Background: Long-term remission is an important treatment goal in schizophrenia. Cariprazine, a dopamine D3/D2 receptor and serotonin 5-HT1A receptor partial agonist, is approved in the United States and Europe to treat adults with schizophrenia.
Methods: Post hoc analyses of data from a long-term cariprazine relapse prevention study (NCT01412060; September 27, 2011-September 3, 2014) investigated the efficacy of cariprazine for maintaining remission in clinically stable patients with DSM-IV-TR-defined schizophrenia. Patients were stabilized with open-label cariprazine (20 weeks), then randomized 1:1 to cariprazine (3, 6, or 9 mg/d) or placebo for double-blind treatment (up to 72 weeks). Symptomatic remission was defined as scores ≤ 3 on 8 items from the General, Positive, and Negative Symptoms subscales of the Positive and Negative Syndrome Scale (PANSS). Sustained remission included meeting remission criteria at the current and all prior double-blind visits or for ≥ 6 consecutive months.
Results: At randomization, 169/200 patients (84.5%) met symptomatic remission criteria. During double-blind treatment, time to loss of sustained remission was significantly longer (P = .0020) for cariprazine versus placebo (hazard ratio = 0.51); 60.5% of cariprazine-treated and 34.9% of placebo-treated patients sustained remission through the final visit (odds ratio [OR] = 2.85; P = .0012; number needed to treat [NNT] = 4). Almost twice as many cariprazine-treated (39.6%) as placebo-treated (21.2%) patients met symptomatic remission criteria at all visits ≥ 6 consecutive months immediately before/including the final double-blind visit (OR = 2.44; P = .0057; NNT = 6). More cariprazine-treated (41.6%) than placebo-treated (27.3%) patients sustained remission for any ≥ 6 consecutive month period (OR = 1.90, P = .0379; NNT = 7).
Conclusions: Cariprazine was associated with significantly longer sustained remission, higher remission rates, and increased likelihood of sustaining remission for ≥ 6 consecutive months versus placebo.
Trial Registration: ClinicalTrials.gov identifier: NCT01412060
This work may not be copied, distributed, displayed, published, reproduced, transmitted, modified, posted, sold, licensed, or used for commercial purposes. By downloading this file, you are agreeing to the publisher’s Terms & Conditions.
Long-Term Remission With Cariprazine Treatment in Patients With Schizophrenia:
A Post Hoc Analysis of a Randomized, Double-Blind, Placebo-Controlled, Relapse Prevention Trial
ABSTRACT
Background: Long-term remission is an important treatment goal in schizophrenia. Cariprazine, a dopamine D3/D2 receptor and serotonin 5-HT1A receptor partial agonist, is approved in the United States and Europe to treat adults with schizophrenia.
Methods: Post hoc analyses of data from a long-term cariprazine relapse prevention study (NCT01412060; September 27, 2011-September 3, 2014) investigated the efficacy of cariprazine for maintaining remission in clinically stable patients with DSM-IV-TR-defined schizophrenia. Patients were stabilized with open-label cariprazine (20 weeks), then randomized 1:1 to cariprazine (3, 6, or 9 mg/d) or placebo for double-blind treatment (up to 72 weeks). Symptomatic remission was defined as scores ≤ 3 on 8 items from the General, Positive, and Negative Symptoms subscales of the Positive and Negative Syndrome Scale (PANSS). Sustained remission included meeting remission criteria at the current and all prior double-blind visits or for ≥ 6 consecutive months.
Results: At randomization, 169/200 patients (84.5%) met symptomatic remission criteria. During double-blind treatment, time to loss of sustained remission was significantly longer (P = .0020) for cariprazine versus placebo (hazard ratio = 0.51); 60.5% of cariprazine-treated and 34.9% of placebo-treated patients sustained remission through the final visit (odds ratio [OR] = 2.85; P = .0012; number needed to treat [NNT] = 4). Almost twice as many cariprazine-treated (39.6%) as placebo-treated (21.2%) patients met symptomatic remission criteria at all visits ≥ 6 consecutive months immediately before/including the final double-blind visit (OR = 2.44; P = .0057; NNT = 6). More cariprazine-treated (41.6%) than placebo-treated (27.3%) patients sustained remission for any ≥ 6 consecutive month period (OR = 1.90, P = .0379; NNT = 7).
Conclusions: Cariprazine was associated with significantly longer sustained remission, higher remission rates, and increased likelihood of sustaining remission for ≥ 6 consecutive months versus placebo.
Trial Registration: ClinicalTrials.gov identifier: NCT01412060
J Clin Psychiatry 2019;80(2):18m12495
To cite: Correll CU, Potkin SG, Zhong Y, et al. Long-term remission with cariprazine treatment in patients with schizophrenia: a post hoc analysis of a randomized, double-blind, placebo-controlled, relapse prevention trial. J Clin Psychiatry. 2019;80(2):18m12495.
To share: https://doi.org/10.4088/JCP.18m12495
© Copyright 2019 Physicians Postgraduate Press, Inc.
aDepartment of Psychiatry, The Zucker Hillside Hospital, Northwell Health, Glen Oaks, New York
bDepartment of Psychiatry and Molecular Medicine, Hofstra Northwell School of Medicine, New York, New York
cDepartment of Child and Adolescent Psychiatry, Charité Universitפtsmedizin, Berlin, Germany
dDepartment of Psychiatry and Human Behavior, University of California Irvine, Irvine, California
eAllergan, Madison, New Jersey
fGedeon Richter Plc, Budapest, Hungary
*Corresponding author: Christoph U. Correll, MD, The Zucker Hillside Hospital, Psychiatry Research, 75-59 263rd St, Glen Oaks, NY 11004 ([email protected]).
