What Is Hypomania? Tetrachoric Factor Analysis and Kernel Estimation of DSM-IV Hypomanic Symptoms
Background: The DSM-IV definition of hypomania, which relies on clinical consensus and historical tradition, includes several “nonspecific” symptoms. The aim of this study was to identify the core symptoms of DSM-IV hypomania.
Method: In an outpatient private practice, 266 bipolar II disorder (BP-II) and 138 major depressive disorder (MDD) remitted patients were interviewed by a bipolar-trained psychiatrist, for different study goals. Patients were questioned, using the Structured Clinical Interview for DSM-IV, about the most common symptoms and duration of recent threshold and subthreshold hypomanic episodes. Data were recorded between 2002 and 2006. Four different samples, assessed with the same methodology, were pooled for the present analyses. Tetrachoric factor analysis was used to identify core hypomanic symptoms. Distribution of symptoms by kernel estimation was inspected for bimodality. Validity of core hypomania was tested by receiver operating characteristic (ROC) analysis.
Results: The distribution of subthreshold and threshold hypomanic episodes did not show bimodality. Tetrachoric factor analysis found 2 uncorrelated factors: factor 1 included the “classic” symptoms elevated mood, inflated self-esteem, decreased need for sleep, talkativeness, and increase in goal-directed activity (overactivity); factor 2 included the “nonspecific” symptoms irritable mood, racing/crowded thoughts, and distractibility. Factor 1 discriminatory accuracy for distinguishing BP-II versus MDD was high (ROC area = 0.94). The distribution of the 5-symptom episodes of factor 1 showed clear-cut bimodality. Similar results were found for episodes limited to 3 behavioral symptoms of factor 1 (decreased need for sleep, talkativeness, and overactivity) and 4 behavioral symptoms of factor 1 (adding elevated mood), with high discriminatory accuracy.
Conclusions: A core, categorical DSM-IV hypomania was found that included 3 to 5 symptoms, ie, behavioral symptoms and elevated mood. Behavioral symptoms (overactivity domain) could be the basic phenotype of hypomania. This finding could help in probing for hypomania and reduce misdiagnosis. Biologic research could focus more on the underpinnings of the overactivity domain specifically.
J Clin Psychiatry 2009;70(11):1514-1521
© Copyright 2009 Physicians Postgraduate Press, Inc.
Submitted: February 1, 2009; accepted March 17, 2009.
Online ahead of print: September 8, 2009 (doi:10.4088/JCP.09m05090).
‘ Deceased.
Corresponding author: Franco Benazzi, MD, Via Pozzetto 17, 48015 Castiglione Cervia RA, Italy ([email protected]).
DSM-IV-TR bipolar II disorder (BP-II) is defined by recurrent hypomanic episodes and major depressive episodes (MDEs).1 The DSM-IV-TR “prototypical” symptom of hypomania (criterion A) is elevated mood (and/or irritable mood).1(p366) ICD-10 criteria for hypomania instead require both elevated mood and increased activity (overactivity),2 while in DSM-IV-TR overactivity (“increase in goal-directed activity”) is one of the criterion B symptoms, optional and of similar weight as other criterion B symptoms. Hypomania is the core feature distinguishing BP-II from major depressive disorder (MDD). “Classic” diagnostic validators3 such as family history4-7 and diagnostic stability7-10 support the diagnostic validity of the DSM-IV-TR category of BP-II and thus of hypomania (ICD-10 has no diagnostic criteria for BP-II).
While the DSM-IV-TR definition of hypomania is supported by “classic” diagnostic validators and has shown clinical utility, its definition derives from clinical consensus among the DSM-IV working group11 and from historical descriptions (eg, Falret, 1854; Hecker, 1898; Kraepelin, 1913).12-14 DSM-IV-TR hypomania is a categorical disorder (ie, it has “defining features” and “clear boundaries,” according to the DSM-IV definition of category). However, recent epidemiologic and clinical sample studies have questioned its categorical nature,6,10,15-24 as subthreshold hypomania was found to be more common than threshold hypomania. Subthreshold hypomania was also found to be not uncommon in MDD, both lifetime and concurrent, ie, in mixed depression.25-34
The questioning of the current definition of hypomania comes also from the presence among its symptoms of several “nonspecific” symptoms that are present in many mood disorders and other Axis I and Axis II disorders (ie, irritability, psychomotor agitation, distractibility, risky and impulsive activities). In mixed depression (of BP-I, BP-II, and MDD), defined by the co-occurrence of depression (MDE) and (usually) subthreshold mania/hypomania,5,6 the “nonspecific” hypomanic symptoms are among the most common symptoms of subthreshold hypomania. Presence of “nonspecific” symptoms among DSM-IV-TR hypomania criteria A and B symptoms is probably the main cause of the finding of a high frequency of subthreshold hypomania in epidemiologic samples and in longitudinal and cross-sectional MDD clinical samples. The presence of threshold and subthreshold hypomania in bipolar samples,15,18,20 fluctuations from subthreshold to threshold hypomania in BP-II,10,19 the not uncommon history of subthreshold hypomania in MDD,5,26 and the concurrent depression and subthreshold hypomania in mixed depression (bipolar disorder and MDD) argue against a categorical hypomania as defined by DSM-IV-TR. “Classic” diagnostic validators, especially family history and diagnostic stability, could, on the other hand, support its categorical nature. The categorical versus dimensional nature of DSM-IV-TR hypomania, and its core “specific” symptoms versus its “nonspecific” symptoms, are strongly interlinked. The identification of the core symptoms of hypomania should help clarify its categorical/dimensional nature and can provide a better tool for the study of its biology and genetics. As the biologic underpinnings of hypomania and the related clinical dimensions are unknown, what can be done is to work on the current definition of hypomania. Compared to ICD-10, the DSM-IV-TR has a “strict” set of symptoms that could be tested.
According to DSM-IV-TR, categories are “types based on criteria sets with defining features”1(pxxxi) (ie, differences are qualitative, and there are clear boundaries), whereas “a dimensional system classifies clinical presentations based on quantification of attributes, describing phenomena that are distributed continuously and that do not have clear boundaries.”1(pxxxii) DSM-IV-TR focuses only on the face validity of hypomania. However, diagnostic validation does not have a “gold standard.”11,35 The most common method to assess diagnostic validity relies on external criteria3: (1) clinical description, (2) laboratory studies, (3) delimitation from other disorders by exclusion criteria, (4) follow-up studies including course and diagnostic stability, and (5) family studies. However, even the most supported of these validators, ie, diagnostic stability and family studies, have shown several limitations. Diagnostic stability is high in BP-II, but threshold and subthreshold hypomania fluctuate, and diagnostic stability varies widely in MDD.8,36-41 The older family histories/studies have shown that BP-II is more common in the relatives of BP-II probands compared to BP-I and MDD probands, but MDD is more common than BP-II in relatives of BP-II probands.4 Family studies using polarity-based phenotypes have led to inconclusive genetic studies,4,42 because genetic liability results from multiple genes, overlapping among different disorders, with complex, bidirectional gene-environment interactions.43,44 Validation has thus turned to psychometrics. Several methods have been suggested. The most used and supported validators of the categorical versus the dimensional nature of a syndrome11,45,46 are the distribution of symptoms and validating variables and, among multivariate statistics, factor analysis.19,21-23,33,34,47-58
A dimensional nature would be supported by (1) a distribution of symptoms without bimodality (ie, no clear boundaries from neighboring syndromes separated by a “zone of rarity” of its “defining characteristics”)44-48,59 and (2) factor analysis showing a single-factor solution or highly correlated factors.11
Factor analysis, by its orthogonal and oblique rotations, can show if factors are correlated or distinct. Apart from factor analysis, other multivariate analyses have been suggested to identify distinct subtypes (eg, admixture analysis, latent class analysis, grade-of-membership analysis, taxometric method).35,60-62 These more advanced statistics are still at an early stage: because of their different and strong assumptions, different conclusions have been found independent of the phenomenon, eg, concurrently supporting a categorical or a dimensional nature of a disorder, supporting dimensions in some studies and categories in others.35,63 Until now, the most supported statistical method for testing dimensions has been factor analysis.11
The aim of the study was to identify the core symptoms of the DSM-IV-TR definition of hypomania and to test the categorical nature of a core hypomania.
