Objective: To evaluate the impact of psychopharmacologic treatments on neuropsychological functioning in bipolar youth.
Method: Participants were 173 children (aged 6-17 years) with DSM-IV bipolar disorder. Participants were comprehensively assessed using structured diagnostic interviews (Schedule for Affective Disorders and Schizophrenia for School-Age Children) and neuropsychological measures (eg, subtests of the Wechsler Intelligence Scale for Children-III and Wechsler Adult Intelligence Scale-III) during the years 2001-2006. Comparisons were made in neuropsychological functioning between medicated and unmedicated youth with bipolar disorder.
Results: Children who were treated with mood stabilizers performed significantly (P < .05) more poorly than untreated children on measures of processing speed and working memory. Treatment with other classes of medication, including second-generation antipsychotics, was not significantly associated with neuropsychological impairments.
Conclusions: Treatment with mood stabilizers may be associated with specific neuropsychological impairments. Cognitive side effects may need to be considered in selecting particular psychopharmacologic treatments for children with bipolar disorder.
Submitted: September 9, 2008; accepted February 13, 2009.
Online ahead of print: June 30, 2009.
Corresponding author: Aude Henin, PhD, Pediatric Psychopharmacology Unit, Massachusetts General Hospital, 185 Alewife Brook Pkwy, Suite 2000, Cambridge, MA 02138 ([email protected]).

Abstract
Objective: To evaluate the impact of psychopharmacologic treatments on neuropsychological functioning in bipolar youth.
Method: Participants were 173 children (aged 6-17 years) with DSM-IV bipolar disorder. Participants were comprehensively assessed using structured diagnostic interviews (Schedule for Affective Disorders and Schizophrenia for School-Age Children) and neuropsychological measures (eg, subtests of the Wechsler Intelligence Scale for Children-III and Wechsler Adult Intelligence Scale-III) during the years 2001-2006. Comparisons were made in neuropsychological functioning between medicated and unmedicated youth with bipolar disorder.
Results: Children who were treated with mood stabilizers performed significantly (P < .05) more poorly than untreated children on measures of processing speed and working memory. Treatment with other classes of medication, including second-generation antipsychotics, was not significantly associated with neuropsychological impairments.
Conclusions: Treatment with mood stabilizers may be associated with specific neuropsychological impairments. Cognitive side effects may need to be considered in selecting particular psychopharmacologic treatments for children with bipolar disorder.
J Clin Psychiatry 2009;70(8):1178-1185
© Copyright 2009 Physicians Postgraduate Press, Inc.
Submitted: September 9, 2008; accepted February 13, 2009.
Online ahead of print: June 30, 2009 (doi:10.4088/JCP.08m04696).
Corresponding author: Aude Henin, PhD, Pediatric Psychopharmacology Unit, Massachusetts General Hospital, 185 Alewife Brook Pkwy, Suite 2000, Cambridge, MA 02138 ([email protected]).
As pediatric bipolar disorder has been increasingly recognized as a valid clinical entity, efforts at defining safe and effective treatments for this disorder have begun to be explored. Open and controlled studies have investigated the safety and efficacy of traditional mood stabilizers as well as second-generation neuroleptics.1,2
However, whereas studies have documented varied degrees of improvement in manic symptomatology, most of these psychopharmacologic studies of children with bipolar disorder have not examined the impact of pharmacologic treatment on cognitive functioning. This issue is particularly important in pediatric bipolar disorder given that several of the medications used to treat bipolar disorder, especially the mood stabilizers, have been associated with cognitive impairments in adults, especially in the realms of processing speed and memory.3,4 In addition, youth with bipolar disorder frequently present with cognitive impairments to begin with, making any potential medication-associated deficits all the more problematic.5-7 However, in one of the few studies to examine this issue, Pavuluri et al8 found that there were no differences in neuropsychological functioning between children with bipolar disorder who were unmedicated and those who were medicated with lithium plus risperidone or divalproex plus risperidone, with both the medicated and unmedicated groups exhibiting impairments in attention, executive functioning, working memory, and verbal memory relative to controls.
A better understanding of the neuropsychological effects of medications in youth with bipolar disorder is critical to inform the selection of one medication versus another. Therefore, the main aim of the current study was to examine the impact of different classes of medications used in the management of bipolar youth on neuropsychological functioning in these youth. On the basis of the prior literature, we hypothesized that treatment with mood stabilizers would be associated with cognitive impairment in the areas of processing speed, sustained attention, and working memory.
METHOD
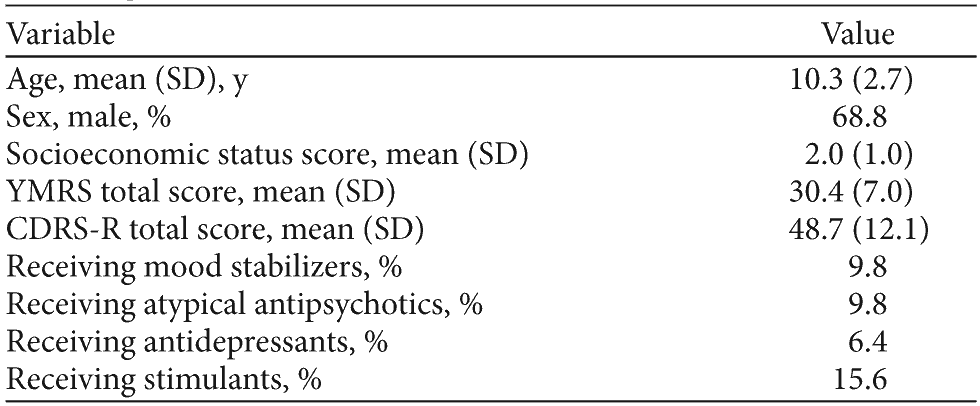
Table 1. Demographic and Clinical Characteristics of Children With Bipolar Disorder (N = 173)
Abbreviations: CDRS-R = Children’s Depression Rating Scale-Revised, YMRS = Young Mania Rating Scale.
