Objective: A relationship between aggression and substance use has been debated for many years. While substance use increases the risk of aggressive behavior, no studies have reported on the relationship between impulsive aggression and substance use/disorder, specifically.
Methods: We analyzed data from the community-based National Comorbidity Survey Replication (N = 9,282 subjects) in order to examine the relationship between current DSM-5 intermittent explosive disorder (IED), a disorder of impulsive aggression, and current substance use disorders (SUDs), overall, and with regard to alcohol, tobacco, and cannabis use disorders and nondisordered use.
Results: Occurrence of current SUD was elevated in current IED versus non-IED adult subjects, and onset of IED preceded that of SUD in 92.5% of comorbid IED + SUD cases. This relationship was not due to the presence, or absence, of current depressive or anxiety disorders. Examination of the severity of IED and of SUD revealed that the presence of IED increases SUD severity but that the presence of SUD does not increase IED severity.
Conclusions: Subjects with IED are at increased risk of developing SUD, compared with those without IED. This suggests that history of recurrent, problematic, impulsive aggression is a risk factor for the later development of SUD rather than the reverse. If so, effective treatment of impulsive aggression, before the onset of substance misuse, may prevent, or delay, the development of SUD in young people.
Intermittent Explosive Disorder and Substance Use Disorder:
Analysis of the National Comorbidity Survey Replication Sample

ABSTRACT
Objective: A relationship between aggression and substance use has been debated for many years. While substance use increases the risk of aggressive behavior, no studies have reported on the relationship between impulsive aggression and substance use/disorder, specifically.
Methods: We analyzed data from the community-based National Comorbidity Survey Replication (N = 9,282 subjects) in order to examine the relationship between current DSM-5 intermittent explosive disorder (IED), a disorder of impulsive aggression, and current substance use disorders (SUDs), overall, and with regard to alcohol, tobacco, and cannabis use disorders and nondisordered use.
Results: Occurrence of current SUD was elevated in current IED versus non-IED adult subjects, and onset of IED preceded that of SUD in 92.5% of comorbid IED + SUD cases. This relationship was not due to the presence, or absence, of current depressive or anxiety disorders. Examination of the severity of IED and of SUD revealed that the presence of IED increases SUD severity but that the presence of SUD does not increase IED severity.
Conclusions: Subjects with IED are at increased risk of developing SUD, compared with those without IED. This suggests that history of recurrent, problematic, impulsive aggression is a risk factor for the later development of SUD rather than the reverse. If so, effective treatment of impulsive aggression, before the onset of substance misuse, may prevent, or delay, the development of SUD in young people.
J Clin Psychiatry 2017;78(6):697-702
https://doi.org/10.4088/JCP.15m10306
© Copyright 2017 Physicians Postgraduate Press, Inc.
aClinical Neuroscience Research Unit, Department of Psychiatry and Behavioral Neuroscience, Pritzker School of Medicine, The University of Chicago, Chicago, Illinois
*Corresponding author: Emil F. Coccaro, MD, Clinical Neuroscience Research Unit, Department of Psychiatry and Behavioral Neuroscience, The University of Chicago, 5841 S Maryland Ave, Chicago, IL 60637 ([email protected]).
Intermittent explosive disorder (IED), as defined in DSM-5, is characterized by recurrent, problematic, impulsive aggressive behavior.1 Aggression in IED may be displayed as high frequency/low intensity aggression that is nondestructive or noninjurious, or as low frequency/high intensity aggression that is destructive and/or injurious.2 However manifest, aggression in IED is impulsive, and/or anger-based. Based on data analyzed for the DSM-5 Task Force, about 70% of individuals with IED display both forms of impulsive aggression, 20% display only the high frequency/low intensity aggression, and 10% display only the low frequency/high intensity aggression.3 Aggressive behavior in IED is most often provoked in social interactions. In IED, hostile cognitive distortions lead to misinterpretations of nonthreatening social-emotional cues as threatening and an inappropriately aggressive response.4
Initially, it was thought that the impulsive aggressive behavior in IED was due to the presence of other psychiatric disorders.5 However, epidemiologic data indicate that the age at onset of IED is earlier than that of most other psychiatric disorders.6,7 Accordingly, when impulsive aggressive outbursts are not limited to episodes of another disorder, the diagnosis of IED may be made.1 In the clinic, IED is often comorbid with other disorders such as depressive, anxiety, and substance use disorders.8,9 We have reported on the comorbidity of IED with depressive and anxiety disorders previously,2 but have not reported on comorbidity of IED with substance use or substance use disorders (SUDs).