Schizophrenia, a chronic and complex neuropsychiatric syndrome, is characterized by multiple episodes of relapse over the course of the illness.1 Frequently associated with treatment nonadherence,2 relapse promotes disease progression through slower and less complete recovery, difficulty regaining the previous level of function, increased rates of hospitalization, and the potential for treatment resistance.2,3 Using a long-term treatment strategy to prevent relapse may allow a patient to achieve remission, with the goal of functional recovery.2 Sustained remission, or the long-term alleviation of symptoms to levels that do not significantly influence behavior,4 is associated with improved outcomes, including social functioning.5,6 Given the importance of maintaining remission in people with schizophrenia, there is a need for treatments that have long-term efficacy and tolerability to minimize the risks of treatment nonadherence and relapse.
The Remission in Schizophrenia Working Group (RSWG) developed a consensus definition and operational criteria for symptomatic remission based on 3 dimensions of schizophrenia psychopathology: psychoticism, disorganization, and negative symptoms.4 The criteria to define remission consist of a symptomatic component and a time component. The symptomatic component requires that patients have scores corresponding to a severity level of mild or better (≤ 3) on 8 individual items of the Positive and Negative Syndrome Scale (PANSS)7 chosen to map the 3 dimensions of psychopathology and the 5 criteria for schizophrenia specified in the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, Text Revision (DSM-IV-TR)8 (ie, delusions, unusual thought content, hallucinatory behavior, conceptual disorganization, mannerisms/posturing, blunted affect, social withdrawal, and lack of spontaneity). The time component requires that symptomatic criteria be met for ≥ 6 consecutive months. Meeting both the symptomatic and time criteria is referred to as sustained remission. Importantly, this definition of remission uses an absolute threshold of symptom severity, not degree of improvement from a baseline score, suggesting that it can be used throughout the course of the disease and may permit between-trial comparisons of different antipsychotics and their efficacy in promoting remission.5
Cariprazine is a potent, orally active, dopamine D3-preferring D3/D2 receptor partial agonist and serotonin 5-HT1A receptor partial agonist approved for the treatment of adults with schizophrenia (United States and Europe) and acute manic or mixed episodes associated with bipolar I disorder (United States). Cariprazine has 2 active metabolites, desmethyl cariprazine and didesmethyl cariprazine. The combined half-life for all 3 active moieties is 1 week,9 which is relatively longer than that of other oral atypical antipsychotics.10,11 This longer half-life may be associated with sustained blood cariprazine levels and some continued effect after antipsychotic treatment interruption or discontinuation, which may have the potential to lessen relapse events in cases of intermittent or partial treatment noncompliance and increase the likelihood of sustained remission. Cariprazine has demonstrated efficacy in several short-term, placebo- and active-controlled trials in patients with acute schizophrenia.12-14 In a long-term, double-blind, placebo-controlled trial to assess the effectiveness of cariprazine in relapse prevention,15 time to relapse, the primary efficacy endpoint, was significantly longer for cariprazine versus placebo (P = .001). The hazard of relapse for cariprazine-treated patients was less than half that of placebo-treated patients (hazard ratio; HR [95% CI] = 0.45 [0.28 to 0.73]). During double-blind treatment, 24.8% of cariprazine- and 47.5% of placebo-treated patients met relapse criteria, with a number needed to treat (NNT) of 5. To investigate the ability of cariprazine to sustain remission in stable patients with schizophrenia, we conducted post hoc analyses of data from this relapse prevention study applying the RSWG remission criteria.4
METHODS
Study Design
Post hoc analyses were performed using data from the long-term (up to 92 weeks), multinational, randomized, double-blind, placebo-controlled relapse prevention study of cariprazine in patients with schizophrenia (NCT01412060, September 27, 2011-September 3, 2014). Detailed methods and results, including a CONSORT diagram and safety outcomes, have been previously published.15 Briefly, patients were initially stabilized with flexible-dose cariprazine (3-9 mg/d) during a 20-week, open-label phase. Patients who met stabilization criteria (PANSS total score ≤ 60; ≥ 20% decrease in PANSS total score from baseline; Clinical Global Impressions-Severity of Illness [CGI-S]16 score ≤ 4; score ≤ 4 on 7 PANSS items [delusions, conceptual disorganization, hallucinatory behavior, suspiciousness/persecution, hostility, uncooperativeness, and poor impulse control]) were randomized 1:1 to fixed-dose cariprazine (3, 6, or 9 mg/d [same dose as in the stabilization phase]) or placebo for up to 72 weeks of double-blind treatment. The study was administratively terminated for all active patients when the last randomized patient completed 26 weeks of treatment. This study was approved by an institutional review board (US study centers) or independent ethics committee (non-US study centers) and was conducted in accordance with the Declaration of Helsinki. All patients gave written informed consent.

- Schizophrenia is a chronic illness that requires effective long-term treatment to improve patient well-being and functional recovery.
- Sustained remission is a critical concept of treatment response that emphasizes symptom stability over prolonged periods of time for patients with schizophrenia.
- These post hoc analyses suggest that cariprazine may be an effective treatment for sustaining stable, prolonged remission in patients with schizophrenia who have previously tolerated and responded to cariprazine.
Patients
The relapse prevention study included male and female inpatients aged 18-60 years, with a DSM-IV-TR8 diagnosis of schizophrenia for a minimum of 1 year and a current psychotic episode of < 4 weeks’ duration. Clinical inclusion criteria included a PANSS total score ≥ 70 and ≤ 120 and a score ≥ 4 (moderately severe) on ≥ 2 of the following PANSS items: delusions, hallucinatory behavior, conceptual disorganization, and suspiciousness/persecution. Patients were excluded if they were experiencing their first psychotic episode or if they were diagnosed with another psychiatric disorder (eg, psychotic disorders, bipolar I or II disorder, substance abuse/dependence within 3 months of study); medical conditions (eg, clinically significant cardiovascular disease, seizure disorder, hypo- or hyperthyroidism) that could interfere with the conduct of the study were also exclusionary.
