ABSTRACT
Objective: To describe lumateperone for the treatment of schizophrenia in adults using number needed to treat (NNT), number needed to harm (NNH), and likelihood to be helped or harmed (LHH).
Methods: Data were obtained from the 3 phase 2/3 lumateperone trials, conducted between 2011 and 2016, in patients with schizophrenia diagnosed using the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, Text Revision, or Fifth Edition. Efficacy was assessed using various response criteria; tolerability was principally assessed using rates of adverse events (AEs).
Results: Pooled data of the 2 informative studies showed statistically significant estimates of NNT versus placebo for lumateperone 42 mg/d for the responder thresholds of ≥ 20% and ≥ 30% improvement on Positive and Negative Syndrome Scale (PANSS) total scores, with NNT for response versus placebo at 4 weeks and endpoint of 9 (95% confidence interval [CI], 5–36) and 8 (95% CI, 5–21), respectively. Pooling all studies, discontinuation because of AEs was uncommon, and the NNH versus placebo was 389 (not statistically significant from placebo [NS]). Rates of individual AEs resulted in NNH versus placebo > 10 except for somnolence/sedation (NNH of 8; 95% CI, 6–12). The occurrence of weight gain ≥ 7% from baseline yielded a NNH estimate of 122 (NS). Rates of akathisia were lower for patients receiving lumateperone compared with placebo. LHH for response versus somnolence/sedation was ~ 1 for lumateperone (similar to the risperidone active control group); otherwise, lumateperone exhibited LHH ratios that were much greater than 1 for all other AEs and that ranged from 13.6 to 48.6 for these other benefit-risk calculations.
Conclusions: In 3 phase 2/3 trials, the benefit-risk assessment of lumateperone was favorable as measured by NNT, NNH, and LHH.
Trial Registration: ClinicalTrials.gov identifiers: NCT01499563, NCT02282761, NCT02469155
J Clin Psychiatry 2023;84(2):22r14631
To cite: Citrome L, Durgam S, Edwards JB, et al. Lumateperone for the treatment of schizophrenia: number needed to treat, number needed to harm, and likelihood to be helped or harmed. J Clin Psychiatry. 2023;84(2):22r14631.
To share: https://doi.org/10.4088/JCP.22r14631
© 2023 Physicians Postgraduate Press, Inc.
aNew York Medical College, Valhalla, New York
bIntra-Cellular Therapies, Inc., New York, New York
*Corresponding author: Leslie Citrome, MD, MPH, 11 Medical Park Drive, Ste 102, Pomona, NY 10970 ([email protected]).
Lumateperone was approved by the United States Food and Drug Administration (FDA) in 2019 for the treatment of adult patients with schizophrenia and in 2021 for the treatment of adults with depressive episodes associated with bipolar I or bipolar II disorder.1 The mechanism of action of lumateperone differs from that of other currently available antipsychotics and involves selective and simultaneous modulation of serotonin, dopamine, and glutamate,2,3 with relatively low dorsal striatal dopamine D2 receptor occupancy (39% at 60 mg occurring 1 hour post-dose).4 Of note, receptor-binding affinity of lumateperone to serotonin 5-HT2A receptors is approximately 60-fold higher than that for dopamine D2 post-synaptic receptors (Ki 0.54 nM vs 32 nM, respectively).1,5
The clinical trial development program of lumateperone for the treatment of schizophrenia included 3 phase 2/3 short-term, acute, placebo-controlled studies—Study 005 (ITI-007-005),6 Study 301 (ITI-007-301),7 and Study 302 (ITI-007-302)8,9—as well as an open-label safety switch study, Study 303 (ITI-007-303).10 In Study 005 and Study 301, both 4 weeks in duration, lumateperone (60 mg ITI-007, equivalent to 42-mg active base) met the primary endpoint with statistically significant superior efficacy over placebo at day 28, as measured by the Positive and Negative Syndrome Scale (PANSS) total score.6,7,11 In Study 302, a 6-week study, neither dose of lumateperone separated from placebo on the primary endpoint in the intent-to-treat population; the study was considered uninformative (failed) due to a high placebo response and a higher discontinuation rate in the risperidone arm.8,9 Across all 3 efficacy trials, lumateperone 42 mg/d improved symptoms of schizophrenia with the same trajectory and same magnitude of improvement from baseline on the PANSS total score. Lumateperone 42 mg/d is the recommended FDA-approved dose for the treatment of patients with schizophrenia.1,8
Lumateperone was well tolerated with a favorable safety profile in all 3 studies.9 In the 2 studies that included risperidone 4 mg/d as an active control for assay sensitivity, lumateperone was statistically significantly better than risperidone on key safety and tolerability outcomes, including on measures of increased prolactin, glucose, lipids, and weight.9 In the open-label safety switching study (Study 303), statistically significant improvements from standard antipsychotic medications were observed in body weight, while cardiometabolic and endocrine parameters worsened again following a switch back to standard of care medication.10
The current analysis evaluates the efficacy and safety of lumateperone using the metrics of evidence-based medicine, namely number needed to treat (NNT) and number needed to harm (NNH), to help practitioners place this intervention into clinical perspective.12–16 NNT and NNH are measures of effect size and indicate how many patients would need to be treated with one agent instead of the comparator to encounter 1 additional outcome of interest, such as response (NNT) or the occurrence of an adverse event (AE) (NNH). NNT and NNH can be further contrasted using the ratio of NNH to NNT, referred to as the likelihood to be helped or harmed (LHH). Similar work has been published for other oral antipsychotics for schizophrenia, including cariprazine,17 brexpiprazole,18 lurasidone,19 iloperidone,20 and asenapine.21
METHODS
Data were extracted from the clinical trial database for 3 double-blind placebo-controlled clinical trials of lumateperone in patients with schizophrenia who experienced an acute exacerbation of psychosis (all studies registered at ClinicalTrials.gov): Study 005 (NCT01499563; phase 2; 4 weeks’ duration; conducted December 2011–November 2013), Study 301 (NCT02282761; phase 3; 4 weeks’ duration; conducted November 2014–July 2015), and Study 302 (NCT02469155; phase 3; 6 weeks’ duration; conducted June 2015–August 2016). Patients were diagnosed according to the Diagnostic Statistical Manual of Mental Disorders, Fourth Edition, Text Revision (DSM-IV-TR),22 or Fifth Edition (DSM-5)23 criteria and confirmed by the Structured Clinical Interview for DSM Disorders–Clinical Trial Version (SCID)24 or modified SCID.25 The study protocols were reviewed and approved by the relevant Institutional Review Board or Independent Ethics Committee at each study site, and informed consent was obtained from all patients.
For the primary efficacy analysis, data were extracted from the informative studies (Study 005, Study 301); data from the failed Study 302 were included in a sensitivity analysis, as has been done previously for a similar evaluation of lurasidone.19 The denominator was the number of patients who had at least 1 post-baseline PANSS measure. Patients who did not complete the 28-day randomized phase of the study were considered non-responders at time points following their discontinuation. Data were collected for those who were classified as responders at day 28 or had early termination. Percentage reduction from baseline PANSS score was calculated as [(endpoint total score – baseline total score)/(baseline total score – 30)].26,27 Data were extracted by study arm, including the number of patients in each treatment arm for the outcome of a ≥ 20%, ≥ 30%, ≥ 40%, and ≥ 50% reduction from baseline on the PANSS total score at week 1, week 2, week 3, week 4, and endpoint (week 4 or early termination).
