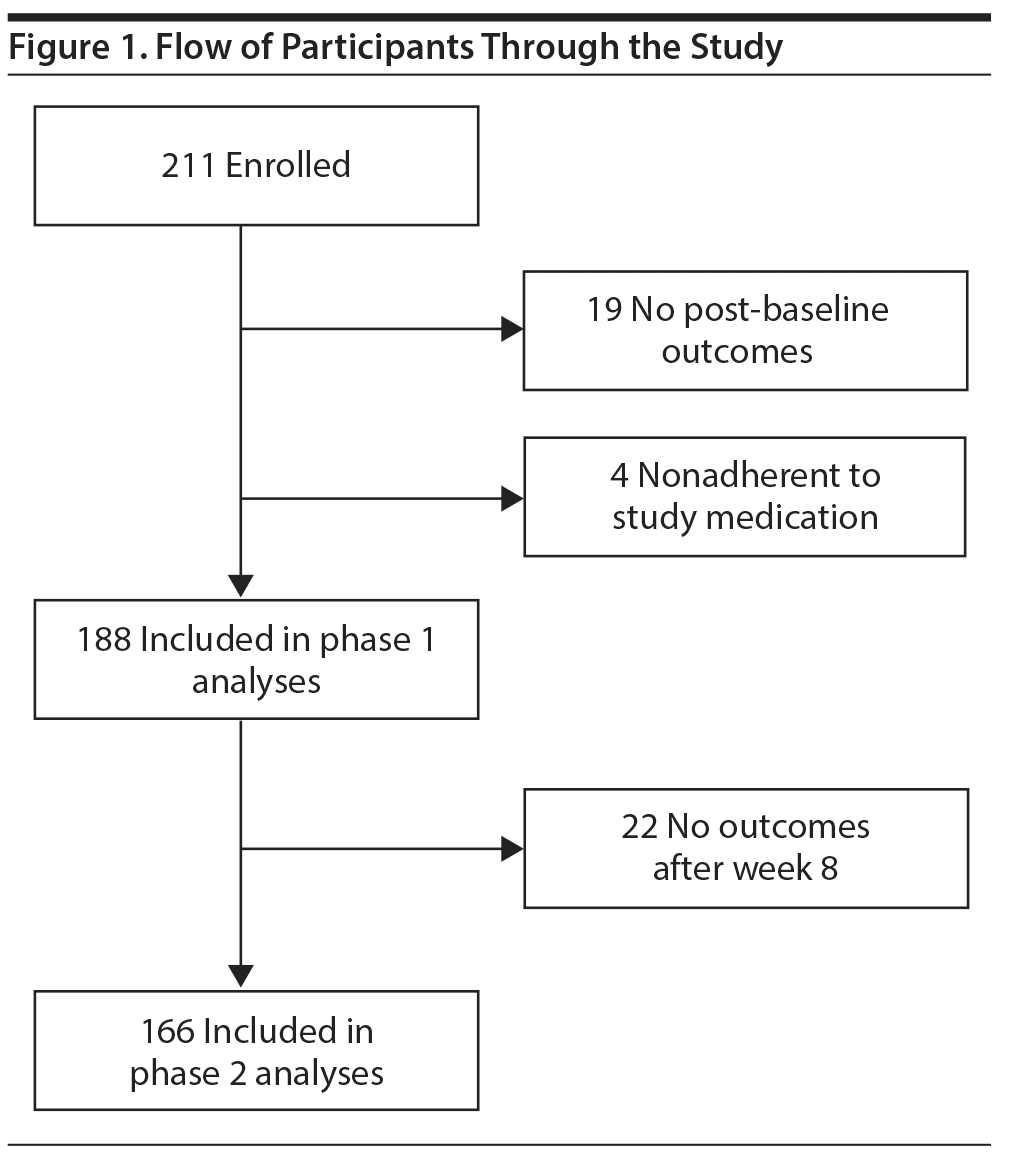
Canadian Biomarker Integration Network in Depression trial 1 (CAN-BIND-1) enrolled 211 adults (78 men and 133 women) with MDD in a multisite, 2-phase, 16-week treatment study. Six study sites in 5 Canadian cities (Vancouver, Calgary, Hamilton, Toronto, Kingston) enrolled participants between August 2013 and December 2016 following the same protocol.23 The study was approved by Research Ethics Boards of participating institutions, written informed consent was obtained from all participants, and the study was registered at ClinicalTrials.gov (identifier: NCT01655706).
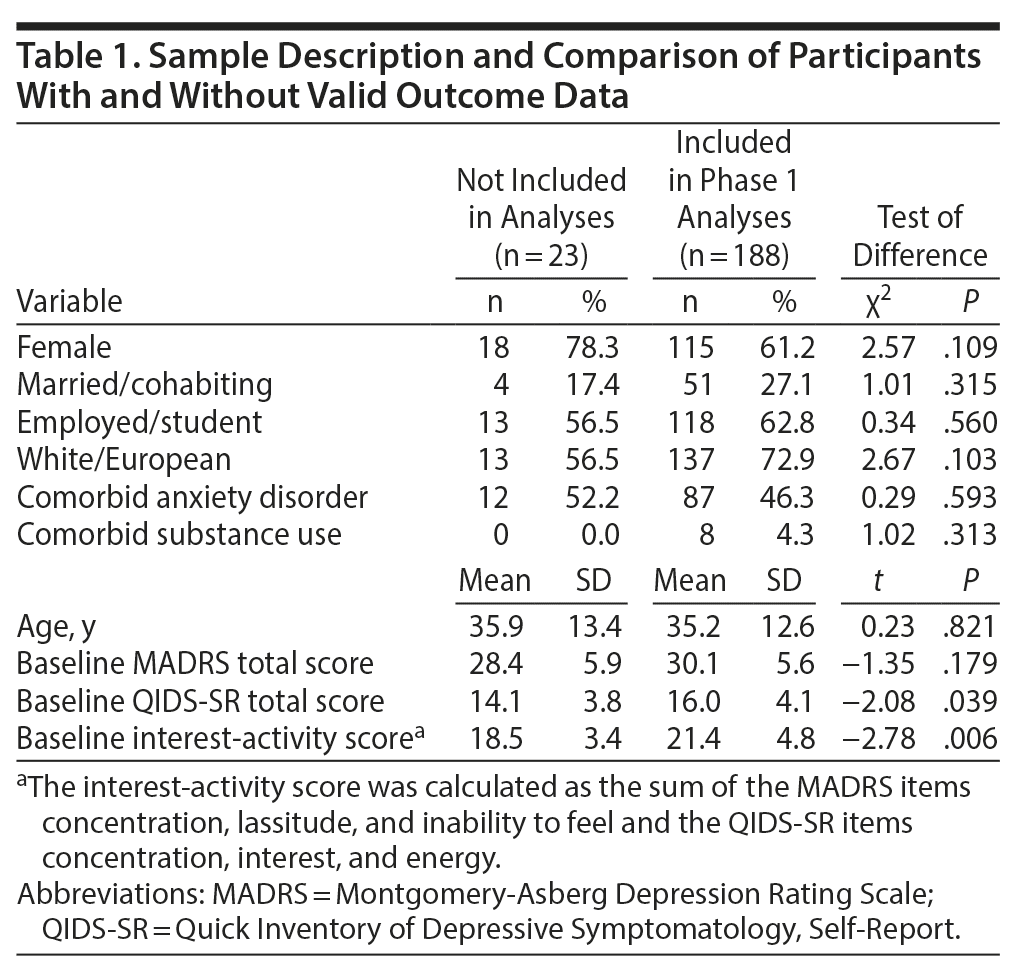
Inclusion criteria were age 18 to 60 years, ability to understand and fluently speak English, diagnosis of MDD confirmed with the Mini-International Neuropsychiatric Interview (MINI),24 current depressive episode lasting 3 months or longer, and a minimum score of 24 on the Montgomery-Åsberg Depression Rating Scale (MADRS).25 Exclusion criteria were lifetime diagnosis of bipolar I or bipolar II disorder, current psychotic symptoms, current substance use disorder, suicide risk incompatible with outpatient treatment, previous unsuccessful trials of study medication (escitalopram, aripiprazole), pregnancy, or breastfeeding.23 The mean (SD) age of participants at baseline was 35.30 (12.65) years (Table 1).