A relationship between aggression and SUDs has been discussed in the literature for many years. In general, substance use increases the risk of aggressive behavior.10-12 Notably, alcohol, cocaine, amphetamines, and cannabis have been found to increase the risk for aggressive behavior in human subjects. This statement belies the complexity of such comorbidity, and there are important issues regarding how aggression and how substance use are defined. For example, there are at least 2 forms of aggressive behavior: (1) aggressive behavior that is impulsive or reactive/defensive in nature13-15 and (2) premeditated aggressive behavior, typically associated with psychopathy.13,16 There are also several ways in which substances may influence aggressive behavior—first, through acute substance intoxication; second, through substance withdrawal; and third, through their association with environments steeped in violence.11
With the advent of a reliable, and valid, clinical entity of impulsive aggression (IED),1 it is timely to examine the relationship between IED and substance use and SUD. In this study, we reanalyzed data from the National Comorbidity Survey Replication (NCS-R)17 project in order to examine the relationship between current IED and current SUD. Based on the literature, and on our experience in working with individuals with recurrent, problematic, impulsive aggressive behavior, we hypothesized that (1) risk of current SUD would be significantly increased in current IED, compared with non-IED subjects; (2) in cases of current IED + SUD comorbidity, age at onset of IED would precede that of SUD; (3) IED + SUD comorbidity would not be influenced by current comorbid depressive or anxiety disorder; (4) among SUDs, risk of alcohol, tobacco, and cannabis use disorder would each be elevated in IED, compared with non-IED subjects; and (5) a “dose (or “use frequency”) effect” would be observed on the risk of specific SUDs in IED versus non-IED subjects.
METHODS
Subjects
The NCS-R sample used in this analysis had 9,282 subjects. Details regarding the design and acquisition of these data have been previously published.17 This study was approved by our Institutional Review Board, the Committee for the Protection of Human Subjects.

- Excessive substance use is common in intermittent explosive disorder (IED). It follows the onset of IED, and the presence of IED enhances the severity of substance misuse.
- Thus, treatment of IED that is comorbid with substance use disorder should include the treatment of aggression as well as the treatment of the substance misuse.
Assignment of Diagnoses
The NCS-R study was designed to assign DSM-IV diagnoses. However, raw NCS-R data allow DSM-IV diagnoses to be updated to those of the DSM-5.1 For the diagnosis of current IED, subjects reported at least 3 “anger attacks” in any given year with at least 1 in the past year (Criterion A2). In addition, “anger attacks” were out of proportion to the circumstances in which they occurred (Criterion B), impulsive in nature (Criterion C), associated with functional impairment and/or distress (Criterion D), with “anger attacks” occurring in the absence of other psychiatric disorders (Criterion F); all subjects were greater than the age of 6 years (Criterion E). Subjects meeting the DSM-IV criteria for drug/alcohol abuse or dependence were assigned a diagnosis of DSM-5 SUD when at least 2 of the DSM-5 SUD criteria were met. Data were available for alcohol, tobacco, cannabis, and cocaine, but not for other substances of abuse. Characteristics of the sample are listed in Table 1.
Focus of Study and Severity of IED, SUD, and Substance Use
We focused on the current disorders of subjects in the sample because it was clear when these disorders were comorbid and because these data were less likely to have retrospective bias compared with lifetime disorders. There were sufficient numbers of subjects with current IED and current alcohol, tobacco, or cannabis use disorder. However, only 1 subject with current IED had current cocaine use disorder and, thus, similar analyses could not be performed for this SUD. Severity of IED was assessed by maximum number of “anger attacks” in any year; severity of SUD was assessed by mean number of DSM-5 criteria met for SUD. Current substance use was assessed by 4 levels of weekly use: “minimal,” “low,” “medium,” and “high.” These levels were defined as follows—alcohol: less than 1, 1 to 6, 7 to 14, and greater than 14 drinks per week; tobacco: less than 10, 10 to 20, 21 to 40, and greater than 40 cigarettes per day; cannabis: less than 1, 1 to 2, 3 to 4, and 5 to 7 times weekly.
Statistical Analysis
Statistical procedures included χ2, Fisher exact test (FET), binary logistic regression, analysis of covariance (ANCOVA), and paired t test, as appropriate. All reported odds ratios (ORs) were adjusted for age, sex, ethnicity, education, and marital status. A 2-tailed α value of .05 was used to denote statistical significance for all analyses.
