J Clin Psychiatry 2023;84(2):23ac14813
To cite: Ercis M, Ozerdem A, Singh B. When and how to use lithium augmentation for treating major depressive disorder. J Clin Psychiatry. 2023;84(2):23ac14813.
To share: https://doi.org/10.4088/JCP.23ac14813
© 2023 Physicians Postgraduate Press, Inc.
aDepartment of Psychiatry and Psychology, Mayo Clinic, Rochester, Minnesota
*Corresponding author: Balwinder Singh, MD, MS, Department of Psychiatry and Psychology, Mayo Clinic, 200 First St SW, Rochester, MN 55905 ([email protected]).
Lithium augmentation was reported in the 1980s to be effective in treating depressed patients who failed to respond to tricyclic antidepressants (TCAs).1,2 Recent studies highlight its effectiveness not only with TCAs, but also with second-generation antidepressants such as selective serotonin reuptake inhibitors (SSRIs).3 Some of the proposed mechanisms for the efficacy of lithium augmentation include the enhancement of serotonin transmission by acting synergistically with antidepressants4 and inhibition of glycogen synthase kinase 3β, which also has a neuroprotective effect.5,6 Whereas lithium remains a treatment of choice for preventing manic and depressive episodes in bipolar disorder,7 it is significantly underutilized as an augmentation agent for the treatment of major depressive disorder (MDD).8
Augmentation in Major Depressive Disorder: Efficacy
Current guidelines for the treatment of MDD recommend using augmentation strategies after the failure of 2 antidepressants or if there is a partial response with a primary antidepressant.9 There are several pharmacologic augmentation strategies available for treatment-resistant depression (TRD), including atypical antipsychotics, stimulants, dopaminergic compounds, ketamine/esketamine, atypical antidepressants, thyroid hormones, and mood stabilizers. Lithium is the most studied mood stabilizer for antidepressant augmentation and has been shown to be effective in many studies.10 It is referred to as a first- or second-line agent for pharmacologic augmentation in the management of TRD in most of the treatment guidelines, similar to atypical antipsychotics, and it is the only mood stabilizer recommended by all major guidelines.11
A comprehensive meta-analysis of 12 randomized controlled trials supported the use of lithium augmentation for MDD, with a significantly higher response rate with adjunctive lithium than with adjunctive placebo (pooled odds ratio = 2.34; 95% confidence interval [CI], 1.57–3.51), and the number needed to treat (NNT) was 5 (3–9), based on random-effects model.10 After limiting the same meta-analysis to TRD patients (9 studies), effect size for response was significantly superior to placebo, with a pooled odds ratio of 3.09 (95% CI, 1.74–5.51).10 A recent network meta-analysis of treatment augmentation strategies in MDD in patients who had failed at least 1 antidepressant also demonstrated that liothyronine, nortriptyline, aripiprazole, brexpiprazole, quetiapine, lithium, modafinil, olanzapine (with fluoxetine), cariprazine, and lisdexamfetamine were all more effective than placebo for treatment response, in order of decreasing efficacy.12 In contrast to the meta-analysis previously discussed,10 the relative effect size for response with lithium was smaller, with a relative risk of 1.25 (1.00–1.56). Moreover, remission rates did not differ significantly from placebo, with a relative risk of 1.03 (0.51–2.08).12
In the Sequenced Treatment Alternatives to Relieve Depression (STAR*D) trial, where several different antidepressant switching and augmentation strategies were examined, nearly two-thirds of the patients required their antidepressant to be switched or augmented with another psychotropic medication.13 The remission rate with lithium in patients who were unresponsive to previous treatments was low, 15.9%, and numerically lower than that observed for triiodothyronine (T3) augmentation, where the response rate was 24.7%.14
As with other strategies, lithium augmentation appears to have a reduced effect when a person is unresponsive to multiple antidepressant treatments.15 Therefore, identifying patients who will benefit most from lithium augmentation earlier in the treatment of depression may lead to better outcomes. It is noteworthy that many of the efficacy trials for lithium augmentation were conducted decades ago and had relatively small sample sizes. The augmentation of lithium with newer antidepressants remains considerably understudied, especially for non-SSRI antidepressants.3 Clinicians should be mindful of these limitations when considering lithium augmentation in patients with TRD.