The study was open-label, and all participants knew what treatment they were receiving. However, both participants and raters were unaware of the hypothesis concerning interest-activity symptoms. Interest-activity symptom scores were calculated only when the entire data collection stage of the study was completed.

- Why some patients with depression improve with a single antidepressant but others require multiple trials or combination treatment is unknown. This study looked at symptom profiles of depressed patients, including loss of interest and reduction in activity.
- Depressed patients with relatively preserved interest and activity will very likely benefit from treatment with escitalopram alone.
- Depressed patients who have lost interest and are inactive may require adjunctive treatment with aripiprazole in addition to escitalopram.
Treatments
In phase 1 (week 0 to week 8), all participants were offered the same treatment with the antidepressant escitalopram for 8 weeks. Escitalopram is an SSRI with a favorable efficacy-tolerability profile in the treatment of MDD.2,26 Escitalopram monotherapy was started immediately after the baseline (week 0) assessment and adjusted during pharmacotherapy visits with a study psychiatrist every 2 weeks. The psychiatrists followed a treatment protocol. Escitalopram was started at 10 mg to be taken once daily with food. In participants who could tolerate escitalopram, the dose was increased to 20 mg once daily at week 2. The maximum tolerated dose (10-20 mg) was continued for the remainder of the trial. At week 8 (end of phase 1), the outcome was evaluated as proportion reduction in the primary outcome measure compared to baseline (week 0) measurement. In phase 2 (week 8 to week 16), participants were offered further treatment depending on the outcome of phase 1. Those who achieved response (defined as 50% reduction on the primary outcome measure) by week 8 continued treatment with escitalopram alone for another 8 weeks.22,23 Those who did not meet response criteria at week 8 were offered augmentation with aripiprazole. Aripiprazole is a partial dopamine agonist, which can enhance dopamine transmission if dopamine availability is low and act as a functional antagonist in conditions of accrued dopamine levels.27-29 Aripiprazole added to serotonin reuptake inhibitor antidepressants is an effective augmentation strategy recommended in cases of partial response or nonresponse to first-line antidepressants.26,30,31 Aripiprazole was started immediately after week 8 assessment (at the beginning of phase 2) at a dose of 2 mg taken once a day. At week 10, aripiprazole could be increased to 5 mg once daily in those who could tolerate it. Further increase to 10 mg was optional, depending on the tolerability and efficacy for the participant.22,23 Benztropine or propranolol was available for the management of treatment-emergent movement-related adverse effects. All participants continued escitalopram throughout phase 2. At weeks 2, 8, and 16, we measured levels of escitalopram, aripiprazole, and their metabolites to confirm compliance with prescribed medication.
Measurement
We assessed the severity of depressive symptoms with 2 scales, 1 clinician-rated and 1 self-report. The primary outcome measure was the clinician-rated MADRS, administered by trained clinical interviewers at baseline and in 2-week intervals through the 16-week trial.25 The second severity rating scale was the Quick Inventory for Depressive Symptomatology, Self-Report (QIDS-SR), completed by participants at baseline and weeks 2, 4, 8, 10, 12, and 16.32 We calculated the interest-activity symptom score as the sum of 6 items that were previously identified through item-response factor analysis and used to score interest-activity in the Genome-based Therapeutic Drugs for Depression (GENDEP) and Sequenced Treatment Alternatives to Relieve Depression (STAR*D) studies.14,33 Specifically, the MADRS items concentration, lassitude, and inability to feel and QIDS-SR items concentration, interest, and energy were used. As in the original interest-activity study,14 the QIDS-SR items with response options ranging from 0 to 3 were doubled to give them a weight equal to that of the MADRS items, whose response options range from 0 to 6. Only baseline measurements were used in calculating the interest-activity score. Higher interest-activity score corresponds to more severe symptoms, including loss of interest and reduction in activity.
Statistical Analysis
The dependent variable was the total MADRS score at weeks 2 to 16. The predictor of interest was the interest-activity symptom score measured at baseline, before participants received study medication. We tested our hypothesis with mixed-effects models for repeated measurements (MMRM) with linear and quadratic effects of time in study. All models were controlled for total depression severity at baseline, participant age and sex, and recruitment site as fixed effects. The random effect was used to model the non-independence of repeated observations from the same individual. All valid outcome measures were used. No data were imputed. Models were fitted using full maximum likelihood with the missing-at-random assumption, which is more realistic and less restrictive than the missing-completely-at-random assumption. The MMRM approach to missing data in clinical trials has been shown to be preferable to both the traditional last-observation-carried-forward and multiple-imputation approaches.34-36 We tested our hypothesis in 3 stages. First, we tested the effects of baseline interest-activity on the outcome of escitalopram monotherapy in phase 1 of the CAN-BIND-1 trial (weeks 2 to 8) to confirm that the previously reported finding that interest-activity predicts worse outcome of escitalopram monotherapy is reproducible in the current dataset. Second, we examined the effect of baseline interest-activity on outcomes in phase 2 (weeks 10 to 16) to assess whether the predictive effect of interest-activity was modified through introduction of the aripiprazole augmentation option. Third, we directly tested the hypothesized differential prediction of outcomes with and without aripiprazole augmentation as an interaction between baseline interest-activity and aripiprazole prescription across the entire trial (weeks 2 to 16). We interpret effect with P values smaller than .05 as statistically significant. We report effect sizes of prediction as number of MADRS points per 1 standard deviation in baseline interest-activity score. In addition, we calculate standardized effect sizes as the number of standard deviations in outcome change per 1 standard deviation difference in the predictor. In all analyses, we used the baseline interest-activity score as a continuous variable. For purposes of visualization only, we stratified the sample by terciles of baseline interest-activity score.