Tolerability data were extracted for all 3 short-term acute studies (005, 301, 302). Specific tolerability and safety outcomes of clinical interest that occurred during the double-blind period were assessed. For Study 302, data were extracted for 2 time periods: start of study to end of week 4 and start of study to end of week 6. The denominator was the number of all randomized patients who had received at least 1 dose of study drug. Data were extracted for the number of patients in each treatment arm for the outcome of early discontinuation from the study due to an AE; the number of patients in each treatment arm with spontaneously reported AEs of somnolence/sedation (combined term), nausea, dry mouth, and dizziness/dizziness postural (selected because the pooled incidence rate for lumateperone 42 mg/d is ≥ 5% as noted in the product label1); the number of patients in each treatment arm with shifts at any time of total cholesterol from < 240 mg/dL to ≥ 240 mg/dL, low-density lipoprotein (LDL) cholesterol from < 160 mg/dL to ≥ 160 mg/dL, fasting glucose from < 126 mg/dL to ≥ 126 mg/dL, fasting triglycerides from < 200 mg/dL to ≥ 200 mg/dL, and electrocardiogram heart rate-corrected QT (ECG QTc) interval from < 450 msec to ≥ 450 msec; and the number of patients in each treatment arm at the last observation carried forward (LOCF) endpoint with weight gain of ≥ 7% from baseline, total cholesterol ≥ 240 mg/dL, LDL cholesterol ≥ 160 mg/dL, fasting glucose ≥ 126 mg/dL, fasting triglycerides ≥ 200 mg/dL, plasma prolactin ≥ 17 ng/mL (men) and ≥ 25 ng/mL (women), and plasma prolactin ≥ 34 ng/mL (men) and ≥ 50 ng/mL (women).
Additional tolerability data regarding sedation (single term), somnolence (single term), and motoric AEs were obtained from the FDA Drug Approval Package8 and the Integrated Summary of Safety, a component of the drug approval regulatory submission (data on file, Intra-Cellular Therapies, Inc.).
NNT and NNH with their respective 95% CIs were calculated for lumateperone versus placebo; first individually for each study and then pooled for the 42 mg/d lumateperone dose. For the active control (risperidone 4 mg/d), analogous analyses compared the active control versus placebo.
LHH was calculated for lumateperone 42 mg/d to illustrate potential trade-offs for efficacy and tolerability outcomes, specifically response versus the most common AEs and for discontinuation because of an AE. Analogous analyses were performed for the active control.
In all instances, if the 95% CI included “infinity,” then the result was considered not statistically significant at the P < .05 threshold. The terms statistically significant and non–statistically significant are used descriptively and not inferentially. The notation NS is used rather than showing the non-continuous 95% CIs generated when statistical significance was not achieved.
Indirect comparisons with other antipsychotics are possible because similar analyses have been done with most other second-generation antipsychotics, including all current branded oral products.17–21 Many of these analyses include the same efficacy and tolerability outcomes.
A brief tutorial and formulae used for calculating NNT, NNH, and LHH can be found in Box 1 and Box 2 in the Supplementary Material in an article by Citrome et al.28
RESULTS
Efficacy
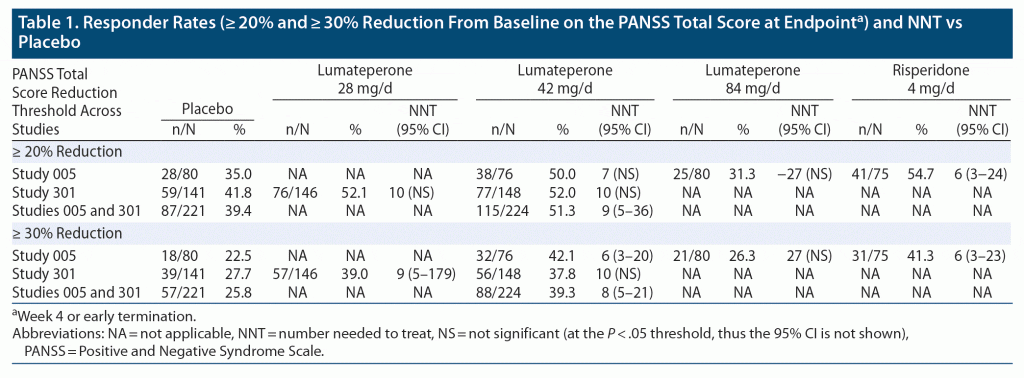
Study 005. Supplementary Table 1a provides the rates of response as defined by different thresholds of PANSS total score improvement from baseline and the respective estimates for NNT versus placebo for each dose of lumateperone assessed in Study 005 (42 mg/d and 84 mg/d) and for the active control (risperidone 4 mg/d). Statistically significant estimates of NNT versus placebo were observed for lumateperone 42 mg/d and risperidone, but none of the outcomes were statistically significant for the comparison of lumateperone 84 mg/d versus placebo. Efficacy at week 4 or endpoint was similar for lumateperone 42 mg/d and risperidone versus placebo for the outcome of PANSS improvement ≥ 20% (7 [NS] and 6 [95% CI, 3–24], respectively) and for the outcome of PANSS improvement ≥ 30% (6 [95% CI, 3–20] and 6 [95% CI, 3–23], respectively), with the NNT values becoming smaller (more robust) as time progressed in the clinical trial. For the PANSS responder thresholds of ≥ 40% and ≥ 50% improvement from baseline, the NNT estimates generally demonstrated small and/or non-significant effect sizes for any of the comparisons.
Study 301. Supplementary Table 1b provides the data for efficacy of lumateperone 28 mg/d and 42 mg/d as assessed in Study 301. Efficacy at week 4 or endpoint was similar for lumateperone 28 mg/d and lumateperone 42 mg/d versus placebo for the outcome of PANSS improvement ≥ 20% or ≥ 30%, with statistical significance achieved for the lumateperone 28 mg/d group versus placebo at the latter threshold (NNT 9 [95% CI, 5–179]). In general, NNT values became smaller (more robust) as time progressed in the clinical trial, although this was not consistent. For the PANSS responder thresholds of ≥ 40% and ≥ 50% improvement from baseline, the NNT estimates were statistically significant at week 4 or endpoint for lumateperone 42 mg/d versus placebo, with NNT values of 10 (95% CI, 6–147) and 10 (95% CI, 6–39) for ≥ 40% and ≥ 50% improvement from baseline, respectively.
Pooled Studies 005 and 301: placebo and lumateperone 42 mg/d. Statistically significant estimates of NNT versus placebo were observed for lumateperone 42 mg/d for most timepoints at all the responder thresholds tested (Supplementary Table 1c). In general, the NNT values became smaller (more robust) as time progressed in the clinical trials. As summarized in Table 1, the NNT for response versus placebo for lumateperone 42 mg/d at endpoint was 9 (95% CI, 5–36) and 8 (95% CI, 5–21) for the responder thresholds of ≥ 20% and ≥ 30% improvement on the PANSS total score, respectively. Supplementary Table 1d shows a sensitivity analysis that pooled together data from week 4 of studies 005 and 301 as well as Study 302 (the failed study in which there was a high placebo response). The NNT estimates for lumateperone 42 mg/d versus placebo remained statistically significant, but were less robust, with 95% CIs that overlapped with those pooled from the studies that were informative regarding efficacy (Studies 005 and 301). In pooled analyses from studies 005 and 302, the analogous NNT estimates for risperidone 4 mg/d versus placebo also demonstrated less robust NNT estimates than from the informative Study 005 alone, and with overlapping 95% CIs. Moreover, the 95% CIs for the risperidone NNT estimates overlapped those for lumateperone 42 mg/d versus placebo for both thresholds.
Tolerability
Study 005. For each lumateperone dose (42 mg/d and 84 mg/d) and the active control (risperidone 4 mg/d) assessed in Study 005, Supplementary Table 2a shows the rates and NNH estimates versus placebo for discontinuation because of an AE, the most common AEs as reported in lumateperone product labeling, shifts in glucose or lipid values, proportions of patients with laboratory tests, ECG QT intervals, or weight gain denoting potentially clinically relevant abnormalities, among other tolerability measures. AEs were more frequent with lumateperone 84 mg/d than with the 42 mg/d dose; thus, the NNH values versus placebo were higher (ie, less problematic) for the 42 mg/d dose. For lumateperone 42 mg/d, the only statistically significant NNH estimate versus placebo was for the AE of nausea (NNH = 17; 95% CI, 9–120,576). Although the NNH for somnolence/sedation was 6 (95% CI, 4–14) for lumateperone 84 mg/d, it was 27 (NS) for lumateperone 42 mg/d, with the latter comparable to what was observed for risperidone (NNH vs placebo = 16; NS) on this outcome. The NNH value for shift of fasting triglycerides from < 200 mg/dL to ≥ 200 mg/dL was a single digit, but a negative value, denoting that the rate of these shifts was higher for placebo than for lumateperone.