Post Hoc Analyses
Post hoc analyses were based on data from the double-blind intent-to-treat (ITT) population (patients who took ≥ 1 dose of study medication and had a baseline and ≥ 1 double-blind postbaseline PANSS total score or CGI-S assessment). Symptomatic remission based on RSWG criteria was defined as a score ≤ 3 (mild) on 8 PANSS items: mannerisms and posturing (G5), unusual thought content (G9), blunted affect (N1), social withdrawal (N4), lack of spontaneity (N6), delusions (P1), conceptual disorganization (P2), and hallucinatory behavior (P3).4
Outcomes
The efficacy of cariprazine was assessed using 4 definitions of sustained remission that were based on recommended RSWG symptomatic and time criteria: (1) meeting symptomatic remission criteria at the visit in question and all previous double-blind visits, (2) meeting symptomatic remission criteria for 6 consecutive months starting at double-blind baseline, (3) meeting symptomatic remission criteria at each visit for 6 consecutive months prior to a patient’s last visit in remission during the double-blind period, and (4) meeting symptomatic remission criteria for 6 consecutive months preceding a patient’s final study visit.
Time to loss of sustained remission, duration of sustained remission, and percentage of patients achieving remission criteria were compared between placebo and pooled cariprazine (3-9 mg/d) treatment groups.
Statistical Analyses
Patients who completed open-label treatment and met stabilization criteria were randomized to double-blind treatment; randomization was considered the double-blind baseline. For randomized patients, time to loss of sustained remission (equivalent to duration of sustained remission) was defined as no longer meeting symptomatic criteria for remission, or as having discontinued due to relapse, using a last-observation-carried-forward (LOCF) approach. Time to loss of sustained remission was estimated using Kaplan-Meier analysis; P values were based on the log-rank test. The HR and 95% CI were based on the Cox proportional hazards regression model, with treatment group as an explanatory variable. The percentage of patients meeting 6-month remission criteria as well as the corresponding odds ratios (ORs) and 95% CIs were calculated using an observed cases approach for each definition of sustained remission; P values were based on the Fisher exact test. The NNT was calculated by dividing 1 by the risk difference and rounding up to the next integer. Treatment compliance (measured by site investigators) was defined as the number of capsules actually taken by a patient during a period divided by the number of capsules prescribed for the same period multiplied by 100; results were presented by descriptive statistics.
RESULTS
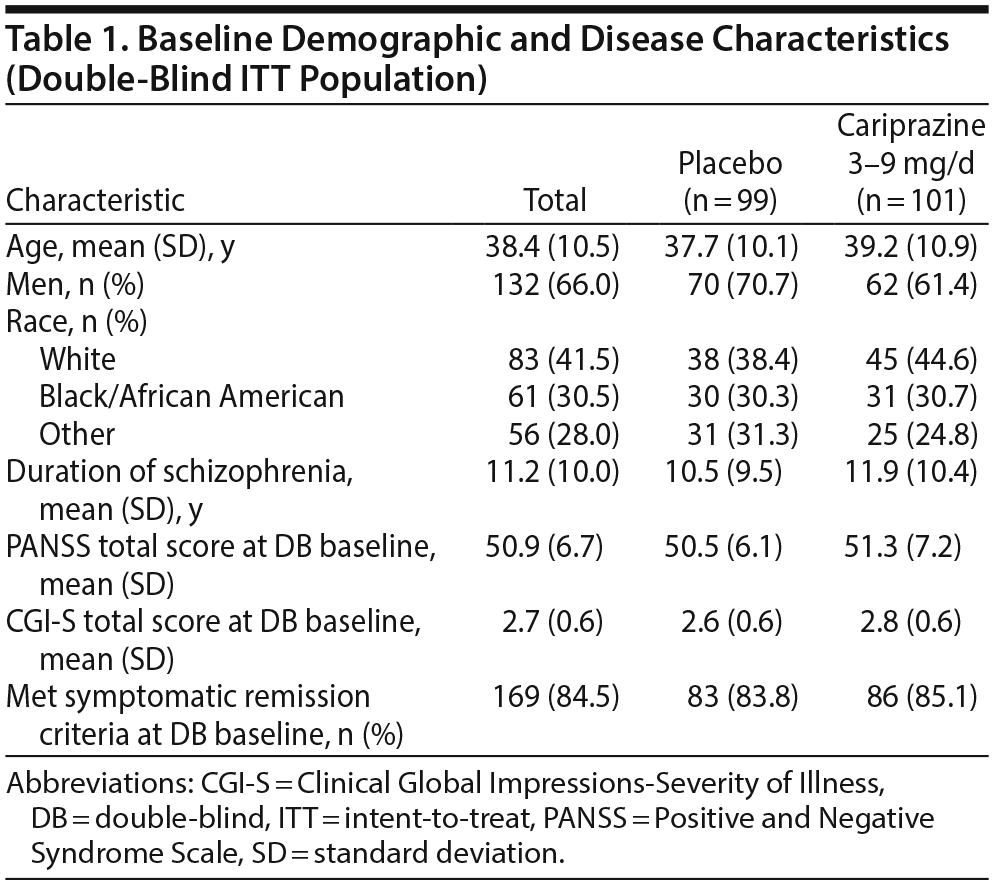
Open-label treatment was completed by 264 of 765 patients (34.5%); the most common reason for premature discontinuation was withdrawal of consent (~20%). The double-blind ITT population comprised 200 stable patients (cariprazine = 101, placebo = 99); of cariprazine-treated patients, 14 were taking 3 mg/d, 37 were taking 6 mg/d, and 50 were taking 9 mg/d. Significantly more placebo than cariprazine patients prematurely discontinued double-blind treatment for reasons other than relapse; the most common reason for discontinuation in both treatment groups was administrative termination per the study protocol. Baseline demographics and disease characteristics were generally similar between the randomized treatment groups (Table 1). At double-blind baseline, high and similar numbers of patients in both treatment groups (84%-85%) met symptomatic remission criteria (Table 1). Overall mean treatment compliance based on pill counts was high in both treatment groups (placebo = 99.3%; cariprazine = 98.5%).