When to Consider
It is recommended that antidepressants should be chosen primarily based on their relative efficacy, their tolerability and safety, comorbid conditions, cost, patient preference, and prior medication history.16 This holds true for augmentation treatments as well. Among many augmentation options, lithium stands out because of its efficacy in preventing relapses and recurrences of mood episodes.3 In addition, lithium augmentation has been found to be superior to antidepressant monotherapy in the continuation treatment phase of unipolar depressed patients who have responded well to electroconvulsive therapy.17
Lithium is known for its effect in reducing suicide and all-cause mortality.18 Its estimated antisuicidal effect is found to be larger than its effect on preventing mood episodes, suggesting that there are possible other mechanisms including a reduction in aggression and impulsivity.18 Lithium is especially recommended for patients who suffer from severe depression with a high risk of suicide.19 It was suggested that patients with more severe depressive symptoms, a history of more than 3 major depressive episodes, weight loss, or psychomotor retardation may have a better response to lithium augmentation.20 Lithium has been shown to be more effective among patients with TRD who have a first-degree relative with mood disorder (MDD or bipolar disorder).21 Age at onset, sex, and presence of atypical features do not appear to be associated with response.20 Regarding cost, lithium augmentation of SSRIs may also be a less expensive option compared to augmentation with atypical antipsychotics.22
Guidance on Lithium Dosing, Monitoring, and Serum Levels
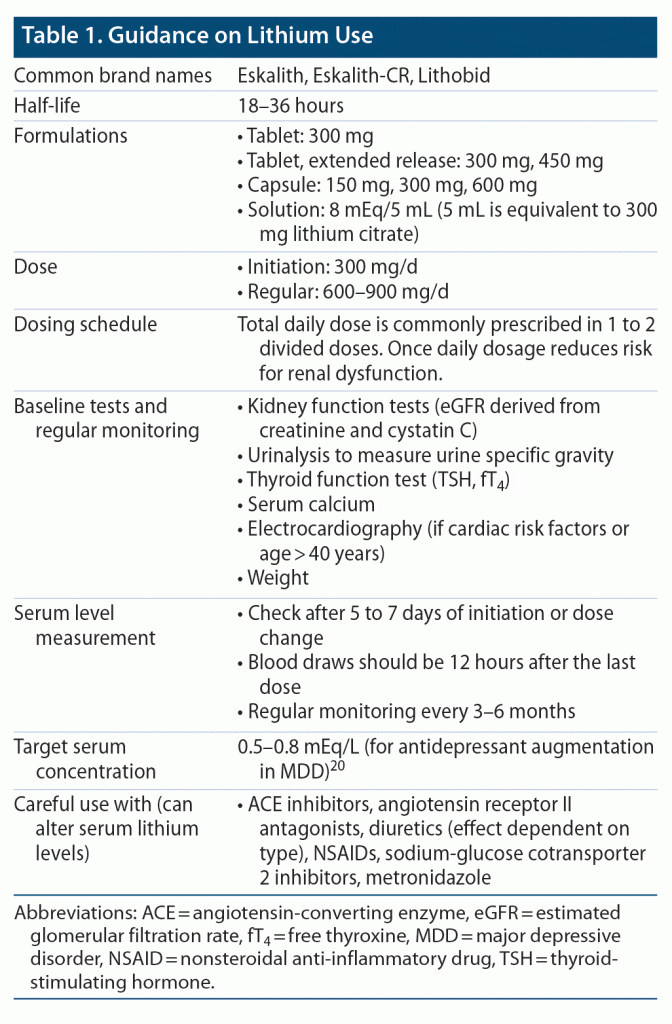
Clinical guidance on lithium use mostly comes from the literature on its use in bipolar disorder. Before initiating treatment with lithium, every patient should undergo a baseline thyroid hormone evaluation with serum thyroid stimulating hormone measurement, kidney function tests (including creatinine/glomerular filtration rate), and serum calcium levels. Additionally, an electrocardiogram is recommended for patients with cardiovascular risk factors or who are 40 years or older.23 It is important to have effective communication with a patient’s primary care physician for effective management of any lithium-related side effects. Guidance on dosing, monitoring, and serum levels is provided in Table 1.