Click figure to enlarge
Subjects
We examined 173 children (aged 6-17 years) with bipolar disorder. Participants were ascertained from among those undergoing a baseline neuropsychological assessment as part of entry into psychopharmacologic clinical trials for bipolar disorder. Children in the sample were a mean (SD) age of 10.3 (2.7) years, and 68.8% of the sample were male. All participants included in the analysis met full DSM-IV criteria for bipolar I disorder and had a Young Mania Rating Scale (YMRS)9,10 score greater than 15. Mean YMRS total score is provided in Table 1. We compared participants who, at the time of neuropsychological assessment (during the years 2001-2006), were receiving naturalistic treatment with different classes of medication (mood stabilizers, second-generation antipsychotics, stimulants, and antidepressants) to children who were psychopharmacologically untreated at the time of assessment. Of the total sample, 12.1% (n = 21) were treated with a mood stabilizer (including carbamazepine, lithium, divalproex, topiramate, and gabapentin), 9.8% (n = 17) were treated with a second-generation antipsychotic (risperidone or olanzapine), 6.4% (n = 11) were treated with an antidepressant (citalopram, paroxetine, venlafaxine, fluoxetine, bupropion, sertraline, or escitalopram), and 15.6% (n = 27) were treated with a stimulant (methylphenidate or amphetamine).
Participants with major sensorimotor handicaps (paralysis, deafness, or blindness), autism, an inadequate command of the English language, or a full-scale IQ11 less than 70 were excluded from the study. The study also excluded participants with any serious, unstable illness, including hepatic, renal, gastroenterologic, respiratory, cardiovascular (including ischemic heart disease), endocrinologic, neurologic, immunologic, or hematologic disease; DSM-IV substance (except nicotine) dependence within the past 6 months (but not substance abuse); or current, serious suicidal risk.
Procedure
The study was conducted in the Pediatric Psychopharmacology Clinical & Research Program of the Massachusetts General Hospital, a Harvard Medical School-affiliated major medical center serving metropolitan Boston and its surrounding areas. Diagnosis of bipolar disorder was made via clinician interviews with parents and directly with all children, conducted by board-certified child psychiatrists with expertise in the diagnosis of childhood bipolar disorder. These clinicians also administered the YMRS9,10 and Children’s Depression Rating Scale-Revised (CDRS-R)12 as part of their assessment. Socioeconomic status was assessed using the Hollingshead Four-Factor Index.13 Structured diagnostic interviews and a neuropsychological assessment battery were subsequently executed by a pool of raters who were blind to study hypotheses. Raters had undergraduate or master’s degrees in psychology and were trained to high levels of interrater reliability. They underwent a training program that required them to (1) learn about DSM-IV criteria, (2) master the diagnostic and neuropsychological instruments, (3) watch training tapes, (4) observe interviews and neuropsychological assessments performed by experienced raters, (5) rate several subjects under the supervision of senior raters, (6) undergo continued supervision of their diagnostic and neuropsychological assessments by senior project staff, and (7) audiotape all assessments for later random checking. For diagnostic purposes, all interviews were then presented for review to a committee of board-certified child and adult psychiatrists and licensed psychologists who were blind to the subject’s ascertainment status, referral source, and neuropsychological data. Diagnoses presented for review were considered positive only if a consensus was achieved that criteria were met to a degree that would be considered clinically meaningful. The administration of the diagnostic interviews was supervised by the lead author (A.H.). The administration of the neuropsychological tests was supervised by a team of licensed neuropsychologists (including R.F.). Written informed consent was obtained from all parents, and written assent was obtained from all children older than 7 years. All study procedures were reviewed and approved by the hospital’s institutional review board.
Measures
Diagnostic assessment. Diagnoses of bipolar disorder were made via clinician assessments with parents and children and were confirmed via the DSM-IV Schedule for Affective Disorders and Schizophrenia for School-Age Children-Epidemiologic Version (K-SADS-E).14 The K-SADS-E is a widely used, semistructured, DSM-IV-based psychiatric diagnostic interview with established psychometric properties.14 The interview inquires about the child’s lifetime history of psychopathology. It was designed for use in clinical and epidemiologic research to obtain a past and current history of Axis I psychiatric disorders in children and adolescents aged 6-17 years. In addition to establishing a lifetime diagnosis, the interview documents the onset and offset of disorders, the degree of impairment associated with each diagnosis, total duration of illness, and the type of treatment obtained. On the structured diagnostic interview, diagnostic information was obtained from interviews with a parent, usually the mother, on all children as well as directly from youth aged 12 years and older. We did not directly interview children younger than 12 years because of the complexity and length of the structured diagnostic interview, as well as findings that maternal reports of psychopathology are highly reliable, even over a 1-year period.15 Separate interviewers conducted the parent and child interviews. Data from these separate interviews were integrated using an algorithm that considered diagnoses as being positive if sufficient DSM-IV criteria were met in either the parent or child interview. Interviews were audiotaped, with permission. Diagnostic uncertainties were resolved by a committee of board-certified child psychiatrists and licensed psychologists who were blind to the subject’s ascertainment group, neuropsychological data, and data from family members. To assess the reliability of diagnostic procedures, randomly selected interviews were rerated by experienced, board certified child and adult psychiatrists and licensed clinical psychologists. Results showed high agreement between the interviewers and these expert clinicians. The κ coefficient for bipolar disorder in children was 0.89.