RESULTS
Comorbidity of Current IED and Current SUD
Despite a prevalence of current SUD of 5.7% (532/9,282) and of current IED of 2.2% (207/9,282), comorbidity of current SUD in current IED subjects was significantly greater than chance compared with non-IED subjects (28.5% [59/207] versus 5.2% [473/9,075]; OR = 5.42 [95% CI, 3.88-7.55], P < .001).
Current IED and SUD Comorbidity: Age at Onset of IED and SUD
Onset of IED preceded onset of SUD in 91.5% of current IED + SUD comorbid subjects (54/59). In IED + SUD subjects for whom IED preceded SUD, onset of IED preceded that of SUD by more than 8 years (8.3 ± 6.9 years; ie, mean + SD age = 11.9 ± 4.1 years for IED vs age = 20.2 ± 6.8 years for SUD, paired t53 = 8.80, P < .001) with a range of 1 to 32 years and a median of 6.5 years. In the remaining 5 IED + SUD subjects, onset of SUD preceded that of IED by less than 1 year (0.6 ± 0.6 years; ie, age = 17.0 ± 3.1 years for SUD vs age = 17.6 ± 2.6 years for IED, paired t4 = 2.45, P = .07) with a range of 0 to 1 year and a median of 0.5 years. For all IED (n = 320) and SUD (n = 532) subjects, respectively, mean ages at onset were 13.2 ± 6.8 years and 21.0 ± 9.0 years (t850 = 13.38, P < .001).
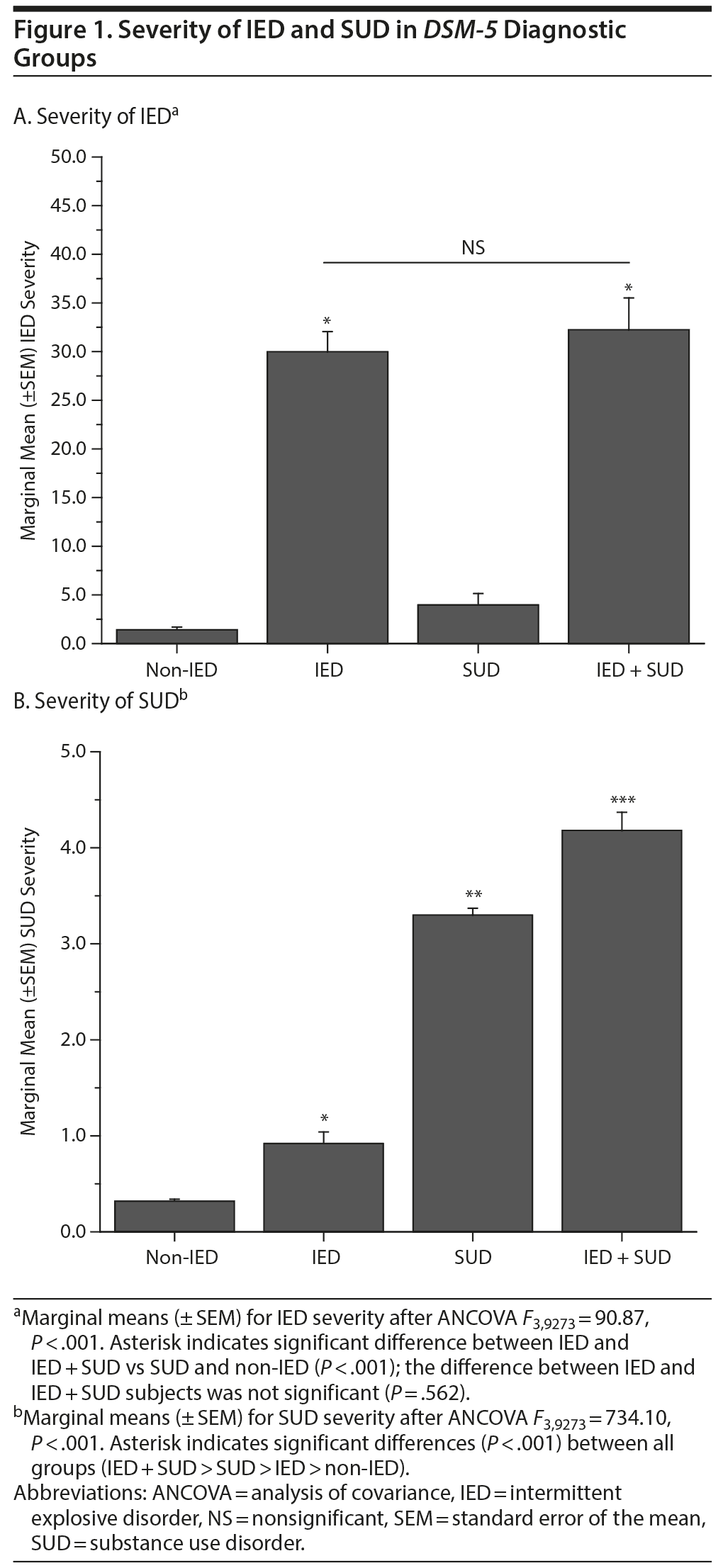
Current IED and SUD Comorbidity: Severity of IED and SUD
ANCOVA revealed that IED and IED + SUD subjects had similar IED severity and significantly higher IED severity compared with SUD (and non-IED) subjects; Figure 1A. Conversely, IED + SUD subjects had significantly higher SUD severity compared with SUD and IED (and non-IED) subjects; Figure 1B.