Titration. Lithium can be started at 300 mg twice per day and the dosage adjusted upward as indicated by plasma lithium levels. The sustained-release formulation may reduce gastric irritation. Lithium level can be obtained 5 days after the dose increase and therapeutic trough level checked about 12 hours after the last dose.
Dosing and duration. There is no direct evidence regarding optimal lithium levels, how long the lithium trial should last, or how long the treatment should continue. Most current knowledge stems from bipolar disorder research, yet extrapolating these findings to MDD has its limitations. Different TRD guidelines have varying lower (0.4 to 0.6 mEq/L) and upper limits (0.6 to 1.2 mEq/L) for target serum levels, while some do not provide a specific dose range.11 Although there is no randomized controlled trial on optimal serum levels of lithium for augmentation in MDD, the efficacy trials typically reported targeting a minimum lithium level of 0.5–0.6 mEq/L, which is similar to that used for maintenance therapy of bipolar disorder.24 In a post hoc analysis of a randomized controlled trial in MDD, patients with serum lithium levels above 0.5 mEq/L showed a greater decrease in suicidality scores, though the clinical response was not different.25 The literature suggests that serum concentrations of 0.5–0.8 mEq/L might be as effective with a lower risk of side effects.20,26 The effects of lithium augmentation may appear within 10 days after reaching a steady level of target plasma concentration, but for an adequate trial, at least 2 weeks are recommended.24 After stabilization, some patients may do best with a once-daily dose at night. A level < 0.6 mEq/L is often advisable in the elderly. Upon achieving remission, both lithium and the antidepressant treatment should be continued since withdrawal of either lithium or antidepressant may result in loss of efficacy and lead to relapse.27 Even so, there is no conclusive evidence on how long augmentation treatment with lithium should last. If the patient is stable on lithium and later on decides to stop, lithium should be tapered gradually over 3 months to manage the risk of relapse.
Monitoring. Lithium levels can be monitored every 3 to 6 months unless another medication that can alter lithium levels is initiated. Particular attention should be paid to comorbid physical conditions and other medications used. The use of angiotensin-converting enzyme inhibitors, diuretics, or non-steroidal anti-inflammatory drugs may cause changes in serum lithium levels, thus requiring more frequent monitoring to prevent lithium toxicity.23 When there is a change in the patient’s daily sodium consumption, lithium levels should be checked since low sodium intake increases the risk for lithium toxicity.
Toxicity (lithium level ≥ 1.5 mEq/L). Symptoms of lithium toxicity include nausea and vomiting, abdominal pain, diarrhea, coarse tremor, weakness, ataxia, dysarthria, and confusion. These symptoms warrant an urgent assessment of lithium levels to determine the course of action, ranging from withholding the next dose and aggressive hydration to hemodialysis (for higher lithium levels, usually ≥ 4 mEq/L). Patients with chronic or acute-on-chronic toxicity are at higher risk of neurotoxicity. Time to reinitiate lithium after acute toxicity can be guided by the patient’s symptoms and serial lithium levels.