Young Mania Rating Scale. The YMRS9,10 is an 11-item clinician interview that queries, over the past week, the core symptoms of mania in the child, including elevated mood, irritability, psychomotor agitation, hypersexuality, and aggressive behavior. It was administered by a board-certified child psychiatrist to the parents of all youth with bipolar disorder. Scores on the 11 items are summed, yielding a total score that ranges from 0 to 60. The YMRS, when used with children, has shown good internal consistency9 and good discriminative validity.16 It is one of the best- established measures of mania in youth that has been widely used in phenomenological17 and treatment outcome studies of youth with bipolar disorder.18-20 Scores above 13 identify possible hypomania or mania. A cutoff score of 15 was used to enter children with bipolar disorder in the study. This cutoff score has frequently been used in psychopharmacologic studies of children with bipolar disorder (for example, see Biederman et al1,21,22 and Wozniak et al23).
Children’s Depression Rating Scale-Revised. The CDRS-R12 is a clinician-rated measure that is modeled after the Hamilton Rating Scale for Depression in adults and assesses the presence and severity of depressive symptoms in children aged 6 years and older. Seventeen symptom areas associated with depression are assessed. Scores on the CDRS-R range from 17 to 113. The CDRS-R was administered by a board-certified child psychiatrist to youth with bipolar disorder. It has been widely used in treatment outcome studies of depression and bipolar disorder in children.19,24
Neuropsychological testing. Tests were administered and scored by psychometricians trained and supervised by a team of licensed neuropsychologists (including R.F.). We estimated full-scale IQ11 from the vocabulary and block design subtests of the Wechsler Intelligence Scale for Children-III, (WISC-III)25 for individuals under 17 years of age and the Wechsler Adult Intelligence Scale-III (WAIS-III)26 for individuals aged 17 years. Achievement testing was conducted using the reading and arithmetic subtests of the Wide Range Achievement Test-Third Edition (WRAT-III).27 The remaining tests in the battery were selected on the basis of the domains of functioning thought to be indirect indices of fronto-limbic systems, although it should be noted that many of these measures may be multifactorial and assess more than one domain of function.
Sustained attention. On the Seidman Continuous Performance Test,28 individuals listen to a series of letters read aloud and are asked to tap their finger in response to various rules. Sustained attention was measured by the “vigilance” section, during which subjects tap after the letter Q if it comes right after the letter A.
Working memory. Working memory was assessed with the arithmetic and digit span subtests from the WISC-III25,26 and with the “memory” and “interference” sections of the Seidman Continuous Performance Test. In the memory section, subjects tap after hearing a Q 4 letters after an A, and in the more difficult interference section (for subjects aged 12 years and older), the same rules as the memory section apply, but there are additional distracter Q‘s that occur between the stimulus (A) and the target (Q).
Processing speed. Processing speed was measured by the word and color naming subtests of the Stroop Color-Word Test29 and by the digit/symbol coding and the symbol search subtests of the WISC-III.
Interference control. Interference control was measured by color-word and interference scores of the Stroop Color-Word Test and the failure to maintain set score of the Wisconsin Card Sorting Test (WCST)-computerized version.30
Abstract problem solving/set shifting. Problem solving was measured by the categories completed, perseverative errors, and nonperseverative errors of the WCST.
Visuospatial organization and learning. Visuospatial organization was assessed with the copy and delay organization scores of the Rey-Osterrieth Complex Figure test.31,32 The Rey-Osterrieth Complex Figure test was scored according to the methods of Bernstein and Waber33 by individuals with master’s degrees in psychology who were blind to subject characteristics. For administration, the figure was reproduced such that the base rectangle measured 8.0 ×— 5.5 cm.
Verbal learning. Verbal learning was assessed with the California Verbal Learning Test-Children’s Version 1 (CVLT).34 In this task, the subject is presented a list of 16 items read to them in 5 study trials, with free recall after each trial. The list contains an embedded semantic structure, in which words can be grouped into 1 of 4 categories (eg, fruits). This structure is not explicitly explained to subjects, and words are presented so that words from the same category never immediately follow one another. An interference list is then administered and recalled after the fifth study trial. Short- and long-delay free and category-cued recall of the original list are subsequently assessed, followed by a recognition test.34