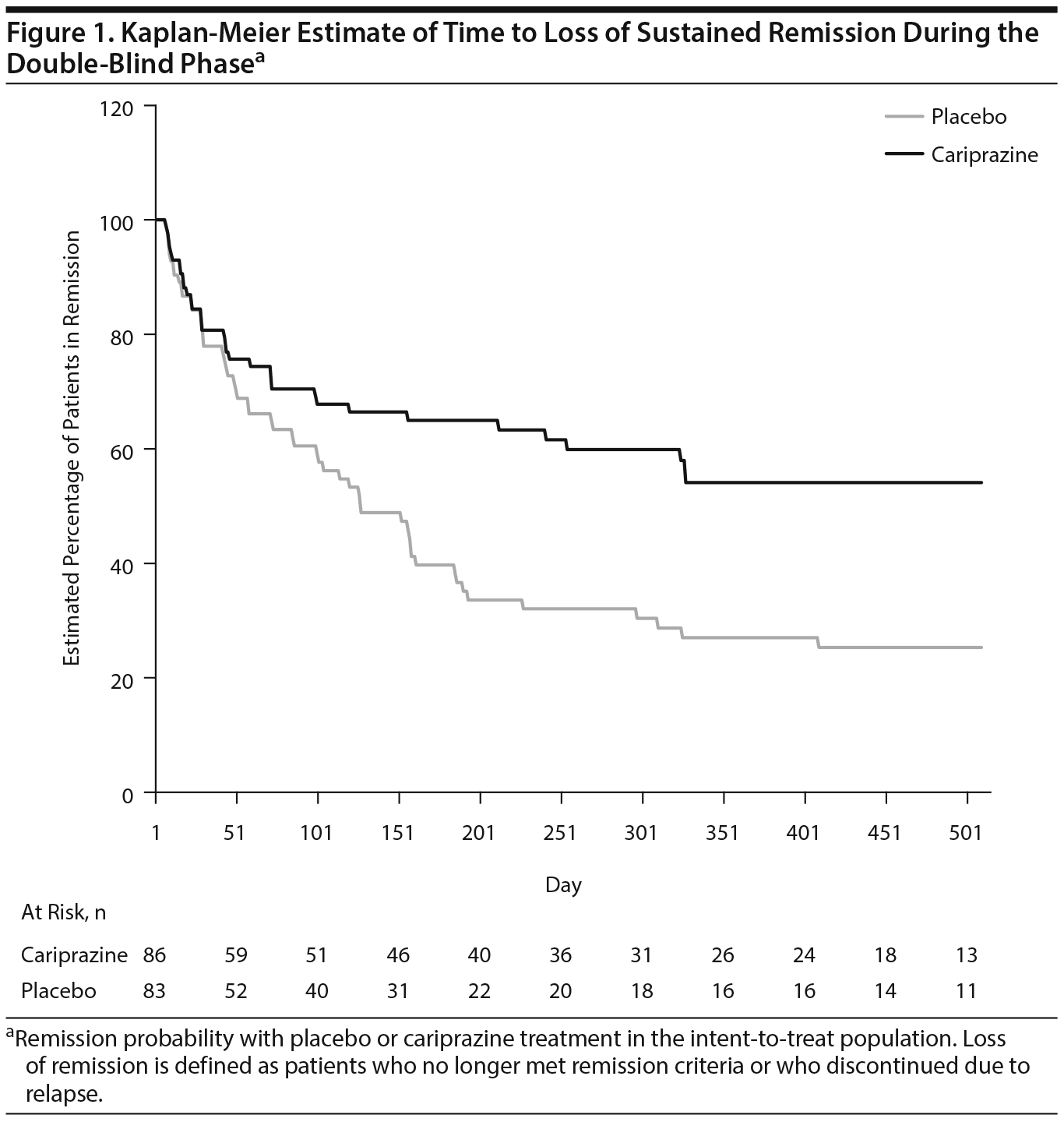
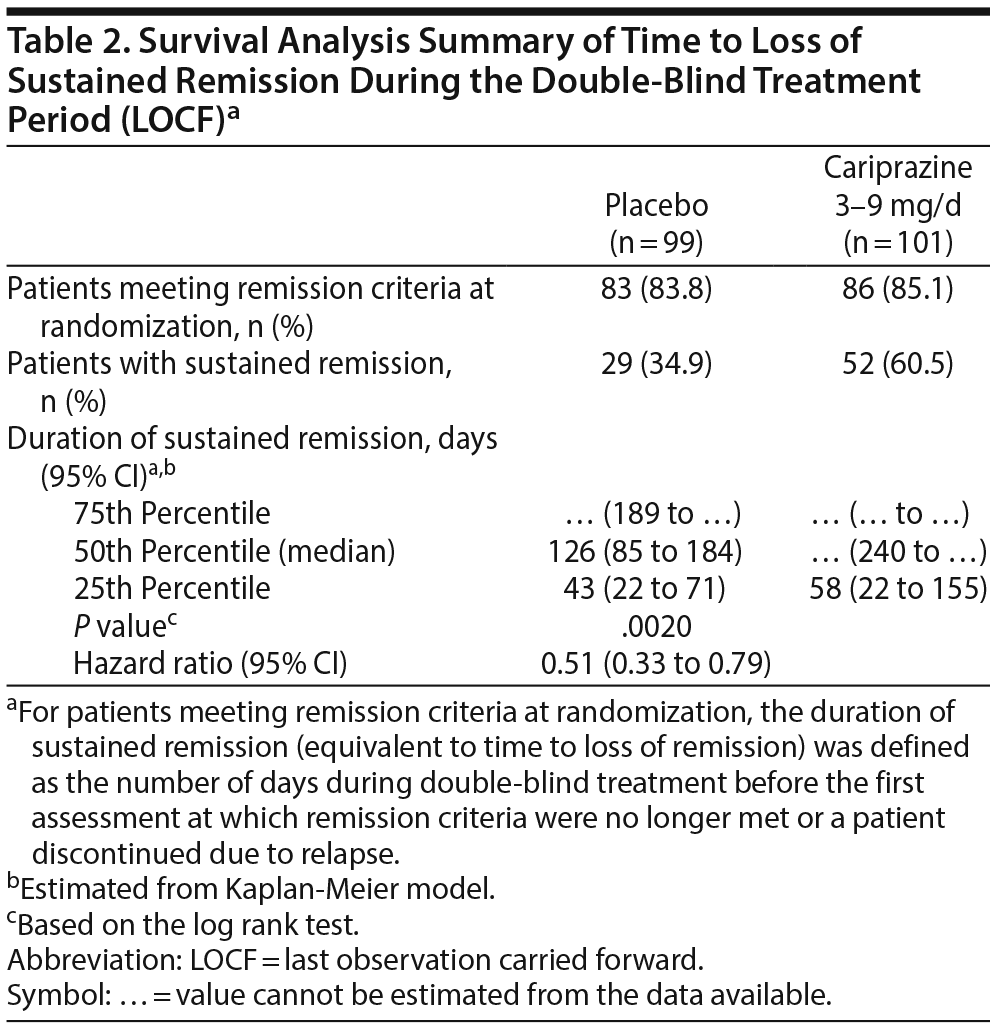
During double-blind treatment, time to loss of sustained remission was significantly longer (P = .0020) in the cariprazine group than in the placebo group (HR [95% CI] = 0.51 [0.33 to 0.79]) (Figure 1); 60.5% of cariprazine- and 34.9% of placebo-treated patients sustained remission through the final visit (OR [95% CI] = 2.85 [1.52 to 5.32]; P = .0012; NNT = 4). This corresponds to a significantly longer time to loss of sustained remission with cariprazine than with placebo. The 25th percentile (95% CI) for time to loss of sustained remission, which signifies that 25% of patients lost sustained remission at or before that time, was 43 (22 to 71) days for placebo- and 58 (22 to 155) days for cariprazine-treated patients. For the 50th percentile, time to loss of sustained remission was 126 (85 to 184) days with placebo, and it could not be estimated for cariprazine because fewer than half of the patients lost sustained remission in this group (Table 2). Although there were too few patients to estimate the time to loss of sustained remission in the cariprazine group for the 50th percentile, based on the estimated lower limit of the 95% confidence