RESULTS
Participants Treatment and Data Completeness
Of the 211 enrolled participants, 19 did not complete any postbaseline assessments and an additional 4 participants were excluded because blood tests suggested that they did not take study medication (Figure 1). The remaining 188 participants were included in phase 1 analyses. Those who did not provide outcome data or did not take study medication were on average less severely depressed according to self-report measures and had milder interest-activity symptoms but did not differ on demographic characteristics from the included participants (Table 1).
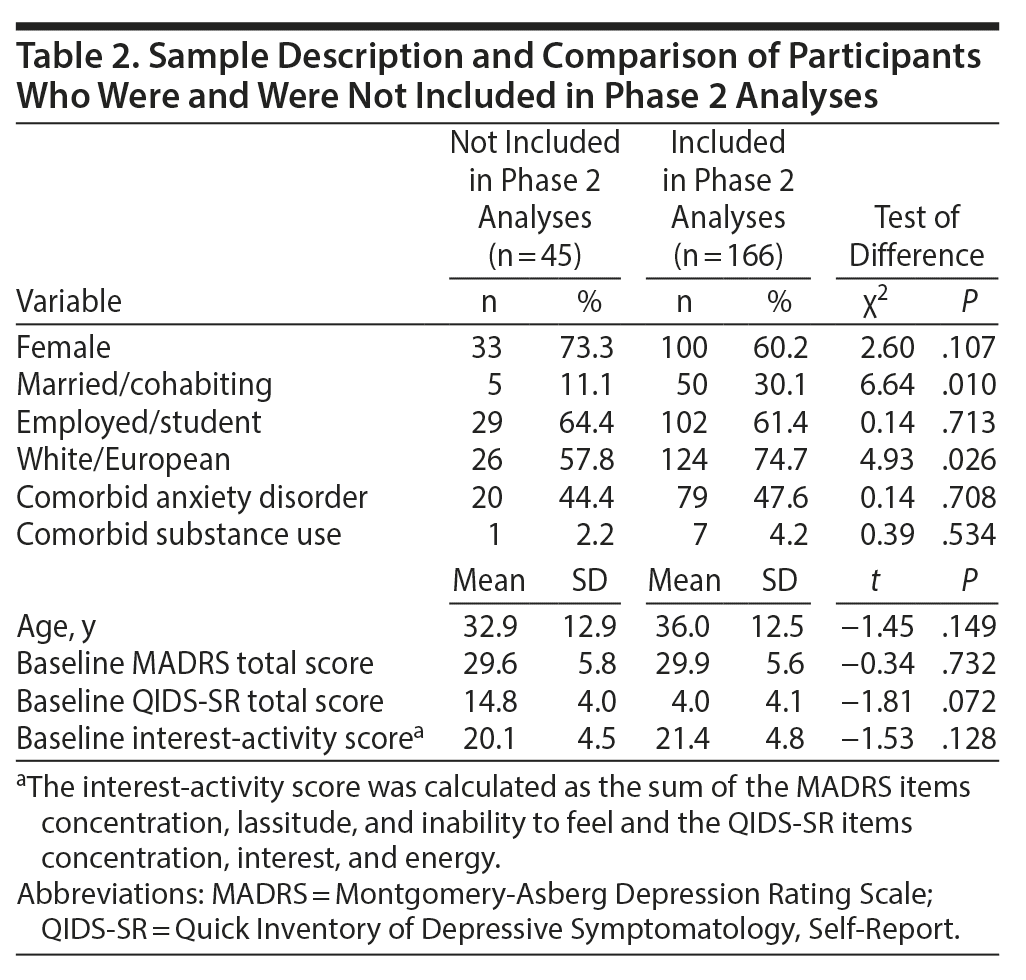
Of the 188 participants who provided outcome data, 22 discontinued participation between weeks 2 and 10. The remaining 166 who provided valid outcome data during at least 1 assessment from week 10 to week 16 were included in phase 2 analyses. Those who did not provide outcome data toward phase 2 analyses were more likely not to be married or in a cohabiting relationship and to report ethnicity other than White/European but were similar on other demographic and clinical characteristics to those included in phase 2 analyses (Table 2).
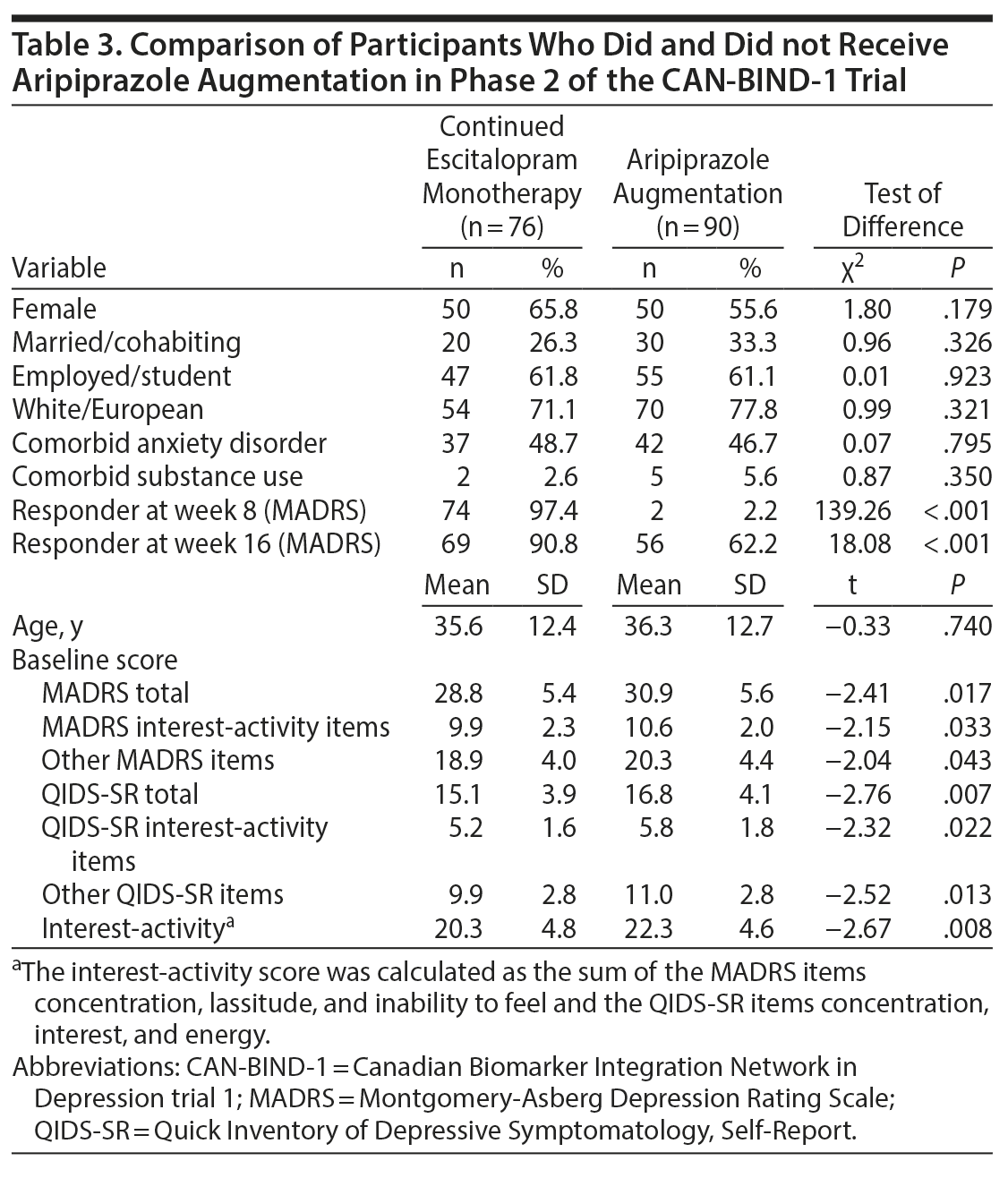
Of the 166 phase 2 participants, 90 received augmentation with aripiprazole. Participants who received aripiprazole augmentation had higher scores on depression rating scales and higher scores of interest-activity at baseline compared to those who continued to receive escitalopram alone (Table 3).
