J Clin Psychiatry 2023;84(2):22br14564
To cite: Philipp-Muller AE, Stephenson CJ, Moghimi E, et al. Combining ketamine and psychotherapy for the treatment of posttraumatic stress disorder: a systematic review and meta-analysis. J Clin Psychiatry. 2023;84(2):22br14564.
To share: https://doi.org/10.4088/JCP.22br14564
© 2023 Physicians Postgraduate Press, Inc.
aCentre for Neuroscience Studies, Queen’s University, Kingston, Ontario, Canada
bDepartment of Psychiatry, Queen’s University, Kingston, Ontario, Canada
*Corresponding author: Aaron E. Philipp-Muller, BScH, Centre for Neuroscience Studies, Queen’s University, Kingston, Ontario, Canada, Hotel Dieu Hospital – 166 Brock St, Kingston, Ontario, Canada K7L 5G2 ([email protected]).
Posttraumatic stress disorder (PTSD) is a chronic and debilitating mental health condition associated with high treatment resistance, where 35%–50% of patients do not respond to established pharmacologic and psychotherapeutic interventions.1–5 Ketamine is an emerging treatment for a number of psychopathologies such as major depressive disorder and PTSD, with a higher patient response than other pharmacologic agents.6–11 Although the clinical data for ketamine in PTSD are preliminary, it is hypothesized to function by rapidly facilitating long-term potentiation, thereby allowing a patient to disengage from an established pattern of thought more readily.10,12–20 However, ketamine has notable side effects, only lasts 1 week for PTSD, and must be administered intravenously in a hospital, rendering it impractical for long-term weekly administration.6,10,21 Pharmacologically enhanced psychotherapy is one potential means of prolonging ketamine’s effects, with the class of psychedelic medications (in which ketamine is included) yielding encouraging results.22–24 Due to the potentially promising nature of this combined treatment model, this brief report will review all literature on the combination of ketamine and psychotherapy for the treatment of PTSD to determine whether it produces a sustained reduction in symptoms of PTSD.
Methods
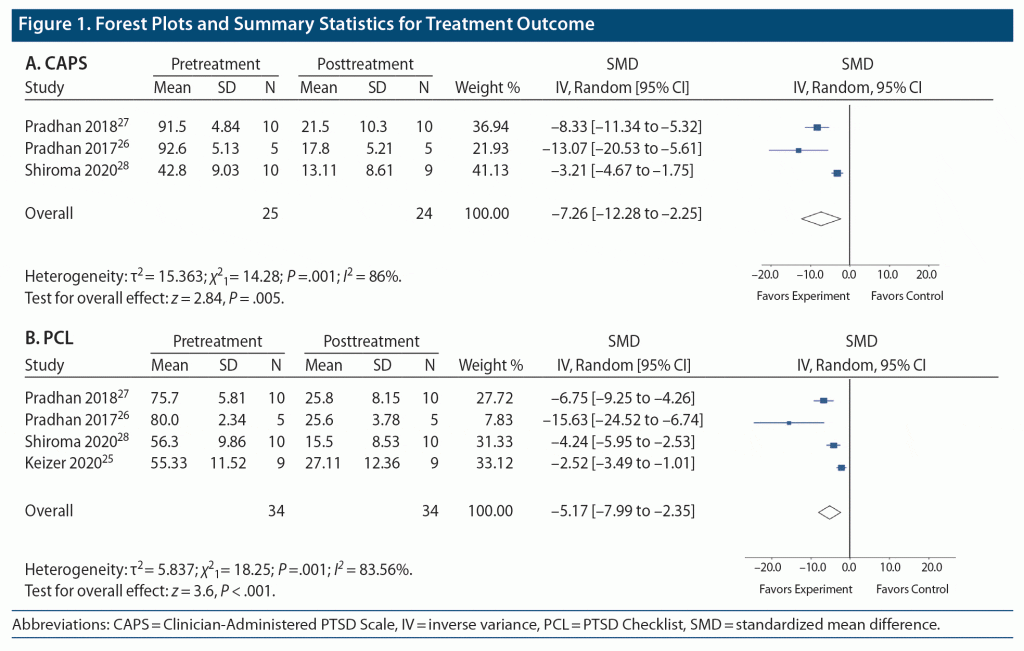
This study consisted of a systematic review and meta-analysis of all published works on this topic,25–28 according to the PRISMA guidelines,29 before June 2021 across 5 databases, including MEDLINE, PsycINFO, Embase, CINAHL, and PTSDpubs. Database search terms were built around 3 themes: PTSD, psychotherapy, and ketamine. After duplicates were removed, search results were screened independently by 2 authors (A.E.P.M. and C.J.S.) at the abstract and full-text levels. The most important criteria were that (1) all studies included patients diagnosed with PTSD, (2) all studies included an intervention involving ketamine alongside any form of psychotherapy, and (3) all patients were assessed before and after treatment using either the Clinician-Administered PTSD Scale (CAPS)30 or the PTSD Checklist (PCL).31 Full-text review was subjected to a quality assessment using the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) checklist.32,33 Data were analyzed with the estimated weighted mean effect size for each measure (standardized mean difference [SMD]), comparing pre- and posttreatment symptom severity scores.