interval, it would be greater than 240 days, more than double the time for the placebo group. Separation of the placebo- and cariprazine-treatment group curves was first evident at approximately 7 weeks after the start of double-blind treatment, and it was maintained until the end of the study.
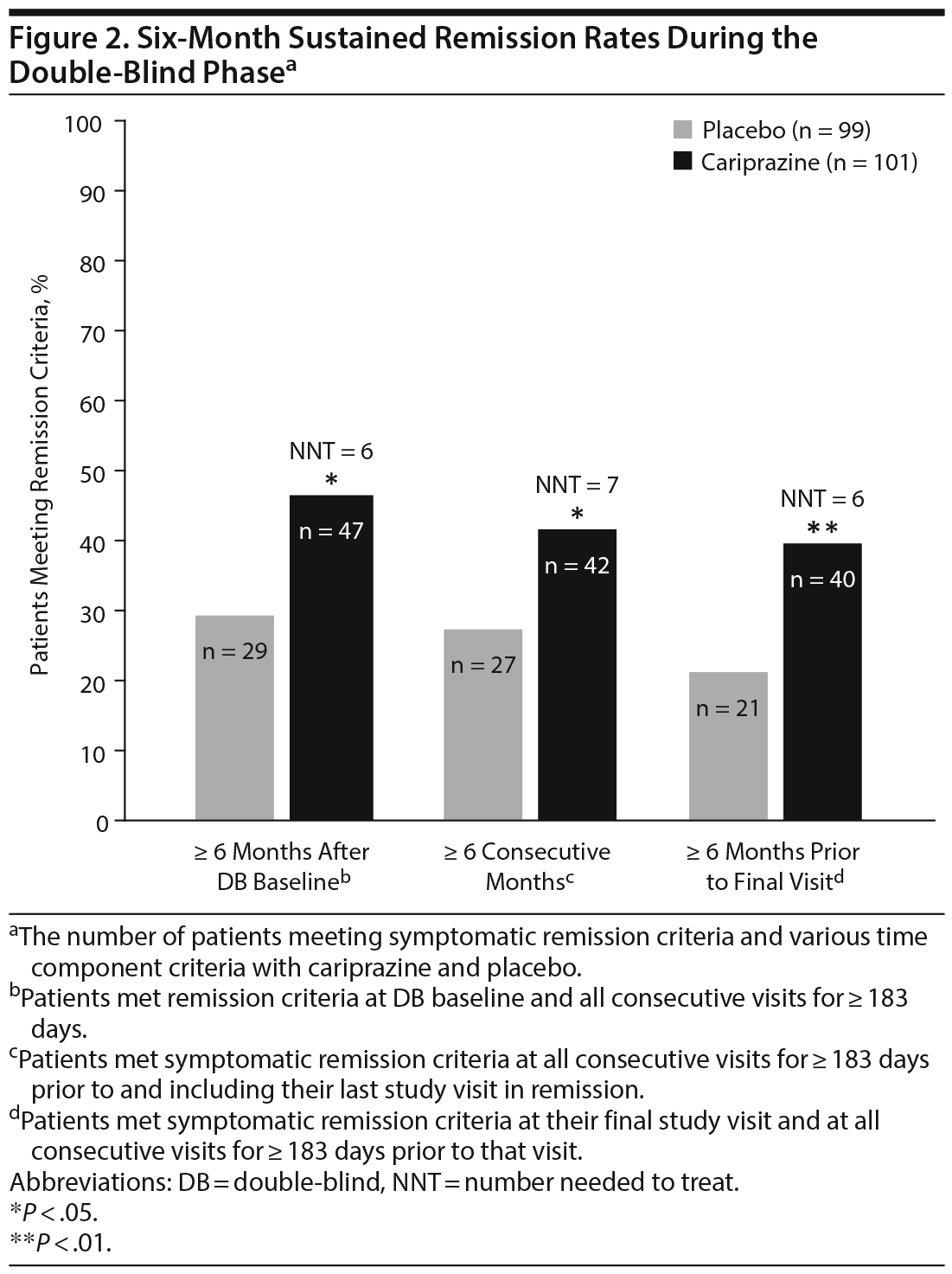
Of patients in the double-blind ITT population, more cariprazine-treated (46.5%) than placebo-treated (29.3%) patients met sustained remission criteria 6 months after the double-blind baseline; the difference between the treatment groups was statistically significant in favor of cariprazine (OR [95% CI] = 2.1 [1.17 to 3.76]; P = .0136; NNT = 6) (Figure 2). Similarly, significantly more cariprazine-treated (41.6%) than placebo-treated (27.3%) patients had sustained remission at all visits for at least 6 consecutive months prior to their last study visit in remission (OR [95% CI] = 1.90 [1.05 to 3.44]; P = .0379; NNT = 7). Almost twice as many cariprazine-treated (39.6%) as placebo-treated (21.2%) patients met symptomatic remission criteria at all visits for ≥ 6 consecutive months prior to the patient’s final visit in the double-blind period (OR [95% CI] = 2.44 [1.30 to 4.55]; P = .0057; NNT = 6).