Side Effects and Special Considerations
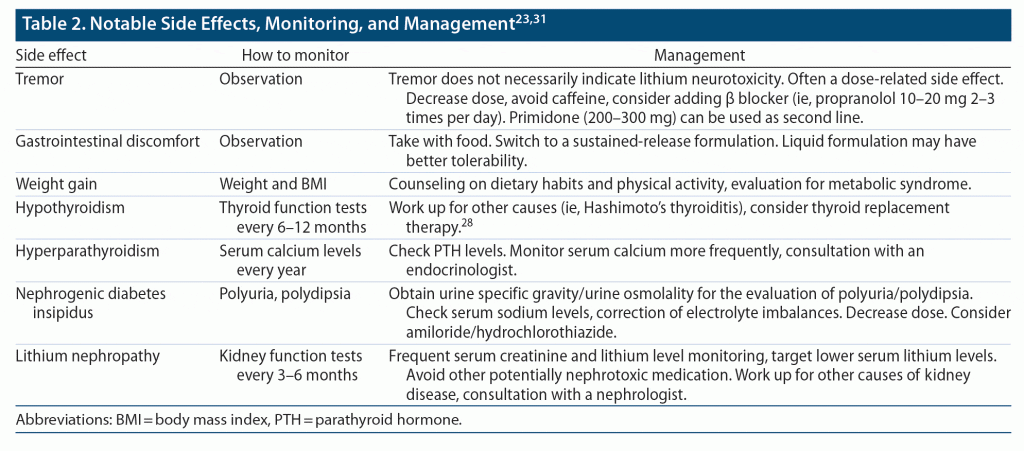
Tremor, gastrointestinal discomfort, polyuria, and polydipsia are some common side effects of lithium and can appear after a short period of time. Long-term side effects include weight gain, thyroid dysfunction, diabetes insipidus, decreased glomerular filtration rate, and hyperparathyroidism. Management of side effects is outlined in Table 2.
Special measures are required in cases of hypothyroidism or decreased kidney functioning if the patient is on long-term lithium treatment. Lithium-treated patients are more likely to develop hypothyroidism if they already have thyroid autoantibodies, are female, are older, or have a family history of hypothyroidism. Lithium-induced hypothyroidism can be managed with thyroid hormone replacement therapy.28,29
If chronic kidney disease (CKD) stage 3 is suspected (ie, estimated glomerular filtration rate < 60 mL/min/1.73 m2), monitor serum creatinine and lithium levels more frequently, target lower serum levels (0.5–0.6 mEq/L), avoid any nephrotoxic medication, and adequately treat potential medical comorbidities (such as hypertension and diabetes). Long-term lithium treatment, older age, previous episodes of lithium toxicity, comorbid medical conditions, and concomitant medications are known risk factors for lithium-induced nephropathy. Once-daily dosing is recommended for reducing the risk of CKD.30 A decrease in glomerular filtration rate does not necessarily indicate the presence of lithium nephropathy or necessitate discontinuing lithium treatment. Workup for other causes of kidney disease and consultation with a nephrologist are recommended.31 The risk-benefit analysis of continuing lithium treatment in the setting of CKD is different in excellent lithium responders. Recent studies have suggested risk of CKD but no clear increased risk of end-stage kidney disease with lithium.32,33 Patients who are stable on long-term lithium therapy are at a high risk of relapse after lithium discontinuation, warranting a need for thorough discussion before its discontinuation, although the majority of these findings are extrapolated from patients with bipolar disorder.34
Conclusion
Lithium augmentation can be helpful in the management of patients with MDD who fail to respond or have a partial response to antidepressant treatments. Lithium augmentation should be considered in TRD when patients have psychomotor retardation and weight loss, increased suicidality, recurrent depressive episodes, or a family history of mood disorder or previously had a good response with ECT. However, it may be less beneficial for patients with atypical features or who are resistant to multiple treatments. Lithium’s antisuicidal effect and low cost make it unique among other augmentation strategies. Lithium can provide an effective alternative to other augmentation strategies such as atypical antipsychotics, antidepressant combinations, thyroid hormone, or esketamine/ketamine.
Published online: March 8, 2023.
Relevant financial relationships: Dr Singh reports grant support from Mayo Clinic. Drs Ercis and Ozerdem report no potential conflicts of interest.
Funding/support: None.
ORCID: Mete Ercis: https://orcid.org/0000-0003-1272-5503; Aysegul Ozerdem: https://orcid.org/0000-0002-9455-5896; Balwinder Singh: https://orcid.org/0000-0001-7062-8192
The ASCP Corner, edited by Leslie L. Citrome, MD, MPH, is a collection of brief peer-reviewed, evidence-based articles, authored by American Society of Clinical Psychopharmacology members, that examine the practice of psychopharmacology through the lens of clinical experience. The information contained herein only represents the opinion of the author(s).