Results
After screening, 4 studies were deemed eligible,25–28 2 of which were of moderate quality and 2 of which were of low quality according to the GRADE assessment. A total of 34 patients were included across all studies, with diverse traumatic experiences. The studies included several ketamine administration protocols, including one used previously for treating depression34 and one used for chronic pain.35,36 In 2 of the studies, patients received 12 sessions over 10 weeks of Trauma Interventions using Mindfulness Based Extinction and Reconsolidation therapy alongside a single dose of ketamine, administered at the time of psychotherapy.26,27,37 In the third study, patients received 10 weekly sessions of Prolonged Exposure therapy alongside 3 weekly doses of ketamine administered 24 hours prior to the first 3 sessions.28,38 In the final study, patients received 5 daily sessions of exposure therapy over the course of a single ketamine infusion.25 For both measures, all studies demonstrated a significant reduction in symptoms. The pooled SMD for the CAPS was −7.26 (P = .005; 95% CI, −12.28 to −2.25), while the pooled SMD for the PCL was −5.17 (P < .001; 95% CI, −7.99 to −2.35) (Figure 1).
Discussion
This study is the first in several years to review the literature on the combined efficacy of ketamine and psychotherapy for PTSD. The results of this meta-analysis indicate that this treatment may be highly effective, as seen by the significant improvements in symptoms on multiple measures. This demonstrates the potential feasibility of this treatment model and corroborates previous work.10,37,39,40 Regarding limitations, due to the novelty of this research area, the sample size was very small. This prompted the inclusion of non-randomized studies to increase the sample, which lowered the quality of the evidence. In conclusion, these preliminary findings indicate the potential of ketamine-assisted psychotherapy for PTSD.
Published online: February 6, 2023.
Relevant financial relationships: Dr Milev has received consulting and speaking honoraria from AbbVie, Allergan, Eisai, Janssen, KYE, Lallemand, Lundbeck, Neonmind, Otsuka, and Sunovion and research grants from Canadian Biomarker Integration Network in Depression (CAN-BIND), Canadian Institute of Health Research, Janssen, Lallemand, Lundbeck, Nubiyota, OBI, and Ontario Mental Health Foundation. No other author has reported any potential conflicts of interest.
Funding/support: This research was indirectly supported by the Internal Faculty Grant from the Department of Psychiatry at Queen’s University. Mr Philipp-Muller also received scholarship support from the Usona Institute, and Mr Stephenson received scholarship support from the Canadian Institute of Health Research over the course of this project.
Role of the sponsor: As described above.
Supplementary material: Available at Psychiatrist.com.