METHOD
Setting
The study setting was an outpatient psychiatry private practice (nontertiary care), which is the first or second line of treatment of depression in the wealthy Italian Emilia-Romagna region. Most people can afford a fee-for-service (not expensive) private visit in this region, limiting a possible selection bias. The interviewer was the author (F.B.), a clinical (26 years in practice) and mood disorder research psychiatrist.
Population
The study population consisted of a consecutive remitted sample of 266 outpatients with BP-II and a consecutive, independent, remitted sample of 138 outpatients with MDD, whose data were previously recorded for different study goals. Exclusion criteria were as follows: comorbid substance-related disorders and borderline personality disorder (uncommon in the study setting64), to avoid confounding the diagnosis of hypomania65; cognitive disorders (assessed by clinical evaluation using a semistructured interview based on DSM-IV-TR criteria); and clinically significant (ie, impairing functioning) general medical illnesses (assessed by clinical evaluation). The interviews had been conducted when the patients were in a state of remission during follow-up visits (which, at the beginning of the remission, included visits every other week), in order to overcome possible biases related to a current episode. Remission was assessed clinically and with the Global Assessment of Functioning (GAF, in the Structured Clinical Interview for DSM-IV Axis I Disorders-Clinician Version [SCID-CV]66), requiring a score > 80 for at least 1 month. Often (in around 70% of cases; no exact figure recorded), family members or close friends supplemented clinical information during the diagnostic interviews.
Data were recorded between 2002 and 2006. All patients meeting study criteria were included, and data were pooled from 4 independent samples for the present analyses (ie, all recorded patients were assessed during remission for a study goal, with the same methodology). All interviewed patients with a mood disorder (BP-II or MDD) were included in the samples.
Subjects gave their informed consent after the procedure was fully explained. Local institutional review board approval was obtained for the investigation.
Diagnostic Interviewing
The SCID-CV was used (F.B. interrater reliability κ for diagnosing past hypomania by the SCID-CV was 0.7367). As BP-II patients rarely present for treatment of a hypomanic episode, interview was based on recall of symptoms of past hypomanic episodes, as in most studies on hypomania.1,4
During follow-up visits, patients had been interviewed and rediagnosed with the SCID-CV. Questioning addressed the most common symptoms and duration of the most recent threshold and subthreshold hypomanic episodes. Relatively few variables were recorded because of the limited time of a follow-up visit in this busy private practice. The SCID-CV is partly semistructured and is based on clinical evaluation (differently from the epidemiologic structured interviews, which use “fixed” questions administered by lay interviewers). Wording of the questions can be changed in order to improve and check the interviewees’ understanding of the questions. This is an important advantage versus fully structured interviews, because this interview method has been shown to increase the correct classification of BP-II.68-70 The skip-out instruction in the stem question on history of mood change (elevated/irritable mood) was not followed, in order to assess all past hypomanic symptoms, as in Dunner and Tay.68 A minimum duration of threshold and subthreshold hypomania of 2 days was required, instead of the DSM-IV-TR minimum duration of 4 days, on the basis of the evidence supporting this cutoff, ie, no differences on “classic” diagnostic validators between BP-II patients whose hypomania lasted 2 to 3 days and patients with DSM-IV-TR BP-II7,15,17,19,71,72; indeed, the DSM-IV-TR cutoff of 4 days is only consensus-based.73 Around 30% of the BP-II samples met the 2-day minimum duration.
Definition of Hypomanic Episode
Threshold hypomanic episodes met DSM-IV-TR criteria (apart from duration). Subthreshold hypomanic episodes did not meet DSM-IV-TR criteria because of the absence of criterion A (mood change) or because the minimum number of symptoms was not met (eg, subthreshold hypomania: 5 symptoms but no mood change; mood change plus 2 symptoms). All of the symptoms of threshold and subthreshold hypomanic episodes were assessed and recorded in both the BP-II and the MDD samples.
Testing Study Aim
The aims of the study were to identify the core symptoms of DSM-IV-TR hypomania and to test the categorical nature of a core hypomania. For testing the categorical nature, and for identifying core symptoms, DSM-IV-TR was followed (ie, categories are “types based on criteria sets with defining features”; “a dimensional system classifies clinical presentations based on quantification of attributes, describing phenomena that are distributed continuously and that do not have clear boundaries”) and the corresponding methods suggested by many authors.11, 21, 22, 33, 34, 46-48, 51-58, 74, 75 These methods included (1) the inspection for bimodality of the cross-sectional distribution of symptoms (all of the constellation of symptoms that define hypomania were concurrently assessed, following Ruscio et al35) and (2) factor analysis, for identifying the factor structure and testing correlations among factors. The validity of the core symptoms of hypomania was tested by assessing the discriminatory accuracy in distinguishing BP-II and MDD by receiver operating characteristic (ROC) analysis.
Statistics
Univariate logistic regression was used to compare the frequency of hypomanic episodes in BP-II versus MDD. The distribution of the frequency of hypomanic episodes was assessed by univariate kernel density estimation. Kernel density estimation has the advantage, compared to the histogram method, that it does not tend to concentrate the observations in the center and in the tails (biasing results).76 Kernel density estimation is smooth and is independent of the choice of origin (the location of bins in a histogram). The distribution of hypomanic episodes was inspected for unimodality or bimodality (ie, modes separated by a “zone of rarity”). Factor analysis was used to identify the “core” symptoms of hypomania, eliminating the “nonspecific” symptoms, which could bias results toward unimodality. Tetrachoric factor analysis for binary variables was used to study the factor structure of hypomania, because symptoms were recorded as binary (0/1) variables.53 Factor analysis is a model for measuring continuous unimodal variables using a Pearson correlation matrix, which may be misleading for Bernoulli-distributed variables (ie, binary variables). In this case, a matrix of tetrachoric correlations is more appropriate. Iterated principal factors and principal-component factors were tested. Varimax orthogonal rotation and oblimin oblique rotation (Kaiser off) were also tested to assess the correlation among factors. The selection of factors relied on an eigenvalue > 1 and on inspection of the scree plot. Item loading had to be > 0.40.
Nonparametric ROC analysis was used to test the accuracy of the hypomanic factor structure in discriminating BP-II versus MDD. A global performance of the discriminatory accuracy is summarized by the area under the ROC curve. This area has a range of 0.5 to 1; the closer it is to 1, the stronger the discriminatory accuracy. STATA Statistical Software, Release 10.1 (StataCorp; College Station, Texas; 2008) was used. P values were 2-tailed; α level was set at .05.