References (34)

- Heninger GR, Charney DS, Sternberg DE. Lithium carbonate augmentation of antidepressant treatment: an effective prescription for treatment-refractory depression. Arch Gen Psychiatry. 1983;40(12):1335–1342. PubMed CrossRef
- Dé Montigny C, Grunberg F, Mayer A, et al. Lithium induces rapid relief of depression in tricyclic antidepressant drug non-responders. Br J Psychiatry. 1981;138(3):252–256. PubMed CrossRef
- Nelson JC, Baumann P, Delucchi K, et al. A systematic review and meta-analysis of lithium augmentation of tricyclic and second generation antidepressants in major depression. J Affect Disord. 2014;168:269–275. PubMed CrossRef
- Bschor T, Lewitzka U, Sasse J, et al. Lithium augmentation in treatment-resistant depression: clinical evidence, serotonergic and endocrine mechanisms. Pharmacopsychiatry. 2003;36(suppl 3):230–234. PubMed CrossRef
- Adli M, Hollinde DL, Stamm T, et al. Response to lithium augmentation in depression is associated with the glycogen synthase kinase 3-beta -50T/C single nucleotide polymorphism. Biol Psychiatry. 2007;62(11):1295–1302. PubMed CrossRef
- Puglisi-Allegra S, Ruggieri S, Fornai F. Translational evidence for lithium-induced brain plasticity and neuroprotection in the treatment of neuropsychiatric disorders. Transl Psychiatry. 2021;11(1):366. PubMed CrossRef
- Yatham LN, Kennedy SH, Parikh SV, et al. Canadian Network for Mood and Anxiety Treatments (CANMAT) and International Society for Bipolar Disorders (ISBD) 2018 guidelines for the management of patients with bipolar disorder. Bipolar Disord. 2018;20(2):97–170. PubMed CrossRef
- Valenstein M, McCarthy JF, Austin KL, et al. What happened to lithium? antidepressant augmentation in clinical settings. Am J Psychiatry. 2006;163(7):1219–1225. PubMed CrossRef
- Kennedy SH, Lam RW, McIntyre RS, et al; CANMAT Depression Work Group. Canadian Network for Mood and Anxiety Treatments (CANMAT) 2016 clinical guidelines for the management of adults with major depressive disorder: section 3. pharmacological treatments. Can J Psychiatry. 2016;61(9):540–560. PubMed CrossRef
- Undurraga J, Sim K, Tondo L, et al. Lithium treatment for unipolar major depressive disorder: systematic review. J Psychopharmacol. 2019;33(2):167–176. PubMed CrossRef
- Taylor RW, Marwood L, Oprea E, et al. Pharmacological augmentation in unipolar depression: a guide to the guidelines. Int J Neuropsychopharmacol. 2020;23(9):587–625. PubMed CrossRef
- Nuñez NA, Joseph B, Pahwa M, et al. Augmentation strategies for treatment resistant major depression: a systematic review and network meta-analysis. J Affect Disord. 2022;302:385–400. PubMed CrossRef
- Warden D, Rush AJ, Trivedi MH, et al. The STAR*D project results: a comprehensive review of findings. Curr Psychiatry Rep. 2007;9(6):449–459. PubMed CrossRef
- Nierenberg AA, Fava M, Trivedi MH, et al. A comparison of lithium and T(3) augmentation following two failed medication treatments for depression: a STAR*D report. Am J Psychiatry. 2006;163(9):1519–1530, quiz 1665. PubMed CrossRef
- Nierenberg AA, Papakostas GI, Petersen T, et al. Lithium augmentation of nortriptyline for subjects resistant to multiple antidepressants. J Clin Psychopharmacol. 2003;23(1):92–95. PubMed CrossRef
- Gelenberg A, Freeman MP, Markowitz JC, et al. Practice Guideline for the Treatment of Patients With Major Depressive Disorder. 3rd ed. American Psychiatric Association; 2010.