Comorbid IED and SUD as a Function of Depressive and Anxiety Disorders
To explore if relationships between current IED and current SUD extend to other disorders often comorbid with IED,8,9 we performed similar analyses with current depressive disorder and with current anxiety disorder (Table 2). Current IED was more prevalent among current SUD in subjects regardless of current depressive disorder or current anxiety disorder. For both the depressive disorder and anxiety disorder comparisons, however, significantly greater ORs of SUD in IED subjects were observed in subjects without anxiety disorder compared to subjects with anxiety disorder.
Comorbid IED With Specific SUDs: Alcohol Use Disorder (AUD), Tobacco Use Disorder (TUD), and Cannabis Use Disorder (CUD)
Frequency of current IED with each of these current SUD disorders was elevated compared with non-IED subjects as follows: AUD 15.5% (32/207) vs 2.1% (188/9,075), OR = 5.78 (95% CI, 3.79-8.85), P < .001; TUD 13.5% (28/207) vs 3.1% (283/9,075), OR = 4.06 (95% CI, 2.65-6.24), P < .001; and CUD 7.2% (15/207) vs 0.6% (52/9,075), OR = 6.65 (95% CI, 3.58-12.35), P < .001. Differences in these ORs were not statistically significant: AUD and TUD (z = 1.08, P = .280); AUD and CUD (z = 0.43, P = .668); CUD and TUD (z = 1.50, P = .134). As with SUD overall, age at onset of IED in subjects with current AUD, TUD, or CUD was lower than the age at onset for AUD (11.7 ± 4.1 vs 19.1 ± 5.8 years, t31 = 7.26, P < .001), TUD (11.9 ± 4.3 vs 22.9 ± 6.7 years, t27 = 7.24, P < .001), or CUD (12.4 ± 3.0 vs 14.1 ± 2.2 years, t14 = 2.19, P < .05).
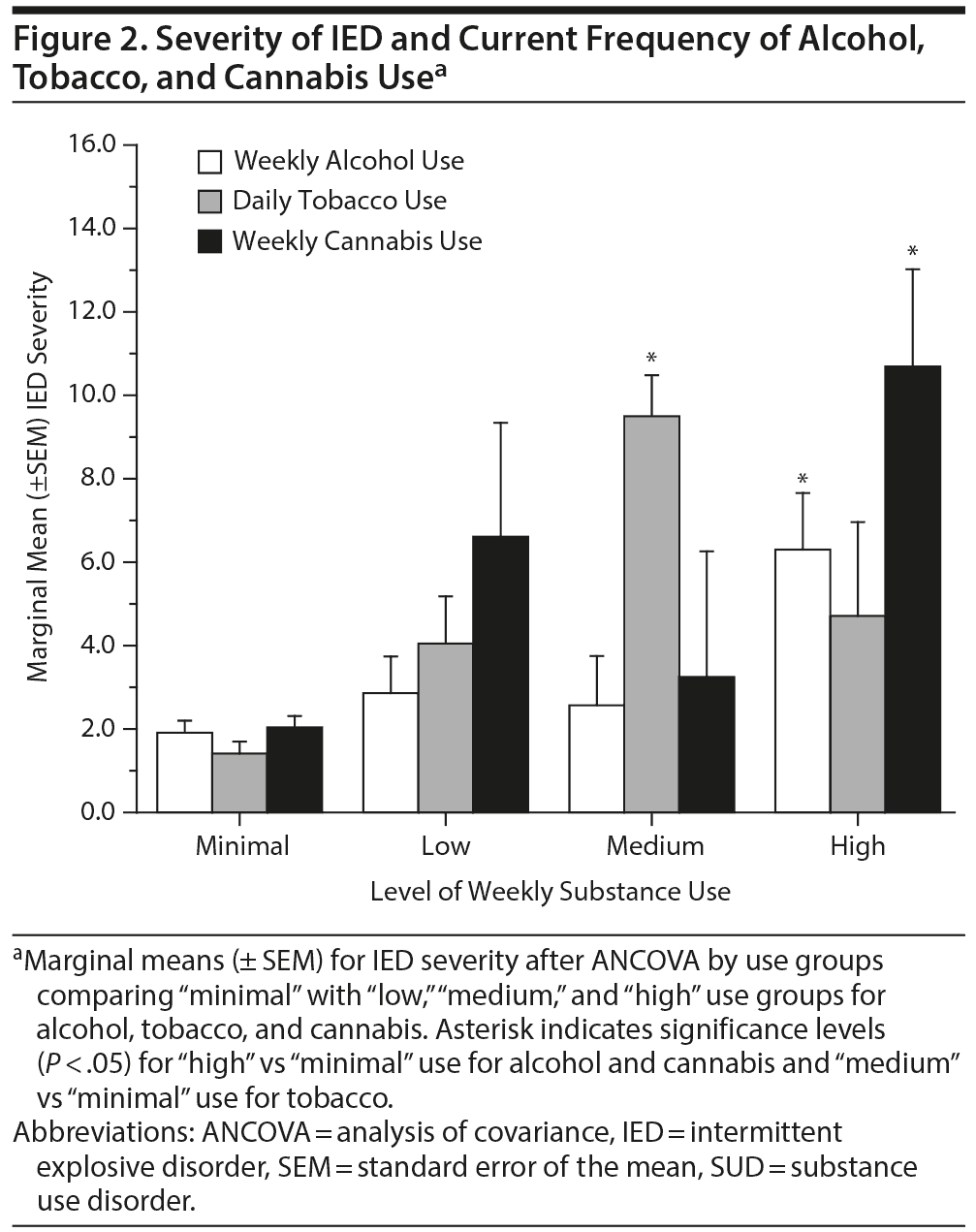
Severity of IED and Current Frequency of Alcohol, Tobacco, and Cannabis Use
In order to determine if these observations held true across different levels of substance use, we examined the relationship of IED severity with weekly current substance use defined as “minimal,” “low,” “medium,” and “high.” Significant differences in IED severity were observed at “high use” for alcohol and cannabis and at “medium use” for tobacco when compared to minimal use (Figure 2). However, even at high current use of alcohol and cannabis, less than a third of these subjects met DSM-5 criteria for current AUD (28.8%) or current CUD (32.2%), respectively, and less than 20% met criteria for TUD at the moderate current use level of tobacco (18.8%), indicating that these relationships are not firmly defined by DSM-5 criteria for SUD.