RESULTS
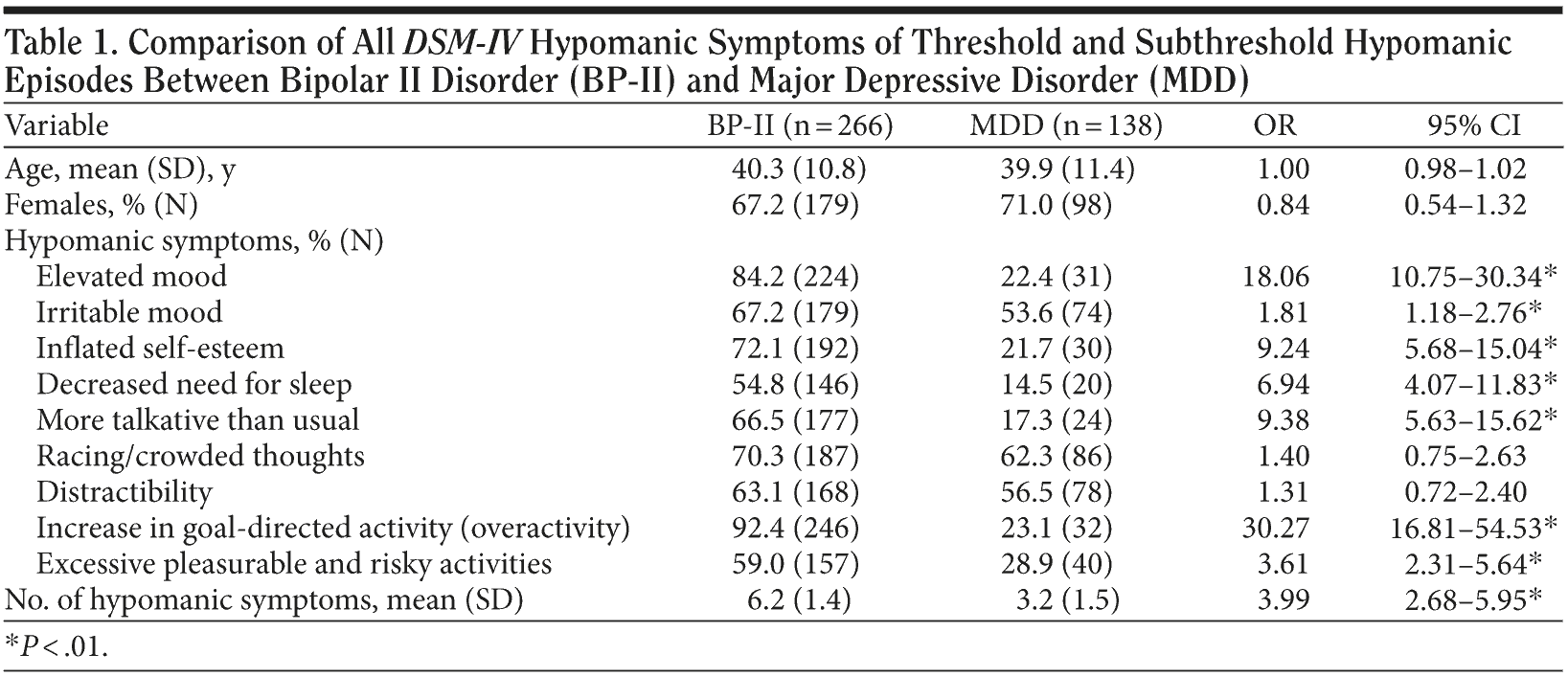
Comparison of symptoms of threshold and subthreshold hypomanic episodes in BP-II versus MDD is presented in Table 1. As expected, BP-II was much more likely to have a higher frequency of hypomanic symptoms (apart from racing thoughts and distractibility), but subthreshold hypomanic episodes were not uncommon in MDD (especially including irritable mood, racing thoughts, and distractibility). Among the symptoms, elevated mood (OR = 18) and overactivity (OR = 30.3) had the highest association with BP-II, and overactivity had the strongest.
Episodes were assessed according to the number of all DSM-IV-TR hypomanic symptoms in BP-II and in MDD: in BP-II, the modal number of symptoms per episode was 6; in MDD, subthreshold episodes of 3 to 4 symptoms were the most common, with a frequency of 53.5%.
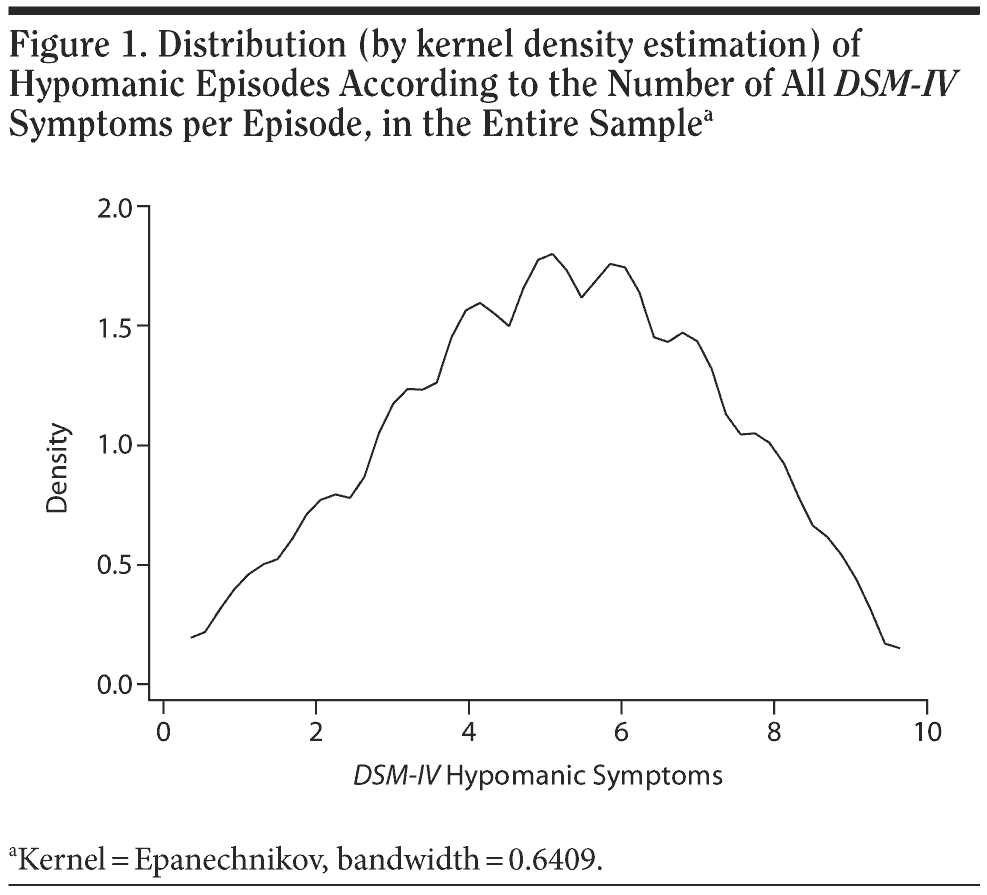
The distribution of all subthreshold and threshold hypomanic episodes was studied in the entire sample. Figure 1 shows the kernel density estimation of the distribution of hypomanic episodes, according to the number of all DSM-IV-TR symptoms. No bimodality was evident, and the curve was normal-like.