NNH versus placebo for risperidone 4 mg/d for the outcome of discontinuation because of an AE was more robust (41; NS) than for lumateperone (83 and −85 for 42 mg/d and 84 mg/d, respectively; NS). Of note, the NNH versus placebo for weight gain ≥ 7% from baseline was 9 (95% CI, 5–49) for risperidone and 17 (NS) for lumateperone 42 mg/d. NNH versus placebo for elevation of prolactin values for risperidone was 2 (95% CI, 2–3) for men and 2 (95% CI, 2–6) for women, in contrast to NNH values of 36 (NS) and –12 (NS) for lumateperone 42 mg/d for men and women, respectively.
Study 301. Tolerability measures for each dose of lumateperone assessed in Study 301 (28 mg/d and 42 mg/d) are shown in Supplementary Table 2b. The only statistically significant NNH estimate was for the AE of somnolence/sedation for which the NNH versus placebo was 9 (95% CI, 6–31) and 5 (95% CI, 4–9) for the lumateperone 28 mg/d and 42 mg/d dose groups, respectively.
Study 302. Tolerability measures for each dose of lumateperone assessed in Study 302 (14 mg/d and 42 mg/d) and the active control (risperidone 4 mg/d) are shown in Supplementary Table 2c (through week 4) and Supplementary Table 2d (through week 6). Safety and tolerability data were similar through week 4 and through week 6. Examining the entire 6 weeks of the study, fewer patients receiving lumateperone 42 mg/d discontinued the study because of an AE compared with patients in the placebo group (NNH = −178). In contrast, the NNH versus placebo on this outcome for the risperidone group was 20 (95% CI, 12–64). All groups experienced AEs of somnolence/sedation at rates higher than placebo, with resultant NNH values versus placebo of 12 (95% CI, 7–72), 8 (95% CI, 5–18), and 7 (95% CI, 5–12), for lumateperone 14 mg/d, lumateperone 42 mg/d, and risperidone 4 mg/d, respectively. Although the AE of dry mouth also yielded a statistically significant NNH estimate versus placebo for lumateperone 42 mg/d (NNH = 20; 95% CI, 12–53), none of the other outcomes did so, other than the negative NNH value observed for weight gain (weight gain ≥ 7% from baseline occurred at a higher rate in the placebo group). For risperidone, the AE of dry mouth yielded a NNH estimate versus placebo of 25 (95% CI, 15–91), a NNH versus placebo for weight gain of 13 (95% CI, 7–174), and a NNH of 2 for elevation in prolactin levels (95% CI, 2–2 for both men and women).
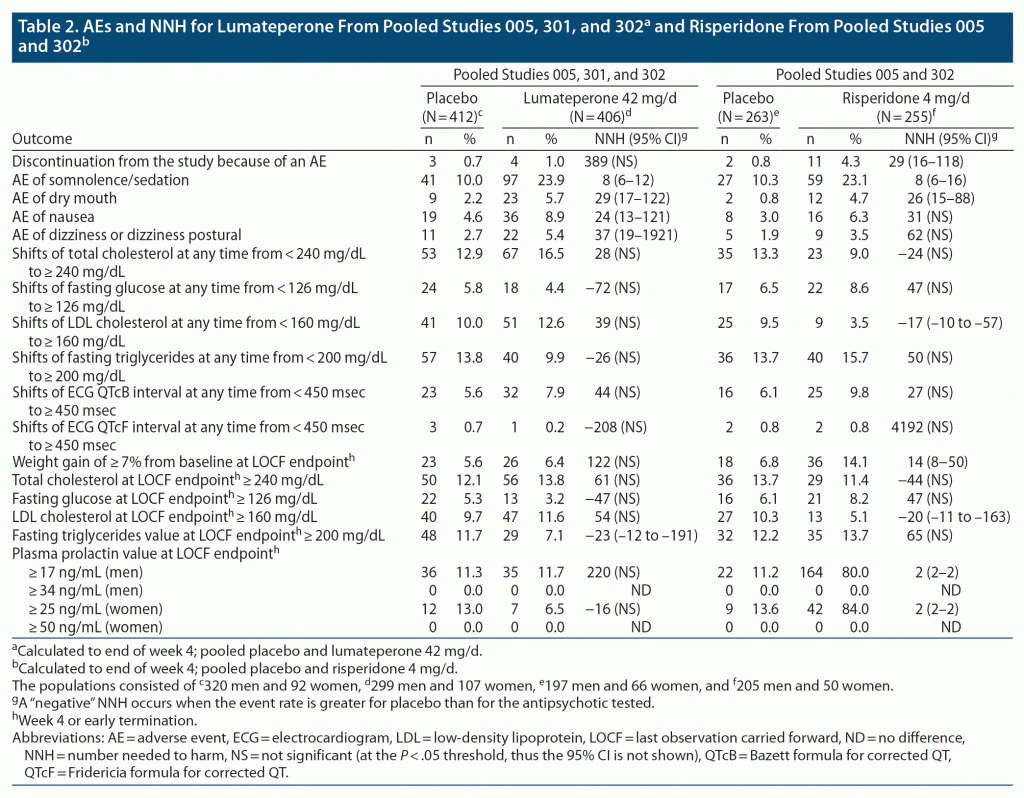
Pooled Studies 005, 301, 302 (to end of week 4): pooled placebo and lumateperone 42 mg/d. Tolerability measures for lumateperone 42 mg/d after up to 4 weeks of exposure for studies 005, 301, and 302 are shown in Table 2. Discontinuation because of an AE was uncommon, and the NNH versus placebo was 389 (NS). Somnolence/sedation was the only AE that resulted in a NNH value < 10 (NNH vs placebo = 8 [95% CI, 6–12]). Although the NNH estimates for the AEs of dry mouth, nausea, and dizziness/dizziness postural were statistically significant, the effect size was much smaller than for somnolence/sedation. No other tolerability outcomes demonstrated a statistically significant NNH estimate other than a negative NNH value for elevation of fasting triglycerides at endpoint (this event was more commonly observed in the placebo group). The occurrence of weight gain ≥ 7% from baseline yielded a NNH estimate of 122 (NS). There was no consistent signal denoting a deleterious effect of lumateperone 42 mg/d on metabolic outcomes, the ECG QT interval, or plasma prolactin levels.
Pooled Studies 005, 302 (to end of week 4): pooled placebo and risperidone 4 mg/d. Tolerability measures for risperidone 4 mg/d after up to 4 weeks of exposure for studies 005 and 302 are shown in Table 2. Discontinuation because of an AE was greater for the risperidone group than for placebo, yielding a NNH versus placebo of 29 (95% CI, 16–118). The NNH versus placebo for the AE of somnolence/sedation was 8 (95% CI, 6–16) which was similar to that observed for the pooled lumateperone 42 mg/d data (Table 2).
The NNH estimate for the AE of dry mouth with risperidone versus placebo was also statistically significant but with a less robust effect size than for somnolence/sedation and similar to that observed for the pooled lumateperone 42 mg/d data (Table 2). Although the NNH estimate versus placebo for weight gain ≥ 7% from baseline for risperidone was statistically significant at 14 (95% CI, 8–50), the remainder of the metabolic outcomes did not demonstrate a consistent signal denoting a deleterious effect.
Prolactin elevation was a common event with risperidone 4 mg/d, with ≥ 80% of men and women meeting or exceeding the pre-specified thresholds of 17 ng/mL and 25 ng/mL, respectively, yielding NNH estimates versus placebo of 2 (95% CI, 2–2) for both sexes. Prolactin elevations ≥ 34 ng/mL (men) or ≥ 50 ng/mL (women) were not observed in any patients in any of the trials.
