Intranasal drug delivery (INDD) systems offer a route to the brain that bypasses problems related to gastrointestinal absorption, first-pass metabolism, and the blood-brain barrier; onset of therapeutic action is rapid, and the inconvenience and discomfort of parenteral administration are avoided. INDD has found several applications in neuropsychiatry, such as to treat migraine, acute and chronic pain, Parkinson disease, disorders of cognition, autism, schizophrenia, social phobia, and depression. INDD has also been used to test experimental drugs, such as peptides, for neuropsychiatric indications; these drugs cannot easily be administered by other routes. This article examines the advantages and applications of INDD in neuropsychiatry; provides examples of test, experimental, and approved INDD treatments; and focuses especially on the potential of intranasal ketamine for the acute and maintenance therapy of refractory depression.
Each month in his online column, Dr Andrade considers theoretical and practical ideas in clinical psychopharmacology with a view to update the knowledge and skills of medical practitioners who treat patients with psychiatric conditions.
- Intranasal drug delivery (INDD) systems allow for improved bioavailability, avoidance of the inconvenience and discomfort of parenteral administration, rapid onset of therapeutic action, and bypassing of the blood-brain barrier.
- INDD has been used for test, experimental, and approved indications to treat conditions such as migraine, acute and chronic pain, Parkinson disease, disorders of cognition, schizophrenia, social phobia, autism, and refractory depression.
- Intranasal ketamine is emerging as a potential alternative to intravenous ketamine infusions for patients with refractory depression; at present, however, the data are limited, and so the treatment remains emphatically experimental.

ABSTRACT
Intranasal drug delivery (INDD) systems offer a route to the brain that bypasses problems related to gastrointestinal absorption, first-pass metabolism, and the blood-brain barrier; onset of therapeutic action is rapid, and the inconvenience and discomfort of parenteral administration are avoided. INDD has found several applications in neuropsychiatry, such as to treat migraine, acute and chronic pain, Parkinson disease, disorders of cognition, autism, schizophrenia, social phobia, and depression. INDD has also been used to test experimental drugs, such as peptides, for neuropsychiatric indications; these drugs cannot easily be administered by other routes. This article examines the advantages and applications of INDD in neuropsychiatry; provides examples of test, experimental, and approved INDD treatments; and focuses especially on the potential of intranasal ketamine for the acute and maintenance therapy of refractory depression.
J Clin Psychiatry 2015;76(5):e628-e631 (doi:10.4088/JCP.15f10026).
© Copyright 2015 Physicians Postgraduate Press, Inc.
Clinical Question
Intravenous ketamine infusion has been found safe1 and effective2,3 as a treatment for medication-refractory depression; suicidal symptoms also attenuate.4 The benefits, however, are transient and seldom persist beyond 1-2 weeks.5 Some data suggest that repeated infusions, such as on alternate days, prolong the duration of response.6 However, frequently repeated intravenous ketamine infusion is not a practical treatment strategy for maintenance therapy in patients who relapse after response to ketamine and subsequent maintenance with conventional antidepressant medication. So, how may ketamine responders be treated in the long term to prolong the treatment response?
Introduction
Medications in neuropsychiatric practice are most commonly administered either orally or parenterally. Intranasal drug delivery (INDD) systems, however, have been available for decades; there is a large body of animal literature on the subject, this route of drug administration has long been used in several medical fields, and, more recently, INDD has gained importance even in neuropsychiatry. This article will examine INDD in neuropsychiatry with particular focus on the use of intranasal ketamine in the treatment of refractory depression.
Why Deliver Drugs Intranasally?