- Sackeim HA, Haskett RF, Mulsant BH, et al. Continuation pharmacotherapy in the prevention of relapse following electroconvulsive therapy: a randomized controlled trial. JAMA. 2001;285(10):1299–1307. PubMed CrossRef
- Cipriani A, Hawton K, Stockton S, et al. Lithium in the prevention of suicide in mood disorders: updated systematic review and meta-analysis. BMJ. 2013;346(jun27 4):f3646. PubMed CrossRef
- Abou-Saleh MT, Müller-Oerlinghausen B, Coppen AJ. Lithium in the episode and suicide prophylaxis and in augmenting strategies in patients with unipolar depression. Int J Bipolar Disord. 2017;5(1):11. PubMed CrossRef
- Bauer M, Adli M, Ricken R, et al. Role of lithium augmentation in the management of major depressive disorder. CNS Drugs. 2014;28(4):331–342. PubMed CrossRef
- Sugawara H, Sakamoto K, Harada T, et al. Predictors of efficacy in lithium augmentation for treatment-resistant depression. J Affect Disord. 2010;125(1–3):165–168. PubMed CrossRef
- Edwards SJ, Hamilton V, Nherera L, et al. Lithium or an atypical antipsychotic drug in the management of treatment-resistant depression: a systematic review and economic evaluation. Health Technol Assess. 2013;17(54):1–190. PubMed CrossRef
- Lithium. In: The American Psychiatric Association Publishing Textbook of Psychopharmacology. American Psychiatric Pub; 2017.
- Bauer M, Dopfmer S. Lithium augmentation in treatment-resistant depression: meta-analysis of placebo-controlled studies. J Clin Psychopharmacol. 1999;19(5):427–434. PubMed CrossRef
- Khan A, Khan SR, Hobus J, et al. Differential pattern of response in mood symptoms and suicide risk measures in severely ill depressed patients assigned to citalopram with placebo or citalopram combined with lithium: role of lithium levels. J Psychiatr Res. 2011;45(11):1489–1496. PubMed CrossRef
- Malhi GS, Bassett D, Boyce P, et al. Royal Australian and New Zealand College of Psychiatrists clinical practice guidelines for mood disorders. Aust N Z J Psychiatry. 2015;49(12):1087–1206. PubMed CrossRef
- Bauer M, Bschor T, Kunz D, et al. Double-blind, placebo-controlled trial of the use of lithium to augment antidepressant medication in continuation treatment of unipolar major depression. Am J Psychiatry. 2000;157(9):1429–1435. PubMed CrossRef
- Singh B, Sundaresh V. Thyroid hormone use in mood disorders: revisiting the evidence. J Clin Psychiatry. 2022;83(5):22ac14590. PubMed CrossRef
- Joseph B, Nunez NA, Pazdernik V, et al. Long-term lithium therapy and thyroid disorders in bipolar disorder: a historical cohort study. Brain Sci. 2023;13(1):133. PubMed CrossRef
- Castro VM, Roberson AM, McCoy TH, et al. Stratifying risk for renal insufficiency among lithium-treated patients: an electronic health record study. Neuropsychopharmacology. 2016;41(4):1138–1143. PubMed CrossRef
- Gitlin M. Lithium side effects and toxicity: prevalence and management strategies. Int J Bipolar Disord. 2016;4(1):27. PubMed CrossRef
- Kessing LV, Gerds TA, Feldt-Rasmussen B, et al. Use of lithium and anticonvulsants and the rate of chronic kidney disease: a nationwide population-based study. JAMA Psychiatry. 2015;72(12):1182–1191. PubMed CrossRef
- Pahwa M, Joseph B, Nunez NA, et al. Long-term lithium therapy and risk of chronic kidney disease in bipolar disorder: a historical cohort study. Bipolar Disord. 2021;23(7):715–723. PubMed CrossRef
- Kumar R, Joseph B, Pazdernik VM, et al. Real-world clinical practice among patients with bipolar disorder and chronic kidney disease on long-term lithium therapy. J Clin Psychopharmacol. 2023;43(1):6–11. PubMed CrossRef
This PDF is free for all visitors!
Save
Cite