DISCUSSION
In this post hoc analysis of data from a long-term, placebo-controlled, relapse prevention study, cariprazine was associated with a statistically significant and clinically meaningful longer time to loss of sustained remission than placebo. Significantly more cariprazine- than placebo-treated patients met sustained remission criteria using multiple time component criteria, with medium-to-large effect sizes (NNT = 6 to 7) that were clinically meaningful. These findings suggest that cariprazine was effective in maintaining remission in stable patients with schizophrenia.
Achieving sustained remission is an important step toward well-being and recovery for patients with schizophrenia. The lifetime nature of schizophrenia necessitates ongoing pharmacologic treatment to prevent relapse in most patients2; as such, identifying treatments that can produce symptom stability over a long period of time is an important component of comprehensive clinical management. Patients who are able to maintain stability for long durations may be less likely to lose remission status or relapse, even when they are partially or intermittently nonadherent to their medication. Treatment nonadherence is frequently associated with failure to maintain remission in patients with schizophrenia,5 and approximately 50%-75% of patients with schizophrenia are only partially adherent.17 The results of our post hoc analyses suggest that cariprazine has the ability to sustain remission for at least 6 months, a timeframe that was selected by the RSWG based on the long-term course of schizophrenia and the characteristics intrinsic to its psychopathology (eg, delusions, hallucinations, disorganization, negative symptoms).4
In this post hoc analysis, 29/99 (29.3%) and 47/101 (46.5%) of placebo- and cariprazine-treated patients, respectively, met sustained remission criteria at 6 months after the double-blind baseline using RSWG remission criteria. These results need to be interpreted in the context of other studies that also used RSWG remission criteria. For example, a post hoc analysis evaluated sustained remission using data from a long-term, randomized, placebo-controlled relapse prevention study of quetiapine extended-release in patients with schizophrenia.18 After an open-label stabilization period, almost all placebo-treated (94/102 [92.2%]) and quetiapine-treated (86/93 [92.5%]) patients were in symptomatic remission at randomization; after 6 months, 6/102 placebo-treated patients (5.9%) and 15/93 quetiapine-treated patients (16.1%) met sustained remission criteria (NNT = 10). Quetiapine compared with placebo significantly increased time to loss of sustained remission and duration of remission, with an HR of 0.39 (P = .009). RSWG criteria have also been used to assess remission in an open-label, active-controlled, 2-year study that compared the efficacy of oral quetiapine and risperidone long-acting injectable (RLAI) for preventing relapse in symptomatically stable patients with schizophrenia or schizoaffective disorder; remission was a secondary, preplanned, prespecified, post hoc outcome. Sustained remission (meeting RSWG symptom severity criteria for ≥ 6 months) was reported for 51% of RLAI-treated patients and 39% of quetiapine-treated patients during the study.19 Additionally, in a pooled, post hoc analysis that used RSWG criteria to analyze data from two 52-week studies, remission rates for ≥ 6 consecutive months were higher for aripiprazole- (32%) than for haloperidol-treated patients (22%), with an NNT of 10.20
Interestingly, separation of the cariprazine and placebo curves for time to loss of remission in our post hoc analysis was not evident until approximately 7 weeks after the start of double-blind treatment. In contrast, separation of the Kaplan-Meier curves estimating time to loss of remission for quetiapine and placebo in the previously summarized post hoc analysis occurred approximately 2-3 weeks after randomization.18 Interestingly, a similar delay until separation of the curves for cariprazine versus placebo was also apparent in the cariprazine relapse prevention study; although different criteria were used to estimate relapse than sustained remission, separation of the cariprazine and placebo curves for cumulative rate of relapse also occurred at approximately 6-7 weeks.15 Of note, other relapse prevention studies of similar design conducted using atypical antipsychotics have estimated comparatively shorter times to separation of the treatment- and placebo-group curves for incidence of relapse than what was observed for cariprazine and placebo, with separation occurring at approximately 1 week for asenapine,21 2-3 weeks for lurasidone22 and olanzapine,23 and 4 weeks for brexpiprazole.24 The longer time to separation of the curves for cariprazine and placebo may be related to the relatively longer half-life of cariprazine compared with other antipsychotics and may suggest some persistent drug effect for patients switched to placebo at randomization. A delayed antipsychotic-placebo separation in placebo-controlled discontinuation trials has also been clearly described comparing the placebo substitution arms after stabilization with oral paliperidone, once-monthly paliperidone, or once-every-3-months paliperidone.25 This effect of a longer maintenance of efficacy even after drug discontinuation, indicated by a delayed drug-placebo separation, could be relevant for preventing relapse and sustaining remission in cases of partial nonadherence, but additional cariprazine investigations are needed to better understand the corollaries related to its combined 1-week half-life.