Interest-Activity Symptoms and Outcome of Escitalopram Monotherapy
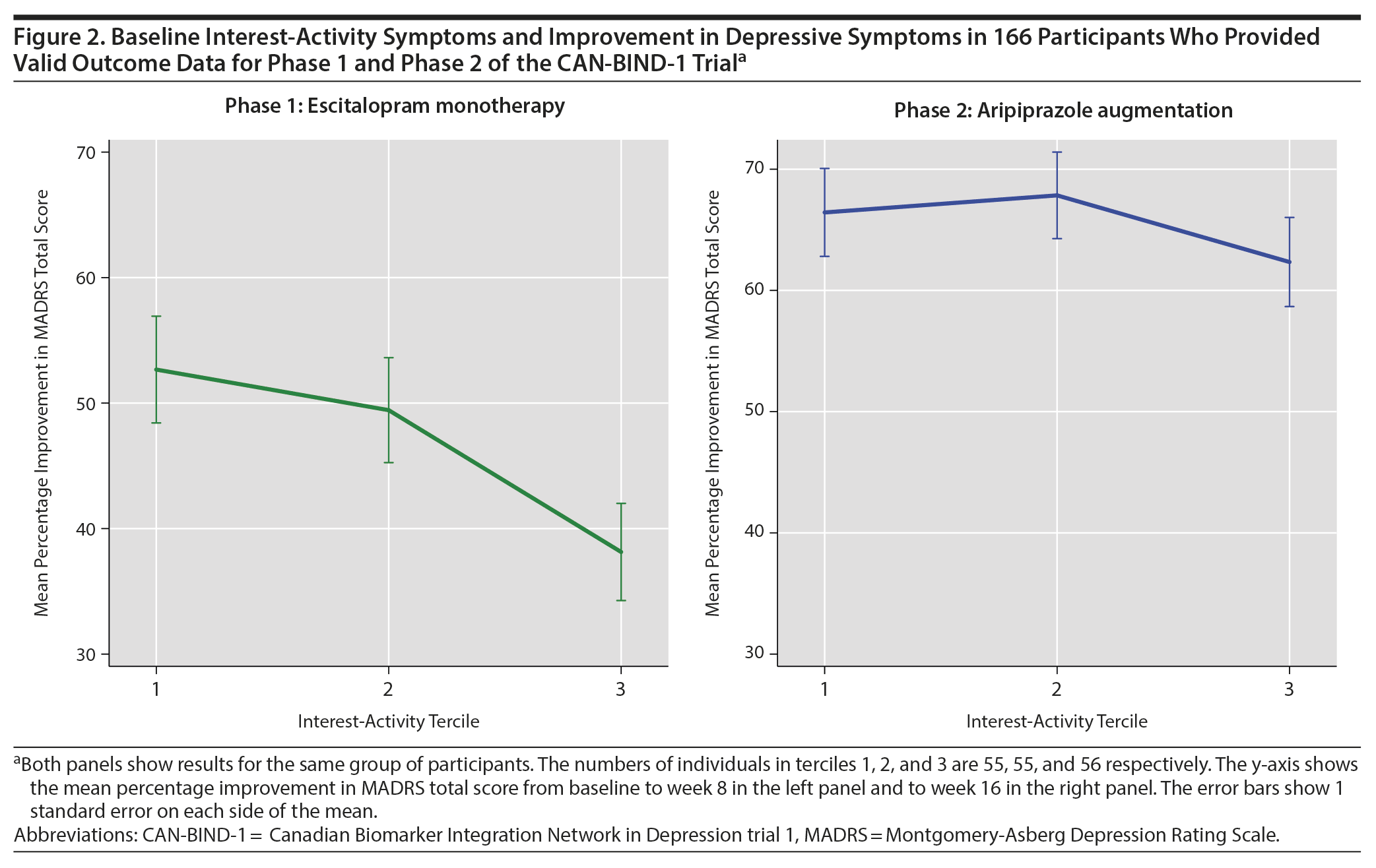
First, we examined how baseline interest-activity symptom score affected response to escitalopram monotherapy over the 8 weeks of treatment. Escitalopram was titrated up to a mean dose of 18.7 mg (range, 10-20 mg), and 188 participants provided valid outcome data while on escitalopram monotherapy. Escitalopram dose was not significantly related to the baseline score of interest-activity (b = 0.34; 95% CI, −0.13 to 0.81; P = .153). After controlling for baseline total MADRS score, age, sex, and site, each 1 standard deviation in baseline interest-activity score was associated with a 1.75-point increase in the MADRS scores during escitalopram treatment (b = 1.75; 95% CI, 0.45 to 3.05; P = .009). The standardized effect size of this prediction was 0.18 (95% CI, 0.05 to 0.32). Overall, individuals who reported greater loss of interest and lack of activity at baseline experienced substantially less reduction in their overall depression scores with escitalopram (Figure 2, left panel).
Interest-Activity Symptoms and Outcome of Aripiprazole Augmentation
Across the 166 participants, the baseline interest-activity symptoms were no longer associated with treatment outcome during phase 2 (b = −0.19; 95% CI, −1.30 to 0.92; P = .739) and the overall degree of improvement was similar for individuals with low and high levels of interest-activity symptoms at baseline (Figure 2, right panel).
Aripiprazole was titrated to a mean dose of 5.0 mg (range, 2-10 mg) daily. Among those who received augmentation, aripiprazole dose was not significantly associated with baseline interest-activity (b = 0.59; 95% CI, −0.01 to 1.18; P = .052).