Current IED and Tobacco and Cannabis Use Disorders as a Function of Alcohol Use Disorder
Since current AUD was significantly comorbid with current TUD (OR = 5.13 [95% CI, 3.47-7.58]; P < .001) and with current CUD (OR = 27.25 [95% CI, 15.88-46.74]; P < .001), it was of interest to determine if the presence of current AUD influenced comorbidity with current IED. Similar to the results above, an elevated OR for current TUD was observed in current IED versus non-IED, but only in subjects without current AUD (Table 3). For current CUD, an elevated OR for IED was observed regardless of current AUD, although the OR for subjects without current AUD was significantly greater than that for those with current AUD (Table 3).
DISCUSSION
The primary finding in this analysis is that current IED, a categorical expression of impulsive aggression, is comorbid with current SUD and represents an overall risk for SUD that may be 5-fold greater than that observed in non-IED subjects. In addition, we found that the age at onset of IED was earlier than that for SUD for the vast majority of IED + SUD comorbid subjects. This finding in a population-based sample of adults supports a previous report that the onset of IED precedes SUD in adolescents.7 Thus, IED may identify a risk factor for the development of maladaptive substance use, and SUD, in later adolescence and adulthood.15
Examination of the comparative severity of IED and SUD in IED + SUD subjects suggests an effect of IED on SUD rather than the reverse. Specifically, we found that comorbid IED + SUD was associated with greater SUD severity (compared with SUD only subjects) in the absence of any difference in IED severity (compared with IED only subjects). In addition, SUD severity in IED only subjects was significantly greater than that for non-IED/non-SUD subjects suggesting that even the presence of IED alone increases the risk of SUD symptoms. It is possible that IED is an early indicator of a more severe impulsive externalizing liability that expresses in later substance use.