References (40)

- Magruder KM, Goldberg J, Forsberg CW, et al. Long-term trajectories of PTSD in Vietnam-Era Veterans: the course and consequences of ptsd in twins. J Trauma Stress. 2016;29(1):5–16. PubMed CrossRef
- Morina N, Wicherts JM, Lobbrecht J, et al. Remission from post-traumatic stress disorder in adults: a systematic review and meta-analysis of long term outcome studies. Clin Psychol Rev. 2014;34(3):249–255. PubMed CrossRef
- Cipriani A, Williams T, Nikolakopoulou A, et al. Comparative efficacy and acceptability of pharmacological treatments for post-traumatic stress disorder in adults: a network meta-analysis. Psychol Med. 2018;48(12):1975–1984. PubMed CrossRef
- Hoskins MD, Bridges J, Sinnerton R, et al. Pharmacological therapy for post-traumatic stress disorder: a systematic review and meta-analysis of monotherapy, augmentation and head-to-head approaches. Eur J Psychotraumatol. 2021;12(1):1802920. PubMed CrossRef
- Stein DJ, Ipser JC, Seedat S. Pharmacotherapy for post traumatic stress disorder (PTSD). Cochrane Database Syst Rev. 2006;2006(1):CD002795. PubMed CrossRef
- Davis MT, DellaGiogia N, Maruff P, et al. Acute cognitive effects of single-dose intravenous ketamine in major depressive and posttraumatic stress disorder. Transl Psychiatry. 2021;11(1):205. PubMed CrossRef
- Mion G. History of anaesthesia: the ketamine story - past, present and future. Eur J Anaesthesiol. 2017;34(9):571–575. PubMed CrossRef
- Sanacora G, Frye MA, McDonald W, et al; American Psychiatric Association (APA) Council of Research Task Force on Novel Biomarkers and Treatments. A consensus statement on the use of ketamine in the treatment of mood disorders. JAMA Psychiatry. 2017;74(4):399–405. PubMed CrossRef
- Varker T, Watson L, Gibson K, et al. Efficacy of psychoactive drugs for the treatment of posttraumatic stress disorder: a systematic review of MDMA, ketamine, LSD and psilocybin. J Psychoactive Drugs. 2021;53(1):85–95. PubMed CrossRef
- Feder A, Parides MK, Murrough JW, et al. Efficacy of intravenous ketamine for treatment of chronic posttraumatic stress disorder: a randomized clinical trial. JAMA Psychiatry. 2014;71(6):681–688. PubMed CrossRef
- Stein MB, Simon NM. Ketamine for PTSD: well, isn’t that special. Am J Psychiatry. 2021;178(2):116–118. PubMed CrossRef
- Aleksandrova LR, Wang YT, Phillips AG. Ketamine and its metabolite, (2R,6R)-HNK, restore hippocampal LTP and long-term spatial memory in the Wistar-Kyoto rat model of depression. Mol Brain. 2020;13(1):92. PubMed CrossRef
- Das RK, Gale G, Walsh K, et al. Ketamine can reduce harmful drinking by pharmacologically rewriting drinking memories. Nat Commun. 2019;10(1):5187. PubMed CrossRef
- Girgenti MJ, Ghosal S, LoPresto D, et al. Ketamine accelerates fear extinction via mTORC1 signaling. Neurobiol Dis. 2017;100:1–8. PubMed CrossRef
- Li N, Lee B, Liu RJ, et al. mTOR-dependent synapse formation underlies the rapid antidepressant effects of NMDA antagonists. Science. 2010;329(5994):959–964. PubMed CrossRef
- Peng Y, Zhao J, Gu QH, et al. Distinct trafficking and expression mechanisms underlie LTP and LTD of NMDA receptor-mediated synaptic responses. Hippocampus. 2010;20(5):646–658. PubMed CrossRef
- Asim M, Wang B, Hao B, et al. Ketamine for post-traumatic stress disorders and it’s possible therapeutic mechanism. Neurochem Int. 2021;146:105044. PubMed CrossRef
- Dwyer JM, Duman RS. Activation of mammalian target of rapamycin and synaptogenesis: role in the actions of rapid-acting antidepressants. Biol Psychiatry. 2013;73(12):1189–1198. PubMed CrossRef
- Pacheco A, Aguayo FI, Aliaga E, et al. Chronic stress triggers expression of immediate early genes and differentially affects the expression of AMPA and NMDA subunits in dorsal and ventral hippocampus of rats. Front Mol Neurosci. 2017;10:244. PubMed CrossRef
- Del Arco A, Mora F. Neurotransmitters and prefrontal cortex-limbic system interactions: implications for plasticity and psychiatric disorders. J Neural Transm (Vienna). 2009;116(8):941–952. PubMed CrossRef
- Hasler G. Toward specific ways to combine ketamine and psychotherapy in treating depression. CNS Spectr. 2020;25(3):445–447. PubMed CrossRef