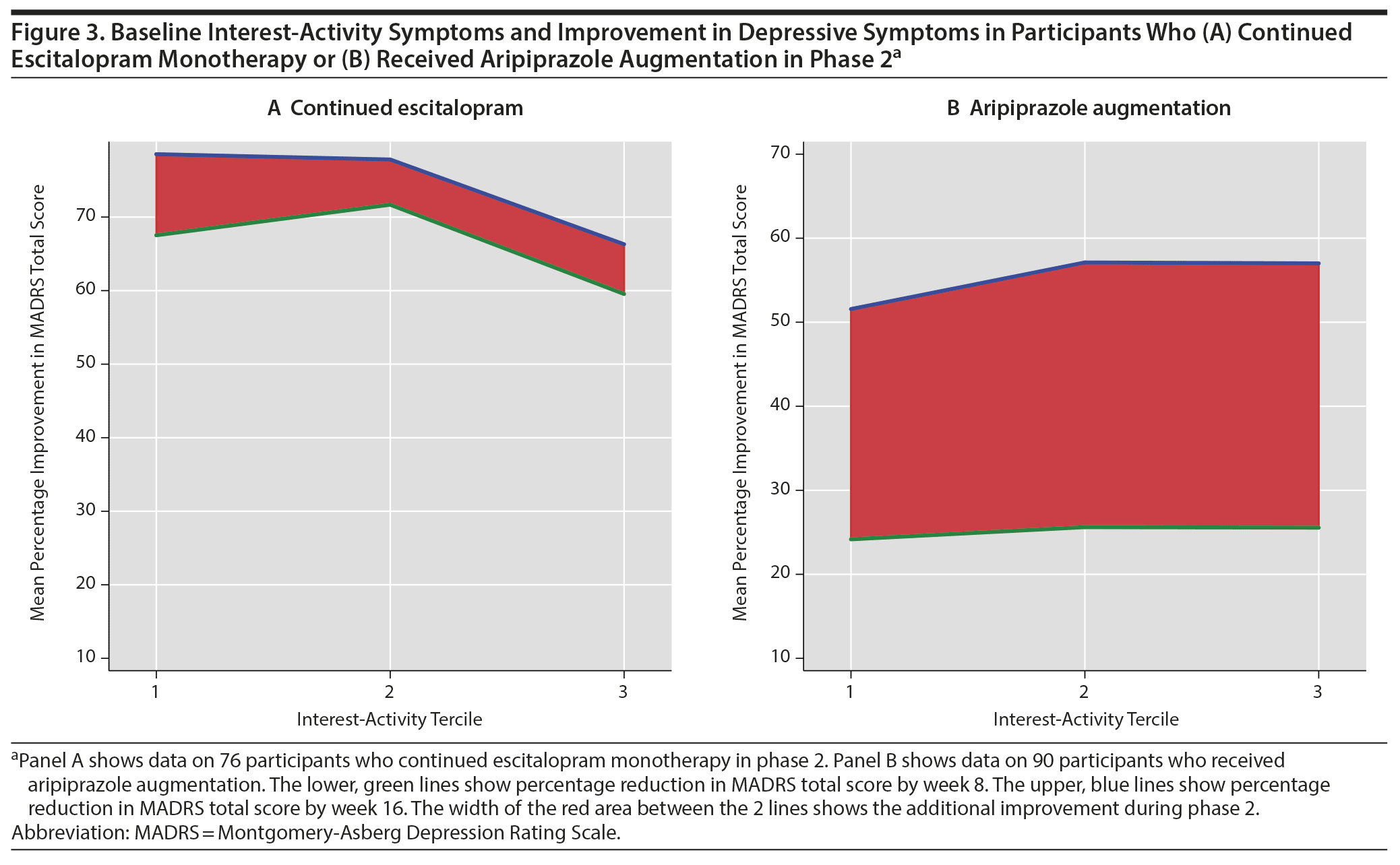
There was a significant interaction between the baseline interest-activity score and aripiprazole in their effect on MADRS score reduction (b = −1.60; 95% CI, −2.35 to −0.84; P < .001). The standardized effect size of this interaction was 0.15 (95% CI, 0.07 to 0.23). Higher baseline interest-activity symptoms were associated with less improvement during escitalopram monotherapy but more improvement during aripiprazole augmentation (Figure 3).
Additional analyses, including the change in self-reported depressive symptoms and change in interest-activity and other depressive symptoms, are reported in Supplementary Appendix 1 and Supplementary Figures 1-5.
DISCUSSION
The domain of symptoms consisting of loss of interest and reduced activity level was previously identified as a strong predictor of poor outcome of treatment with antidepressant monotherapy. In addition to replicating this earlier finding, we found that the same domain of symptoms is associated with a relatively good outcome of augmentation with aripiprazole, a partial dopamine agonist. This new finding suggests that an easily obtainable predictor, considered until now as a predictor of a negative outcome, may also be used as an indicator or predictor of a positive outcome for an add-on treatment with a potential of improving outcomes of depression in clinical practice.
The symptom dimension of interest-activity was identified in a combined item factor analysis of clinician-rated and self-report measures of depression severity as a grouping of symptoms including loss of interest, lack of motivation, low energy, and reduced activity.33 Individuals with MDD who reported more severe interest-activity symptoms responded less well to treatment with citalopram, escitalopram, and nortriptyline in 2 large depression treatment trials.14,37 The predictive effect of interest-activity symptoms was robust to statistical control for overall severity of symptoms, suggesting that among individuals with similar overall depression severity, those who endorse other depressive symptoms (eg, depressed mood, worthlessness, insomnia, suicidal ideation) derive more benefits from antidepressants than those with loss of interest and reduced activity. The predictive effect of interest-activity was replicated in the first 8 weeks of the CAN-BIND-1 study, when all participants received treatment with escitalopram monotherapy. The consistency of findings across 3 multicenter trials conducted in Europe, the United States, and Canada suggests that the symptom dimension of interest-activity is a generalizable predictor of treatment outcome with frequently prescribed antidepressants.
Generalizable predictors of treatment outcome may be useful in clinical practice as clinicians consider more intensive treatment options earlier for individuals with predicted poor outcomes.10 However, predictors become even more useful if there is a defined alternative treatment that is demonstrably more effective for those who have a predicted poor outcome with standard treatment. The present study suggests that aripiprazole augmentation may be an effective adjunctive treatment for individuals with depression who experience profound loss of interest and reduced activity. While individuals with this symptom profile had a poorer response to escitalopram monotherapy, they benefited more from aripiprazole augmentation than individuals with preserved interest and activity levels. The effect size of the prediction is approximately one half of the effect of augmentation with atypical antipsychotics for treatment-resistant depression compared to placebo.38 This finding was apparent in observed improvement (Figure 2) and robust to statistical control for a number of baseline characteristics, including overall severity of depression. Taken together, these observations suggest that in addition to being a predictor of poor outcome with monotherapy, high score on interest-activity may be used as an indicator for augmentation with aripiprazole.