However, other pharmacologic factors may also affect reported relapse rates. For example, differences in receptor binding affinity may affect symptom stability during periods of dose adjustment and medication switching. Namely, symptom destabilization can occur when agents that have strong antihistaminergic or anticholinergic effects (eg, quetiapine, olanzapine, asenapine) are changed too quickly to agents with lower affinity to these receptors or to placebo.26 This abrupt change in receptor binding can result in rebound effects and symptoms (eg, anxiety, agitation) that could be interpreted as relapse. As such, patients undergoing abrupt discontinuation of a histaminergic or cholinergic antipsychotic, whether in the context of a switch to placebo in a clinical trial or for purposes of clinical management, should be monitored for rebound symptoms, and care should be taken so as not to confound relapse and rebound phenomena.
These analyses are subject to several limitations, including their post hoc nature and lack of an active comparator. As is common in post hoc analyses, P values were not controlled for multiple comparisons; additionally, the study was not powered to examine differences between cariprazine doses. The long open-label period in the cariprazine relapse-prevention study required patients to become stable and maintain symptom stability on cariprazine; as such, patients for whom cariprazine was not effective were discontinued from the study before randomization. The requirement to meet strict stability criteria during the open-label stabilization phase resulted in a low number of completers during this phase, which may have led to a bias in the double-blind phase in favor of patients who improved on and tolerated cariprazine. These findings may not be generalizable to the overall patient population due to strict inclusion/exclusion criteria. Given these limitations, assessing sustained remission rates in real-world patients with significant comorbidities would be warranted to determine the naturalistic value of cariprazine for sustaining long-term remission in an effectiveness trial.
Despite these limitations, post hoc analyses of data from a long-term relapse prevention trial demonstrated that cariprazine was effective in maintaining stable, long-term remission in patients with schizophrenia who had previously responded to cariprazine in open-label treatment. Patients treated with cariprazine compared with placebo had significantly longer time to loss of remission and significantly higher rates of sustained remission for 6 consecutive months starting at double-blind baseline, at each visit for 6 consecutive months prior to a patient’s last visit in remission during the double-blind period, and for 6 consecutive months preceding the patient’s final study visit. These findings suggest that cariprazine may be an effective long-term treatment option for patients with schizophrenia, given that sustained remission is a necessary component of patient wellness and eventual functional recovery.
Submitted: July 31, 2018; accepted November 5, 2018.
Published online: January 8, 2019.
Author contributions: Drs Correll, Potkin, Harsányi, Szatmári, and Earley were involved with the study design, analysis, and interpretation of data. Dr Zhong performed statistical analyses. All authors met ICMJE (International Committee of Medical Journal Editors) authorship criteria and contributed to and approved the final manuscript; they are accountable for all aspects of the work and the ability to identify the contributions of each coauthor and ensure the integrity of their contributions. Neither honoraria nor payments were made for authorship.
Potential conflicts of interest: Dr Correll has been a consultant and/or advisor to or has received honoraria from Alkermes, Allergan, Angelini, Gerson Lehrman Group, IntraCellular Therapies, Janssen/J&J, LB Pharma, Lundbeck, Medavante, Medscape, Merck, Neurocrine, Otsuka, Pfizer, ROVI, Servier, Sunovion, Takeda, and Teva; provided expert testimony for Bristol-Myers Squibb, Janssen, and Otsuka; served on data safety monitoring boards for Lundbeck, ROVI, and Teva; received royalties from UpToDate and grant support from Janssen and Takeda; and is a shareholder of LB Pharma. Dr Potkin has received grant support, funding, or honoraria from or served as a consultant for the following companies that conducted scientific or medical research and/or marketed medications related to psychiatric and neurodegenerative disorders: Alkermes, Allergan, Baylor University, Eisai, Eli Lilly, Forest, FORUM Pharmaceuticals, Lundbeck, Merck, National Institutes of Health, Novartis, Otsuka, Roche/Genentech, Sunovion, Takeda, Teva, Toyama, University of Southern California, University of California San Diego, University of California San Francisco, Vanda, and the Gerson Lehrman Group. Drs Zhong and Earley are employees of Allergan. Drs Harsányi and Szatmári are employees of Gedeon Richter.
Funding/support: This analysis and manuscript were sponsored by Allergan Plc and Gedeon Richter Plc.
Role of the sponsor: Forest Research Institute, Inc, an Allergan affiliate, and Gedeon Richter Plc participated in the study design, study conduct, and interpretation of the data, and they reviewed and approved the manuscript for publication.
Previous presentation: Poster presented at the International Congress on Schizophrenia Research, March 24-28, 2017, San Diego, California; the Annual Meeting of the American Society of Clinical Psychopharmacology, May 29-June 3, 2017, Miami Beach, Florida; the European College of Neuropsychopharmacology Annual Congress, September 2-5, 2017, Paris, France; and the Psych Congress, September 16-19, 2017, New Orleans, Louisiana.
Acknowledgments: Writing and editorial assistance were provided by Jennifer Fetting, PhD, of Prescott Medical Communications Group (Chicago, Illinois), a contractor of Allergan.