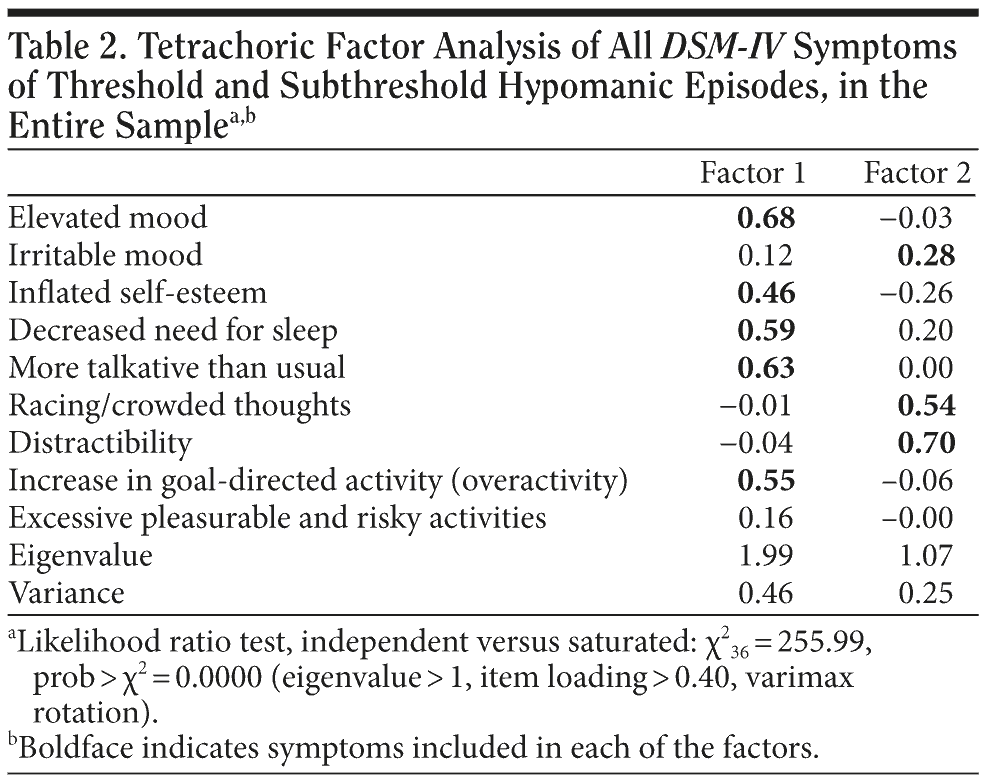
To determine the core (ie, more specific) symptoms of hypomania, all hypomanic symptoms of the entire sample were included in a tetrachoric factor analysis (Table 2). Factor analysis found 2 factors. Iterated principal factors and principal-component factors were tested. Both identified the same factors, apart from a slight difference in item loadings. Factor analysis is presented using the iterated principal factors, because the assumption of the principal-component factors was not met (uniqueness, ie, the percentage of variance for the item that is not explained by the common factors has to be 0, while in the present analysis the range of uniqueness of items was 0.27-0.91) and because the variance it explained was 0.44, while the iterated principal factors explained 0.71 of the variance. Rotation was carried out in order to find more interpretable (clear) factors. Both varimax orthogonal rotation (Kaiser off) and oblimin oblique rotation (Kaiser off) (which also tests correlations among factors) were used. It was found that the correlation between factor 1 and factor 2 was very small (-0.098), suggesting that the 2 factors were independent. The results of the tetrachoric factor analysis, using the iterated principal factors, and oblimin oblique rotation, are presented in Table 2. Factor 1 explained most of the variance (0.46) and included 5 “classic” hypomanic symptoms, ie, elevated mood, inflated self-esteem, decreased need for sleep, talkativeness, and increase in goal-directed activity (overactivity). Factor 2 included the “nonspecific” symptoms irritable mood (loading < 0.40), racing/crowded thoughts, and distractibility. These 2 factors were not correlated (-0.098).
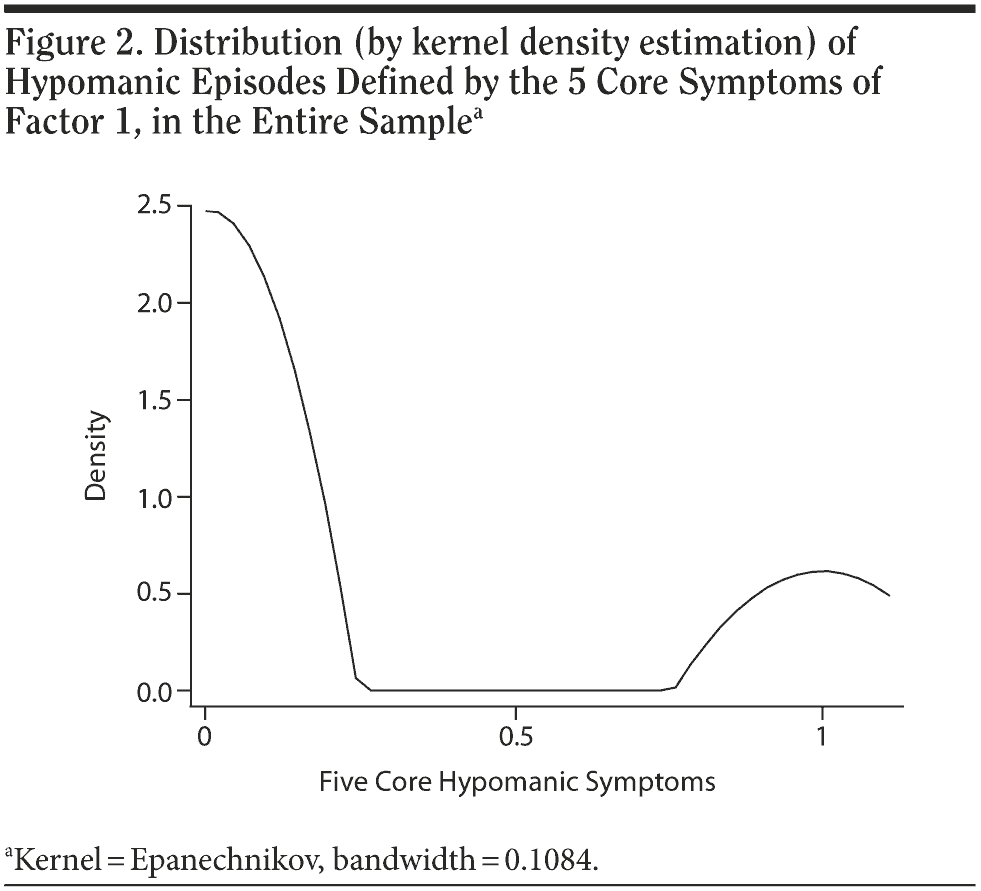
In order to test the validity of the core symptoms of factor 1, discriminatory accuracy for distinguishing DSM-IV-TR BP-II versus MDD was tested by ROC analysis. The area under the ROC curve was 0.94 (95% CI, 0.91-0.96), suggesting a very high discriminatory accuracy. To further test the validity of factor 1 as the core cluster of symptoms of DSM-IV-TR hypomania, the distribution of episodes including the 5 symptoms of factor 1 (5-symptom episode) was assessed by kernel density estimation (Figure 2) in the entire sample. Differently from the nonbimodal distribution previously found by testing hypomanic episodes including all DSM-IV-TR symptoms, in this case a clear-cut bimodality (with a “zone of rarity”) was found. To further test the core symptoms of DSM-IV-TR hypomania, a definition that included only 4 of the 5 symptoms of factor 1 (4-symptom episode) was tested, by deleting “inflated self-esteem.” This was carried out in order to focus more on behavioral symptoms (3 of 4) (elevated mood, decreased need for sleep, talkativeness, and overactivity), following previous reports.5,16,17 The distribution of the 4-symptom episodes was found to be again clearly bimodal, and very similar to Figure 2. The ROC area was 0.93 (95% CI, 0.91-0.96), very similar to the previous one. Then, a definition of DSM-IV-TR core hypomanic symptoms (3-symptom episode) including only the 3 behavioral symptoms of factor 1 (decreased need for sleep, talkativeness, and overactivity) was tested. The distribution of these 3-symptom episodes was found to be again clearly bimodal, and very similar to Figure 2. The ROC area was 0.90 (95% CI, 0.87-0.93), similar to the previous values.