Statistical Analyses
All statistical analyses were 2-tailed, with statistical significance set at the .05 level. Linear regression models were used for continuous outcomes (ie, performance on neuropsychological tests). Because groups were not mutually exclusive (ie, participants could be treated with more than 1 class of medication), we simultaneously entered all 4 classes of medication (mood stabilizers, atypical antipsychotics, stimulants, and antidepressants) into our regression models. Thus, results for each class of medication control for the presence of all other medications. All analyses controlled for child sex and age. To examine the impact of severity of illness on our findings, we also reran these analyses with YMRS and CDRS-R scores included in the models. Data were analyzed using the statistical software package STATA.35
RESULTS
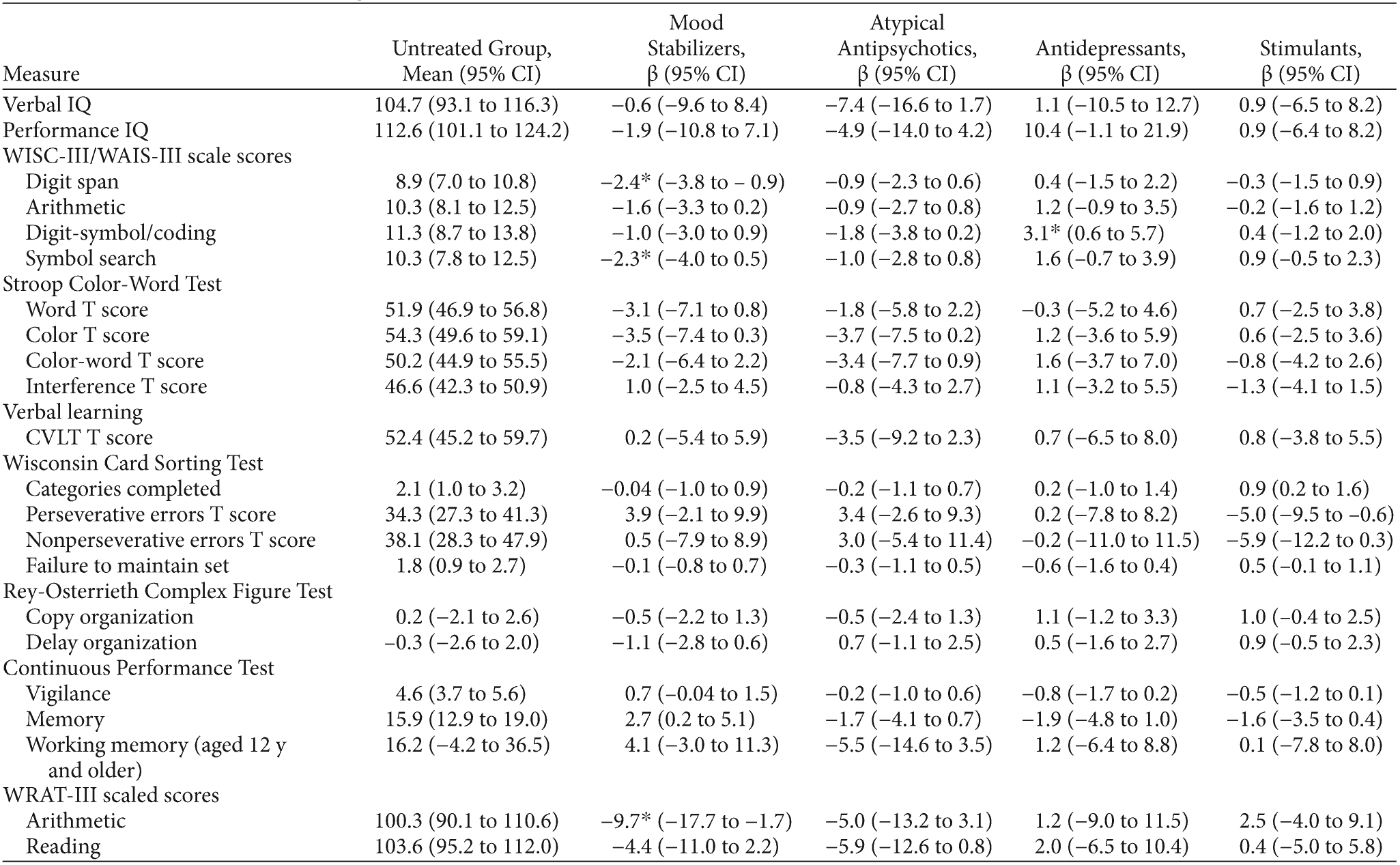
As seen in Table 2, there were no differences between children who were and were not treated with different medications on verbal and performance IQ, with all groups scoring in the average range. Children who were treated with mood stabilizers performed more poorly than those who were untreated on tests of processing speed (WISC-III/WAIS-III symbol search subtest: scaled score of 7.8 vs 10.0, P < .05), working memory (WISC-III/WAIS-III digit span subtest: scaled score of 7.1 vs 9.3, P < .05), and achievement on a timed math test (WRAT-III arithmetic subtest: scaled score of 88.1 vs 97.0, P < .05). There was no impact of treatment with mood stabilizers on interference control, verbal learning, problem-solving, visuospatial memory, or reading achievement. Treatment with second-generation antipsychotics, stimulants, or antidepressants was not significantly associated with impairments on neuropsychological measures. Treatment with antidepressants was associated with improved performance on a measure of processing speed (WISC-III/WAIS-III digit-symbol/coding subtest: scaled score of 10.7 vs 7.8 for treated vs untreated, P < .05).
Table 2. Medication Effects on Cognitive Performance in Children With Bipolar Disordera
aThe mean reported for the untreated group is the intercept (constant). Because groups were not mutually exclusive, we report the mean change in each score associated with each class of medication, controlling for all other medications as well as child age and sex.
*P < .05.
Abbreviations: CVLT = California Verbal Learning Test-Children’s Version, WAIS-III = Wechsler Adult Intelligence Scale-III, WISC-III = Wechsler Intelligence Scale for Children-III, WRAT-III = Wide Range Achievement Test-III.
Click figure to enlarge
To examine whether our findings were impacted by severity of illness, we reran our analyses with YMRS and CDRS-R scores included in the models. Including these variables did not change our results, except in 1 respect. With severity of illness included as covariates, stimulant treatment improved performance on the WCST, including categories completed (β = 0.9 [SE = 0.4], P = .01), perseverative errors (β = −5.4 [SE = 2.3], P = .02), nonperseverative errors (β = −6.8 [SE = 3.2], P = .03), and failure to maintain set (β = 0.6 [SE = 0.3], P = .04).
DISCUSSION
This study compared the neuropsychological functioning of unmedicated outpatient children with bipolar disorder to that of children with bipolar disorder who were medicated with mood stabilizers, second-generation antipsychotics, stimulants, or antidepressants. There were few differences between the groups. However, children who were currently treated with mood stabilizing medications were more impaired, relative to untreated children, on the WISC-III/WAIS-III symbol search and digit span subtests, as well as on the WRAT-III arithmetic subtest. These findings suggest that mood stabilizers may have a specific negative impact on speed of processing and working memory. Children treated with antidepressant medication performed better on the WISC-III/WAIS-III digit-symbol/coding subtest.
Few prior studies have specifically examined the neuropsychological impact of medications in youth with bipolar disorder. However, our findings differ from those of Pavuluri et al,8 who observed that there were no differences between youth with bipolar disorder who were and who were not treated with a combination of mood stabilizers and second-generation antipsychotics, with both groups showing impairments on a range of neuropsychological measures.