REFERENCES
1. Kahn RS, Sommer IE, Murray RM, et al. Schizophrenia. Nat Rev Dis Primers. 2015;1:15067. PubMed CrossRef
2. Kane JM, Kishimoto T, Correll CU. Non-adherence to medication in patients with psychotic disorders: epidemiology, contributing factors and management strategies. World Psychiatry. 2013;12(3):216-226. PubMed CrossRef
3. Carbon M, Correll CU. Clinical predictors of therapeutic response to antipsychotics in schizophrenia. Dialogues Clin Neurosci. 2014;16(4):505-524. PubMed
4. Andreasen NC, Carpenter WT Jr, Kane JM, et al. Remission in schizophrenia: proposed criteria and rationale for consensus. Am J Psychiatry. 2005;162(3):441-449. PubMed CrossRef
5. Lambert M, Karow A, Leucht S, et al. Remission in schizophrenia: validity, frequency, predictors, and patients’ perspective 5 years later. Dialogues Clin Neurosci. 2010;12(3):393-407. PubMed
6. Wunderink L, Nienhuis FJ, Sytema S, et al. Predictive validity of proposed remission criteria in first-episode schizophrenic patients responding to antipsychotics. Schizophr Bull. 2007;33(3):792-796. PubMed CrossRef
7. Kay SR, Fiszbein A, Opler LA. The Positive and Negative Syndrome Scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13(2):261-276. PubMed CrossRef
8. American Psychiatric Association. Diagnostic and Statistical Manual for Mental Disorders. Fourth Edition, Text Revision. Washington, DC: American Psychiatric Association; 2000.
9. Nakamura T, Kubota T, Iwakaji A, et al. Clinical pharmacology study of cariprazine (MP-214) in patients with schizophrenia (12-week treatment). Drug Des Devel Ther. 2016;10:327-338. PubMed CrossRef
10. Frankel JS, Schwartz TL. Brexpiprazole and cariprazine: distinguishing two new atypical antipsychotics from the original dopamine stabilizer aripiprazole. Ther Adv Psychopharmacol. 2017;7(1):29-41. PubMed CrossRef
11. Mauri MC, Paletta S, Maffini M, et al. Clinical pharmacology of atypical antipsychotics: an update. EXCLI J. 2014;13:1163-1191. PubMed
12. Durgam S, Cutler AJ, Lu K, et al. Cariprazine in acute exacerbation of schizophrenia: a fixed-dose, phase 3, randomized, double-blind, placebo- and active-controlled trial. J Clin Psychiatry. 2015;76(12):e1574-e1582. PubMed CrossRef
13. Durgam S, Starace A, Li D, et al. An evaluation of the safety and efficacy of cariprazine in patients with acute exacerbation of schizophrenia: a phase II, randomized clinical trial. Schizophr Res. 2014;152(2-3):450-457. PubMed CrossRef
14. Kane JM, Zukin S, Wang Y, et al. Efficacy and safety of cariprazine in acute exacerbation of schizophrenia: results from an international, phase III clinical trial. J Clin Psychopharmacol. 2015;35(4):367-373. PubMed
15. Durgam S, Earley W, Li R, et al. Long-term cariprazine treatment for the prevention of relapse in patients with schizophrenia: a randomized, double-blind, placebo-controlled trial. Schizophr Res. 2016;176(2-3):264-271. PubMed CrossRef
16. Guy W. The Clinical Global Severity and Impression scales. ECDEU Assessment Manual for Psychopharmacology. Rockville, MD: National Institute of Mental Health; 1976:218-222.
17. Leucht S, Heres S. Epidemiology, clinical consequences, and psychosocial treatment of nonadherence in schizophrenia. J Clin Psychiatry. 2006;67(suppl 5):3-8. PubMed
18. Peuskens J, Trivedi JK, Brecher M, et al; Study 4 Investigators. Long-term symptomatic remission of schizophrenia with once-daily extended release quetiapine fumarate: post-hoc analysis of data from a randomized withdrawal, placebo-controlled study. Int Clin Psychopharmacol. 2010;25(3):183-187. PubMed CrossRef
19. Smeraldi E, Cavallaro R, Folnegović-Šmalc V, et al. Long-term remission in schizophrenia and schizoaffective disorder: results from the risperidone long-acting injectable versus quetiapine relapse prevention trial (ConstaTRE). Ther Adv Psychopharmacol. 2013;3(4):191-199. PubMed CrossRef
20. Kane JM, Crandall DT, Marcus RN, et al. Symptomatic remission in schizophrenia patients treated with aripiprazole or haloperidol for up to 52 weeks. Schizophr Res. 2007;95(1-3):143-150. PubMed CrossRef
21. Kane JM, Mackle M, Snow-Adami L, et al. A randomized placebo-controlled trial of asenapine for the prevention of relapse of schizophrenia after long-term treatment. J Clin Psychiatry. 2011;72(3):349-355. PubMed CrossRef
22. Tandon R, Cucchiaro J, Phillips D, et al. A double-blind, placebo-controlled, randomized withdrawal study of lurasidone for the maintenance of efficacy in patients with schizophrenia. J Psychopharmacol. 2016;30(1):69-77. PubMed CrossRef
23. Beasley CM Jr, Sutton VK, Hamilton SH, et al; Olanzapine Relapse Prevention Study Group. A double-blind, randomized, placebo-controlled trial of olanzapine in the prevention of psychotic relapse. J Clin Psychopharmacol. 2003;23(6):582-594. PubMed CrossRef
24. Fleischhacker WW, Hobart M, Ouyang J, et al. Efficacy and safety of brexpiprazole (OPC-34712) as maintenance treatment in adults with schizophrenia: a randomized, double-blind, placebo-controlled study. Int J Neuropsychopharmacol. 2017;20(1):11-21. PubMed CrossRef
25. Weiden PJ, Kim E, Bermak J, et al. Does half-life matter after antipsychotic discontinuation? a relapse comparison in schizophrenia with 3 different formulations of paliperidone. J Clin Psychiatry. 2017;78(7):e813-e820. PubMed CrossRef
26. Correll CU. From receptor pharmacology to improved outcomes: individualising the selection, dosing, and switching of antipsychotics. Eur Psychiatry. 2010;25(suppl 2):S12-S21. PubMed CrossRef
This PDF is free for all visitors!
Save
Cite