Another notable observation is the influence of current comorbidity of depressive and anxiety disorders in their association of IED and SUD. In this study, ORs for subjects with, or without, current depressive or anxiety disorders were each greater than chance, although to different degrees. Specifically, the risk of IED + SUD in subjects with current anxiety (but not depressive) disorder was lower than that for subjects without current anxiety disorder by about 5-fold. This is because the presence of current depressive disorder increased IED + SUD comorbidity more so than the presence of current anxiety disorder. The reason for this is unknown, as depressive and anxiety disorders are highly comorbid with each other and both are known to be associated with behavioral irritability. However, these data are unable to shed any further light on this observation.
The NCS-R data set did not allow an analysis of all possible SUDs; we were only able to examine SUDs related to alcohol, tobacco, and cannabis. For these substances, current IED was associated with a 4-fold to a nearly 7-fold increased risk for current AUD, TUD, and CUD, although these risk estimates did not significantly differ from each other.
Examination of the association between current IED and the individual substances investigated revealed a significant difference in IED severity between “minimal” and “highest” use levels for alcohol and cannabis; a similar difference was observed at the “medium” use level for tobacco. This approach complements that of the DSM categorical approach and suggests, further, that IED is also associated with increases in the subclinical use of these substances.
While these data cannot provide definitive insight on the direction of these relationships, these data are consistent with other relevant data. For example, human laboratory studies demonstrate that alcohol administration increases aggression particularly in individuals with higher scores on aggression measures compared with individuals with lower scores on these measures.18 Similar interpretations for the relationship between aggression and tobacco use and cannabis use, however, are not likely to be as straightforward. Specifically, the possibility that increasing use of cannabis leads to increasing impulsive aggressive behavior is just as plausible as the possibility that increasing levels of aggressive behavior lead to greater cannabis use. While studies in this area are few, acute administration of cannabis has been shown to reduce aggressive responding in some,19 although not all,20 laboratory aggression tasks. Conversely, it is also known that irritability and aggressive behavior can emerge during cannabis withdrawal within 1 to 5 days of the absence of further cannabis intake.21-24 Thus, it is unknown if an association between current IED and current CUD (or cannabis use) is due to an attempt by an individual with IED to reduce aggression/irritability by using cannabis19,25 or if the aggression observed in those with IED is associated with acute cannabis withdrawal21-24 during intermittent periods of cannabis use. A third possibility is that heavy cannabis use has deleterious effects on brain function that lead to the development of IED. However, since the onset of IED was earlier than that of cannabis use disorder in IED + CUD subjects, cannabis may well be being used for its antiaggressive properties19,25 rather than the converse (ie, IED emerging as a consequence of cannabis withdrawal or cannabis-related neurologic damage). The same may be true for the relationship between tobacco use and tobacco use disorder and aggression. Laboratory studies of human aggression report that smoking tobacco, in cigarette smokers, reduces aggressive responding to provocation.26 In contrast, acute abstinence from smoking tobacco is associated with increased aggressive responding to provocation that is then blocked by administration of nicotine gum.27
The relationship between the 3 substances of abuse in this study is also noteworthy. Specifically, the risk of comorbid current IED and current TUD is lower in subjects with current AUD compared with those without current AUD. This may be due to the fact that alcohol use is widely known to increase aggressive behavior in humans10,11 and, thus, aggressive events would be expected to be higher even in non-IED subjects. That said, the risk of current IED is elevated above chance in subjects with current TUD and CUD regardless of current AUD.