DISCUSSION
The main study findings were the following: (1) subthreshold hypomanic episodes were common in MDD (modal number of symptoms, 3 to 4; frequency, 54%); (2) the distribution of all DSM-IV-TR hypomanic symptoms between BP-II and MDD showed no evidence of bimodality; (3) tetrachoric factor analysis found 2 independent factors, one including the core domains of elevated mood and overactivity (elevated mood, inflated self-esteem, decreased need for sleep, talkativeness, and increase in goal-directed activity) and one including “nonspecific” symptoms (irritable mood, distractibility, racing/crowded thoughts); (4) the distribution of episodes with core symptoms of DSM-IV-TR hypomania showed a clear-cut bimodality between BP-II and MDD; (5) the episodes with core symptoms had a high discriminatory accuracy for distinguishing BP-II from MDD (ROC area > 90); and (6) by a series of symptom distribution studies and ROC analyses of the core symptoms, it was found that an episode including the 3 symptoms of the overactivity domain of factor 1 had a similar bimodality and discriminatory accuracy compared to that of all the other episodes of the core symptoms of factor 1 (4- and 5-symptom episodes).
We found that MDD had a high frequency of past subthreshold hypomanic episodes, mainly including 3 to 4 symptoms. This finding replicates previous studies of lifetime manic/hypomanic symptoms and episodes in MDD, and concurrent/superimposed subthreshold hypomania in bipolar (types I and II) depression and in MDD (ie, mixed depression).21,22,25,26,30-32,77-80 In these studies, the superimposed subthreshold hypomanic symptoms present in MDD and in bipolar mixed depressions were mainly the “nonspecific” symptoms of hypomania. Our finding in the present analyses of no bimodality in the distribution of subthreshold and threshold hypomanic episodes between BP-II and MDD (the expected finding was a clear bimodality with a loading of episodes on the BP-II side of the distribution) suggests that this finding could be related to the “nonspecific” hypomanic symptoms (ie, irritable mood, distractibility, and racing/crowded thoughts), which made the samples too heterogeneous.
The nonbimodal distribution of DSM-IV-TR subthreshold and threshold hypomanic episodes, including all DSM-IV-TR symptoms, suggests a dimensional nature of hypomania. These findings complement recent epidemiologic and clinical sample studies15-24,74,75 reporting that subthreshold hypomania is more common than threshold hypomania and that subthreshold hypomania is not uncommon in MDD, both lifetime21,22 and concurrent in mixed depression.25,26,30-32
However, these studies and the first part of the present analyses were questioned by the results of our tetrachoric factor analysis. Using tetrachoric factor analysis of all DSM-IV-TR symptoms, 2 independent factors were found. Factor 1, explaining most of the variance, included the “classic” manic/hypomanic symptoms elevated mood, inflated self-esteem, decreased need for sleep, talkativeness, and increase in goal-directed activity (overactivity). Factor 2 included instead the “nonspecific” symptoms irritable mood, racing/crowded thoughts, and distractibility.
The symptoms of factor 2 were the “nonspecific” symptoms, as they are common in many mood and other Axis I and Axis II disorders. The “nonspecific” symptoms of DSM-IV-TR hypomania are irritable mood, distractibility, racing/crowded thoughts, and excessive involvement in pleasurable activities with a high potential for painful consequences, which represent 44% of its symptoms (4 of 9 symptoms). In DSM-IV-TR, irritable mood is also reported to be common in MDD. Distractibility may arise from external stimuli (according to DSM-IV-TR hypomania criteria, to distinguish it from the distractibility of MDD), but it may also arise from mental overactivity (racing/crowded thoughts, found to be common in lifetime and concurrent subthreshold hypomania in MDD, can make it difficult to focus attention because of the constant flow of thoughts). Our finding that a “typical” bipolar symptom such as racing thoughts was as common in MDD as in BP-II may arise from the difficulty that the patient may have in distinguishing among the several (artificial) variants of mental overactivity (lying along a continuum of severity from obsessions/ruminations, to racing/crowded thoughts, to flight of ideas). Kraepelin described the milder variants of mental overactivity in phrases like, “Meditate so much, fresh thoughts always coming, too much in the head, no settled thoughts, constantly things come crowding into the head.”14(pp14,75,108) Excessive involvement in pleasurable activities with a high potential for painful consequences (impulsive, risky activities) (exemplified in DSM-IV-TR by buying sprees, sexual indiscretions, foolish investments, reckless driving, substance abuse) can be found in many Axis I and Axis II disorders. Factor analysis results suggest that factor 1 could represent the core symptoms of DSM-IV-TR hypomania.
Our next step was to test the categorical nature of factor 1. We found that the distribution of hypomanic episodes including all 5 symptoms of factor 1 was bimodal with a clear-cut “zone of rarity” (Figure 2). This finding would support a categorical nature of hypomania defined by the 5 symptoms of factor 1. In order to validate this definition, its discriminatory accuracy in distinguishing DSM-IV-TR BP-II and MDD was tested by ROC analysis, which showed a high accuracy (ROC area > 90). In trying to find the basic, core symptoms of DSM-IV-TR hypomania, inflated self-esteem was eliminated, and the cluster of the 4 symptoms of factor 1 was tested (elevated mood, decreased need for sleep, talkativeness, and overactivity). Inspection of the distribution of the 4-symptom hypomanic episodes, and ROC area, yielded results very similar to those in Figure 2. Then, a definition of DSM-IV-TR hypomania was tested that included only the 3 behavioral signs (decreased need for sleep, talkativeness, and overactivity) of factor 1 (3-symptom episodes). Findings showed results similar to the previous analyses. In conclusion, these analyses showed that these 3 behavioral signs could represent the core symptom of a categorical hypomania deriving from the DSM-IV-TR definition of hypomania. Analyses of definitions different from that of DSM-IV-TR hypomania should be carried out in order to find the “core” symptoms of hypomania, even if based only on face validity and multivariate analyses, and not on the phenomenological domains of its biologic underpinnings (as they should be).