Although studies of adults with bipolar disorder have yielded inconsistent findings, our finding that children treated with mood stabilizers had poorer performance on tests of processing speed and working memory is consistent with several previous adult studies that have observed that treatment with medications such as lithium or valproate is associated with poorer performance on tests of processing speed and memory. For example, an older, controlled, blinded study of lithium discontinuation and resumption among euthymic adults with bipolar disorder found that scores on memory measures, tests of tapping speed, and associative productivity all improved significantly during the time off treatment with lithium.4
Similar findings have also been reported among healthy adult controls when they are exposed to lithium. For example, in one discontinuation study of normal controls, participants who were randomly assigned to receive lithium exhibited poorer performance on short-term memory tasks on treatment with lithium than they did after discontinuing this medication (although, unlike our study, this study did not find an effect on processing speed).36 Similarly, in a randomized, double-blind crossover design of 59 healthy adults, Meador et al,37 found that there were significant effects of antiepileptic drugs on tests of cognitive speed and verbal memory, as well as on tests of inhibition (Stroop), sustained attention, and concentration.
Finally, the cognitive effects of antiepileptic drugs have also been examined among children and adults with seizure disorders.38,39 Some studies of adults with epilepsy have not found differences between adults with epilepsy on and off treatment with medications such as valproate or carbamazepine.40-42 However, a number of studies have suggested findings that are similar to ours. For example, in a study of 100 children with epilepsy, withdrawing antiseizure medication was associated with improvement on a measure of psychomotor speed.40 In one recent study of 139 adults with epilepsy who were treated with 1 antiepileptic and were seizure free, participants who were randomly assigned to discontinue their medication displayed improved performance on tests that required complex cognitive processing under time pressure, including divided attention, rapid language and form discrimination, and reaction time.43 In fact, in a review of the literature on the cognitive effects of lithium, Pachet and Wisniewski44 concluded that, among both clinical and nonclinical populations, treatment with lithium carbonate was associated with definite negative effects on psychomotor speed, and possibly on verbal memory.
Our finding that the second-generation antipsychotics were not significantly associated with neuropsychological impairments in children with bipolar disorder has not been reported elsewhere. However, our findings are different from those of a few adult studies that have found that treatment with antipsychotic medication is associated with poorer cognitive performance among individuals with bipolar disorder. For example, Donaldson et al45 reported that among adults with bipolar disorder, current treatment with antipsychotic medication, including both typical and second-generation antipsychotics, was associated with lower current full-scale IQ, lower general memory scores, and lower working memory scores. This was not simply attributable to greater illness severity among those treated with antipsychotic medication, as 1 marker of severity (duration of illness) had no effect on either IQ or working memory measures. Recent studies also suggested that treatment with olanzapine was associated with impairments in several aspects of psychomotor function (including psychomotor speed), as well as verbal memory.46
Our finding that current treatment with antidepressant medication was associated with improved processing speed is an intriguing one that needs replication. One potential explanation for this finding is that treatment with these medications may have reduced the neurovegetative symptoms of depression, including psychomotor or cognitive slowing. Indeed, a few studies in adult populations suggest that depression is associated with slower processing speed, whereas treatment with antidepressant medication improves speed of processing.47-50 For example, one recent study suggested that, among adults with depression, those who responded to antidepressant treatment performed better on several tests of neuropsychological functioning (including tests of executive functioning, processing speed, and attention) than depressed adults who were untreated.47 However, caution is needed in interpreting this literature because of the small number of studies, exclusive focus on adults, and use of very different measures of processing speed, ranging from simple reaction time tests to tasks requiring complex processing. Clearly, more work is needed to elucidate this issue, especially in children.
Disentangling the neuropsychological deficits associated with medication treatment in pediatric bipolar disorder has important clinical implications. The areas of neuropsychological dysfunction observed in our study have important implications for the psychosocial functioning of youth with bipolar disorder. Processing speed and working memory are critical to the development of emotion regulation and self-control,51 as well as to academic functioning. Thus, the neuropsychological impact of different classes of medication may need to be considered in selecting one medication over another. It may be that for some youth, especially those who are already compromised in neuropsychological functioning, additional consideration of neuropsychological impact of medications should be given. In addition, from a scientific perspective, it is critical to clarify, in neuropsychological studies of bipolar youth, which impairments are due to the illness itself versus its treatment. This is especially important given that most studies have included mixed samples of medicated and unmedicated youth.
The findings from the current study must be considered in light of several weaknesses. Although our total sample size was quite large, the samples of children treated with each class of medication were smaller, limiting our ability to detect smaller effects. In addition, it should be noted that, because this was not a randomized study, there may have been differences between groups, including differences in illness severity between those treated with different classes of medications or not treated, that may have impacted our findings. Future clinical trials of medications should examine neuropsychological outcomes to further examine this issue. Our sample of children with bipolar disorder may have been a cognitively higher-functioning group, in that, despite being in an acute mood state, they obtained a group mean IQ in the average range. This may have contributed to their relatively unimpaired performance on neuropsychological tests.
This study did not distinguish between specific medications within each class. It is possible that some mood stabilizers have a more negative impact than others. For example, Meador et al52 found that subjects taking carbamazepine performed more poorly than those taking lamotrigine on measures of attention, cognitive speed, memory, and graphomotor coding. Similarly, in a study of 33 adults with bipolar disorder, those treated with lamotrigine had better performance than patients treated with other anticonvulsants on a task of verbal fluency and had moderate (though nonsignificant) effect sizes on the CVLT verbal memory.53 Gallassi et al54 also found that subjects taking valproate performed more poorly than those taking carbamazepine on tasks of visuomotor function and memory. Whether these findings are replicable in children with bipolar disorder deserves additional scrutiny.
Given that children with bipolar disorder were acutely symptomatic, in some instances despite mood stabilizing treatment, at the time of neuropsychological testing, it is possible that the impairments they exhibited were due to mood state effects. This possibility is made less likely by our finding that, even after controlling for severity of manic and depressive symptoms, our results remained largely the same. However, it is possible that their acute symptomatic status obscured more subtle medication effects. It would be important for future studies to examine whether the neuropsychological impairments observed in this study are also found during euthymia. Future clinical trials of medications for pediatric bipolar disorder should also examine whether there are pretreatment and posttreatment differences in specific aspects of cognitive functioning among these youth and whether there are relationships between treatment response and changes in cognitive performance.