Strengths and Limitations
The major strengths of the current study are that these data were collected as part of a large, nationally representative, community sample, using state of the art assessment tools, and that these data were blind to any of our hypotheses. Additionally, updating psychiatric diagnoses to meet DSM-5 criteria makes these data currently useful to researchers and clinicians alike. As with any investigation, a number of limitations must be noted. First, there are a modest number of subjects in some subanalyses, and this increases the risk of type 1 and 2 error depending on the finding. Second, these data are from a cross-sectional examination, and this limits our interpretation for the causation of comorbidity. That said, the age-at-onset data strongly suggest that IED first occurs prior to the onset of SUD by several years. Third, these data were collected more than a decade ago and may not fully reflect more recent population-wide changes, particularly in regard to SUD. However, there are no other epidemiologic data sets with more recently collected data with which to address these questions. Fourth, the NCS-R data set did not contain data regarding high-frequency/low-intensity aggressive outbursts (ie, Criterion A1 for DSM-5 IED), and so, information about subjects with IED of this type,28,29 which might include an additional 10% of IED subjects,9 was not available in this data set. However, about 75% of IED subjects with low-frequency/high-intensity aggressive outbursts (ie, Criterion A2 for DSM-5 IED), as assessed in the NCS-R project, also have this form of aggressive outburst, and IED subjects who only meet Criterion A1 do not differ from those who meet Criterion A2.3
CONCLUSION
The current presence of IED is associated with a statistically significant increase in the risk of current SUD. At the same time, the onset of IED precedes that of SUD in more than 90% of comorbid cases. This relationship between IED and SUD did not appear to be due to the presence, or absence, of current depressive or anxiety disorders. Accordingly, individuals with IED are at increased risk of developing SUD at some time after the onset of IED. This may be because IED is an early indicator of a more severe impulsive externalizing liability that expresses in later substance use. If so, effective treatment of impulsive aggression, before the onset of substance misuse, may prevent, or delay, the development of SUD in young people.
Submitted: August 6, 2015; accepted March 29, 2016.
Online first: February 28, 2017.
Potential conflicts of interest: Dr Coccaro reports being on the Scientific Advisory Board of Azevan Pharmaceuticals. Dr Lee reports being the recipient of a research grant from Azevan Pharmaceuticals. Dr Fanning has nothing to disclose.
Funding/support: This work was supported in part by a grant from the National Institute of Mental Health (RO1MH104673: Dr Coccaro).
Role of the sponsor: The funding agency had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; or preparation, review, and approval of the manuscript.
REFERENCES
1. American Psychiatric Association. Diagnostic and Statistical Manual for Mental Disorders. Fifth Edition. Washington, DC: American Psychiatric Association; 2013.
2. Coccaro EF. Intermittent explosive disorder as a disorder of impulsive aggression for DSM-5. Am J Psychiatry. 2012;169(6):577-588. PubMed doi:10.1176/appi.ajp.2012.11081259
3. Coccaro EF, Lee R, McCloskey MS. Validity of the new A1 and A2 criteria for DSM-5 intermittent explosive disorder. Compr Psychiatry. 2014;55(2):260-267. PubMed doi:10.1016/j.comppsych.2013.09.007
4. Coccaro EF, Noblett KL, McCloskey MS. Attributional and emotional responses to socially ambiguous cues: validation of a new assessment of social/emotional information processing in healthy adults and impulsive aggressive patients. J Psychiatr Res. 2009;43(10):915-925. PubMed doi:10.1016/j.jpsychires.2009.01.012
5. Felthous AR, Bryant SG, Wingerter CB, et al. The diagnosis of intermittent explosive disorder in violent men. Bull Am Acad Psychiatry Law. 1991;19(1):71-79. PubMed
6. Kessler RC, Coccaro EF, Fava M, et al. The prevalence and correlates of DSM-IV intermittent explosive disorder in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2006;63(6):669-678. PubMed doi:10.1001/archpsyc.63.6.669
7. McLaughlin KA, Green JG, Hwang I, et al. Intermittent explosive disorder in the National Comorbidity Survey Replication Adolescent Supplement. Arch Gen Psychiatry. 2012;69(11):1131-1139. PubMed doi:10.1001/archgenpsychiatry.2012.592
8. Coccaro EF, Kavoussi RJ, Berman ME, et al. Intermittent explosive disorder-revised: development, reliability, and validity of research criteria. Compr Psychiatry. 1998;39(6):368-376. PubMed doi:10.1016/S0010-440X(98)90050-5
9. Coccaro EF. Intermittent explosive disorder: development of integrated research criteria for Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition. Comprehensive Psychiatry. 2011;52(2):119-125.