The core symptoms of DSM-IV-TR hypomania found in the present study are supported by the classic observations by Falret, Hecker, and Kraepelin.12-14 Regarding Kraepelin’s description of hypomania, he seems to have given more weight to behavioral manifestations than to elevated mood. Regarding “pressure of activity” and “increased busyness,” he stated that “perhaps this is even to be regarded as the fundamental manifestation” of mania/hypomania.14(pp 28-29) Following Helzer et al11 and DSM-IV-TR, the dimensional nature of DSM-IV-TR hypomania was supported by no bimodality in the distribution of subthreshold and threshold hypomanic episodes between BP-II and MDD (ie, no clear boundaries, no “zone of rarity” from neighboring syndromes of its “defining characteristics”). The categorical nature of the core symptoms of DSM-IV-TR hypomania was supported by a clear-cut bimodality in the distribution of hypomanic episodes including 3 symptoms, 4 symptoms, and the 5 symptoms of factor 1 (3-, 4-, 5-symptom hypomanic episode). The entire constellation of symptoms that define hypomania was assessed concurrently in the distribution analysis, as suggested by Ruscio et al.35 The selection of symptoms is important for the correct assessment of a unimodal-bimodal distribution: bimodality may result from too homogeneous samples (such as including mainly highly “specific” symptoms), and unimodality may result from too heterogeneous samples (such as including many overlapping, “nonspecific” symptoms).35 These 2 biases could have occurred in the present analyses, but this was the result of the DSM-IV-TR definition of hypomania, not a personal choice. However, apart from bimodality, the results of ROC analysis added further support to the study findings. The definition of DSM-IV-TR hypomania based on the symptoms of factor 1 showed a high discriminatory accuracy by ROC analysis, validating this core definition of DSM-IV-TR hypomania based on behavioral symptoms involving the domain of overactivity. Our findings correspond to several multivariate analyses which found that “activation” was the core feature of mania/hypomania.5,6,53
Apart from factor analysis, other multivariate analyses have been suggested to identify distinct subtypes of mania/hypomania (eg, admixture analysis, latent class analysis, grade-of-membership analysis, taxometric method).35,60-62 These more advanced statistics are still at an early stage: by having different and strong assumptions, different conclusions have been found independent of the phenomenon.35,63 Factor analysis still remains the most supported multivariate statistical method for testing dimensions.11
CONCLUSIONS
A core, categorical DSM-IV-TR hypomania was found that included 3 to 5 symptoms, ie, behavioral symptoms (overactivity) and elevated mood. Behavioral symptoms could be its basic phenotype. This could help in probing for hypomania and reduce misdiagnosis. It could also further research on the biologic underpinnings of hypomania.
Limitations
The single interviewer (F.B.) was experienced in diagnosing BP-II.5 Study samples were part of pooled databases previously recorded for different study goals, making interviewer bias unlikely. The description of past hypomania may be limited by recall bias, but it is not possible to examine hypomania directly, in a large enough sample, because individuals with BP-II rarely present to the clinician for hypomania.1,4 Only long-term follow-ups would provide the opportunity to observe hypomanic episodes.19 The present author does not have the resources to go beyond cross-sectional and retrospective studies. Assessment during remission, focus on recent episodes, and often concurrent interviewing of key informants should have reduced the impact of recall bias. A methodological advantage of this study is that BP-II and MDD patients were studied in a state of remission. This provides assurance that the phenomenology of the hypomania was not contaminated by depression, in particular its cognitive bias for remembering the “positive” experiences of hypomania.81 The systematic collection of data by validated instruments should also have reduced any unintended biases. Assessing all past hypomanic symptoms (the present study method), following Dunner and Tay,68 was shown to increase the correct classification of BP-II.
Author affiliations: Hecker Psychiatry Research Center, a University of California at San Diego Collaborating Center, Forli, Italy; Department of Psychiatry, University of Szeged, Szeged, Hungary; and Department of Psychiatry, National Health Service, Forli, Italy.
Financial disclosure: None reported.
Funding/support: None reported.
References
1. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, Text Revision (DSM-IV-TR). Washington, DC: American Psychiatric Association; 2000.
2. World Health Organization. The ICD-10 Classification of Mental and Behavioural Disorders. Geneva, Switzerland: World Health Organization; 1992.
3. Robins E, Guze SB. Establishment of diagnostic validity in psychiatric illness: its application to schizophrenia. Am J Psychiatry. 1970;126:983-987. PubMed
4. Goodwin FK, Jamison KR. Manic-Depressive Illness. 2nd ed. New York, NY: Oxford University Press; 2007.
5. Benazzi F. Bipolar disorder—focus on bipolar II disorder and mixed depression. Lancet. 2007;369(9565):935-945. PubMed doi:10.1016/S0140-6736(07)60453-X
6. Benazzi F. Is there a continuity between bipolar and depressive disorders? Psychother Psychosom. 2007;76:70-76. PubMed doi:10.1159/000097965
7. Vieta E, Suppes T. Bipolar II disorder: arguments for and against a distinct diagnostic entity. Bipolar Disord. 2008;10(1, pt 2):163-178. PubMed doi:10.1111/j.1399-5618.2007.00561.x
8. Coryell W, Endicott J, Maser JD, et al. Long-term stability of polarity distinctions in the affective disorders. Am J Psychiatry. 1995;152(3):385-390. PubMed
9. Akiskal HS, Maser JD, Zeller PJ, et al. Switching from “unipolar” to bipolar II: an 11-year prospective study of clinical and temperamental predictors in 559 patients. Arch Gen Psychiatry. 1995;52(2):114-123. PubMed
10. Suppes T, Mintz J, McElroy SL, et al. Mixed hypomania in 908 patients with bipolar disorder evaluated prospectively in the Stanley Foundation Bipolar Treatment Network: a sex-specific phenomenon. Arch Gen Psychiatry. 2005;62(10):1089-1096. PubMed doi:10.1001/archpsyc.62.10.1089
11. Helzer JE, Kraemer HC, Krueger RF, et al, eds. Dimensional Approaches in Diagnostic Classification. Refining the Research Agenda for DSM-V. Arlington, VA: American Psychiatric Association; 2008.
12. Sedler MJ. Falret’s discovery: the origin of the concept of bipolar affective illness. Am J Psychiatry. 1983;140(9):1127-1133. PubMed
13. Koukopoulos A. Ewald Hecker’s description of cyclothymia as a cyclical mood disorder: its relevance to the modern concept of bipolar II. J Affect Disord. 2003;73(1-2):199-205. PubMed doi:10.1016/S0165-0327(02)00326-9