Motoric adverse events. According to data extracted from the lumateperone Drug Approval Package,8 an AE of akathisia was observed in 8 (2.0%) of 406 patients randomized to lumateperone 42 mg/d versus 12 (2.9%) of 412 among those randomized to placebo across the 3 studies,9 yielding a NNH of –107 (NS). Rates of akathisia for the other doses of lumateperone—14 mg/d (1/172; 0.6%), 28 mg/d (2/150; 1.3%), and 84 mg/d (2/83; 2.4%)—were similar but were somewhat higher for risperidone 4 mg/d at 12 (4.7%) of 255 and for the latter yielding a NNH versus placebo of 56 (NS). The frequency of reported events related to extrapyramidal symptoms, including akathisia, extrapyramidal disorder, muscle spasms, restlessness, musculoskeletal stiffness, dyskinesia, dystonia, muscle twitching, tardive dyskinesia, tremor, drooling, and involuntary muscle contractions, was 6.7% for lumateperone 42 mg/d, 10.6% for risperidone 4 mg/d, and 6.3% for placebo (lumateperone product label1 and data on file, Intra-Cellular Therapies, Inc.), resulting in NNH values versus placebo of 295 (NS) for lumateperone 42 mg/d and 24 (NS) for risperidone 4 mg/d. There were no clinically meaningful changes from baseline on the Barnes Akathisia Rating Scale, Simpson Angus Scale, or Abnormal Involuntary Movement Scale in any treatment arm (placebo, lumateperone, or risperidone) in the 3 placebo-controlled studies.9
Indirect Comparisons
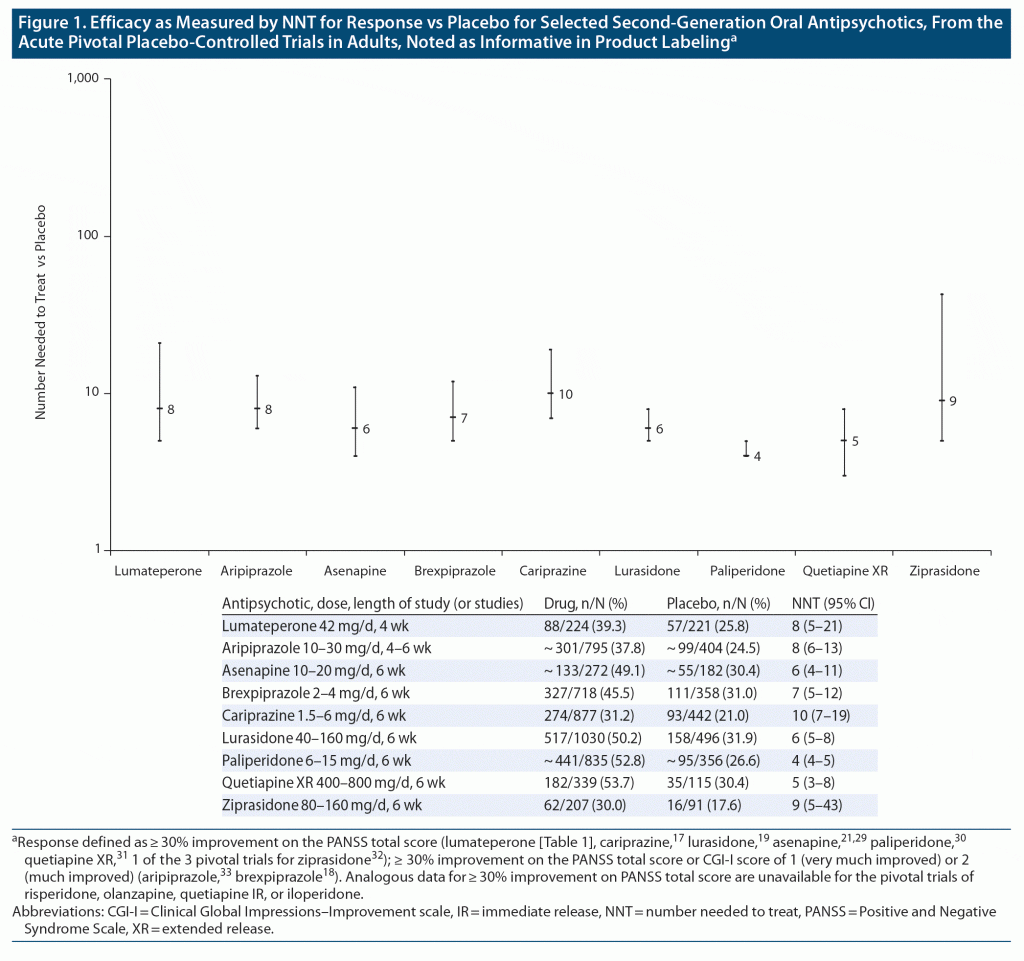
Efficacy. Figure 1 describes the NNT for response versus placebo for selected oral, first-line, second-generation antipsychotics, as calculated from acute pivotal placebo-controlled trials in adults that were noted as informative in product labeling (Table 1).17–19,21,29–33 The 95% CI for NNT for response versus placebo for lumateperone 42 mg/d overlaps with that found in analogous analyses of several oral, first-line, second-generation antipsychotics.
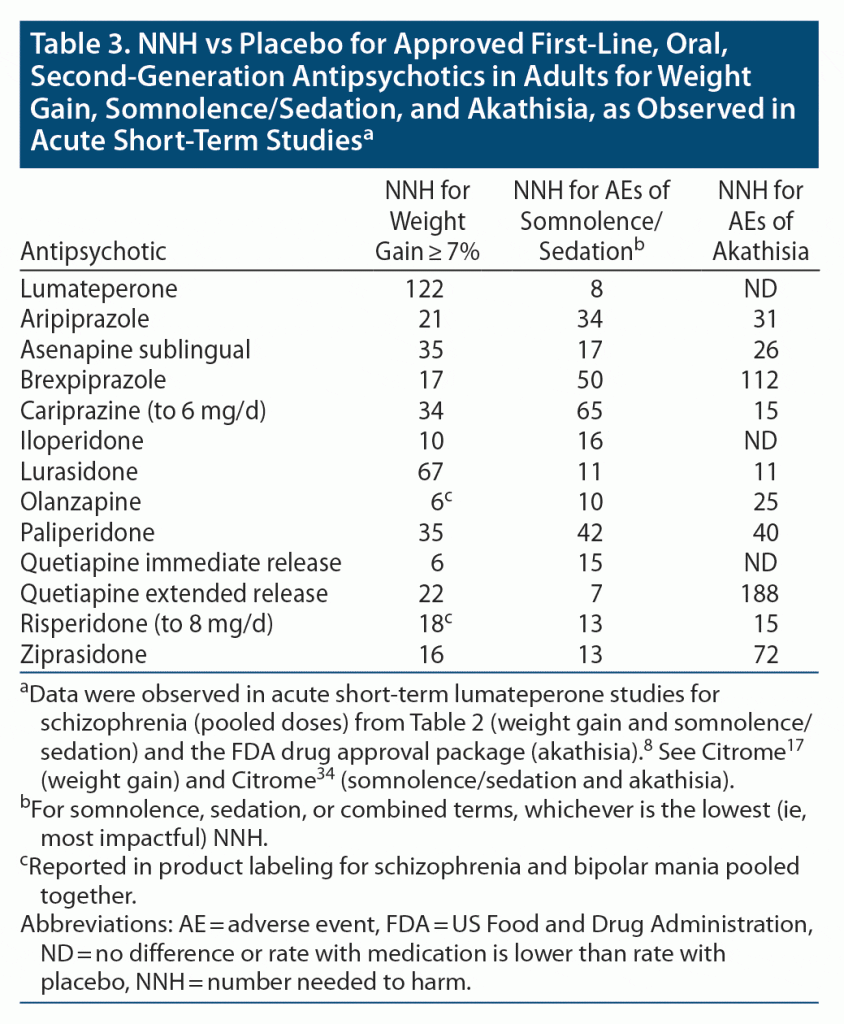
Tolerability: overview of NNH for weight gain, somnolence/sedation, and akathisia. The NNH versus placebo for approved first-line, oral, second-generation antipsychotics in adults for weight gain, somnolence/sedation, and akathisia is shown in Table 3.8,17,34 The values for risperidone differ from what was observed during the lumateperone clinical trial program, as the pivotal studies for risperidone were done in the past with differences in study designs, doses, and study populations. Heterogeneity of study designs and populations can also account for the differing estimates seen for quetiapine immediate release and quetiapine extended release. Nonetheless, these NNH values can be helpful in providing a broad overview of medication tolerability.