- Mithoefer MC, Wagner MT, Mithoefer AT, et al. The safety and efficacy of +/−3,4-methylenedioxymethamphetamine-assisted psychotherapy in subjects with chronic, treatment-resistant posttraumatic stress disorder: the first randomized controlled pilot study. J Psychopharmacol. 2011;25(4):439–452. PubMed CrossRef
- Mithoefer MC, Wagner MT, Mithoefer AT, et al. Durability of improvement in post-traumatic stress disorder symptoms and absence of harmful effects or drug dependency after 3,4-methylenedioxymethamphetamine-assisted psychotherapy: a prospective long-term follow-up study. J Psychopharmacol. 2013;27(1):28–39. PubMed CrossRef
- Dore J, Turnipseed B, Dwyer S, et al. Ketamine Assisted Psychotherapy (KAP): patient demographics, clinical data and outcomes in three large practices administering ketamine with psychotherapy. J Psychoactive Drugs. 2019;51(2):189–198. PubMed CrossRef
- Keizer BM, Roache JD, Jones JR, et al. Continuous ketamine infusion for pain as an opportunity for psychotherapy for PTSD: a case series of ketamine-enhanced psychotherapy for PTSD and pain (KEP-P2). Psychother Psychosom. 2020;89(5):326–329. PubMed CrossRef
- Pradhan B, Wainer I, Moaddel R, et al. Trauma Interventions using Mindfulness Based Extinction and Reconsolidation (TIMBER) psychotherapy prolong the therapeutic effects of single ketamine infusion on post-traumatic stress disorder and comorbid depression: a pilot randomized, placebo-controlled. Asia Pac J Clin Trials Nerv Syst Dis. 2017;2(3):80–90. CrossRef
- Pradhan B, Mitrev L, Moaddell R, et al. d-Serine is a potential biomarker for clinical response in treatment of post-traumatic stress disorder using (R,S)-ketamine infusion and TIMBER psychotherapy: A pilot study. Biochim Biophys Acta Proteins Proteomics. 2018;1866(7):831–839. PubMed CrossRef
- Shiroma PR, Thuras P, Wels J, et al. A proof-of-concept study of subanesthetic intravenous ketamine combined with prolonged exposure therapy among veterans with posttraumatic stress disorder. J Clin Psychiatry. 2020;81(6):20l13406. PubMed CrossRef
- Moher D, Liberati A, Tetzlaff J, et al; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. PubMed CrossRef
- Weathers FW, Bovin MJ, Lee DJ, et al. The Clinician-Administered PTSD Scale for DSM-5 (CAPS-5): development and initial psychometric evaluation in military veterans. Psychol Assess. 2018;30(3):383–395. PubMed CrossRef
- Weathers F, Litz B, Herman D, et al. The PTSD Checklist (PCL): reliability, validity, and diagnostic utility. Presented at the Annual Convention of the International Society for Traumatic Stress Studies. San Antonio, Texas: 1993.
- Guyatt G, Oxman AD, Akl EA, et al. GRADE guidelines: 1. Introduction-GRADE evidence profiles and summary of findings tables. J Clin Epidemiol. 2011;64(4):383–394. PubMed CrossRef
- Meader N, King K, Llewellyn A, et al. A checklist designed to aid consistency and reproducibility of GRADE assessments: development and pilot validation. Syst Rev. 2014;3(1):82. PubMed CrossRef
- Correll GE, Maleki J, Gracely EJ, et al. Subanesthetic ketamine infusion therapy: a retrospective analysis of a novel therapeutic approach to complex regional pain syndrome. Pain Med. 2004;5(3):263–275. PubMed CrossRef
- Kiefer RT, Rohr P, Ploppa A, et al. Efficacy of ketamine in anesthetic dosage for the treatment of refractory complex regional pain syndrome: an open-label phase II study. Pain Med. 2008;9(8):1173–1201. PubMed CrossRef
- Krystal JH, Karper LP, Seibyl JP, et al. Subanesthetic effects of the noncompetitive NMDA antagonist, ketamine, in humans. Psychotomimetic, perceptual, cognitive, and neuroendocrine responses. Arch Gen Psychiatry. 1994;51(3):199–214. PubMed CrossRef
- Pradhan BK, Pinninti N. Yoga & mindfulness based cognitive therapy for psychosis (Y-MBCTp): A pilot study on its efficacy as brief therapy. In: Brief Interventions for Psychosis: A Clinical Compendium. Springer Publishers; 2016.
- Foa EB, Hembree EA, Rothbaum BO. Prolonged Exposure Therapy for PTSD: Emotional Processing of Traumatic Experiences: Therapist Guide. Oxford University Press; 2007:viii,146.
- Pradhan BK. Yoga and Mindfulness Based Cognitive Therapy: A Clinical Guide. Springer International Publishing; 2015.
- Gasperi M, Afari N, Goldberg J, et al. Pain and trauma: the role of criterion A trauma and stressful life events in the pain and PTSD relationship. J Pain. 2021;22(11):1506–1517. PubMed CrossRef
This PDF is free for all visitors!
Save
Cite