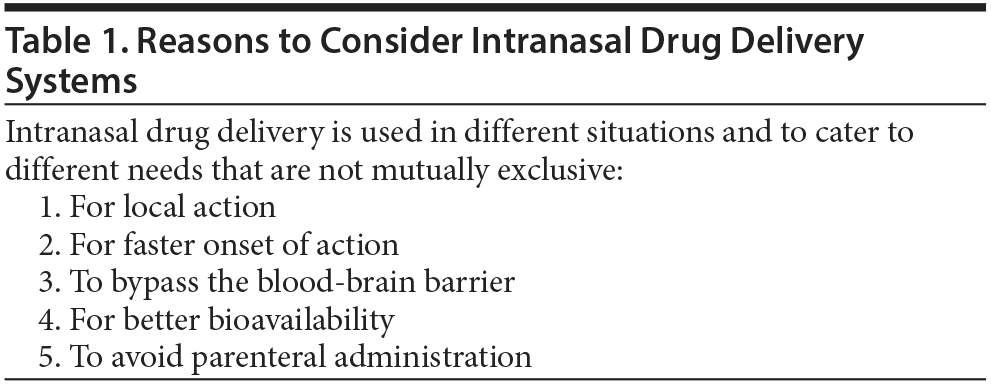
INDD systems cater to different situations and needs that are not necessarily mutually exclusive (Table 1). These are briefly discussed below.
Local action. In otorhinolaryngologic practice, medications have for long been administered intranasally (as drops or sprays) for local action. In neuropsychiatry, a patient may require a nasal decongestant if a stuffy nose results from the use of sildenafil or a tricyclic antidepressant drug.7,8
Rapid onset of action. INDD is associated with a fast onset of action. This is because there is quick drug absorption from the rich, intranasal vascular bed. Peak blood levels are rapidly attained.9 As examples, sumatriptan nasal spray10 and intranasal lidocaine11 both afford rapid relief from acute migraine. Nicotine nasal spray affords rapid relief from craving in nicotine-dependent individuals.12
Bypassing the blood-brain barrier. INDD can deliver drugs directly to the central nervous system, bypassing the blood-brain barrier. Absorption occurs through the olfactory epithelium, and transport through the cribriform plate, via the olfactory pathways, into the brain.9 An example is the use of insulin spray as an experimental treatment for cognitive decline and Alzheimer’s disease.13 INDD can also be used to study brain functioning. For example, a peptide that interferes with the interaction between D1 and D2 receptors was shown to have antidepressant action in the forced swim test in rodents for up to 2 hours after intranasal administration. Inhibition of D1-D2 receptor interaction was demonstrated in the prefrontal cortex.14
Improvement of bioavailability. INDD can improve bioavailability of drugs such as peptides that may be digested rather than absorbed after oral administration. Examples of approved and experimental treatments include desmopressin for pediatric15 and geriatric16 enuresis, insulin for disorders of cognition,13 and oxytocin for a variety of experimental indications (see below).
Avoidance of parenteral administration. INDD can improve the convenience of drug administration, as with intranasally administered ketamine17 in place of intravenously infused ketamine for patients with refractory depression.
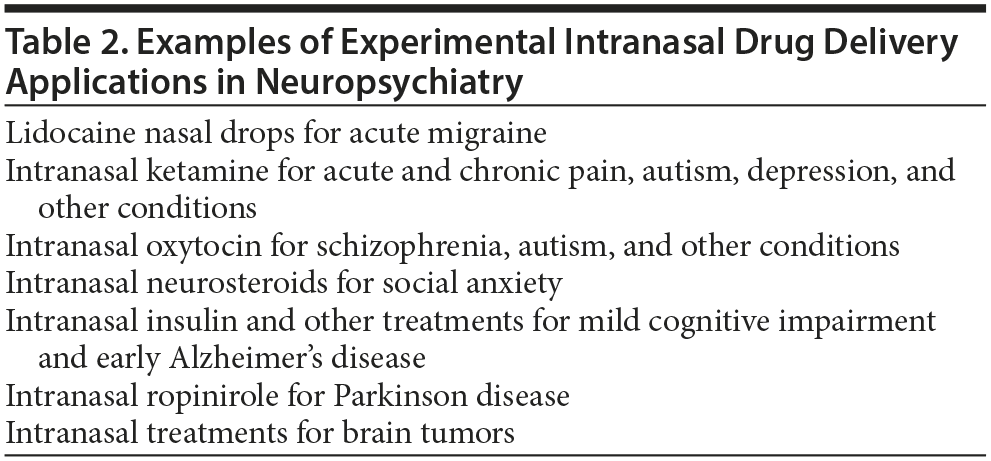
Table 2 lists a few examples of INDD applications in neuropsychiatry; in this regard, ketamine and oxytocin are perhaps the best-studied agents. Aqueous (4%) lidocaine nasal drops have demonstrated rapid efficacy in episodes of acute migraine.11 Intranasal ropinirole18 and other intranasal treatments are being studied for Parkinson disease, and intranasal insulin and other treatments are being studied for mild cognitive impairment and early Alzheimer’s disease.13,19 A neurosteroid, PH94B, was successfully trialled for social anxiety in women.20 INDD is also being studied for brain neoplasms.21 This list is not exhaustive.