Lumateperone 42 mg/d appears best-in-class on the outcome of weight gain ≥ 7% from baseline with a NNH estimate versus placebo of 122, followed by lurasidone (NNH = 67).
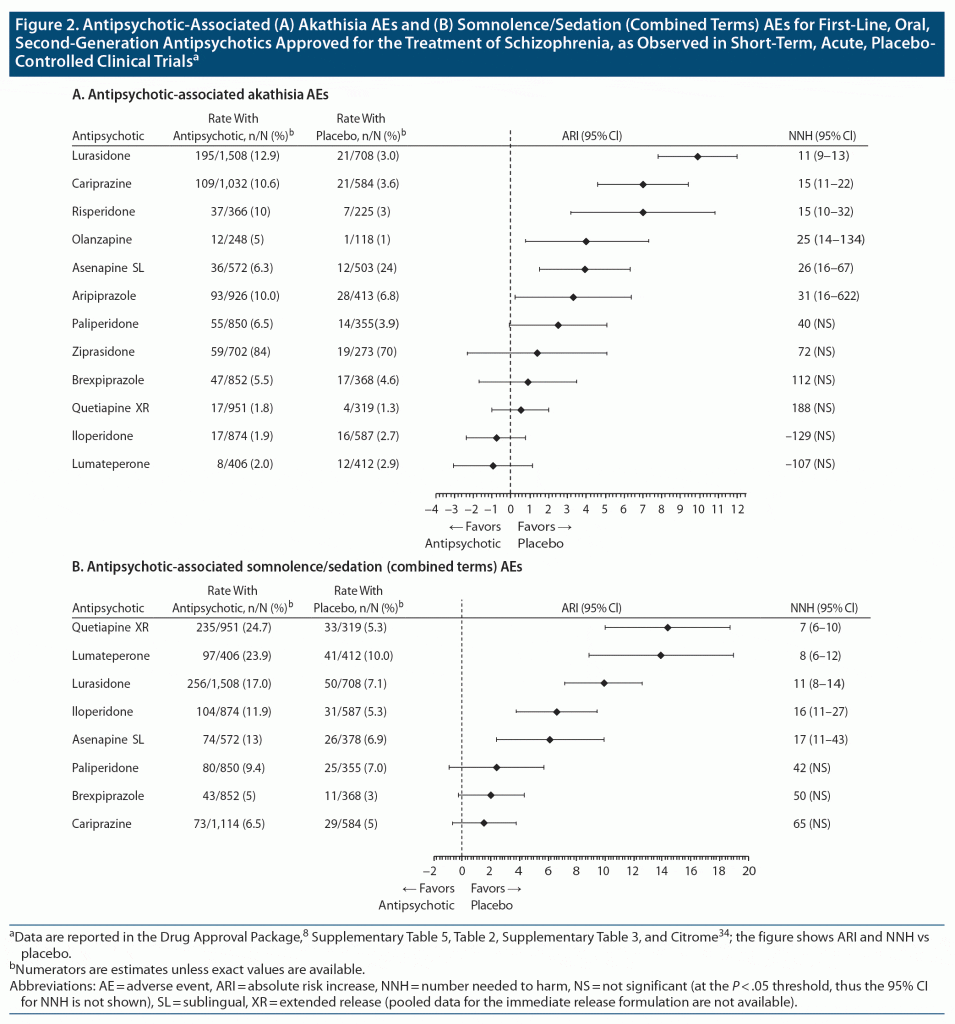
More patients receiving placebo experienced akathisia than patients receiving lumateperone 42 mg/d, thus the notation of no difference from placebo; this has also been observed for iloperidone and quetiapine immediate release. In addition, NNH estimates versus placebo are > 100 for brexpiprazole and quetiapine extended release, indicating a favorable profile regarding akathisia for those interventions as well. Figure 2A illustrates a forest plot of akathisia AEs versus placebo for the first-line, oral, second-generation antipsychotics approved for the treatment of schizophrenia based on available information.34
The NNH estimate versus placebo for somnolence/sedation for lumateperone 42 mg/d is 8, approximately the same as that observed for quetiapine extended release (Figure 2B). On this AE outcome, cariprazine has the most favorable NNH value versus placebo (NNH = 65), followed by brexpiprazole (NNH = 50).34
Supplementary Table 3 outlines antipsychotic-associated somnolence and/or sedation AEs, as observed in short-term, acute, placebo-controlled clinical trials, for lumateperone 42 mg/d and for first-line, oral, second-generation antipsychotics approved for the treatment of schizophrenia, using the metric of absolute risk increase versus placebo.34 Forest plots for these rates of somnolence/sedation (combined terms) are shown in Figure 2B, and forest plots for these rates of somnolence (single term) and sedation (single term) are provided in Supplementary Figures 1 and 2, respectively. There is significant overlap in the 95% CIs for lumateperone 42 mg/d with the aforementioned AEs for many of the other antipsychotics.
Discontinuation because of AEs. Overall tolerability/acceptability can be measured by NNH versus placebo for discontinuation because of an AE. Supplementary Table 4 summarizes this outcome for lumateperone (NNH = 389) and for other oral second-generation antipsychotics, from the acute pivotal placebo-controlled trials in adults, as noted in product labeling and Drug Approval Packages.8,35–46 The underlying discontinuation rates vary greatly, rendering interpretation difficult. All the listed antipsychotics appear reasonably tolerable, with the lowest NNH (least favorable) evidenced for risperidone with a NNH versus placebo of 41 (NS). Several of the antipsychotics (aripiprazole, asenapine, brexpiprazole, cariprazine, iloperidone, olanzapine, paliperidone, and quetiapine extended release) have negative values for NNH versus placebo on this outcome, as more patients randomized to placebo than to the antipsychotic medication discontinued because of an AE in the clinical trials of those compounds.
Likelihood to be helped or harmed (LHH). Supplementary Figure 3 illustrates benefits versus harms for lumateperone 42 mg/d benefit by plotting NNT and NNH versus placebo with 95% CIs, using pooled data from Tables 1 and 2. Except for the AE of somnolence/sedation, effect sizes are consistently larger for the efficacy outcomes than for the tolerability outcomes.
Supplementary Table 5 provides the LHH for lumateperone 42 mg/d by contrasting the NNTs versus placebo for therapeutic response (defined as a ≥ 20% and ≥ 30% improvement from baseline on the PANSS total score) and NNHs versus placebo for tolerability (as defined by rate of discontinuation because of an AE, rate of weight gain ≥ 7% from baseline, somnolence/sedation AE rate, and akathisia AE rate). Similar analyses were performed for risperidone 4 mg/d from the trials in which it was included as an active control. LHH for response versus somnolence/sedation was ~ 1 for both lumateperone and risperidone; thus, an encounter of a therapeutic response or an AE of somnolence/sedation is equally likely for these 2 drugs. For the other LHH calculations (response vs discontinuation because of an AE, response vs weight gain, and response vs akathisia), the LHH values for lumateperone 42 mg/d ranged from 13.6 to 48.6, meaning that lumateperone 42 mg/d is 13.6 to 48.6 times more likely to result in a therapeutic response (note: akathisia was excluded from the LHH calculation for lumateperone because the rate of akathisia was higher in the placebo group). In contrast, for risperidone, the analogous LHH values appear to be about 1 order of magnitude smaller (less desirable), ranging from 2.3 to 9.3 (excluding the LHH for somnolence/sedation), meaning that risperidone 4 mg/d is about 2.3 to 9.3 times more likely to result in a therapeutic response than a discontinuation because of an AE, weight gain ≥ 7% from baseline, or an AE of akathisia.
Because a negative value for NNH denotes an event that occurs more frequently with placebo than with the antipsychotic, LHH cannot be meaningfully calculated under these circumstances. This was observed for lumateperone for akathisia, for which the NNH versus placebo was −107 (NS). In the case of zero or negative absolute risk difference for a tolerability assessment, some authors have imputed the absolute risk difference as 0.001 (resulting in a NNH estimate of 1,000).47,48 A NNH of 1,000 for akathisia would give a LHH for response versus akathisia of ~ 100 for lumateperone 42 mg/d.