This study did not consider the impact of dosing or blood serum level on neuropsychological functioning. However, prior studies have suggested that there may be a relationship between serum levels of these medications and cognitive effects. For example, some studies of adults with epilepsy and those with mood disorders have suggested that compromised cognitive functioning is dose related,55 although others have reported no correlation between neuropsychological impairments and serum level.41
Despite these limitations, the findings from this study suggest that, among children with bipolar disorder, treatment with mood stabilizers is associated with specific impairments in processing speed and working memory. Thus, potential cognitive side effects should be considered in selecting treatments for bipolar disorder in youth.
Drug names: bupropion (Aplenzin, Wellbutrin, and others), carbamazepine (Carbatrol, Equetro, and others), citalopram (Celexa and others), divalproex (Depakote and others), escitalopram (Lexapro and others), fluoxetine (Prozac and others), gabapentin (Neurontin and others), lamotrigine (Lamictal and others), lithium (Eskalith, Lithobid, and others), methylphenidate (Daytrana, Ritalin, and others), olanzapine (Zyprexa), paroxetine (Paxil, Pexeva, and others), risperidone (Risperdal and others), sertraline (Zoloft and others), topiramate (Topamax and others), valproate (Depacon and others), venlafaxine (Effexor and others).
Author affiliations: Pediatric Psychopharmacology Clinical & Research Program (all authors) and Department of Psychiatry (Drs Henin, Mick, Biederman, Fried, Hirshfeld-Becker, Micco, and Wozniak), Massachusetts General Hospital (MGH), Boston.
Financial disclosure: Dr Henin has received grant/research support from the National Institute of Mental Health (NIMH) and NARSAD; has received honoraria from Shire and Abbott Neuroscience; and has received other financial or material support from Oxford University Press. Dr Henin has also received honoraria from Reed Medical Education (a company working as a logistics collaborator for the MGH Psychiatry Academy). The education programs conducted by the MGH Psychiatry Academy were partially supported through Independent Medical Education grants from pharmaceutical companies including Shire, AstraZeneca, Lilly, McNeil Pediatrics, Janssen, Bristol-Myers Squibb, Pfizer, Forest, and Sanofi Aventis. Dr Mick is a consultant to Shire; has received grant/research support from Ortho-McNeil Janssen and Pfizer; has received honoraria from Ortho-McNeil Janssen; and is a member of the advisory boards for Shire and Validus. Dr Biederman has received grant/research support from Alza, AstraZeneca, Bristol-Myers Squibb, Eli Lilly, Janssen, McNeil, Merck, Organon, Otsuka America, Shire, National Institute of Child Health and Human Development, and NIMH; is a consultant or member of the advisory board for Janssen, McNeil, Novartis, and Shire; and is a member of the speakers bureau for Janssen, McNeil, Novartis, Shire, and UCB Pharma. Dr Hirshfeld-Becker has received grant/research support from NIMH, and her spouse/partner has a noncontrolling interest in a family limited partnership that holds stocks in the following companies: Amgen, Boston Scientific, Eli Lilly, Pfizer, Sepracor, Wyeth, and United Health. Dr Micco has received grant/research support from NIMH and NARSAD. Dr Wozniak has received grant/research support from Boehringer-Ingelheim, GlaxoSmithKline, UCB Pharma, and Sepracor; is a member of the speakers/advisory boards for Boehringer-Ingelheim, Sepracor, GlaxoSmithKline, Sanofi-Aventis, Takeda, Axon Labs, Jazz, Novartis, Neurogen, Pfizer, and UCB Pharma; and has received other financial or material support from Bantam Books. Her spouse/partner has received grant/research support from Boehringer-Ingelheim, GlaxoSmithKline, UCB (Schwarz) Pharma, and Sepracor; is a member of the speakers bureau for Boehringer- Ingelheim, GlaxoSmithKline, Sanofi-Aventis, Sepracor, and Takeda; and is a member of the advisory boards for Axon Labs, Boehringer-Ingelheim, GlaxoSmithKline, Jazz, Novartis, Neurogen, Pfizer, UCB (Schwarz) Pharma, and Takeda. Dr Fried and Mss Miller and Rycyna report no additional financial or other relationships relevant to the subject of this article.
Funding/support: Clinical trials were supported by Janssen, Pfizer, Bristol-Myers Squibb, Ortho-McNeil Janssen Scientific Affairs, and the Stanley Medical Research Institute. Analyses of these data were supported by a NARSAD Junior Investigator Award (Dr Henin) and by a research grant from the NIMH (K01MH065523; Dr Mick). Support also came, in part, from the Neal-Kimmerly Fund for the Study of Cognition, Boston, Massachusetts.
Previous presentation: These data were presented at the 6th Annual Collaborative Pediatric Bipolar Disorder Conference; March 28-29, 2008; Boston, Massachusetts.