14. Kraepelin E. Manic-Depressive Insanity and Paranoia. Edinburgh, Scotland: Livingstone E & S; 1921.
15. Angst J, Gamma A, Benazzi F, et al. Toward a re-definition of subthreshold bipolarity: epidemiology and proposed criteria for bipolar-II, minor bipolar disorders and hypomania. J Affect Disord. 2003;73(1-2):133-146. PubMed doi:10.1016/S0165-0327(02)00322-1
16. Angst J. The bipolar spectrum. Br J Psychiatry. 2007;190:189-191. PubMed doi:10.1192/bjp.bp.106.030957
17. Angst J. Bipolar disorder—methodological problems and future perspectives. Dialogues Clin Neurosci. 2008;10(2):129-139. PubMed
18. Judd LL, Akiskal HS. The prevalence and disability of bipolar spectrum disorders in the US population: re-analysis of the ECA database taking into account subthreshold cases. J Affect Disord. 2003;73(1-2):123-131. PubMed doi:10.1016/S0165-0327(02)00332-4
19. Judd LL, Akiskal HS, Schettler PJ, et al. A prospective investigation of the natural history of the long-term weekly symptomatic status of bipolar II disorder. Arch Gen Psychiatry. 2003;60(3):261-269. PubMed doi:10.1001/archpsyc.60.3.261
20. Merikangas KR, Akiskal HS, Angst J, et al. Lifetime and 12-month prevalence of bipolar spectrum disorder in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2007;64(5):543-552. PubMed doi:10.1001/archpsyc.64.5.543
21. Cassano GB, Frank E, Miniati M, et al. Conceptual underpinnings and empirical support for the mood spectrum. Psychiatr Clin North Am. 2002;25(4):699-712. PubMed doi:10.1016/S0193-953X(02)00025-4
22. Cassano GB, Rucci P, Frank E, et al. The mood spectrum in unipolar and bipolar disorder: arguments for a unitary approach. Am J Psychiatry. 2004;161(7):1264-1269. PubMed doi:10.1176/appi.ajp.161.7.1264
23. Judd LL, Akiskal HS, Schettler PJ, et al. The long-term natural history of the weekly symptomatic status of bipolar I disorder. Arch Gen Psychiatry. 2002;59(6):530-537. PubMed doi:10.1001/archpsyc.59.6.530
24. Benazzi F. Frequency of bipolar spectrum in 111 private practice depression outpatients. Eur Arch Psychiatry Clin Neurosci. 2003;253(4):203-208. PubMed doi:10.1007/s00406-003-0433-6
25. Maj M, Pirozzi R, Magliano L, et al. Agitated depression in bipolar I disorder: prevalence, phenomenology, and outcome. Am J Psychiatry. 2003;160(12):2134-2140. PubMed doi:10.1176/appi.ajp.160.12.2134
26. Maj M, Pirozzi R, Magliano L, et al. Agitated “unipolar” major depression: prevalence, phenomenology, and outcome. J Clin Psychiatry. 2006;67(5):712-719. PubMed
27. Benazzi F. Which could be a clinically useful definition of depressive mixed state? Prog Neuropsychopharmacol Biol Psychiatry. 2002;26(6):1105-1111. PubMed doi:10.1016/S0278-5846(02)00244-0
28. Benazzi F. Depressive mixed state: dimensional versus categorical definitions. Prog Neuropsychopharmacol Biol Psychiatry. 2003;27(1):129-134. PubMed doi:10.1016/S0278-5846(02)00343-3
29. Benazzi F. Bipolar II depressive mixed state: finding a useful definition. Compr Psychiatry. 2003;44(1):21-27. PubMed doi:10.1053/comp.2003.50000
30. Benazzi F. Defining mixed depression. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32(4):932-939. PubMed doi:10.1016/j.pnpbp.2007.12.019
31. Benazzi F. Reviewing the diagnostic validity and utility of mixed depression (depressive mixed states). Eur Psychiatry. 2008;23(1):40-48. PubMed doi:10.1016/j.eurpsy.2007.07.003
32. Benazzi F. A tetrachoric factor analysis validation of mixed depression. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32(1):186-192. PubMed doi:10.1016/j.pnpbp.2007.08.005
33. Bauer MS, Simon GE, Ludman E, et al. “Bipolarity” in bipolar disorder: distribution of manic and depressive symptoms in a treated population. Br J Psychiatry. 2005;187:87-88. PubMed doi:10.1192/bjp.187.1.87
34. Swann AC, Gerard Moeller F, Steinberg JL, et al. Manic symptoms and impulsivity during bipolar depressive episodes. Bipolar Disord. 2007;9(3):206-212. PubMed doi:10.1111/j.1399-5618.2007.00357.x
35. Ruscio J, Haslam N, Ruscio AM. Introduction to the Taxometric Method. A Practical Guide. Mahwah, NJ: Lawrence Erlbaum Associates, Publishers; 2006.
36. Mueller TI, Leon AC, Keller MB, et al. Recurrence after recovery from major depressive disorder during 15 years of observational follow-up. Am J Psychiatry. 1999;156(7):1000-1006. PubMed
37. Goldberg JF, Harrow M, Whiteside JE. Risk for bipolar illness in patients initially hospitalized for unipolar depression. Am J Psychiatry. 2001;158(8):1265-1270. PubMed doi:10.1176/appi.ajp.158.8.1265
38. Angst J, Sellaro R, Stassen HH, et al. Diagnostic conversion from depression to bipolar disorders: results of a long-term prospective study of hospital admissions. J Affect Disord. 2005;84(2-3):149-157. PubMed doi:10.1016/S0165-0327(03)00195-2
39. Kessing LV. Diagnostic stability in depressive disorder as according to ICD-10 in clinical practice. Psychopathology. 2005;38(1):32-37. PubMed doi:10.1159/000083968
40. Mattisson C, Bogren M, Horstmann V, et al. The long-term course of depressive disorders in the Lundby Study. Psychol Med. 2007;37(6):883-891. PubMed doi:10.1017/S0033291707000074
41. Holma KM, Melartin TK, Holma IAK, et al. Predictors for switch from unipolar major depressive disorder to bipolar disorder type I or II: a 5-year prospective study. J Clin Psychiatry. 2008;69(8):1267-1275. PubMed doi:10.4088/JCP.v69n0809
42. Escamilla MA, Zavala JM. Genetics of bipolar disorder. Dialogues Clin Neurosci. 2008;10(2):141-152. PubMed
43. Kendler KS, Greenspan RJ. The nature of genetic influences on behavior: lessons from “simpler” organisms. Am J Psychiatry. 2006;163(10):1683-1694. PubMed doi:10.1176/appi.ajp.163.10.1683
44. Zachar P, Kendler KS. Psychiatric disorders: a conceptual taxonomy. Am J Psychiatry. 2007;164(4):557-565. PubMed doi:10.1176/appi.ajp.164.4.557
45. Kendell RE. Clinical validity. Psychol Med. 1989;19(1):45-55. PubMed doi:10.1017/S0033291700011016
46. Kendell R, Jablensky A. Distinguishing between the validity and utility of psychiatric diagnoses. Am J Psychiatry. 2003;160(1):4-12. PubMed doi:10.1176/appi.ajp.160.1.4
47. Kessler RC, Zhao S, Blazer DG, et al. Prevalence, correlates, and course of minor depression and major depression in the National Comorbidity Survey. J Affect Disord. 1997;45(1-2):19-30. PubMed doi:10.1016/S0165-0327(97)00056-6
48. Kendler KS, Gardner CO Jr. Boundaries of major depression: an evaluation of DSM-IV criteria. Am J Psychiatry. 1998;155(2):172-177. PubMed