DISCUSSION
The efficacy profile of lumateperone 42 mg/d is similar to those of most other first-line, oral, second-generation antipsychotics; however, it is unique in not being associated with clinically relevant effects on body weight, metabolic variables, motoric function, the ECG QT interval, or prolactin levels,1 with a LHH much greater than 1 comparing therapeutic response to these tolerability/safety outcomes. The most prominent AE of lumateperone is somnolence/sedation. In the clinical trials examined here, AE rates for somnolence/sedation were similar to that observed with the active control, risperidone 4 mg/d. Clinically, additional considerations include the time to onset of the AE versus time to onset of a therapeutic response, as well as the severity and duration of the AE. The AE in question may be easily manageable if it is non-serious and short-lived. In the placebo-controlled clinical trials included in this analysis, lumateperone was administered in the morning, which increased patients’ likelihood of experiencing an AE of somnolence/sedation. Nonetheless, the majority of somnolence/sedation events were of mild intensity for all treatment groups, and none were severe.49 In an open-label antipsychotic switch study in adult outpatients with stable schizophrenia who were switched from previous antipsychotics to lumateperone 42 mg/d for 6 weeks,10 medication was administered in the evening, and the rate of somnolence was lower (6.6%).
The study has several limitations, including that the data analyzed were limited to dichotomous outcomes. The metrics of NNT and NNH are not appropriate for continuous outcomes, such as mean change in PANSS total score, and require dichotomization for NNT to be directly calculated. In addition, the results may not be generalizable to patients outside the confines of a clinical trial; this is always a concern for results of registration trials because of the strict inclusion/exclusion criteria that these studies require. Reasons for clinical trial discontinuation can be complex; thus, NNH for discontinuation due to AEs in a study may not always generalize to overall tolerability in clinical practice. The brief durations of the available controlled studies of lumateperone limit the sensitivity of calculating NNH for delayed adverse outcomes, and the relatively small sample sizes of the studies limit sensitivity of calculating NNH for uncommon adverse outcomes and subpopulation effects. Attention to the underlying percentages used to calculate NNT or NNH is a relevant consideration because a NNT of 10 may result from a difference in responder rates of 20% versus 10% or 90% versus 80%, which represent very different clinical scenarios.
Indirect comparisons of NNT and NNH with other antipsychotics as calculated from other studies of these agents versus placebo must be approached with caution because of heterogeneity in study design, including age of participants, dosing, and duration. For example, there may be differences in the time when study medication was administered and in the timing of when the assessments were made. Heterogeneity is exemplified by the large variation in discontinuation rates because of AEs for patients randomized to placebo, ranging from as low as 0.7% for the lumateperone clinical trials to as high as 14.7% for the brexpiprazole clinical trials (Supplementary Table 4). Product labels routinely have disclaimers stating that “because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.”1 Although NNH calculations involve taking into account any underlying placebo AE rate, studies differ in terms of settings (inpatient, outpatient) and expectations on the part of both investigators and patients regarding AEs in general, their specific type, and their anticipated severity.
An additional limitation is that most of the pivotal trials used in the calculation of NNT and NNH for the other agents were 6 weeks in duration versus 4 weeks for the positive lumateperone trials. Placebo response rates have historically been a source of controversy, and rates varied from a low of 11.3% for the lumateperone clinical trials to as high as 31.9% for the clinical trials of lurasidone.50 A further caveat is that baseline psychopathology was generally less severe in the clinical trials of lumateperone, as observed by mean baseline PANSS total score (mean range, 84.6–90.4, moderately-to-markedly ill)8,51 compared with clinical trial populations for other agents (markedly ill) including brexpiprazole (94.6–96.3),40,52 lurasidone (89.6–97.9),53 cariprazine (94.1–98.1),54–57 aripiprazole (92.6–100.2),58,59 asenapine (92.2–96.5),39 paliperidone (91.6–94.6),60–62 and iloperidone (90.3–96.0).42
Enumeration of responder rates for other antipsychotics was limited to when data for a ≥ 30% reduction from baseline on the PANSS were available, including when it was part of the definition of response together with a Clinical Global Impressions–Improvement scale score of very much improved or much improved (a measure not used in the lumateperone placebo-controlled clinical trials described here). For other agents, it was not always noted if responder rate calculations were made by subtracting 30 from the measured PANSS total scores. Eliminated from further consideration for indirect efficacy comparisons were the pivotal trials of risperidone, olanzapine, quetiapine immediate release, and iloperidone, for which categorical response data either were not available, used different thresholds (eg, ≥ 20% improvement), or used different rating scales (eg, Brief Psychiatric Rating Scale or only the positive subscale of the PANSS). Lastly, only informative (positive) trials were included in the indirect efficacy comparisons; adding trials that did not demonstrate superiority to placebo on the primary outcome measure would weaken the effect size estimates (as has been demonstrated here in Supplementary Table 1d and seen with lurasidone in a similar NNT analysis19 and with asenapine as described in a meta-analysis conducted by its manufacturer63).
CONCLUSION
Lumateperone 42 mg/d is efficacious in treating schizophrenia and is not associated with clinically relevant effects on body weight, metabolic variables, motoric function, the ECG QT interval, or prolactin levels, with a LHH much greater than 1 comparing therapeutic response to these tolerability/safety outcomes. However, lumateperone appears to be associated with somnolence/sedation, and LHH approaches 1 when contrasting therapeutic response versus a somnolence/sedation AE. In indirect comparisons with other antipsychotics, this somnolence/sedation AE profile is not entirely dissimilar to that of many other antipsychotic medications; however, the avoidance of weight gain, glucose/lipid abnormalities, and akathisia and the absence of prolactin elevations render lumateperone unique.
Submitted: September 6, 2022; accepted December 16, 2022.
Published online: March 6, 2023.
Relevant financial relationships: Dr Citrome serves as consultant to AbbVie/Allergan, Acadia, Adamas, Alkermes, Angelini, Astellas, Avanir, Axsome, BioXcel, Boehringer Ingelheim, Cadent Therapeutics, Cerevel, Clinilabs, COMPASS, Eisai, Enteris BioPharma, HLS Therapeutics, Idorsia, INmune Bio, Impel, Intra-Cellular Therapies, Janssen, Karuna, Lundbeck, Lyndra, Medavante-ProPhase, Marvin, Merck, Neurocrine, Neurelis, Novartis, Noven, Otsuka, Ovid, Praxis, Recordati, Relmada, Reviva, Sage, Sunovion, Supernus, Teva, and University of Arizona and has done one-off ad hoc consulting for individuals/entities conducting marketing, commercial, or scientific scoping research; has been a speaker for AbbVie/Allergan, Acadia, Alkermes, Angelini, Axsome, BioXcel, Eisai, Idorsia, Intra-Cellular Therapies, Janssen, Lundbeck, Neurocrine, Noven, Otsuka, Recordati, Sage, Sunovion, Takeda, Teva, and Continuing Medical Education (CME) activities organized by medical education companies such as Medscape, North American Center for Continuing Medical Education (NACCME), Neuroscience Education Institute (NEI), Vindico, and Universities and Professional Organizations/Societies; owns stocks (small number of shares of common stock) in Bristol-Myers Squibb, Eli Lilly, Johnson & Johnson, Merck, Pfizer purchased > 10 years ago; has stock options for Reviva; and receives royalties/publishing income from Taylor & Francis (Editor-in-Chief, Current Medical Research and Opinion, 2022 to date), Wiley (Editor-in-Chief, International Journal of Clinical Practice, through end 2019), UpToDate (reviewer), Springer Healthcare (book), and Elsevier (Topic Editor, Psychiatry, Clinical Therapeutics). Drs Durgam, Edwards, and Davis are employees of Intra-Cellular Therapies, Inc.
Funding/support: This study was funded by Intra-Cellular Therapies, Inc., New York, NY.
Role of the sponsor: Although personnel at Intra-Cellular Therapies, Inc., reviewed the manuscript, final approval for submission of the manuscript was the sole decision of the authors.