Intranasal Oxytocin
Intranasal oxytocin may influence social relationships and has been much studied in this regard; notwithstanding the media hype over this so-called love hormone, research has not resulted in straightforward conclusions.22,23
There has been much investigation of the possible benefits of intranasal oxytocin for schizophrenia,24 with improvements recorded in domains such as clinical symptom ratings25 and social cognition.26 Intranasal oxytocin has also been studied for autism. For example, 15 children and adolescents with autism spectrum disorder showed improvement during 12 weeks of treatment in the domains of social functioning, repetitive behaviors, and anxiety; some improvements persisted as long as 3 months later.27 A randomized controlled trial, however, failed to demonstrate efficacy.28 Some benefits with intranasal oxytocin have also been recorded in social anxiety.29 Intranasal oxytocin is also being studied for the prevention of posttraumatic stress disorder in persons who experience trauma.30
Intranasal Ketamine
Intranasal ketamine attenuates pain in the emergency room in children31 and adults.32 Intranasal ketamine also reduces the severity of pain in migraine.33 Wink et al34 reported a 29-year-old woman with autism who was treated with intranasal ketamine (20-60 mg) on 12 dosing occasions across 6 weeks. She showed improvements in mood, social interactions, flexibility, tolerance of changes in routine, motivation, and concentration. Adverse events were mostly mild; the most prominent was headache, which lasted for up to 10 hours after a treatment. A case report also showed benefits with intranasal ketamine in depression.35
In a randomized, double-blind, saline-controlled, crossover trial conducted in 20 patients with major depression, Lapidus et al17 found that a single intranasal dose of ketamine (50 mg) outperformed saline by 7.6 points on the Montgomery-Asberg Depression Rating Scale as assessed 24 hours after dosing; the response rate was 44% vs 6%, respectively. Anxiety ratings also decreased significantly more with ketamine. However, there was no significant separation between ketamine and saline at 3 and 7 days posttreatment. In this study, intranasal ketamine was well tolerated, with few, mild, and very transient adverse effects such as feelings of unreality. There was also a small and transient increase in systolic blood pressure (by 7.6 mm Hg at 40 minutes).
Maintenance treatment with intravenous ketamine infusion maintains treatment gains.6 Because the antidepressant benefits of intranasal ketamine wear off within 3 days,17 might maintenance treatment with intranasal ketamine be a viable treatment strategy to extend treatment gains? Regrettably, this has not been investigated in the context of depression. However, in a retrospective chart review of 12 treatment-refractory bipolar youth (10 male; age, 6-19 years) with fear of harm phenotype, Papolos et al36 reported that maintenance therapy with intranasal ketamine (30-120 mg) resulted in improvements in anxiety, aggression, fear of harm, cognition, behavior, sleep, and other symptoms. Interestingly, hypomanic symptoms also attenuated with intranasal ketamine. Adverse events were mostly dissociative in nature, and all remitted within an hour, without medical intervention. Intranasal ketamine dosing had to be repeated every 3-7 days to maintain the experienced benefits. In many patients, other medications could be tapered or discontinued. No patient dropped out of treatment, and, at the time of writing, 1 patient had been receiving maintenance intranasal ketamine for over 4 years.