REFERENCES
1. Biederman J, Mick E, Hammerness P, et al. Open-label, 8-week trial of olanzapine and risperidone for the treatment of bipolar disorder in preschool-age children. Biol Psychiatry. 2005;58(7):589-594. PubMed doi:10.1016/j.biopsych.2005.03.019
2. DelBello MP, Kowatch RA, Adler CM, et al. A double-blind randomized pilot study comparing quetiapine and divalproex for adolescent mania. J Am Acad Child Adolesc Psychiatry. 2006;45(3):305-313. PubMed
3. Honig A, Arts BM, Ponds RW, et al. Lithium-induced cognitive side-effects in bipolar disorder: a qualitative analysis and implications for daily practice. Int Clin Psychopharmacol. 1999;14(3):167-171. PubMed doi:10.1097/00004850-199905002-00003
4. Kocsis JH, Shaw ED, Stokes PE, et al. Neuropsychologic effects of lithium discontinuation. J Clin Psychopharmacol. 1993;13(4):268-275. PubMed doi:10.1097/00004714-199308000-00007
5. Dickstein DP, Treland JE, Snow J, et al. Neuropsychological performance in pediatric bipolar disorder. Biol Psychiatry. 2004;55(1):32-39. PubMed doi:10.1016/S0006-3223(03)00701-7
6. Doyle AE, Wilens TE, Kwon A, et al. Neuropsychological functioning in youth with bipolar disorder. Biol Psychiatry. 2005;58(7):540-548. PubMed doi:10.1016/j.biopsych.2005.07.019
7. McCarthy J, Arrese D, McGlashan A, et al. Sustained attention and visual processing speed in children and adolescents with bipolar disorder and other psychiatric disorders. Psychol Rep. 2004;95(1):39-47. PubMed doi:10.2466/PR0.95.5.39-47
8. Pavuluri MN, Schenkel LS, Aryal S, et al. Neurocognitive function in unmedicated manic and medicated euthymic pediatric bipolar patients. Am J Psychiatry. 2006;163(2):286-293. PubMed doi:10.1176/appi.ajp.163.2.286
9. Youngstrom EA, Danielson CK, Findling RL, et al. Factor structure of the Young Mania Rating Scale for use with youths ages 5 to 17 years. J Clin Child Adolesc Psychol. 2002;31(4):567-572. PubMed
10. Young RC, Biggs JT, Ziegler VE, et al. A rating scale for mania: reliability, validity and sensitivity. Br J Psychiatry. 1978;133:429-435. PubMed doi:10.1192/bjp.133.5.429
11. Sattler J. Assessment of Children’s Intelligence. San Diego, CA: Jerome M. Sattler; 1988.
12. Poznanski EO, Freeman LN, Mokros HB. Children’s Depression Rating Scale-Revised. Psychopharmacol Bull. 1985;21(4):979-989.
13. Hollingshead AB, ed. Four-Factor Index of Social Status. New Haven, CT: Yale Press; 1975.
14. Orvaschel H. Schedule for Affective Disorders and Schizophrenia for School-Age Children-Epidemiologic Version. 5th ed. Ft Lauderdale, FL: Nova Southeastern University, Center for Psychological Studies; 1994.
15. Fallon T, Schwab-Stone M. Determinants of reliability in psychiatric surveys of children aged 6-11 years: a test-retest study. J Child Psychol Psychiatry. 1994;35:1391-1408. PubMed doi:10.1111/j.1469-7610.1994.tb01282.x
16. Gracious BL, Youngstrom EA, Findling RL, et al. Discriminative validity of a parent version of the Young Mania Rating Scale. J Am Acad Child Adolesc Psychiatry. 2002;41(11):1350-1359. PubMed doi:10.1097/00004583-200211000-00017
17. Rajeev J, Srinath S, Reddy YC, et al. The index manic episode in juvenile-onset bipolar disorder: the pattern of recovery. Can J Psychiatry. 2003;48(1):52-55. PubMed
18. Chang KD, Dienes K, Blasey C, et al. Divalproex monotherapy in the treatment of bipolar offspring with mood and behavioral disorders and at least mild affective symptoms. J Clin Psychiatry. 2003;64(8):936-942. PubMed
19. Findling RL, McNamara NK, Gracious BL, et al. Combination lithium and divalproex sodium in pediatric bipolarity. J Am Acad Child Adolesc Psychiatry. 2003;42(8):895-901. PubMed doi:10.1097/01.CHI.0000046893.27264.53
20. Frazier JA, Biederman J, Tohen M, et al. A prospective open-label treatment trial of olanzapine monotherapy in children and adolescents with bipolar disorder. J Child Adolesc Psychopharmacol. 2001;11(3):239-250. PubMed doi:10.1089/10445460152595568
21. Biederman J, Mick E, Wozniak J, et al. An open-label trial of risperidone in children and adolescents with bipolar disorder. J Child Adolesc Psychopharmacol. 2005;15(2):311-317. PubMed doi:10.1089/cap.2005.15.311
22. Biederman J, Mick E, Spencer T, et al. An open-label trial of aripiprazole monotherapy in children and adolescents with bipolar disorder. CNS Spectr. 2007;12(9):683-689. PubMed
23. Wozniak J, Biederman J, Mick E, et al. Omega-3 fatty acid monotherapy for pediatric bipolar disorder: a prospective open-label trial. Eur Neuropsychopharmacol. 2007;17(6-7):440-447. PubMed doi:10.1016/j.euroneuro.2006.11.006
24. Wagner KD, Ambrosini P, Rynn M, et al. Efficacy of sertraline in the treatment of children and adolescents with major depressive disorder: two randomized controlled trials. JAMA. 2003;290(8):1033-1041. PubMed doi:10.1001/jama.290.8.1033
25. Wechsler D. Wechsler Intelligence Scale for Children-III (WISC-III). 3rd ed. San Antonio, TX: Harcourt Assessment, Inc; 1991.
26. Wechsler D. Wechsler Adult Intelligence Scale III. 3rd ed. San Antonio, TX: The Psychological Corporation; 1997. [manual].
27. Wilkinson GS. Wide Range Achievement Test-Revision 3. Wilmington, DE: Jastak Associates; 1993.
28. Seidman LJ, Breiter HC, Goodman JM, et al. A functional magnetic resonance imaging study of auditory vigilance with low and high information processing demands. Neuropsychology. 1998;12(4):505-518. PubMed doi:10.1037/0894-4105.12.4.505
29. Golden CJ. Stroop Color and Word Test: A Manual for Clinical and Experimental Use. Chicago, IL: Stoelting, Co.; 1978.
30. Heaton RK, Chelune GJ, Talley JL, et al. Wisconsin Card Sort Test Manual: Revised and Expanded. Odessa, FL: Psychological Assessment Resources, Inc.; 1993.