We are not aware of other reports suggesting that anhedonia, low interest, and/or reduced activity may predict good response to aripiprazole augmentation in MDD. However, the present finding is consistent with previous pharmacologic knowledge, results of animal experiments, effects observed in individuals with other disorders, and effects of other dopaminergic agents in individuals with MDD. Dopamine is the key neuromediator involved in the processes that underlie interest and activity, such as reinforcement learning and incentive motivation.39 Consequently, it has been suggested that dopaminergic agents may be advantageous in treating the types of depression that are marked by profound loss of interest (anhedonia) and reduced activity.15,16 As predicted by these models, aripiprazole has shown efficacy in improving reward-directed activity in an animal model of anhedonia.40 Aripiprazole also led to improvement in interest in humans with bipolar depression and with schizophrenia.41,42 In addition to partial agonism on dopamine D2 receptors, aripiprazole may induce the release of endogenous dopamine through its action on serotonergic 5-HT1A and 5-HT7 receptors.43-45 Other psychotropic agents that enhance dopaminergic transmission, including the dopamine reuptake inhibitor/releaser bupropion and the D3/D2 receptor agonist pramipexole, have been noted to improve interest and motivation in individuals with MDD.18,20 In combination, these findings point to dopaminergic transmission as the mediator of pharmacologic effects on depression marked by profound reduction of interest and activity. In addition to aripiprazole as implicated by the current study, bupropion and pramipexole may be explored as add-on treatments for individuals with high scores on the interest-activity symptom dimension in future studies.
Interest-activity symptoms provide a potentially widely applicable tool for personalized treatment indication as they can be easily measured in routine clinical settings. We envisage two potential ways to apply the present findings. First, interest-activity symptoms can be used as an indicator for using aripiprazole augmentation in individuals who have not responded to antidepressant monotherapy. Since aripiprazole augmentation is one of the best evidence-based options in case of partial response or nonresponse,26 the threshold for this application may be relatively low, and the present level of indicative evidence may be appropriate. Second, it is possible that individuals with profound loss of interest and severe reduction in activity may obtain the best benefit-to-risk balance from combination treatment with an antidepressant and aripiprazole early in the treatment course, without waiting for a nonresponse. Such an accelerated approach would constitute a significant departure from present clinical practice, although the advantages in potentially improved efficacy as well as risk in terms of increased side effect burden should be explicitly tested in a new randomized controlled trial before adoption in clinical practice is considered. The adoption of interest-activity in any application should include at least one self-report and at least one clinician-rated depression measurement scale, as the two ways of measuring depression complement one another.46,47 Interest-activity symptoms may be usefully combined with other predictors of response to aripiprazole augmentation, such as cognitive testing, anxiety, and illness history.48-50
While the prediction of antidepressant treatment outcome by interest-activity symptoms has been robustly replicated across studies, the new finding that individuals with severe interest-activity symptoms respond well to aripiprazole augmentation relies on a single study and should be interpreted in the context of its limitations. First, our study did not include random allocation, and the differential prediction of response to escitalopram monotherapy and aripiprazole augmentation partly depends on a within-individual contrast comparing weeks before and after adding aripiprazole to escitalopram monotherapy. The nonrandomized design does not allow distinguishing between the effect of aripiprazole augmentation and delayed response to escitalopram. Previous studies found that the predictive effect of interest-activity symptoms lasted for at least 12 weeks, suggesting that the change in this trend at week 8 in the present study is most likely the result of augmentation with aripiprazole. However, the comparative efficacy of aripiprazole and alternative augmentation or switching procedure in escitalopram nonresponders with high interest-activity symptoms remains to be established in randomized studies. Second, the study was open-label, and there was no attempt to blind participants or raters to the treatment they were receiving. This could have led to a degree of bias with participants or raters being more inclined to report improvement after a treatment was started or added. However, neither participants nor raters were aware of the interest-activity hypothesis, and, therefore, the lack of blinding is an unlikely explanation for the unique prediction from interest-activity that is independent of overall depression severity. Third, while the sample size was sufficient to replicate previously reported prediction and find a significant interaction between predictor and treatment, the effect size estimates come with relatively broad confidence intervals. More accurate estimates of the prediction effect size and threshold level of interest-activity symptoms for considering aripiprazole augmentation will require a larger study. Finally, our study is limited to the comparison of escitalopram monotherapy and aripiprazole augmentation of escitalopram. It is possible that aripiprazole monotherapy may also be effective for MDD with pronounced interest-activity symptoms, but the evidence of aripiprazole efficacy in MDD is primarily as an augmentation agent and the use of aripiprazole monotherapy remains an experimental option.51-53
In conclusion, we report that loss of interest and reduction of activity in MDD may predict poor response to antidepressant monotherapy and indicate the need for aripiprazole augmentation. Future studies may evaluate accelerated use of augmentation with dopaminergic agents for depression with prominent interest-activity symptoms earlier in the course of treatment.
Submitted: January 1, 2020; accepted April 27, 2020.
Published online: June 16, 2020.
Potential conflicts of interest: Dr Blier has received honoraria for advisory board participation, giving lectures, and/or scientific expertise from Allergan, Bristol-Myers Squibb, Janssen, Lundbeck, Otsuka, and Pierre Fabre Medicaments and research grants from Allergan, Canadian Biomarker Integration Network in Depression (CAN-BIND), Janssen, Lundbeck, and Otsuka. Dr Frey has received a research grant from Pfizer. Dr Kennedy has received research funding or honoraria from Abbott, Alkermes, Allergan, Bristol-Myers Squibb, Brain Canada, Canadian Institutes for Health Research (CIHR), Janssen, Lundbeck, Lundbeck Institute, Ontario Brain Institute (OBI), Ontario Research Fund (ORF), Otsuka, Pfizer, Servier, Sunovion, and Xian-Janssen. Dr Lam has received honoraria for ad hoc speaking or advising/consulting, or received research funds, from Akili, Allergan, Asia-Pacific Economic Cooperation, BC Leading Edge Foundation, CIHR, Canadian Network for Mood and Anxiety Treatments, Canadian Psychiatric Association, CME Institute, Hansoh, Healthy Minds Canada, Janssen, Lundbeck, Lundbeck Institute, Medscape, Mind.Me, MITACS, Movember Foundation, OBI, Otsuka, Pfizer, St Jude Medical, University Health Network Foundation, and VGH-UBCH Foundation. Dr MacQueen has received honoraria for ad hoc speaking or advising/consulting, or received research funds, from Ontario Brain Institute, CIHR, Pfizer, Lundbeck, Janssen, Johnson & Johnson, and Allergan. Dr Milev has received consulting and speaking honoraria from Allergan, Janssen, KYE, Lundbeck, Otsuka, Pfizer, and Sunovion and research grants from CAN-BIND, CIHR, Janssen, Lallemand, Lundbeck, Nubiyota, OBI, Ontario Mental Health Foundation, and Pfizer. Dr Parikh is a consultant to Assurex, Takeda, and Mensante; has received honoraria from Sunovion and Mensante; and had received research funding from Assurex and Takeda. Dr Ravindran has received grants and/or honoraria from AstraZeneca, Bristol-Myers Squibb, Cephalon, Eli Lilly, GlaxoSmithKline, Janssen-Ortho, Lundbeck, Pfizer, Roche, Servier, and Wyeth. Dr Rotzinger holds a patent “‘ Teneurin C-Terminal Associated Peptides (TCAP) and methods and uses thereof.” Inventors: David Lovejoy, R. B. Chewpoy, Dalia Barsyte, Susan Rotzinger. Dr Soares has acted as a consultant for Servier, Sunovion, Lundbeck, and Otsuka. Drs Uher, Quilty, Müller, Foster, and Turecki report no relationships with financial interests.