This author (Andrade, unpublished data) has personal experience with using intranasal ketamine in the dose of 50-80 mg per treatment occasion, once in 2-3 days, as maintenance therapy for a 25-year-old, medication- and electroconvulsive therapy-refractory, functionally impaired man with severe depression. The treatment has been ongoing for the past 26 months and keeps depression at bay only when punctually administered. It has been the only intervention to have helped the patient during a 10-year span of life-crippling depressive illness.
One hopes that there will soon be parallel-group, randomized controlled trials examining the safety and efficacy of repeated dosing with intranasal ketamine, followed by trials of its safety and efficacy during maintenance therapy in treatment-refractory depressed patients. Until data from such trials become available, intranasal ketamine will remain an experimental treatment.
Parting Notes
- The oral bioavailability of ketamine is only 8%-17% because of extensive first-pass metabolism37,38; bioavailability is slightly higher, at 29%, when the drug is administered sublingually.39 Interestingly, both oral and sublingual ketamine have been trialled in depression. In a 4-week, proof-of-concept study, Irwin et al40 administered oral ketamine (0.5 mg/kg/d) to 14 mildly anxious and depressed patients in hospice care. Four patients dropped out because of nonresponse, and 2, for reasons unrelated to ketamine. All 8 treatment completers showed improvements in anxiety and depression, the former occurring earlier than the latter. Lara et al41 administered very low dose (10 mg) sublingual ketamine every 2-3 days or weekly to 26 outpatients with refractory unipolar or bipolar depression. Rapid and sustained improvement occurred in mood, cognition, and sleep in 20 patients (77%). The treatment was very well tolerated, with mild, transient light-headedness as the only common adverse effect. Other reports have also been published.42-44 Oral ketamine has also been used to treat chronic pain.45,46
- Inhalational drug delivery systems, such as those that deliver bronchodilators or steroids to the lungs, have long been used in medicine. These have found recent application in psychiatry, as well. Inhaled loxapine is approved in the United States and the European Union for acute agitation in schizophrenia and bipolar disorder. It is administered using a hand-held, single-dose, single-use device. The drug enters pulmonary alveoli and is quickly absorbed into the systemic circulation. Peak blood levels are attained in 2 minutes, and clinical benefits are evident as early as 10 minutes after drug administration. Inhalational loxapine is contraindicated in the presence of acute respiratory disease and in patients at risk of bronchospasm.47
- Djupesland et al48 provided an extensive review on anatomic, physiologic, and delivery technology issues related to INDD systems.
 Each month in his online column, Dr Andrade considers theoretical and practical ideas in clinical psychopharmacology with a view to update the knowledge and skills of medical practitioners who treat patients with psychiatric conditions.
Each month in his online column, Dr Andrade considers theoretical and practical ideas in clinical psychopharmacology with a view to update the knowledge and skills of medical practitioners who treat patients with psychiatric conditions.
Department of Clinical Psychopharmacology and Neurotoxicology, National Institute of Mental Health and Neurosciences, Bangalore, India ([email protected]).
Financial disclosure and more about Dr Andrade.