10. Beck A, Heinz AJ, Heinz A. Translational clinical neuroscience perspectives on the cognitive and neurobiological mechanisms underlying alcohol-related aggression. Curr Top Behav Neurosci. 2014;17:443-474. PubMed doi:10.1007/7854_2013_258
11. Hoaken PN, Stewart SH. Drugs of abuse and the elicitation of human aggressive behavior. Addict Behav. 2003;28(9):1533-1554. PubMed doi:10.1016/j.addbeh.2003.08.033
12. Boles SM, Miotto K. Substance abuse and violence: a review of the literature. Aggress Violent Behav. 2003;8:155-174. doi:10.1016/S1359-1789(01)00057-X
13. Cornell DG, Warren J, Hawk G, et al. Psychopathy in instrumental and reactive violent offenders. J Consult Clin Psychol. 1996;64(4):783-790. PubMed doi:10.1037/0022-006X.64.4.783
14. Stanford MS, Houston RJ, Mathias CW, et al. Characterizing aggressive behavior. Assessment. 2003;10(2):183-190. PubMed doi:10.1177/1073191103010002009
15. Kockler TR, Stanford MS, Nelson CE, et al. Characterizing aggressive behavior in a forensic population. Am J Orthopsychiatry. 2006;76(1):80-85. PubMed doi:10.1037/0002-9432.76.1.80
16. Coccaro EF, Lee R, McCloskey MS. Relationship between psychopathy, aggression, anger, impulsivity, and intermittent explosive disorder. Aggress Behav. 2014;40(6):526-536. PubMed doi:10.1002/ab.21536
17. Kessler RC, Merikangas KR. The National Comorbidity Survey Replication (NCS-R): background and aims. Int J Methods Psychiatr Res. 2004;13(2):60-68. PubMed doi:10.1002/mpr.166
18. Parrott DJ, Zeichner A. Effects of alcohol and trait anger on physical aggression in men. J Stud Alcohol. 2002;63(2):196-204. PubMed doi:10.15288/jsa.2002.63.196
19. Myerscough R, Taylor S. The effects of marijuana on human physical aggression. J Pers Soc Psychol. 1985;49(6):1541-1546. PubMed doi:10.1037/0022-3514.49.6.1541
20. Cherek DR, Dougherty DM. Provocation frequency and its role in determining the effects of smoked marijuana on human aggressive responding. Behav Pharmacol. 1995;6(4):405-412. PubMed doi:10.1097/00008877-199506000-00011
21. Kouri EM, Pope HGJ Jr, Lukas SE. Changes in aggressive behavior during withdrawal from long-term marijuana use. Psychopharmacology (Berl). 1999;143(3):302-308. PubMed doi:10.1007/s002130050951
22. Budney AJ, Hughes JR, Moore BA, et al. Marijuana abstinence effects in marijuana smokers maintained in their home environment. Arch Gen Psychiatry. 2001;58(10):917-924. PubMed doi:10.1001/archpsyc.58.10.917
23. Budney AJ, Moore BA, Vandrey RG, et al. The time course and significance of cannabis withdrawal. J Abnorm Psychol. 2003;112(3):393-402. PubMed doi:10.1037/0021-843X.112.3.393
24. Haney M, Ward AS, Comer SD, et al. Abstinence symptoms following smoked marijuana in humans. Psychopharmacology (Berl). 1999;141(4):395-404. PubMed doi:10.1007/s002130050849
25. Arendt M, Rosenberg R, Fjordback L, et al. Testing the self-medication hypothesis of depression and aggression in cannabis-dependent subjects. Psychol Med. 2007;37(7):935-945. PubMed doi:10.1017/S0033291706009688
26. Cherek DR. Effects of smoking different doses of nicotine on human aggressive behavior. Psychopharmacology (Berl). 1981;75(4):339-345. PubMed doi:10.1007/BF00435849
27. Cherek DR, Bennett RH, Grabowski J. Human aggressive responding during acute tobacco abstinence: effects of nicotine and placebo gum. Psychopharmacology (Berl). 1991;104(3):317-322. PubMed doi:10.1007/BF02246030
28. Koelsch S, Sammler D, Jentschke S, et al. EEG correlates of moderate intermittent explosive disorder. Clin Neurophysiol. 2008;119(1):151-162. PubMed doi:10.1016/j.clinph.2007.09.131
29. Koelsch S. P3a and mismatch negativity in individuals with moderate intermittent explosive disorder. Neurosci Lett. 2009;460(1):21-26. PubMed doi:10.1016/j.neulet.2009.05.047
Please sign in or purchase this PDF for $40.00.
Save
Cite