Funding/support: This research was conducted as part of the Canadian Biomarker Integration Network in Depression (CAN-BIND), an Integrated Discovery Program supported by the Ontario Brain Institute, which is an independent nonprofit corporation, funded partially by the Ontario Government. Additional funding was provided by the Canadian Institutes of Health Research (CIHR), Lundbeck, Bristol-Myers Squibb, and Servier. Funding and/or in-kind support was also provided by the investigators’ universities and academic institutions. Dr Uher has received additional support from the Canada Research Chairs Program (award number 231397), the Canadian Institutes of Health Research (Grant reference number 148394), and the Dalhousie Medical Research Foundation.
Role of the sponsor: The supporters had no role in the design, analysis, interpretation, or publication of this study.
Disclaimer: The opinions, results and conclusions are those of the authors, and no endorsement by the Ontario Brain Institute is intended or should be inferred.
Previous presentation: Presented at the 10th Conference of the International Society for Affective Disorders (ISAD); November 14, 2019; London, United Kingdom.
Supplementary material: See accompanying pages.
REFERENCES
1.Rehm J, Shield KD. Global burden of disease and the impact of mental and addictive disorders. Curr Psychiatry Rep. 2019;21(2):10. PubMed CrossRef
2.Cipriani A, Furukawa TA, Salanti G, et al. Comparative efficacy and acceptability of 21 antidepressant drugs for the acute treatment of adults with major depressive disorder: a systematic review and network meta-analysis. Lancet. 2018;391(10128):1357-1366. PubMed CrossRef
3.Rush AJ, Trivedi MH, Wisniewski SR, et al. Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: a STAR*D report. Am J Psychiatry. 2006;163(11):1905-1917. PubMed CrossRef
4.Thornicroft G, Chatterji S, Evans-Lacko S, et al. Undertreatment of people with major depressive disorder in 21 countries. Br J Psychiatry. 2017;210(2):119-124. PubMed CrossRef
5.Wells JE, Browne MO, Aguilar-Gaxiola S, et al. Drop out from out-patient mental healthcare in the World Health Organization’s World Mental Health Survey initiative. Br J Psychiatry. 2013;202(1):42-49. PubMed CrossRef
6.Oluboka OJ, Katzman MA, Habert J, et al. Functional recovery in major depressive disorder: providing early optimal treatment for the individual patient. Int J Neuropsychopharmacol. 2018;21(2):128-144. PubMed CrossRef
7.Kessler RC. The potential of predictive analytics to provide clinical decision support in depression treatment planning. Curr Opin Psychiatry. 2018;31(1):32-39. PubMed CrossRef
8.Simon GE, Perlis RH. Personalized medicine for depression: can we match patients with treatments? Am J Psychiatry. 2010;167(12):1445-1455. PubMed CrossRef
9.Nanni V, Uher R, Danese A. Childhood maltreatment predicts unfavorable course of illness and treatment outcome in depression: a meta-analysis. Am J Psychiatry. 2012;169(2):141-151. PubMed CrossRef
10.Perlis RH. Abandoning personalization to get to precision in the pharmacotherapy of depression. World Psychiatry. 2016;15(3):228-235. PubMed CrossRef
11.Williams LM, Debattista C, Duchemin AM, et al. Childhood trauma predicts antidepressant response in adults with major depression: data from the randomized international study to predict optimized treatment for depression. Transl Psychiatry. 2016;6(5):e799. PubMed CrossRef
12.McGrath CL, Kelley ME, Holtzheimer PE, et al. Toward a neuroimaging treatment selection biomarker for major depressive disorder. JAMA Psychiatry. 2013;70(8):821-829. PubMed CrossRef
13.Uher R, Tansey KE, Dew T, et al. An inflammatory biomarker as a differential predictor of outcome of depression treatment with escitalopram and nortriptyline. Am J Psychiatry. 2014;171(12):1278-1286. PubMed CrossRef
14.Uher R, Perlis RH, Henigsberg N, et al. Depression symptom dimensions as predictors of antidepressant treatment outcome: replicable evidence for interest-activity symptoms. Psychol Med. 2012;42(5):967-980. PubMed CrossRef
15.Lane RM. Restoration of positive mood states in major depression as a potential drug development target. J Psychopharmacol. 2014;28(6):527-535. PubMed CrossRef
16.Nutt D, Demyttenaere K, Janka Z, et al. The other face of depression, reduced positive affect: the role of catecholamines in causation and cure. J Psychopharmacol. 2007;21(5):461-471. PubMed CrossRef
17.Jamerson BD, Krishnan KR, Roberts J, et al. Effect of bupropion SR on specific symptom clusters of depression: analysis of the 31-item Hamilton Rating Scale for depression. Psychopharmacol Bull. 2003;37(2):67-78. PubMed
18.Tomarken AJ, Dichter GS, Freid C, et al. Assessing the effects of bupropion SR on mood dimensions of depression. J Affect Disord. 2004;78(3):235-241. PubMed CrossRef
19.Hudson R, Zhou Y, Leri F. The combination of escitalopram and aripiprazole: investigation of psychomotor effects in rats. J Psychopharmacol. 2017;31(12):1605-1614. PubMed CrossRef
20.Fawcett J, Rush AJ, Vukelich J, et al. Clinical experience with high-dosage pramipexole in patients with treatment-resistant depressive episodes in unipolar and bipolar depression. Am J Psychiatry. 2016;173(2):107-111. PubMed CrossRef
21.Mohamed S, Johnson GR, Chen P, et al; VAST-D Investigators. Effect of antidepressant switching vs augmentation on remission among patients with major depressive disorder unresponsive to antidepressant treatment: the VAST-D randomized clinical trial. JAMA. 2017;318(2):132-145. PubMed CrossRef
22.Kennedy SH, Lam RW, Rotzinger S, et al; CAN-BIND Investigator Team. Symptomatic and functional outcomes and early prediction of response to escitalopram monotherapy and sequential adjunctive aripiprazole therapy in patients with major depressive disorder: a CAN-BIND-1 report. J Clin Psychiatry. 2019;80(2):18m12202. PubMed CrossRef
23.Lam RW, Milev R, Rotzinger S, et al; CAN-BIND Investigator Team. Discovering biomarkers for antidepressant response: protocol from the Canadian biomarker integration network in depression (CAN-BIND) and clinical characteristics of the first patient cohort. BMC Psychiatry. 2016;16(1):105. PubMed CrossRef