REFERENCES
1. Wan LB, Levitch CF, Perez AM, et al. Ketamine safety and tolerability in clinical trials for treatment-resistant depression. J Clin Psychiatry. 2015;76(3):247-252. PubMed doi:10.4088/JCP.13m08852
2. Rao TS, Andrade C. Innovative approaches to treatment-refractory depression: the ketamine story. Indian J Psychiatry. 2010;52(2):97-99. PubMed doi:10.4103/0019-5545.64573
3. Lee EE, Della Selva MP, Liu A, et al. Ketamine as a novel treatment for major depressive disorder and bipolar depression: a systematic review and quantitative meta-analysis. Gen Hosp Psychiatry. 2015;37(2):178-184. PubMed doi:10.1016/j.genhosppsych.2015.01.003
4. Reinstatler L, Youssef NA. Ketamine as a potential treatment for suicidal ideation: a systematic review of the literature. Drugs R D. 2015;15(1):37-43. PubMed doi:10.1007/s40268-015-0081-0
5. Köhler S, Betzler F. Ketamine—a new treatment option for therapy-resistant depression. Fortschr Neurol Psychiatr. 2015;83(2):91-97. doi:10.1055/s-0034-1398967 PubMed
6. Shiroma PR, Johns B, Kuskowski M, et al. Augmentation of response and remission to serial intravenous subanesthetic ketamine in treatment resistant depression. J Affect Disord. 2014;155:123-129. PubMed doi:10.1016/j.jad.2013.10.036
7. Kiroglu AF, Bayrakli H, Yuca K, et al. Nasal obstruction as a common side-effect of sildenafil citrate. Tohoku J Exp Med. 2006;208(3):251-254. PubMed doi:10.1620/tjem.208.251
8. Levin A. Mianserin and clomipramine in the treatment of depression. S Afr Med J. 1982;61(19):701-704. PubMed
9. Henkin RI. Intranasal delivery to the brain. Nat Biotechnol. 2011;29(6):480. PubMed doi:10.1038/nbt.1866
10. Derry CJ, Derry S, Moore RA. Sumatriptan (intranasal route of administration) for acute migraine attacks in adults. Cochrane Database Syst Rev. 2012;2:CD009663. PubMed doi:/10.1002/14651858.cd009663
11. Maizels M, Scott B, Cohen W, et al. Intranasal lidocaine for treatment of migraine: a randomized, double-blind, controlled trial. JAMA. 1996;276(4):319-321. PubMed doi:10.1001/jama.1996.03540040063034
12. Stead LF, Perera R, Bullen C, et al. Nicotine replacement therapy for smoking cessation. Cochrane Database Syst Rev. 2012;11:CD000146. PubMed doi:10.1002/14651858.cd000146.pub4
13. Claxton A, Baker LD, Hanson A, et al. Long-acting intranasal insulin detemir improves cognition for adults with mild cognitive impairment or early-stage Alzheimer’s disease dementia. J Alzheimers Dis. 2015;44(3):897-906. PubMed
14. Brown V, Liu F. Intranasal delivery of a peptide with antidepressant-like effect. Neuropsychopharmacology. 2014;39(9):2131-2141. PubMed doi:10.1038/npp.2014.61
15. Robson WL, Leung AK, Norgaard JP. The comparative safety of oral versus intranasal desmopressin for the treatment of children with nocturnal enuresis. J Urol. 2007;178(1):24-30. PubMed doi:10.1016/j.juro.2007.03.015
16. Suchowersky O, Furtado S, Rohs G. Beneficial effect of intranasal desmopressin for nocturnal polyuria in Parkinson’s disease. Mov Disord. 1995;10(3):337-340. PubMed doi:10.1002/mds.870100318
17. Lapidus KA, Levitch CF, Perez AM, et al. A randomized controlled trial of intranasal ketamine in major depressive disorder. Biol Psychiatry. 2014;76(12):970-976. PubMed doi:10.1016/j.biopsych.2014.03.026
18. Jafarieh O, Md S, Ali M, et al. Design, characterization, and evaluation of intranasal delivery of ropinirole-loaded mucoadhesive nanoparticles for brain targeting [published online ahead of print December 11, 2014]. Drug Dev Ind Pharm. 2014:1-8. PubMed doi:10.3109/03639045.2014.991400