Previous presentations: Data partially presented at the Psych Congress; New Orleans, Louisiana; September 17–20, 2022 • the Neuroscience Education Institute Congress; Colorado Springs, Colorado; November 3–6, 2022.
Acknowledgments: Kendall Foote, PhD, and Adam Ruth, PhD, of Medical Expressions (Chicago, Illinois) provided editorial support for the manuscript, which was funded by Intra-Cellular Therapies, Inc.
Supplementary material: Available at Psychiatrist.com.
CLINICAL POINTS
- Many pharmacologic options exist for the treatment of schizophrenia.
- Using number needed to treat and number needed to harm can help place new antipsychotics, such as lumateperone, into clinical perspective.
References (63)

- CAPLYTA. (lumateperone) capsules, for oral use. Product label. Intra-Cellular Therapies, Inc website. https://www.intracellulartherapies.com/docs/caplyta_pi.pdf. Revised December 2019. Accessed July 4, 2021.
- Zhang L, Hendrick JP. The presynaptic D2 partial agonist lumateperone acts as a postsynaptic D2 antagonist. Matters (Zur). 2018;4(3):e201712000006. CrossRef
- Snyder GL, Vanover KE, Zhu H, et al. Functional profile of a novel modulator of serotonin, dopamine, and glutamate neurotransmission. Psychopharmacology (Berl). 2015;232(3):605–621. PubMed CrossRef
- Vanover KE, Davis RE, Zhou Y, et al. Dopamine D2 receptor occupancy of lumateperone (ITI-007): a Positron Emission Tomography Study in patients with schizophrenia. Neuropsychopharmacology. 2019;44(3):598–605. PubMed CrossRef
- Snyder GL, Vanover KE, Davis RE, et al. A review of the pharmacology and clinical profile of lumateperone for the treatment of schizophrenia. Adv Pharmacol. 2021;90:253–276. PubMed CrossRef
- Lieberman JA, Davis RE, Correll CU, et al. ITI-007 for the treatment of schizophrenia: a 4-week randomized, double-blind, controlled trial. Biol Psychiatry. 2016;79(12):952–961. PubMed CrossRef
- Correll CU, Davis RE, Weingart M, et al. Efficacy and safety of lumateperone for treatment of schizophrenia: a randomized clinical trial. JAMA Psychiatry. 2020;77(4):349–358. PubMed CrossRef
- US Food and Drug Administration. Drug Approval Package. Caplyta (lumateperone), Company: Intra-Cellular Therapies, Inc. Application No.: 209500. Approval Date: 12/20/2019. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2019/209500Orig1s000TOC.cfm. Created January 21, 2020. Accessed January 23. 2021.
- Kane JM, Durgam S, Satlin A, et al. Safety and tolerability of lumateperone for the treatment of schizophrenia: a pooled analysis of late-phase placebo- and active-controlled clinical trials. Int Clin Psychopharmacol. 2021;36(5):244–250. PubMed CrossRef
- Correll CU, Vanover KE, Davis RE, et al. Safety and tolerability of lumateperone 42 mg: an open-label antipsychotic switch study in outpatients with stable schizophrenia. Schizophr Res. 2021;228:198–205. PubMed CrossRef
- Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13(2):261–276. PubMed CrossRef
- Citrome L. Compelling or irrelevant? using number needed to treat can help decide. Acta Psychiatr Scand. 2008;117(6):412–419. PubMed CrossRef
- Citrome L. Relative vs absolute measures of benefit and risk: what’s the difference? Acta Psychiatr Scand. 2010;121(2):94–102. PubMed CrossRef
- Citrome L. Quantifying risk: the role of absolute and relative measures in interpreting risk of adverse reactions from product labels of antipsychotic medications. Curr Drug Saf. 2009;4(3):229–237. PubMed CrossRef
- Citrome L, Ketter TA. When does a difference make a difference? Interpretation of number needed to treat, number needed to harm, and likelihood to be helped or harmed. Int J Clin Pract. 2013;67(5):407–411. PubMed CrossRef
- Citrome L, Kantrowitz J. Antipsychotics for the treatment of schizophrenia: likelihood to be helped or harmed, understanding proximal and distal benefits and risks. Expert Rev Neurother. 2008;8(7):1079–1091. PubMed CrossRef
- Citrome L. Cariprazine for the treatment of schizophrenia: a review of this dopamine D3-preferring D3/D2 receptor partial agonist. Clin Schizophr Relat Psychoses. 2016;10(2):109–119. PubMed CrossRef
- Citrome L. Brexpiprazole for schizophrenia and as adjunct for major depressive disorder: a systematic review of the efficacy and safety profile for this newly approved antipsychotic - what is the number needed to treat, number needed to harm and likelihood to be helped or harmed? Int J Clin Pract. 2015;69(9):978–997. PubMed CrossRef
- Citrome L. Lurasidone for the acute treatment of adults with schizophrenia: what is the number needed to treat, number needed to harm, and likelihood to be helped or harmed? Clin Schizophr Relat Psychoses. 2012;6(2):76–85. PubMed CrossRef
- Citrome L. Iloperidone for schizophrenia: a review of the efficacy and safety profile for this newly commercialised second-generation antipsychotic. Int J Clin Pract. 2009;63(8):1237–1248. PubMed CrossRef
- Citrome L. Asenapine for schizophrenia and bipolar disorder: a review of the efficacy and safety profile for this newly approved sublingually absorbed second-generation antipsychotic. Int J Clin Pract. 2009;63(12):1762–1784. PubMed CrossRef
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. Fourth Edition, Text Revision. American Psychiatric Association; 2000.
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. Fifth Edition. American Psychiatric Association; 2013.
- First MB, Williams JBW, Spitzer RL, et al. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Clinical Trials Version (SCID-CT). New York: Biometrics Research, New York State Psychiatric Institute; 2007.
- First MB, Williams JB, Karg RS, et al. Structured Clinical Interview for DSM-5 Disorders - Clinical Trials Version (SCID-5-CT, Modified for ITI-007-301). Arlington, VA: American Psychiatric Association; 2014.
- Obermeier M, Mayr A, Schennach-Wolff R, et al. Should the PANSS be rescaled? Schizophr Bull. 2010;36(3):455–460. PubMed CrossRef
- Leucht S, Kissling W, Davis JM. The PANSS should be rescaled. Schizophr Bull. 2010;36(3):461–462. PubMed CrossRef
- Citrome L, Juday T, Frech F, et al. Lemborexant for the treatment of insomnia: direct and indirect comparisons with other hypnotics using number needed to treat, number needed to harm, and likelihood to be helped or harmed. J Clin Psychiatry. 2021;82(4):20m13795. PubMed
- Citrome L. Role of sublingual asenapine in treatment of schizophrenia. Neuropsychiatr Dis Treat. 2011;7:325–339. PubMed CrossRef
- Kantrowitz J, Citrome L. Paliperidone: the evidence of its therapeutic value in schizophrenia. Core Evid. 2008;2(4):261–271. PubMed CrossRef
- Kahn RS, Schulz SC, Palazov VD, et al; Study 132 Investigators. Efficacy and tolerability of once-daily extended release quetiapine fumarate in acute schizophrenia: a randomized, double-blind, placebo-controlled study. J Clin Psychiatry. 2007;68(6):832–842. PubMed CrossRef
- Daniel DG, Zimbroff DL, Potkin SG, et al; Ziprasidone Study Group. Ziprasidone 80 mg/day and 160 mg/day in the acute exacerbation of schizophrenia and schizoaffective disorder: a 6-week placebo-controlled trial. Neuropsychopharmacology. 1999;20(5):491–505. PubMed CrossRef
- Citrome L. The ABC’s of dopamine receptor partial agonists—aripiprazole, brexpiprazole and cariprazine: the 15-min challenge to sort these agents out. Int J Clin Pract. 2015;69(11):1211–1220. PubMed CrossRef
- Citrome L. Activating and sedating adverse effects of second-generation antipsychotics in the treatment of schizophrenia and major depressive disorder: absolute risk increase and number needed to harm. J Clin Psychopharmacol. 2017;37(2):138–147. PubMed CrossRef
- LATUDA. (lurasidone hydrochloride) tablets, for oral use. Product label. Sunovion Pharmaceuticals, Inc. Latuda website. https://www.latuda.com/LatudaPrescribingInformation.pdf. Revised December 2019. Accessed January 23, 2021.