49. Judd LL, Akiskal HS, Maser JD, et al. Major depressive disorder: a prospective study of residual subthreshold depressive symptoms as predictor of rapid relapse. J Affect Disord. 1998;50(2-3):97-108. PubMed doi:10.1016/S0165-0327(98)00138-4
50. Calabrese JR, Hirschfeld RM, Reed M, et al. Impact of bipolar disorder on a US community sample. J Clin Psychiatry. 2003;64(4):425-432. PubMed
51. Goldberg D. Plato versus Aristotle: categorical and dimensional models for common mental disorders. Compr Psychiatry. 2000;41(suppl 1):8-13. PubMed doi:10.1016/S0010-440X(00)80002-4
52. Angst J, Merikangas KR. Multi-dimensional criteria for the diagnosis of depression. J Affect Disord. 2001;62(1-2):7-15. PubMed doi:10.1016/S0165-0327(00)00346-3
53. Cassano GB, Mula M, Rucci P, et al. The structure of lifetime manic-hypomanic spectrum. J Affect Disord. 2009;112(1-3):59-70. PubMed doi:10.1016/j.jad.2008.04.019
54. Melzer D, Tom BD, Brugha TS, et al. Common mental disorder symptom counts in populations: are there distinct case groups above epidemiological cut-offs? Psychol Med. 2002;32(7):1195-1201. PubMed doi:10.1017/S0033291702006049
55. Dikeos DG, Wickham H, McDonald C, et al. Distribution of symptom dimensions across Kraepelinian divisions. Br J Psychiatry. 2006;189:346-353. PubMed doi:10.1192/bjp.bp.105.017251
56. Poulton R, Caspi A, Moffitt TE, et al. Children’s self-reported psychotic symptoms and adult schizophreniform disorder: a 15-year longitudinal study. Arch Gen Psychiatry. 2000;57(11):1053-1058. PubMed doi:10.1001/archpsyc.57.11.1053
57. Reeger EJ, Krabbendam L, De Graaf R, et al. A prospective study of the transition rates of subthreshold (hypo)mania and depression in the general population. Psychol Med. 2006;36(5):619-627. PubMed doi:10.1017/S0033291705006823
58. Kaymaz N, van Os J, de Graaf R, et al. The impact of subclinical psychosis on the transition from subclinicial mania to bipolar disorder. J Affect Disord. 2007;98(1-2):55-64. PubMed doi:10.1016/j.jad.2006.06.028
59. Kendler KS. Reflections on the relationship between psychiatric genetics and psychiatric nosology. Am J Psychiatry. 2006;163(7):1138-1146. PubMed doi:10.1176/appi.ajp.163.7.1138
60. Cassidy F, Yatham LN, Berk M, et al. Pure and mixed manic subtypes: a review of diagnostic classification and validation. Bipolar Disord. 2008;10(1, pt 2):131-143. PubMed doi:10.1111/j.1399-5618.2007.00558.x
61. Jablensky A. Categories, dimensions and prototypes: critical issues for psychiatric classification. Psychopathology. 2005;38(4):201-205. PubMed doi:10.1159/000086092
62. Kraemer HC, Shrout PE, Rubio-Stipec M. Developing the Diagnostic and Statistical Manual V: what will “statistical” mean in DSM-V? Soc Psychiatry Psychiatr Epidemiol. 2007;42(4):259-267. PubMed doi:10.1007/s00127-007-0163-6
63. Klein DN, Shankman SA, McFarland BR. Classification of mood disorders. In: Stein DJ, Kupfer DJ, Schatzberg AF, eds. Textbook of Mood Disorders. Arlington, VA: American Psychiatric Publishing; 2006:17-32.
64. Benazzi F. Borderline personality disorder and bipolar II disorder in private practice depressed outpatients. Compr Psychiatry. 2000;41(2):106-110. PubMed doi:10.1016/S0010-440X(00)90142-1
65. Akiskal HS, Akiskal KK, Lancrenon S, et al. Validating the soft bipolar spectrum in the French National EPIDEP Study: the prominence of BP-II½. J Affect Disord. 2006;96(3):207-213. PubMed doi:10.1016/j.jad.2006.03.011
66. First MB, Spitzer RL, Gibbon M, et al. Structured Clinical Interview for DSM-IV Axis I Disorders-Clinician Version (SCID-CV). Washington, DC: American Psychiatric Press; 1997.
67. Benazzi F. Depression with racing thoughts. Psychiatry Res. 2003;120(3):273-282. PubMed doi:10.1016/S0165-1781(03)00198-7
68. Dunner DL, Tay KL. Diagnostic reliability of the history of hypomania in bipolar II patients and patients with major depression. Compr Psychiatry. 1993;34(5):303-307. PubMed doi:10.1016/0010-440X(93)90015-V
69. Simpson SG, McMahon FJ, McInnis MG, et al. Diagnostic reliability of bipolar II diagnosis. Arch Gen Psychiatry. 2002;59(8):736-740. PubMed doi:10.1001/archpsyc.59.8.736
70. Benazzi F. Diagnosis of bipolar II disorder: a comparison of structured versus semistructured interviews. Prog Neuropsychopharmacol Biol Psychiatry. 2003;27(6):985-991. PubMed doi:10.1016/S0278-5846(03)00158-1
71. Benazzi F. Is 4 days the minimum duration of hypomania in bipolar II disorder? Eur Arch Psychiatry Clin Neurosci. 2001;251(1):32-34. PubMed doi:10.1007/s004060170065
72. Benazzi F, Akiskal H. The duration of hypomania in bipolar-II disorder in private practice: methodology and validation. J Affect Disord. 2006;96(3):189-196. PubMed doi:10.1016/j.jad.2004.04.006
73. Dunner DL. Clinical consequences of under-recognized bipolar spectrum disorder. Bipolar Disord. 2003;5(6):456-464. PubMed doi:10.1046/j.1399-5618.2003.00073.x
74. Benazzi F. The continuum/spectrum concept of mood disorders: is mixed depression the basic link? Eur Arch Psychiatry Clin Neurosci. 2006;256(8):512-515. PubMed doi:10.1007/s00406-006-0672-4
75. Benazzi F. A continuity between bipolar II depression and major depressive disorder? Prog Neuropsychopharmacol Biol Psychiatry. 2006;30(6):1043-1050. PubMed doi:10.1016/j.pnpbp.2006.03.037
76. Salgado-Ugarte IH, Shimizu M, Taniuchi T. Exploring the shape of univariate data using Kernel density estimators. STATA Tech Bull. 1994;16:8-19.
77. Goldberg JF, Perlis RH, Ghaemi SN, et al. Adjunctive antidepressant use and symptomatic recovery among bipolar depressed patients with concomitant manic symptoms: findings from the STEP-BD. Am J Psychiatry. 2007;164(9):1348-1355. PubMed doi:10.1176/appi.ajp.2007.05122032
78. Frye MA, Helleman G, McElroy SL, et al. Minimal manic symptoms in bipolar depression associated with antidepressant treatment-emergent mania/hypomania. Am J Psychiatry. 2009; In press. PubMed
79. Benazzi F, Berk M, Frye MA, et al. Olanzapine/fluoxetine combination for the treatment of mixed depression in bipolar I disorder: a post hoc analysis. J Clin Psychiatry. 2009;70(10):1424-1431.
80. Sato T, Bottlender R, Schroter A, et al. Frequency of manic symptoms during a depressive episode and unipolar “depressive mixed state” as bipolar spectrum. Acta Psychiatr Scand. 2003;107(4):268-274. PubMed doi:10.1034/j.1600-0447.2003.00051.x
81. Peselow ED, Sanfilipo MP, Fieve RR. Relationship between hypomania and personality disorders before and after successful treatment. Am J Psychiatry. 1995;152(2):232-238. PubMed