31. Osterrieth PA. Le test de copie d’ une figure complexe. Arch Psychol. 1944;30:206-356.
32. Rey A. L’ examen psychologique dans les cas d’ encephalopathie traumatique. Arch Psychol. 1941;28:286-340.
33. Bernstein JH, Waber DP. Developmental Scoring System for the Rey-Osterrieth Complex Figure. Odessa, FL: Psychological Assessment Resources, Inc; 1996. ([manual])
34. Delis DC, Kramer JH, Kaplan E, et al. California Verbal Learning Test, Children’s Version. San Antonio, TX: Psychological Corporation; 1998.
35. Stata Corporation. Stata User’s Guide: Release 7.0. College Station, TX: Stata Corporation; 2001.
36. Stip E, Dufresne J, Lussier I, et al. A double-blind, placebo-controlled study of the effects of lithium on cognition in healthy subjects: mild and selective effects on learning. J Affect Disord. 2000;60(3):147-157. PubMed doi:10.1016/S0165-0327(99)00178-0
37. Meador KJ, Loring DW, Moore EE, et al. Comparative cognitive effects of phenobarbital, phenytoin, and valproate in healthy adults. Neurology. 1995;45(8):1494-1499. PubMed
38. Loring DW, Marino S, Meador KJ. Neuropsychological and behavioral effects of antiepilepsy drugs. Neuropsychol Rev. 2007;17(4):413-425. PubMed doi:10.1007/s11065-007-9043-9
39. Loring DW, Meador KJ. Cognitive side effects of antiepileptic drugs in children. Neurology. 2004;62(6):872-877. PubMed
40. Aldenkamp AP, Alpherts WC, Blennow G, et al. Withdrawal of antiepileptic medication in children-effects on cognitive function: The Multicenter Holmfrid Study. Neurology. 1993;43(1):41-50. PubMed
41. Prevey ML, Delaney RC, Cramer JA, et al, and The Department of Veterans Affairs Epilepsy Cooperative Study 264 Group. Effect of valproate on cognitive functioning: comparison with carbamazepine. Arch Neurol. 1996;53(10):1008-1016. PubMed
42. Thompson P, Huppert F, Trimble M. Anticonvulsant drugs, cognitive function and memory. Acta Neurol Scand Suppl. 1980;80:75-81. PubMed
43. Hessen E, Lossius MI, Reinvang I, et al. Influence of major antiepileptic drugs on attention, reaction time, and speed of information processing: results from a randomized, double-blind, placebo-controlled withdrawal study of seizure-free epilepsy patients receiving monotherapy. Epilepsia. 2006;47(12):2038-2045. PubMed doi:10.1111/j.1528-1167.2006.00805.x
44. Pachet AK, Wisniewski AM. The effects of lithium on cognition: an updated review. Psychopharmacology (Berl). 2003;170(3):225-234. PubMed doi:10.1007/s00213-003-1592-x
45. Donaldson S, Goldstein LH, Landau S, et al. The Maudsley Bipolar Disorder Project: the effect of medication, family history, and duration of illness on IQ and memory in bipolar I disorder. J Clin Psychiatry. 2003;64(1):86-93. PubMed
46. Morrens M, Wezenberg E, Verkes RJ, et al. Psychomotor and memory effects of haloperidol, olanzapine, and paroxetine in healthy subjects after short-term administration. J Clin Psychopharmacol. 2007;27(1):15-21. PubMed doi:10.1097/jcp.0b013e31802dfff0
47. Gualtieri CT, Johnson LG, Benedict KB. Neurocognition in depression: patients on and off medication versus healthy comparison subjects. J Neuropsychiatry Clin Neurosci. 2006;18(2):217-225. PubMed
48. Kalb R, Dorner M, Kalb S. Opposite effects of depression and antidepressants on processing speed and error rate. Prog Neuropsychopharmacol Biol Psychiatry. 2006;30(2):244-250. PubMed doi:10.1016/j.pnpbp.2005.10.009
49. Loubinoux I, Tombari D, Pariente J, et al. Modulation of behavior and cortical motor activity in healthy subjects by a chronic administration of a serotonin enhancer. Neuroimage. 2005;27(2):299-313. PubMed doi:10.1016/j.neuroimage.2004.12.023
50. Tsourtos G, Thompson JC, Stough C. Evidence of an early information processing speed deficit in unipolar major depression. Psychol Med. 2002;32(2):259-265. PubMed doi:10.1017/S0033291701005001
51. Leibenluft E, Charney DS, Pine DS. Researching the pathophysiology of pediatric bipolar disorder. Biol Psychiatry. 2003;53(11):1009-1020. PubMed doi:10.1016/S0006-3223(03)00069-6
52. Meador KJ, Loring DW, Ray PG, et al. Differential cognitive and behavioral effects of carbamazepine and lamotrigine. Neurology. 2001;56(9):1177-1182. PubMed
53. Daban C, Martinez-Aran A, Torrent C, et al. Specificity of cognitive deficits in bipolar disorder versus schizophrenia: a systematic review. Psychother Psychosom. 2006;75(2):72-84. PubMed doi:10.1159/000090891
54. Gallassi R, Morreale A, Di Sarro R, et al. Cognitive effects of antiepileptic drug discontinuation. Epilepsia. 1992;33(suppl 6):S41-S44. PubMed doi:10.1111/j.1528-1157.1992.tb06226.x
55. Meador KJ. Newer anticonvulsants: dosing strategies and cognition in treating patients with mood disorders and epilepsy. J Clin Psychiatry. 2003;64(suppl 8):30-34. PubMed
Editor’s Note: We encourage authors to submit papers for consideration as a part of our Focus on Childhood and Adolescent Mental Health section. Please contact Karen D. Wagner, MD, PhD, at [email protected].
Please sign in or purchase this PDF for $40.00.
Save
Cite