- RISPERDAL. (risperidone) tablets, for oral use; RISPERDAL (risperidone) oral solution; RISPERDAL M-TAB (risperidone) orally disintegrating tablets. Product label. Janssen Pharmaceutical Companies website. https://www.janssenlabels.com/package-insert/product-monograph/prescribing-information/RISPERDAL-pi.pdf. Revised January 2020. Accessed January 23, 2021.
- GEODON. (ziprasidone) capsules, for oral use; GEODON (ziprasidone mesylate) for injection, for intramuscular use. Product label. Pfizer, Inc website. https://labeling.pfizer.com/showlabeling.aspx?id=584. Revised October 2020. Accessed January 23, 2021.
- US Food and Drug Administration. Drug Approval Package. Abilify (aripiprazole) tablets, Company: Otsuka Pharmaceutical Co., Ltd. Application No.: 21-436. Approval Date November 15, 2002. Created March 7, 2003. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2002/21-436_Abilify.cfm. Accessed January 23, 2021.
- US Food and Drug Administration. Drug Approval Package. Saphris (asenapine) tablet, Company: Organon USA Inc. Application No.: 022117. Approval Date August 13, 2009. Created October 2, 2009. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2009/022117s000TOC.cfm. Accessed January 23, 2021.
- US Food and Drug Administration. Drug Approval Package. Rexulti (brexpiprazole) tablets, Company: Otsuka Pharmaceutical Company Ltd. Application No.: 205422Orig1s000 and 205422Orig2s000. Approval Date July 10, 2015. Created August 19, 2015. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2015/205422s000TOC.cfm. Accessed January 23, 2021.
- US Food and Drug Administration. Drug Approval Package. Vraylar (cariprazine), Company: Forest Laboratories, LLC. Application No.: 204370 Orig1 and Orig2. Approval Date September 17, 2015. Created September 17, 2015. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2015/204370Orig2s000TOC.cfm. Accessed January 23, 2021.
- US Food and Drug Administration. Drug Approval Package. Fanapt (iloperidone) tablets, Company: Vanda Pharmaceuticals. Application No.: 022192. Approval Date May 6, 2009. Created June 12, 2009. Accessed January 23, 2021. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2009/022192s000TOC.cfm.
- US Food and Drug Administration. Drug Approval Package. Zyprexa (olanzapine), Company: Lilly. NDA 20-592. FDA website. https://www.accessdata.fda.gov/drugsatfda_docs/nda/96/020592_Original_Approval_Pkg%20.pdf. Accessed January 23, 2021.
- US Food and Drug Administration. Drug Approval Package. Invega (paliperidone) Extended-Release Tablets, Company: Janssen. Application No.: 021999. Approval Date December 19, 2006. Created 03/30/2007. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2006/021999s000_TOC.cfm. Accessed January 23, 2021.
- US Food and Drug Administration. Drug Approval Package. Seroquel XR Extended-Release Tablets, Company: AstraZeneca Pharmaceuticals LP. Application No: 022047s000. Approval Date May 17, 2007. Created September 27, 2011. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2007/022047s000TOC.cfm. Accessed January 23, 2021.
- US Food and Drug Administration. Drug Approval Package. Seroquel/Quetiapine Fumarate, Company: Zeneca Pharmaceuticals. Application # 20639. Approval Date September 26, 1997. Created July 17, 2007. https://www.accessdata.fda.gov/drugsatfda_docs/nda/97/20639_seroquel_toc.cfm. Accessed January 23, 2021.
- Vo P, Wen S, Martel M-J, et al. Benefit-risk assessment of erenumab and current migraine prophylactic treatments using the likelihood of being helped or harmed. Cephalalgia. 2019;39(5):608–616. PubMed CrossRef
- Citrome L, Sánchez Del Rio M, Dong Y, et al. Benefit-risk assessment of galcanezumab versus placebo for the treatment of episodic and chronic migraine using the metrics of number needed to treat and number needed to harm. Adv Ther. 2021;38(8):4442–4460. PubMed CrossRef
- Rowe W, Durgam S, Davis RE, et al. Somnolence and sedation with lumateperone treatment: a comparison of morning and evening administration. Presented at the Psych Congress 2020 Virtual Experience. September 10–13, 2020.
- Gopalakrishnan M, Zhu H, Farchione TR, et al. The trend of increasing placebo response and decreasing treatment effect in schizophrenia trials continues: an update from the US Food and Drug Administration. J Clin Psychiatry. 2020;81(2):19r12960. PubMed CrossRef
- Leucht S, Kane JM, Kissling W, et al. What does the PANSS mean? Schizophr Res. 2005;79(2–3):231–238. PubMed CrossRef
- REXULTI. (brexpiprazole) tablets, for oral use. Product label. Otsuka Pharmaceutical Company Ltd. FDA website. https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/205422s003lbl.pdf. Revised December 2021. Accessed January 31, 2022.
- US Food and Drug Administration. Drug Approval Package. Latuda (lurasidone hydrochloride), Company: Sunovion Pharmaceuticals, Inc. Application No.: 200603. Approval Date October 28, 2010. Created December 3, 2010. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2010/200603Orig1s000TOC.cfm. Accessed January 31, 2022.
- Durgam S, Litman RE, Papadakis K, et al. Cariprazine in the treatment of schizophrenia: a proof-of-concept trial. Int Clin Psychopharmacol. 2016;31(2):61–68. PubMed CrossRef
- Durgam S, Cutler AJ, Lu K, et al. Cariprazine in acute exacerbation of schizophrenia: a fixed-dose, phase 3, randomized, double-blind, placebo- and active-controlled trial. J Clin Psychiatry. 2015;76(12):e1574–e1582. PubMed CrossRef
- Kane JM, Zukin S, Wang Y, et al. Efficacy and safety of cariprazine in acute exacerbation of schizophrenia: results from an international, phase III clinical trial. J Clin Psychopharmacol. 2015;35(4):367–373. PubMed CrossRef
- Durgam S, Starace A, Li D, et al. An evaluation of the safety and efficacy of cariprazine in patients with acute exacerbation of schizophrenia: a phase II, randomized clinical trial. Schizophr Res. 2014;152(2–3):450–457. PubMed CrossRef
- Kane JM, Carson WH, Saha AR, et al. Efficacy and safety of aripiprazole and haloperidol versus placebo in patients with schizophrenia and schizoaffective disorder. J Clin Psychiatry. 2002;63(9):763–771. PubMed CrossRef
- Potkin SG, Saha AR, Kujawa MJ, et al. Aripiprazole, an antipsychotic with a novel mechanism of action, and risperidone vs placebo in patients with schizophrenia and schizoaffective disorder. Arch Gen Psychiatry. 2003;60(7):681–690. PubMed CrossRef
- Kane J, Canas F, Kramer M, et al. Treatment of schizophrenia with paliperidone extended-release tablets: a 6-week placebo-controlled trial. Schizophr Res. 2007;90(1–3):147–161. PubMed CrossRef
- Marder SR, Kramer M, Ford L, et al. Efficacy and safety of paliperidone extended-release tablets: results of a 6-week, randomized, placebo-controlled study. Biol Psychiatry. 2007;62(12):1363–1370. PubMed CrossRef
- Davidson M, Emsley R, Kramer M, et al. Efficacy, safety and early response of paliperidone extended-release tablets (paliperidone ER): results of a 6-week, randomized, placebo-controlled study. Schizophr Res. 2007;93(1–3):117–130. PubMed CrossRef
- Szegedi A, Verweij P, van Duijnhoven W, et al. Meta-analyses of the efficacy of asenapine for acute schizophrenia: comparisons with placebo and other antipsychotics. J Clin Psychiatry. 2012;73(12):1533–1540. PubMed CrossRef
Save
Cite
Advertisement
GAM ID: sidebar-top