19. Sood S, Jain K, Gowthamarajan K. Intranasal therapeutic strategies for management of Alzheimer’s disease. J Drug Target. 2014;22(4):279-294. PubMed doi:10.3109/1061186X.2013.876644
20. Liebowitz MR, Salman E, Nicolini H, et al. Effect of an acute intranasal aerosol dose of PH94B on social and performance anxiety in women with social anxiety disorder. Am J Psychiatry. 2014;171(6):675-682. PubMed doi:10.1176/appi.ajp.2014.12101342
21. Peterson A, Bansal A, Hofman F, et al. A systematic review of inhaled intranasal therapy for central nervous system neoplasms: an emerging therapeutic option. J Neurooncol. 2014;116(3):437-446. PubMed doi:10.1007/s11060-013-1346-5
22. Wudarczyk OA, Earp BD, Guastella A, et al. Could intranasal oxytocin be used to enhance relationships? research imperatives, clinical policy, and ethical considerations. Curr Opin Psychiatry. 2013;26(5):474-484. PubMed doi:10.1097/YCO.0b013e3283642e10
23. Zik JB, Roberts DL. The many faces of oxytocin: Implications for psychiatry. Psychiatry Res. 2015;226(1):31-37. PubMed doi:10.1016/j.psychres.2014.11.048
24. De Berardis D, Marini S, Iasevoli F, et al. The role of intranasal oxytocin in the treatment of patients with schizophrenia: a systematic review. CNS Neurol Disord Drug Targets. 2013;12(2):252-264. PubMed doi:10.2174/1871527311312020012
25. Modabbernia A, Rezaei F, Salehi B, et al. Intranasal oxytocin as an adjunct to risperidone in patients with schizophrenia: an 8-week, randomized, double-blind, placebo-controlled study. CNS Drugs. 2013;27(1):57-65. PubMed doi:10.1007/s40263-012-0022-1
26. Woolley JD, Chuang B, Lam O, et al. Oxytocin administration enhances controlled social cognition in patients with schizophrenia. Psychoneuroendocrinology. 2014;47:116-125. PubMed doi:10.1016/j.psyneuen.2014.04.024
27. Anagnostou E, Soorya L, Brian J, et al. Intranasal oxytocin in the treatment of autism spectrum disorders: a review of literature and early safety and efficacy data in youth. Brain Res. 2014;1580:188-198. PubMed doi:10.1016/j.brainres.2014.01.049
28. Guastella AJ, Gray KM, Rinehart NJ, et al. The effects of a course of intranasal oxytocin on social behaviors in youth diagnosed with autism spectrum disorders: a randomized controlled trial. J Child Psychol Psychiatry. 2015;56(4):444-452. PubMed doi:10.1111/jcpp.12305
29. Guastella AJ, Howard AL, Dadds MR, et al. A randomized controlled trial of intranasal oxytocin as an adjunct to exposure therapy for social anxiety disorder. Psychoneuroendocrinology. 2009;34(6):917-923. PubMed doi:10.1016/j.psyneuen.2009.01.005
30. Frijling JL, van Zuiden M, Koch SB, et al. Efficacy of oxytocin administration early after psychotrauma in preventing the development of PTSD: study protocol of a randomized controlled trial. BMC Psychiatry. 2014;14(1):92. PubMed doi:10.1186/1471-244X-14-92
31. Graudins A, Meek R, Egerton-Warburton D, et al. The PICHFORK (Pain in Children Fentanyl or Ketamine) trial: a randomized controlled trial comparing intranasal ketamine and fentanyl for the relief of moderate to severe pain in children with limb injuries. Ann Emerg Med. 2015;65(3):248-254, e1. PubMed doi:10.1016/j.annemergmed.2014.09.024
32. Yeaman F, Meek R, Egerton-Warburton D, et al. Sub-dissociative-dose intranasal ketamine for moderate to severe pain in adult emergency department patients. Emerg Med Australas. 2014;26(3):237-242. PubMed doi:10.1111/1742-6723.12173