24.Sheehan DV, Lecrubier Y, Sheehan KH, et al. The Mini-International Neuropsychiatric Interview (MINI): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. 1998;59(suppl 20):22-33, quiz 34-57. PubMed
25.Montgomery SA, Asberg M. A new depression scale designed to be sensitive to change. Br J Psychiatry. 1979;134(4):382-389. PubMed CrossRef
26.Kennedy SH, Lam RW, McIntyre RS, et al; CANMAT Depression Work Group. Canadian Network for Mood and Anxiety Treatments (CANMAT) 2016 Clinical Guidelines for the Management of Adults with Major Depressive Disorder, section 3: pharmacological treatments. Can J Psychiatry. 2016;61(9):540-560. PubMed CrossRef
27.Tadori Y, Forbes RA, McQuade RD, et al. Characterization of aripiprazole partial agonist activity at human dopamine D3 receptors. Eur J Pharmacol. 2008;597(1-3):27-33. PubMed CrossRef
28.Wood M, Reavill C. Aripiprazole acts as a selective dopamine D2 receptor partial agonist. Expert Opin Investig Drugs. 2007;16(6):771-775. PubMed CrossRef
29.Oosterhof CA, El Mansari M, Blier P. Acute effects of brexpiprazole on serotonin, dopamine, and norepinephrine systems: an in vivo electrophysiologic characterization. J Pharmacol Exp Ther. 2014;351(3):585-595. PubMed CrossRef
30.Casey DE, Laubmeier KK, Eudicone JM, et al. Response and remission rates with adjunctive aripiprazole in patients with major depressive disorder who exhibit minimal or no improvement on antidepressant monotherapy. Int J Clin Pract. 2014;68(11):1301-1308. PubMed CrossRef
31.Nelson JC, Thase ME, Bellocchio EE, et al. Efficacy of adjunctive aripiprazole in patients with major depressive disorder who showed minimal response to initial antidepressant therapy. Int Clin Psychopharmacol. 2012;27(3):125-133. PubMed CrossRef
32.Rush AJ, Trivedi MH, Ibrahim HM, et al. The 16-Item Quick Inventory of Depressive Symptomatology (QIDS), clinician rating (QIDS-C), and self-report (QIDS-SR): a psychometric evaluation in patients with chronic major depression. Biol Psychiatry. 2003;54(5):573-583. PubMed CrossRef
33.Uher R, Farmer A, Maier W, et al. Measuring depression: comparison and integration of three scales in the GENDEP study. Psychol Med. 2008;38(2):289-300. PubMed CrossRef
34.Lane P. Handling drop-out in longitudinal clinical trials: a comparison of the LOCF and MMRM approaches. Pharm Stat. 2008;7(2):93-106. PubMed CrossRef
35.Siddiqui O, Hung HM, O’ Neill R. MMRM vs LOCF: a comprehensive comparison based on simulation study and 25 NDA datasets. J Biopharm Stat. 2009;19(2):227-246. PubMed CrossRef
36.Siddiqui O. MMRM versus MI in dealing with missing data—a comparison based on 25 NDA data sets. J Biopharm Stat. 2011;21(3):423-436. PubMed CrossRef
37.Uher R, Maier W, Hauser J, et al. Differential efficacy of escitalopram and nortriptyline on dimensional measures of depression. Br J Psychiatry. 2009;194(3):252-259. PubMed CrossRef
38.Strawbridge R, Carter B, Marwood L, et al. Augmentation therapies for treatment-resistant depression: systematic review and meta-analysis. Br J Psychiatry. 2019;214(1):42-51. PubMed CrossRef
39.Pizzagalli DA. Depression, stress, and anhedonia: toward a synthesis and integrated model. Annu Rev Clin Psychol. 2014;10(1):393-423. PubMed CrossRef
40.Scheggi S, Pelliccia T, Gambarana C, et al. Aripiprazole relieves motivational anhedonia in rats. J Affect Disord. 2018;227:192-197. PubMed CrossRef
41.Mazza M, Squillacioti MR, Pecora RD, et al. Effect of aripiprazole on self-reported anhedonia in bipolar depressed patients. Psychiatry Res. 2009;165(1-2):193-196. PubMed CrossRef
42.de Boer MK, Liemburg EJ, Wiersma D, et al. P.3.c.035 Differential effects of aripiprazole versus risperidone on sexual performance, negative symptoms or emotional flatness (anhedonia). Eur Neuropsychopharmacol. 2011;21(suppl 3):S488-S489. CrossRef
43.de Bartolomeis A, Tomasetti C, Iasevoli F. Update on the mechanism of action of aripiprazole: translational insights into antipsychotic strategies beyond dopamine receptor antagonism. CNS Drugs. 2015;29(9):773-799. PubMed CrossRef
44.Huang M, Horiguchi M, Felix AR, et al. 5-HT1A and 5-HT7 receptors contribute to lurasidone-induced dopamine efflux. Neuroreport. 2012;23(7):436-440. PubMed
45.Ichikawa J, Ishii H, Bonaccorso S, et al. 5-HT(2A) and D(2) receptor blockade increases cortical DA release via 5-HT(1A) receptor activation: a possible mechanism of atypical antipsychotic-induced cortical dopamine release. J Neurochem. 2001;76(5):1521-1531. PubMed CrossRef
46.Dunlop BW, McCabe B, Eudicone JM, et al. How well do clinicians and patients agree on depression treatment outcomes? implications for personalized medicine. Hum Psychopharmacol. 2014;29(6):528-536. PubMed CrossRef
47.Uher R, Perlis RH, Placentino A, et al. Self-report and clinician-rated measures of depression severity: can one replace the other? Depress Anxiety. 2012;29(12):1043-1049. PubMed CrossRef
48.Kaneriya SH, Robbins-Welty GA, Smagula SF, et al. Predictors and moderators of remission with aripiprazole augmentation in treatment-resistant late-life depression: an analysis of the IRL-GRey randomized clinical trial. JAMA Psychiatry. 2016;73(4):329-336. PubMed CrossRef
49.Sugawara H, Sakamoto K, Harada T, et al. A retrospective study of predictive factors for effective aripiprazole augmentation of antidepressant therapy in treatment-resistant depression. Neuropsychiatr Dis Treat. 2016;12:1151-1156. PubMed
50.Zisook S, Johnson GR, Tal I, et al. General predictors and moderators of depression remission: a VAST-D report. Am J Psychiatry. 2019;176(5):348-357. PubMed CrossRef
51.Hou YC, Lai CH. Aripiprazole monotherapy can relieve ruminations in a case with nonpsychotic depression. J Neuropsychiatry Clin Neurosci. 2014;26(4):E32-E33. PubMed CrossRef
52.Lai CH, Wu YT, Chen CY, et al. Gray matter increases in fronto-parietal regions of depression patients with aripiprazole monotherapy: an exploratory study. Medicine (Baltimore). 2016;95(34):e4654. PubMed CrossRef
53.Suzuki H, Hibino H, Inoue Y, et al. Effect of aripiprazole monotherapy in a patient presenting with delayed sleep phase syndrome associated with depressive symptoms. Psychiatry Clin Neurosci. 2018;72(5):375-376. PubMed CrossRef
This PDF is free for all visitors!
Save
Cite