33. Afridi SK, Giffin NJ, Kaube H, et al. A randomized controlled trial of intranasal ketamine in migraine with prolonged aura. Neurology. 2013;80(7):642-647. PubMed doi:10.1212/WNL.0b013e3182824e66
34. Wink LK, O’ Melia AM, Shaffer RC, et al. Intranasal ketamine treatment in an adult with autism spectrum disorder. J Clin Psychiatry. 2014;75(8):835-836. PubMed doi:10.4088/JCP.13cr08917
35. Clark P. Treatment-refractory depression: a case of successful treatment with intranasal ketamine 10%. Ann Clin Psychiatry. 2014;26(2):145. PubMed
36. Papolos DF, Teicher MH, Faedda GL, et al. Clinical experience using intranasal ketamine in the treatment of pediatric bipolar disorder/fear of harm phenotype. J Affect Disord. 2013;147(1-3):431-436. PubMed doi:10.1016/j.jad.2012.08.040
37. Clements JA, Nimmo WS, Grant IS. Bioavailability, pharmacokinetics, and analgesic activity of ketamine in humans. J Pharm Sci. 1982;71(5):539-542. PubMed doi:10.1002/jps.2600710516
38. Fanta S, Kinnunen M, Backman JT, et al. Population pharmacokinetics of S-ketamine and norketamine in healthy volunteers after intravenous and oral dosing. Eur J Clin Pharmacol. 2015;71(4):441-447. PubMed doi:10.1007/s00228-015-1826-y
39. Rolan P, Lim S, Sunderland V, et al. The absolute bioavailability of racemic ketamine from a novel sublingual formulation. Br J Clin Pharmacol. 2014;77(6):1011-1016. PubMed doi:10.1111/bcp.12264
40. Irwin SA, Iglewicz A, Nelesen RA, et al. Daily oral ketamine for the treatment of depression and anxiety in patients receiving hospice care: a 28-day open-label proof-of-concept trial. J Palliat Med. 2013;16(8):958-965. PubMed doi:10.1089/jpm.2012.0617
41. Lara DR, Bisol LW, Munari LR. Antidepressant, mood stabilizing and procognitive effects of very low dose sublingual ketamine in refractory unipolar and bipolar depression. Int J Neuropsychopharmacol. 2013;16(9):2111-2117. PubMed doi:10.1017/S1461145713000485
42. Irwin SA, Iglewicz A. Oral ketamine for the rapid treatment of depression and anxiety in patients receiving hospice care. J Palliat Med. 2010;13(7):903-908. PubMed doi:10.1089/jpm.2010.9808
43. Paslakis G, Gilles M, Meyer-Lindenberg A, et al. Oral administration of the NMDA receptor antagonist S-ketamine as add-on therapy of depression: a case series. Pharmacopsychiatry. 2010;43(1):33-35. PubMed doi:10.1055/s-0029-1237375
44. De Gioannis A, De Leo D. Oral ketamine augmentation for chronic suicidality in treatment-resistant depression. Aust N Z J Psychiatry. 2014;48(7):686. PubMed doi:10.1177/0004867414520754
45. Blonk MI, Koder BG, van den Bemt PM, et al. Use of oral ketamine in chronic pain management: a review. Eur J Pain. 2010;14(5):466-472. PubMed doi:10.1016/j.ejpain.2009.09.005
46. Marchetti F, Coutaux A, Bellanger A, et al. Efficacy and safety of oral ketamine for the relief of intractable chronic pain: a retrospective 5-year study of 51 patients [published online ahead of print November 10, 2014]. Eur J Pain. 10.1002/ejp.624 PubMed
47. Keating GM. Loxapine inhalation powder: a review of its use in the acute treatment of agitation in patients with bipolar disorder or schizophrenia. CNS Drugs. 2013;27(6):479-489. PubMed doi:10.1007/s40263-013-0075-9
48. Djupesland PG, Messina JC, Mahmoud RA. The nasal approach to delivering treatment for brain diseases: an anatomic, physiologic, and delivery technology overview. Ther Deliv. 2014;5(6):709-733. PubMed doi:10.4155/tde.14.41
Save
Cite
Advertisement
GAM ID: sidebar-top